Detection of SO2F2 Using a Photoacoustic Two-Chamber Approach
Abstract
:1. Introduction
2. Materials and Methods
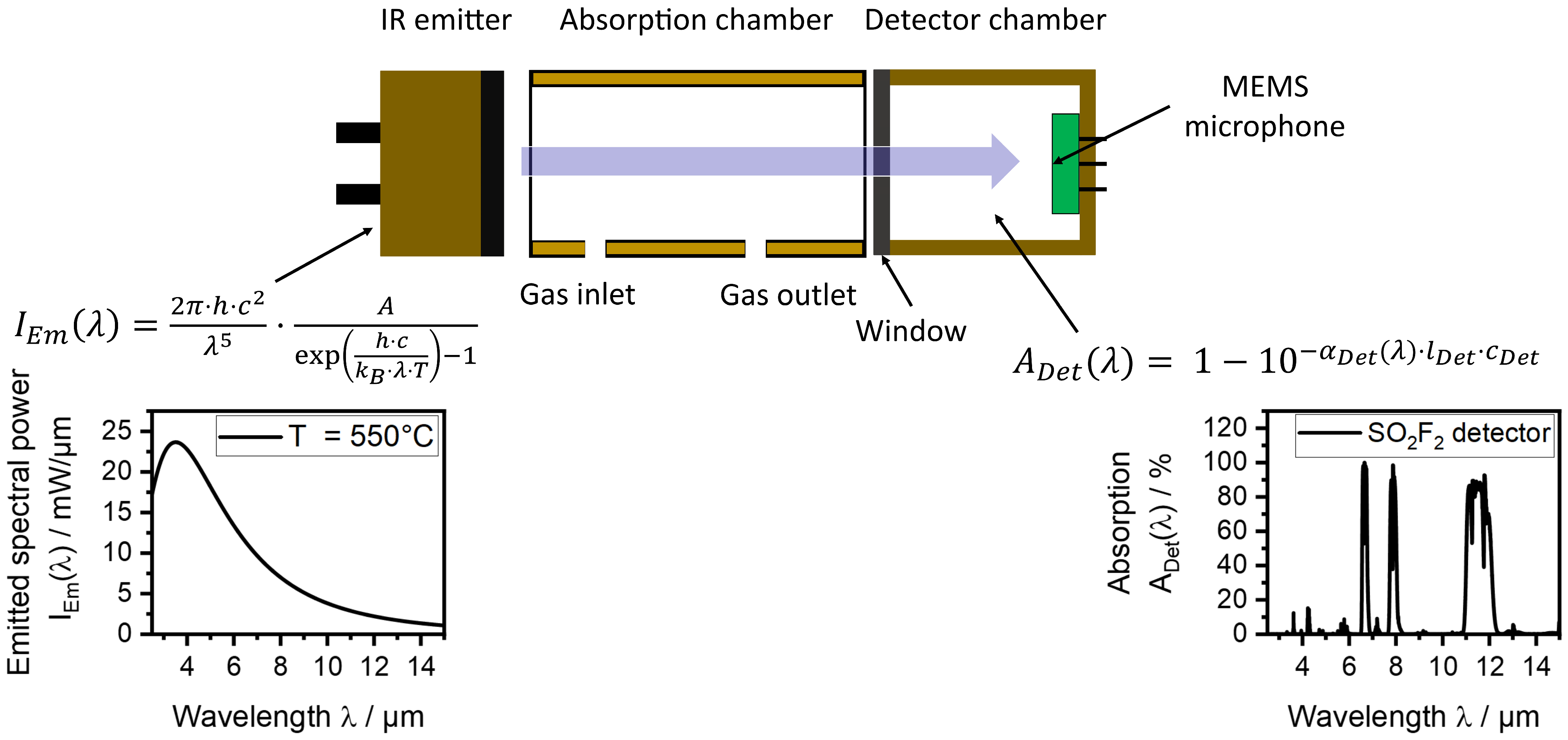
2.1. Photoacoustic Sensors Setups
2.2. SO2F2 Gas Measurements
3. Results
3.1. Sensor Setup for the Measuring Range 0–1 vol.-% SO2F2
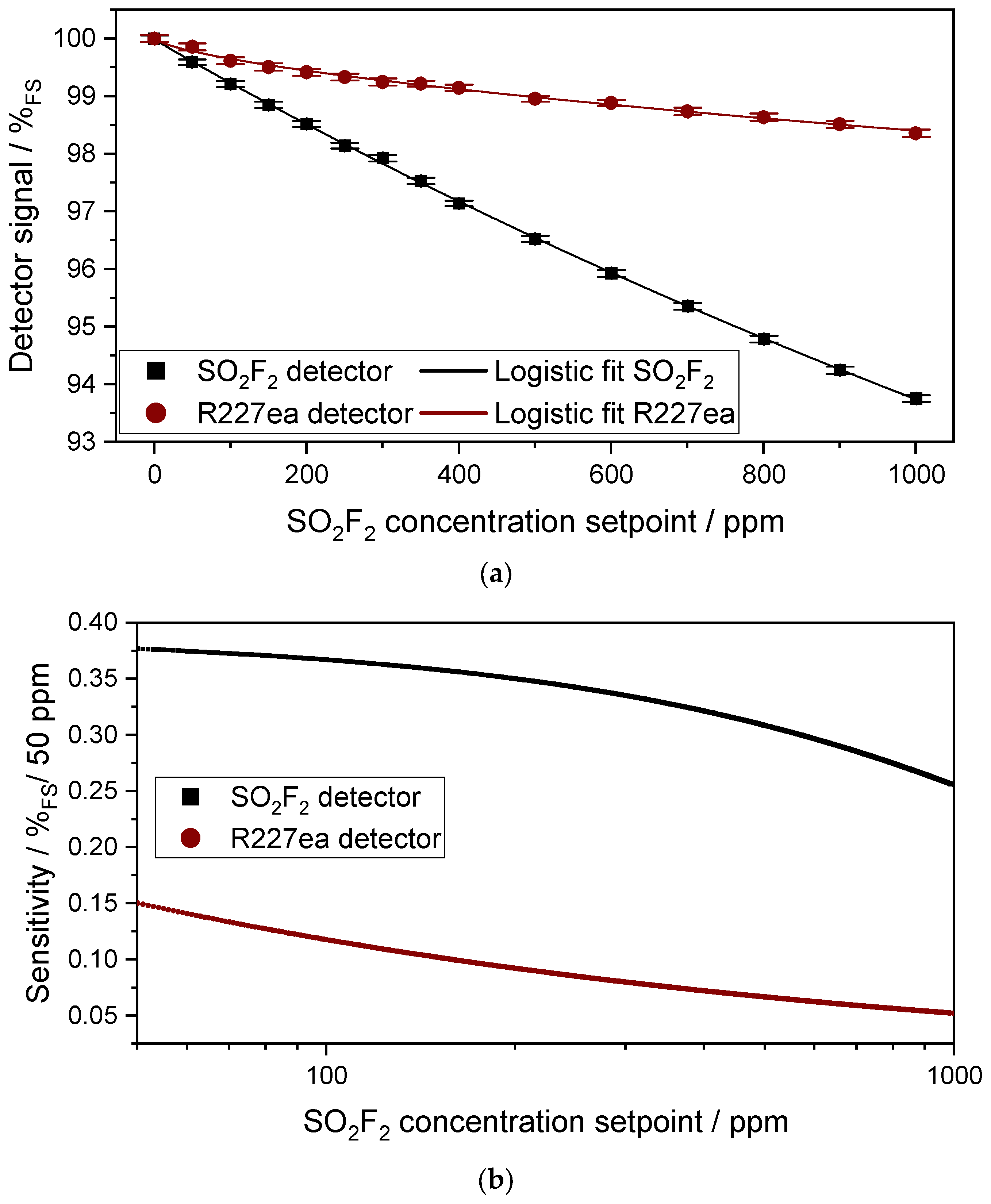
3.1.1. Characterization of the Sensitivity of the Sensors to SO2F2
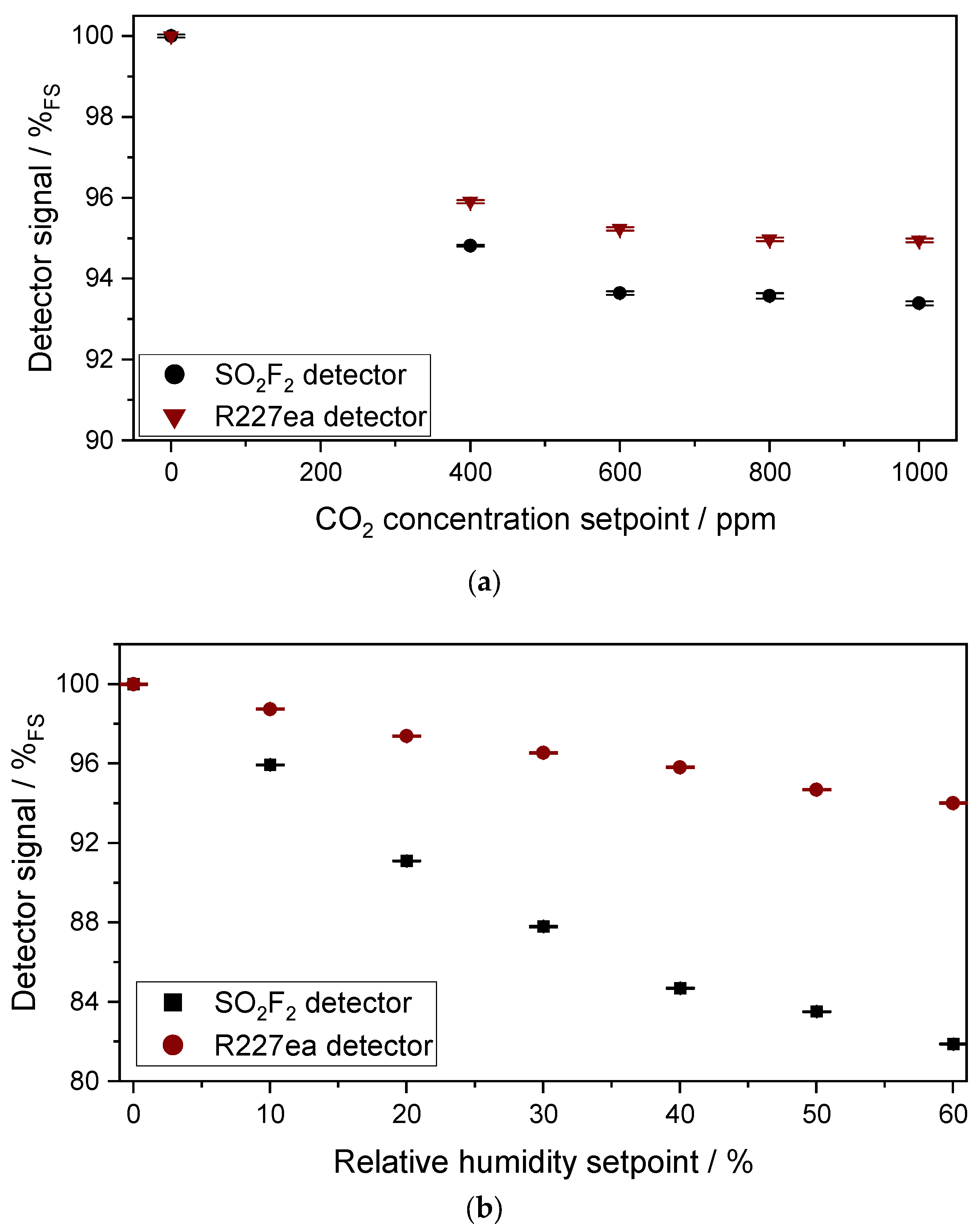
3.1.2. Characterization of the Cross Sensitivity of the Sensors to CO2 and H2O
3.2. Sensor for the Measuring Range 0–100 ppm SO2F2
3.2.1. Characterization of the Sensitivity of the Sensors to SO2F2
3.2.2. Characterization of the Cross-Sensitivity of the Sensors to CO2 and H2O
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, D.; Deng, L.; Yao, B.; Vollmer, M.K.; Chen, L.; Li, Y.; Xu, H.; O’Doherty, S.; Song, Q.; Ning, S. Atmospheric mixing ratios and emissions of sulfuryl fluoride (SO2F2) in China. Atmos. Res. 2022, 275, 106222. [Google Scholar] [CrossRef]
- Gressent, A.; Rigby, M.; Ganesan, A.L.; Prinn, R.G.; Manning, A.J.; Mühle, J.; Salameh, P.K.; Krummel, P.B.; Fraser, P.J.; Steele, L.P.; et al. Growing atmospheric emissions of sulfuryl fluoride. J. Geophys. Res. Atmos. 2021, 126, e2020JD034327. [Google Scholar] [CrossRef]
- NIOSH Pocket Guide to Chemical Hazards; National Institute for Occupational Safety and Health (NIOSH): Washington, DC, USA, 2019.
- Papadimitriou, V.C.; Portmann, R.W.; Fahey, D.W.; Mühle, J.; Weiss, R.F.; Burkholder, J.B. Experimental and theoretical study of the atmospheric chemistry and global warming potential of SO2F2. J. Phys. Chem. A 2008, 112, 12657–12666. [Google Scholar] [CrossRef] [PubMed]
- Sulbaek Andersen, M.P.; Blake, D.R.; Rowland, F.S.; Hurley, M.D.; Wallington, T.J. Atmospheric chemistry of sulfuryl fluoride: Reaction with OH radicals, Cl atoms and O3, atmospheric lifetime, IR spectrum, and global warming potential. Environ. Sci. Technol. 2009, 43, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Deng, J.; Xie, Z.; Pan, Z.; Zhang, J.; Zhou, H. Adsorption and gas sensing properties of the Pt3-MoSe2 monolayer to SOF2 and SO2F2. ACS Omega 2020, 5, 7722–7728. [Google Scholar] [CrossRef] [PubMed]
- Sang, T.Y.; Li, T.; Yang, Y.; Song, Y.; Tian, H.; Song, R.; Wang, C.; Hu, X.; Yang, Z.; Lu, Y.; et al. Pd, Rh-decorated Se-vacancy MoSe2 monolayer: A promising candidate for sensing and detecting SO2F2, SOF2, H2S and SO2. Surf. Interfaces 2022, 33, 102269. [Google Scholar] [CrossRef]
- Ali, N.H.; Al-Badry, L.F. Improvement of sensing performance of SO2, SOF2, and SO2F2 gases on monolayer and bilayer HfSe2 under electric field. Micro Nanostruct. 2023, 182, 207654. [Google Scholar] [CrossRef]
- Zeng, X.; Jiang, L.; Pang, S.; Hao, J.; Gui, Y. Adsorption properties of CuO/ZnO modified HfSe2 monolayer upon SF6 decomposition gases (H2S, SO2, SOF2 and SO2F2). Colloids Surf. A Physicochem. Eng. Asp. 2023, 679, 132562. [Google Scholar] [CrossRef]
- Din, S.U.; ul Haq, M.; Baohui, D.; Zhu, L. Design and synthesis of α-Bi2Mo3O12/CoSO4 composite nanofibers for high-performance SO2F2 sensors at room temperature. Mater. Today Nano 2022, 17, 100154. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Q.; Zhang, Q.; Hong, C.; Xu, L.; Jin, L.; Chen, W. Synthesis, characterization and enhanced sensing properties of a NiO/ZnO p–n junctions sensor for the SF6 decomposition byproducts SO2, SO2F2, and SOF2. Sensors 2017, 17, 913. [Google Scholar] [CrossRef]
- Li, L.; Din, S.U.; ul Haq, M.; Tang, N.; Zhang, M.; Rahman, N.; Zhu, L. Room temperature monitoring of SF6 decomposition byproduct SO2F2 based on TiO2/NiSO4 composite nanofibers. Nanotechnology 2021, 32, 305705. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gui, Y.; He, S.; Yang, J. Hybrid nanogenerator driven self-powered SO2F2 sensing system based on TiO2/Ni/C composites at room temperature. Sens. Actuators B Chem. 2023, 377, 133053. [Google Scholar] [CrossRef]
- Liu, Z.; Gui, Y.; Xu, L.; Chen, X. Adsorption and sensing performances of transition metal (Ag, Pd, Pt, Rh, and Ru) modified WSe2 monolayer upon SF6 decomposition gases (SOF2 and SO2F2). Appl. Surf. Sci. 2022, 581, 152365. [Google Scholar] [CrossRef]
- Gui, Y.; Wang, Y.; Duan, S.; Tang, C.; Zhou, Q.; Xu, L.; Zhang, X. Ab initio study of SOF2 and SO2F2 adsorption on Co-MoS2. ACS Omega 2019, 4, 2517–2522. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.V.; Choi, I.Y.; Son, Y.S.; Kim, J.C. A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction. Sens. Actuators B 2016, 231, 529–538. [Google Scholar] [CrossRef]
- Bogue, R. Detecting gases with light: A review of optical gas sensor technologies. Sens. Rev. 2015, 35, 133–140. [Google Scholar] [CrossRef]
- Naik, R.C.; Shroff, R.D. NDIR based SO2F2 detector for fumigation monitoring. In Proceedings of the 9th International Conference on Controlled Atmosphere and Fumigation in Stored Products, Antalya, Turkey, 15–19 October 2012; pp. 374–379. [Google Scholar]
- Yao, Q.; Yan, X.; He, S.; Qi, R.; Li, X.; Zeng, X.-Z.; Wang, X.; Zhang, S.; Zi, J.; Yue, Y. Detection of SO2F2 concentration of SF6 decomposition product in GIS gas chamber based on ICL-TDLAS. Adv. Sens. Syst. Appl. XI 2021, 11901, 179–188. [Google Scholar]
- Zhang, S.; Qiang, Y. Study on the suitable intermediate infrared spectrum optical detection applied to SO2F2 and SOF2. In Proceedings of the 6th International Workshop on Advanced Algorithms and Control Engineering (IWAACE 2022), Qingdao, China, 8–10 July 2022; Volume 12350, pp. 445–453. [Google Scholar]
- Zhang, X.; Zhu, Z.; Zhang, Y.; Huang, Y.; Zhang, X.; Lv, S.; Yan, J.; Hu, J. The detection of SF6 decomposition components SO2F2, SOF2 using mid-infrared TDLAS at 6.635 µm and 7.462 µm. Vib. Spectrosc. 2022, 123, 103459. [Google Scholar] [CrossRef]
- Bozóki, Z.; Pogany, A.; Szabo, G. Photoacoustic instruments for practical applications: Present, potentials, and future challenges. Appl. Spectrosc. Rev. 2011, 46, 1–37. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Tatam, R.P. Optical gas sensing: A review. Meas. Sci. Technol. 2012, 24, 012004. [Google Scholar] [CrossRef]
- Bian, C.; Dai, F.; Cheng, J.; Chen, X.; Gan, Q.; Zhang, Z.; Tan, T.; Yang, B.; Wang, C.; Cui, G.; et al. Detection of SF6 decomposition components SO2F2 and SOF2 based on mid-infrared laser photoacoustic spectroscopy. In Proceedings of the 22nd International Symposium on High Voltage Engineering (ISH 2021), Xi’an, China, 21–26 November 2021; pp. 1949–1953. [Google Scholar]
- Minini, K.M.S.; Bueno, S.C.E.; da Silva, M.G.; Sthel, M.S.; Vargas, H.; Angster, J.; Miklós, A. Quantum cascade laser-based photoacoustic sulfuryl fluoride sensing. Appl. Phys. B 2017, 123, 61. [Google Scholar] [CrossRef]
- Shiling, Z.; Long, L.; Qiang, Y. Application of Gas Photoacoustic Spectroscopy Optical Detection Technology to SO2F2 Gas Concentration Detection. In Proceedings of the 2022 2nd International Conference on Big Data Engineering and Education (BDEE), Chengdu, China, 5–7 August 2022; pp. 183–188. [Google Scholar]
- Zhang, Y.; Wang, M.; Yu, P.; Liu, Z. Optical gas sensing of sub-ppm SO2F2 and SOF2 from SF6 decomposition based on photoacoustic spectroscopy. IET Optoelectron. 2022, 16, 277–282. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Yu, P.; Liu, Z. Optical Gas-Cell Dynamic Adsorption in a Photoacoustic Spectroscopy-Based SOF2 and SO2F2 Gas Sensor. Sensors 2022, 22, 7949. [Google Scholar] [CrossRef] [PubMed]
- Miklós, A.; Hess, P.; Bozóki, Z. Application of acoustic resonators in photoacoustic trace gas analysis and metrology. Rev. Sci. Instrum. 2001, 72, 1937–1955. [Google Scholar] [CrossRef]
- Yassine, H.; Weber, C.; Brugger, N.; Wöllenstein, J.; Schmitt, K. Towards a Miniaturized Photoacoustic Detector for the Infrared Spectroscopic Analysis of SO2F2 and Refrigerants. Sensors 2022, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Baehr, H.D.; Stephan, K. Wärme-und Stoffübertragung; Springer: Berlin/Heidelberg, Germany, 1994; Volume 7. [Google Scholar]
- JSIR 350-4; HermeSEAL Technology Product Datasheet. Micro-Hybrid-Electronic GmbH: Hermsdorf, Germany, 2020.
- Demtröder, W. Experimentalphysik 2: Elektrizität und Optik; Springer: Berlin/Heidelberg, Germany, 2018; Volume 2. [Google Scholar]
- White, J.U. Long Optical Paths of Large Aperture. J. Opt. Soc. Am. 1942, 32, 285–288. [Google Scholar] [CrossRef]
- Weber, C.; Kapp, J.; Wöllenstein, J.; Schmitt, K. Novel approach for efficient resonance tracking in photoacoustic gas sensor systems based on a light-induced wall signal. Photoacoustics 2023, 31, 100495. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yassine, H.; Weber, C.; Eberhardt, A.; El-Safoury, M.; Wöllenstein, J.; Schmitt, K. Detection of SO2F2 Using a Photoacoustic Two-Chamber Approach. Sensors 2024, 24, 191. https://doi.org/10.3390/s24010191
Yassine H, Weber C, Eberhardt A, El-Safoury M, Wöllenstein J, Schmitt K. Detection of SO2F2 Using a Photoacoustic Two-Chamber Approach. Sensors. 2024; 24(1):191. https://doi.org/10.3390/s24010191
Chicago/Turabian StyleYassine, Hassan, Christian Weber, Andre Eberhardt, Mahmoud El-Safoury, Jürgen Wöllenstein, and Katrin Schmitt. 2024. "Detection of SO2F2 Using a Photoacoustic Two-Chamber Approach" Sensors 24, no. 1: 191. https://doi.org/10.3390/s24010191




