Non-Invasive Blood Pressure Tracking of Spontaneous Hypertension Rats Using an Electronic Nose
Abstract
:1. Introduction
2. Material and Methods
2.1. Hypertensive Animal Model
2.2. Test Methodology
2.2.1. Weight and Blood Pressure Determination
2.2.2. Collection of Fecal Samples
2.2.3. Odor Information Collection
2.3. Statistics
3. Results
3.1. Blood Pressure and Body Weight of SHRs
3.2. Characteristic Response of E-Nose to Fecal Samples of SHRs
3.3. Effect of Blood Pressure on Fecal Odor Information of SHRs
3.4. Qualitative Discrimination of SHRs with Different Blood Pressure Levels
3.5. Quantitative Predictive Model of Blood Pressure
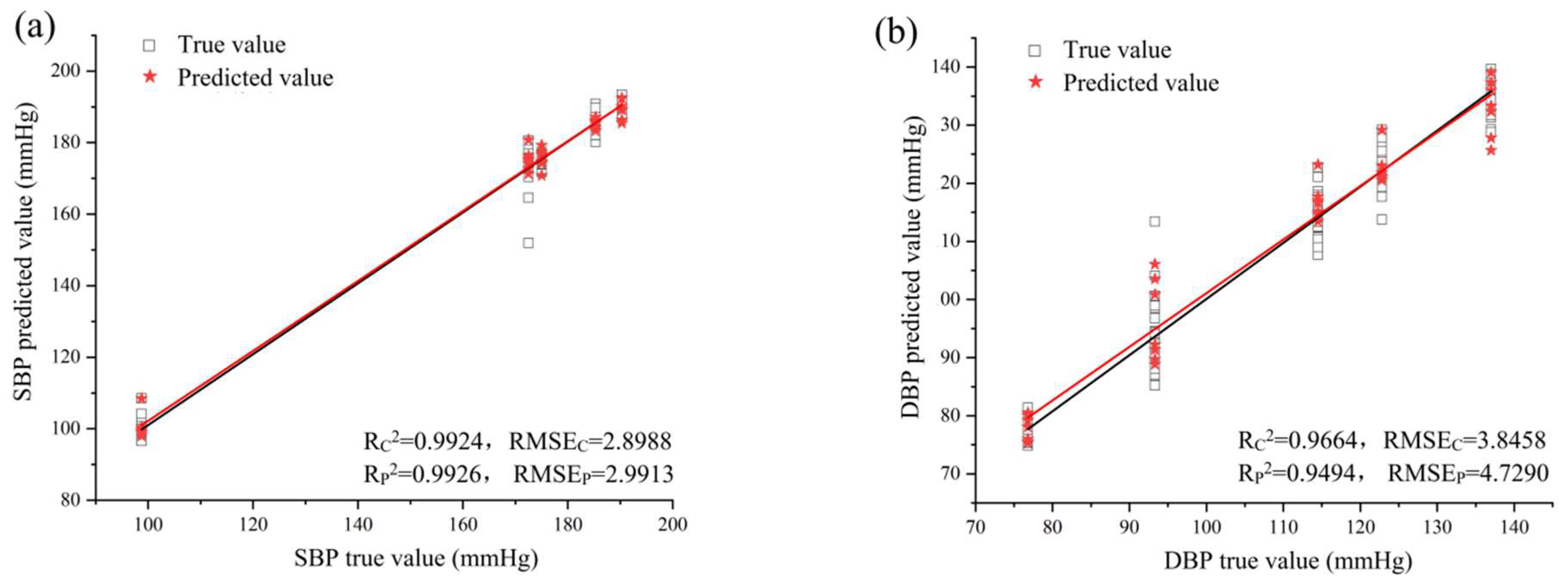
3.5.1. Male SHRs
3.5.2. Female SHRs
3.5.3. No Distinction of Gender
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| E-nose | Electronic nose |
| VOCs | Volatile organic compounds |
| SHR | Spontaneous hypertension rat |
| BP | Blood pressure |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| PCA | Principal component analysis |
| CDA | Canonical discriminant analysis |
| MLR | Multiple linear regression |
| MLP | Multilayer perception neural networks |
| R2 | Coefficients of determination |
| RMSE | Root mean square error |
References
- Huiyu, S.; Qinxue, C.; Changping, L.; Yuanyuan, L.; Zhuang, C. Association between hypertension and cardiovascular disease under the latest changes in blood pressure standards. Mod. Prev. Med. 2022, 49, 3848–3852+3888. [Google Scholar]
- Flynn, J.T.; Urbina, E.M.; Brady, T.M.; Baker-Smith, C.; Daniels, S.R.; Hayman, L.L.; Mitsnefes, M.; Tran, A.; Zachariah, J.P. Ambulatory Blood Pressure Monitoring in Children and Adolescents: 2022 Update: A Scientific Statement From the American Heart Association. Curr. Hypertens. Rep. 2022, 79, e114–e124. [Google Scholar] [CrossRef]
- Nagae, A.; Fujita, M.; Kawarazaki, H.; Matsui, H.; Ando, K.; Fujita, T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation 2009, 119, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Saha, C.; Eckert, G.J.; Ambrosius, W.T.; Chun, T.-Y.; Wagner, M.A.; Zhao, Q.; Pratt, J.H. Improvement in blood pressure with inhibition of the epithelial sodium channel in blacks with hypertension. Hypertension 2005, 46, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, J.; Wei, J.; Jiang, S.; Xu, L.; Qu, L.; Yang, K.; Wang, L.; Buggs, J.; Cheng, F. New mechanism for the sex differences in salt-sensitive hypertension: The role of macula densa NOS1β-mediated tubuloglomerular feedback. Hypertension 2020, 75, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Salman, L.A.; Cohen, J.B. Updates in hypertension: New trials, targets and ways of measuring blood pressure. Curr. Opin. Nephrol. Hypertens. 2022, 31, 258. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.; Liu, H.; Jain, Z.; Settels, J.; Bohringer, C. Intraoperative Invasive Blood Pressure Monitoring and the Potential Pitfalls of Invasively Measured Systolic Blood Pressure. Cureus 2021, 13, e17610. [Google Scholar] [CrossRef]
- Saugel, B.; Dueck, R.; Wagner, J.Y. Measurement of blood pressure. Best Pract. Res. Clin. Anaesthesiol. 2014, 28, 309–322. [Google Scholar] [CrossRef]
- Bin, L.; Jun, Z.; Fang, L. Probe into using Doppler and electronic sphygmomanometer to measure ankle-brachial blood pressure. Chin. Convalescent Med. 2012, 21, 242–243. [Google Scholar]
- Yimin, X.; Tingfeng, H.; Meiqi, C.; Qiaolian, F.; Fenghui, L.; Feng, C. Fluid resuscitation with guidance of pulse pressure variability measured by continuous non-invasive arterial pressure monitor in postoperative hypovolemic patients: A prospective randomized controlled trial. J. Army Med. Univ. 2022, 44, 595–600. [Google Scholar]
- Bause, G.S.; Weintraub, A.C.; Tanner, G.E. Skin avulsion during oscillometry. J. Clin. Monit. Comput. 1986, 2, 262–263. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.R.; Chockalingam, A.; Fodor, J.G.; McKay, D.W. Accurate, reproducible measurement of blood pressure. Can. Med. Assoc. J. 1990, 143, 19. [Google Scholar]
- Lin, C.C.; Jawan, B.; de Villa, M.V.H.; Chen, F.C.; Liu, P.P. Blood pressure cuff compression injury of the radial nerve. J. Clin. Anesth. 2001, 13, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xin, S.; Supeng, Y.; Liang, W.; Yushun, G.; Yongqin, L. Noninvasive blood pressure measurement model based on characteristic parameters of PGG systolic upstroke waveform. Chin. J. Med. 2022, 39, 333–340. [Google Scholar]
- Martínez, G.; Howard, N.; Abbott, D.; Lim, K.; Ward, R.; Elgendi, M. Can photoplethysmography replace arterial blood pressure in the assessment of blood pressure? J. Clin. Med. 2018, 7, 316. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.K.; Leggett, C.L.; Wang, K.K. Diagnosing gastrointestinal illnesses using fecal headspace volatile organic compounds. World J. Gastroenterol. 2016, 22, 1639. [Google Scholar] [CrossRef] [PubMed]
- Lanza, E.; Di Rocco, M.; Schwartz, S.; Caprini, D.; Milanetti, E.; Ferrarese, G.; Teresa Lonardo, M.; Pannone, L.; Ruocco, G.; Martinelli, S.; et al. elegans-based chemosensation strategy for the early detection of cancer metabolites in urine samples. Sci. Rep. 2021, 11, 17133. [Google Scholar] [CrossRef]
- Bosch, S.; Wintjens, D.S.; Wicaksono, A.; Pierik, M.; Covington, J.A.; de Meij, T.G.; de Boer, N.K. Prediction of Inflammatory Bowel Disease Course Based on Fecal Scent. Sensors 2022, 22, 2316. [Google Scholar] [CrossRef]
- Coronel Teixeira, R.; IJdema, D.; Gómez, C.; Arce, D.; Roman, M.; Quintana, Y.; González, F.; Jimenez de Romero, N.; Perez Bejarano, D.; Aguirre, S.; et al. The electronic nose as a rule-out test for tuberculosis in an indigenous population. J. Intern. Med. 2021, 290, 386–391. [Google Scholar] [CrossRef]
- Vishinkin, R.; Busool, R.; Mansour, E.; Fish, F.; Esmail, A.; Kumar, P.; Gharaa, A.; Cancilla, J.C.; Torrecilla, J.S.; Skenders, G.; et al. Profiles of volatile biomarkers detect tuberculosis from skin. Adv. Sci. 2021, 8, 2100235. [Google Scholar] [CrossRef]
- Golden, G.J.; Grady, M.J.; McLean, H.E.; Shriner, S.A.; Hartwig, A.; Bowen, R.A.; Kimball, B.A. Biodetection of a specific odor signature in mallard feces associated with infection by low pathogenic avian influenza A virus. PLoS ONE 2021, 16, e0251841. [Google Scholar] [CrossRef]
- de Jesús Beleño-Sáenz, K.; Cáceres-Tarazona, J.M.; Nol, P.; Jaimes-Mogollón, A.L.; Gualdrón-Guerrero, O.E.; Durán-Acevedo, C.M.; Barasona, J.A.; Vicente, J.; Torres, M.J.; Welearegay, T.G. Non-invasive method to detect infection with Mycobacterium tuberculosis complex in wild boar by measurement of volatile organic compounds obtained from feces with an electronic nose system. Sensors 2021, 21, 584. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.K.; Stahl, R.S.; Nol, P.; Waters, W.R.; Palmer, M.V.; Rhyan, J.C.; VerCauteren, K.C.; McCollum, M.; Salman, M.D. A pilot study exploring the use of breath analysis to differentiate healthy cattle from cattle experimentally infected with Mycobacterium bovis. PLoS ONE 2014, 9, e89280. [Google Scholar] [CrossRef] [PubMed]
- Wlodzimirow, K.A.; Abu-Hanna, A.; Schultz, M.J.; Maas, M.A.W.; Bos, L.D.J.; Sterk, P.J.; Knobel, H.H.; Soers, R.J.T.; Chamuleau, R.A. Exhaled breath analysis with electronic nose technology for detection of acute liver failure in rats. Biosens. Bioelectron. 2014, 53, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Filianoti, A.; Costantini, M.; Bove, A.M.; Anceschi, U.; Brassetti, A.; Ferriero, M.; Mastroianni, R.; Misuraca, L.; Tuderti, G.; Ciliberto, G. Volatilome Analysis in Prostate Cancer by Electronic Nose: A Pilot Monocentric Study. Cancers 2022, 14, 2927. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhu, T.; Tan, Z.; Chen, S.; Fang, Z. Role of Gut Microbiota in Pulmonary Arterial Hypertension. Front. Cell. Infect. Microbiol. 2022, 12, 493. [Google Scholar] [CrossRef]
- Jiayuan, Z.; Tao, Z.; Yong, Y.; Zhoujin, T. Research progress on the relationship between intestinal flora and its metabolites and hypertension. Chin. J. Microecol. 2022, 34, 347–351. [Google Scholar]
- Ge, X.; Zheng, L.; Zhuang, R.; Yu, P.; Xu, Z.; Liu, G.; Xi, X.; Zhou, X.; Fan, H. The gut microbial metabolite trimethylamine N-oxide and hypertension risk: A systematic review and dose–response meta-analysis. Adv. Nutr. 2020, 11, 66–76. [Google Scholar] [CrossRef]
- Yang, F.; Chen, H.; Gao, Y.; An, N.; Li, X.; Pan, X.; Yang, X.; Tian, L.; Sun, J.; Xiong, X. Gut microbiota-derived short-chain fatty acids and hypertension: Mechanism and treatment. Biomed. Pharmacother. 2020, 130, 110503. [Google Scholar] [CrossRef]
- Calderón-Pérez, L.; Gosalbes, M.J.; Yuste, S.; Valls, R.M.; Pedret, A.; Llauradó, E.; Jimenez-Hernandez, N.; Artacho, A.; Pla-Pagà, L.; Companys, J. Gut metagenomic and short chain fatty acids signature in hypertension: A cross-sectional study. Sci. Rep.-UK 2020, 10, 6436. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Han, Q.; Yang, J. Trimethylamine-N-oxide (TMAO) increased aquaporin-2 expression in spontaneously hypertensive rats. Clin. Exp. Hypertens. 2019, 41, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Jin, J.; Su, X.; Yin, X.; Gao, J.; Wang, X.; Zhang, S.; Bu, P.; Wang, M.; Zhang, Y. Intestinal flora modulates blood pressure by regulating the synthesis of intestinal-derived corticosterone in high salt-induced hypertension. Circ. Res. 2020, 126, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.; Peng, Z.; Yuanlin, L.; Xiaoqin, B.; Fumei, Z.; Xiaojing, T.; Hong, C.; Xueyan, Z.; Zhongren, M.; Li, L. Identification of functional effects of bovine whey protein based on odor information of mouse feces. Zhejiang J. Agric. Sci. 2021, 33, 714. [Google Scholar]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [PubMed]
- Jun, C.; Yingxian, S.; Yuming, L.; Yaling, H.; Shengshou, H. Chinese Clinical Practice Guidelines for Hypertension. Chin. J. Cardiol. 2022, 50, 1050–1095. [Google Scholar]
- Elmarakby, A.A.; Sullivan, J.C. Sex differences in hypertension: Lessons from spontaneously hypertensive rats (SHR). Clin. Sci. 2021, 135, 1791–1804. [Google Scholar] [CrossRef]
- Maranon, R.; Reckelhoff, J.F. Sex and gender differences in control of blood pressure. Clin. Sci. 2013, 125, 311–318. [Google Scholar] [CrossRef]
- Ashton, N.; Balment, R.J. Sexual dimorphism in renal function and hormonal status of New Zealand genetically hypertensive rats. Eur. J. Endocrinol. 1991, 124, 91–97. [Google Scholar] [CrossRef]
- Guoying, Q. Analysis on the antihypertensive effect of aerobic exercise on weight loss in young and middle-aged hypertension. Knowl. Cardiovasc. Dis. Prev. Treat. 2018, 7, 13–15. [Google Scholar]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.M.; Quigley, E.M.; Cryan, J.F.; Dinan, T.G. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Pilleron, S.; Aboyans, V.; Mbelesso, P.; Ndamba-Bandzouzi, B.; Desormais, I.; Lacroix, P.; Preux, P.M.; Guerchet, M.; Desormais, I.; Desport, J.C. Prevalence, awareness, treatment, and control of hypertension in older people in Central Africa: The EPIDEMCA study. J. Am. Soc. Hypertens. 2017, 11, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Xiaogang, L.; Lihua, S. Effects of low-intensity aerobic exercise on blood pressure and its ACE2 and Ang II levels in spontaneously hypertensive rats. Chin. J. Gerontol. 2022, 42, 1175–1178. [Google Scholar]
- Jinjin, R.; Simin, P.; Jingsi, C.; Menglan, L.; Zhongxiao, W.; Jiaying, X.; Liqiang, Q. Effects of lactoferrin on blood pressure and gut microbiota in spontaneously hypertensive rats. J. Hyg. Res. 2022, 51, 260–265. [Google Scholar]
- de Meij, T.G.; Larbi, I.B.; van der Schee, M.P.; Lentferink, Y.E.; Paff, T.; Terhaar sive Droste, J.S.; Mulder, C.J.; van Bodegraven, A.A.; de Boer, N.K. Electronic nose can discriminate colorectal carcinoma and advanced adenomas by fecal volatile biomarker analysis: Proof of principle study. Int. J. Cancer 2014, 134, 1132–1138. [Google Scholar] [CrossRef]
- Ma, Z. Study on the Regulation of Intestinal Flora Structure and Anti-Aging Effect of Whey Powder in D-Galactose-Induced Aging Mice. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2021. [Google Scholar]
- Montag, S.; Frank, M.; Ulmer, H.; Wernet, D.; Göpel, W.; Rammensee, H.G. “Electronic nose” detects major histocompatibility complex-dependent prerenal and postrenal odor components. Proc. Natl. Acad. Sci. USA 2001, 98, 9249–9254. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Picca, A.; Tosato, M.; Martone, A.M.; Ortolani, E.; Sisto, A.; D’Angelo, E.; Serafini, E.; Desideri, G. Body mass index is strongly associated with hypertension: Results from the longevity check-up 7+ study. Nutrients 2018, 10, 1976. [Google Scholar] [CrossRef]
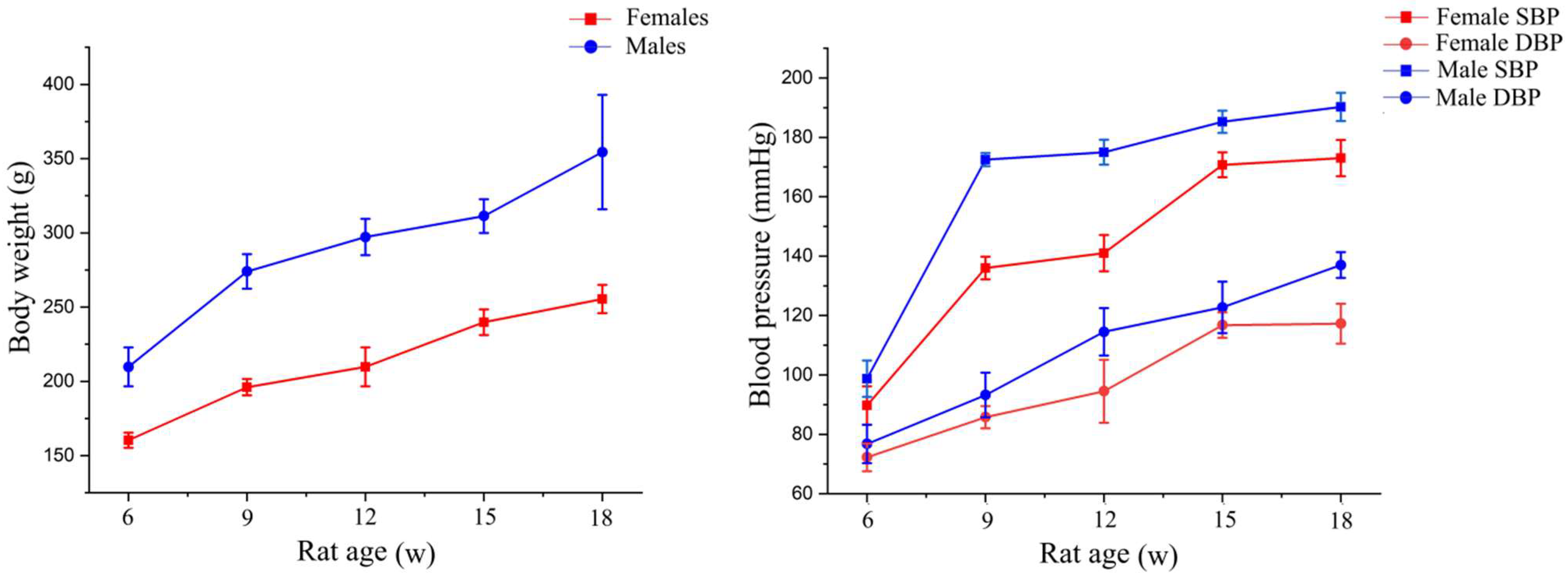
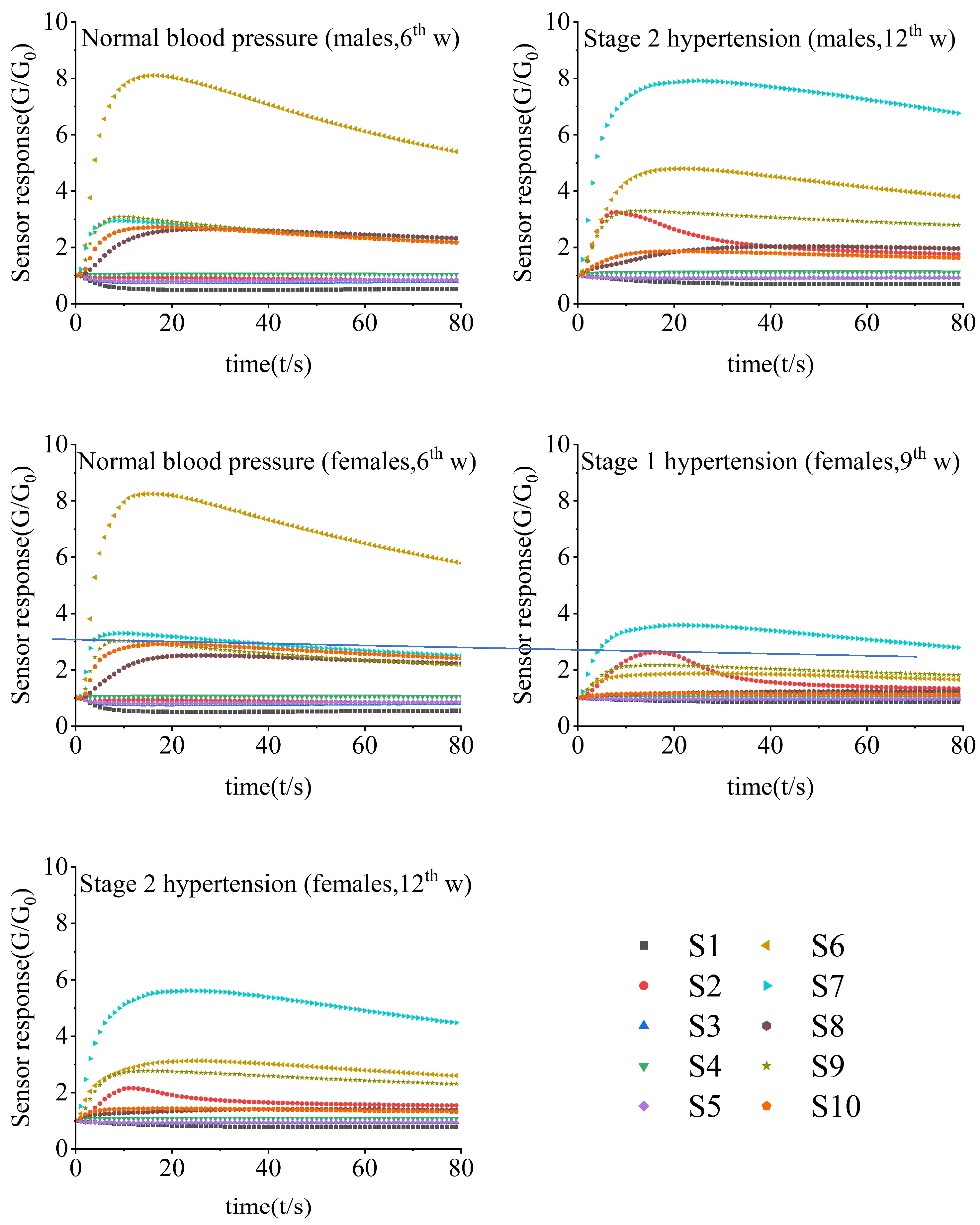


| BP Category | SBP/mmHg | DBP/mmHg | Age of SHRs (Weeks) | ||
|---|---|---|---|---|---|
| Male | Female | ||||
| Normal | ≤130 | ≤80 | 6th | 6th | |
| Hypertension | Stage 1 | 130~139 | 80~89 | 9th | |
| Stage 2 | ≥140 | ≥90 | 9th–18th | 12th–18th | |
| Gender | Sensor Response | Age of SHRs (Weeks) | ||||
|---|---|---|---|---|---|---|
| 6 | 9 | 12 | 15 | 18 | ||
| Males | S1 | 0.54 ± 0.01 d | 0.68 ± 0.01 c | 0.78 ± 0.01 a | 0.73 ± 0.01 b | 0.65 ± 0.01 c |
| S2 | 0.83 ± 0.01 c | 0.99 ± 0.03 c | 1.37 ± 0.09 b | 1.69 ± 0.19 a | 1.42 ± 0.15 a | |
| S3 | 0.80 ± 0.01 d | 0.91 ± 0.01 c | 0.96 ± 0.01 a | 0.93 ± 0.01 b | 0.91 ± 0.01 c | |
| S4 | 1.04 ± 0.01 d | 1.07 ± 0.01 c | 1.07 ± 0.01 c | 1.13 ± 0.01 b | 1.19 ± 0.01 a | |
| S5 | 0.86 ± 0.01 d | 0.93 ± 0.01 b | 0.96 ± 0.01 a | 0.93 ± 0.01 b | 0.91 ± 0.01 c | |
| S6 | 5.09 ± 0.22 a | 3.38 ± 0.11 b | 2.54 ± 0.08 d | 3.64 ± 0.06 b | 5.15 ± 0.18 a | |
| S7 | 2.36 ± 0.08 c | 3.61 ± 0.26 b | 3.87 ± 0.34 b | 3.88 ± 0.30 b | 6.72 ± 0.27 a | |
| S8 | 2.21 ± 0.06 b | 1.88 ± 0.04 c | 1.45 ± 0.03 d | 1.92 ± 0.02 c | 2.42 ± 0.05 a | |
| S9 | 2.24 ± 0.07 c | 2.22 ± 0.06 c | 2.20 ± 0.08 c | 2.72 ± 0.14 b | 3.26 ± 0.16 a | |
| S10 | 2.11 ± 0.05 a | 1.42 ± 0.02 c | 1.25 ± 0.01 d | 1.62 ± 0.01 c | 1.86 ± 0.03 b | |
| Females | S1 | 0.49 ± 0.06 b | 0.81 ± 0.07 a | 0.79 ± 0.10 a | 0.79 ± 0.07 a | 0.80 ± 0.07 a |
| S2 | 0.84 ± 0.08 c | 1.16 ± 0.27 c | 1.04 ± 0.15 c | 1.70 ± 0.67 b | 2.78 ± 1.41 a | |
| S3 | 0.75 ± 0.05 b | 0.96 ± 0.03 a | 0.95 ± 0.04 a | 0.95 ± 0.02 a | 0.96 ± 0.02 a | |
| S4 | 1.07 ± 0.04 b | 1.02 ± 0.03 b | 1.08 ± 0.07 b | 1.10 ± 0.37 b | 1.25 ± 0.38 a | |
| S5 | 0.81 ± 0.05 b | 0.96 ± 0.02 a | 0.96 ± 0.03 a | 0.95 ± 0.02 a | 0.95 ± 0.02 a | |
| S6 | 7.41 ± 1.70 a | 2.10 ± 0.53 c | 2.47 ± 1.13 bc | 2.70 ± 0.71 bc | 3.12 ± 1.24 b | |
| S7 | 2.80 ± 0.36 b | 2.91 ± 0.54 b | 3.01 ± 0.79 b | 5.38 ± 2.51 a | 6.32 ± 4.55 a | |
| S8 | 2.62 ± 0.38 a | 1.46 ± 0.26 b | 1.58 ± 0.41 b | 1.51 ± 0.28 b | 1.54 ± 0.26 b | |
| S9 | 2.69 ± 0.46 a | 1.90 ± 0.16 b | 1.94 ± 0.31 b | 2.46 ± 0.51 a | 2.58 ± 1.00 a | |
| S10 | 2.74 ± 0.39 a | 1.24 ± 0.13 c | 1.28 ± 0.22 c | 1.34 ± 0.13 bc | 1.42 ± 0.13 b | |
| Gender | BP Status | Males N | Males H | Females N | Females H | N | H | Correct Classification Rate |
|---|---|---|---|---|---|---|---|---|
| Male | N | 26 | 9 | 0 | 0 | 60.49% | ||
| H | 7 | 78 | 57 | 1 | ||||
| Female | N | 0 | 12 | 27 | 1 | |||
| H | 1 | 2 | 55 | 91 | ||||
| No distinction of gender | N | 75 | 0 | 100% | ||||
| H | 0 | 292 |
| Input Data | Gender | BP Status | Accuracy | Precision | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|---|---|
| E-nose responses | Male | N | 95.36% | 74.29% | 76.47% | 97.30% | 87.03% |
| H | 76.02% | 54.55% | 77.23% | 75.56% | 76.40% | ||
| Female | N | 65.94% | 87.18% | 19.42% | 94.30% | 56.86% | |
| H | 83.65% | 61.07% | 97.85% | 78.83% | 88.34% | ||
| No distinction of gender | N | 100% | 100% | 100% | 100% | 100% | |
| H | 100% | 100% | 100% | 100% | 100% |
| Prediction Methods | Group | BP | Training Set | Test Set | ||
|---|---|---|---|---|---|---|
| R2 | RMESC | R2 | RMESP | |||
| MLR | Male | SBP | 0.9382 | 8.1777 | 0.9509 | 7.4234 |
| DBP | 0.9279 | 5.6242 | 0.9248 | 5.9631 | ||
| Female | SBP | 0.9421 | 9.4059 | 0.9100 | 9.4539 | |
| DBP | 0.8357 | 7.1069 | 0.8036 | 8.1333 | ||
| No distinction | SBP | 0.8252 | 12.3431 | 0.7779 | 12.4202 | |
| DBP | 0.7983 | 10.1148 | 0.7392 | 10.2085 | ||
| PLS | Male | SBP | 0.9371 | 8.2486 | 0.9551 | 7.0407 |
| DBP | 0.9276 | 5.6451 | 0.9284 | 5.7763 | ||
| Female | SBP | 0.9231 | 8.4741 | 0.9192 | 8.7428 | |
| DBP | 0.8326 | 7.1899 | 0.8196 | 7.4555 | ||
| No distinction | SBP | 0.8240 | 14.2090 | 0.7848 | 15.5468 | |
| DBP | 0.7947 | 9.1503 | 0.7552 | 10.0995 | ||
| MLP | Male | SBP | 0.9924 | 2.8988 | 0.9926 | 2.9913 |
| DBP | 0.9664 | 3.8458 | 0.9494 | 4.7290 | ||
| Female | SBP | 0.9358 | 7.8121 | 0.8892 | 10.1845 | |
| DBP | 0.9103 | 5.2558 | 0.6987 | 9.8762 | ||
| No distinction | SBP | 0.9161 | 9.8250 | 0.9325 | 8.9503 | |
| DBP | 0.9213 | 5.6714 | 0.8765 | 7.1162 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Yang, L.; Wei, J.; Tian, X. Non-Invasive Blood Pressure Tracking of Spontaneous Hypertension Rats Using an Electronic Nose. Sensors 2024, 24, 238. https://doi.org/10.3390/s24010238
Zhang F, Yang L, Wei J, Tian X. Non-Invasive Blood Pressure Tracking of Spontaneous Hypertension Rats Using an Electronic Nose. Sensors. 2024; 24(1):238. https://doi.org/10.3390/s24010238
Chicago/Turabian StyleZhang, Fumei, Lijing Yang, Jia Wei, and Xiaojing Tian. 2024. "Non-Invasive Blood Pressure Tracking of Spontaneous Hypertension Rats Using an Electronic Nose" Sensors 24, no. 1: 238. https://doi.org/10.3390/s24010238
APA StyleZhang, F., Yang, L., Wei, J., & Tian, X. (2024). Non-Invasive Blood Pressure Tracking of Spontaneous Hypertension Rats Using an Electronic Nose. Sensors, 24(1), 238. https://doi.org/10.3390/s24010238





