Enhancing the Responsiveness of Thermoelectric Gas Sensors with Boron-Doped and Thermally Annealed SiGe Thin Films via Low-Pressure Chemical Vapor Deposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of TE Thin Films
2.2. Characterization and Electrical Property Measurements of the Thin Films
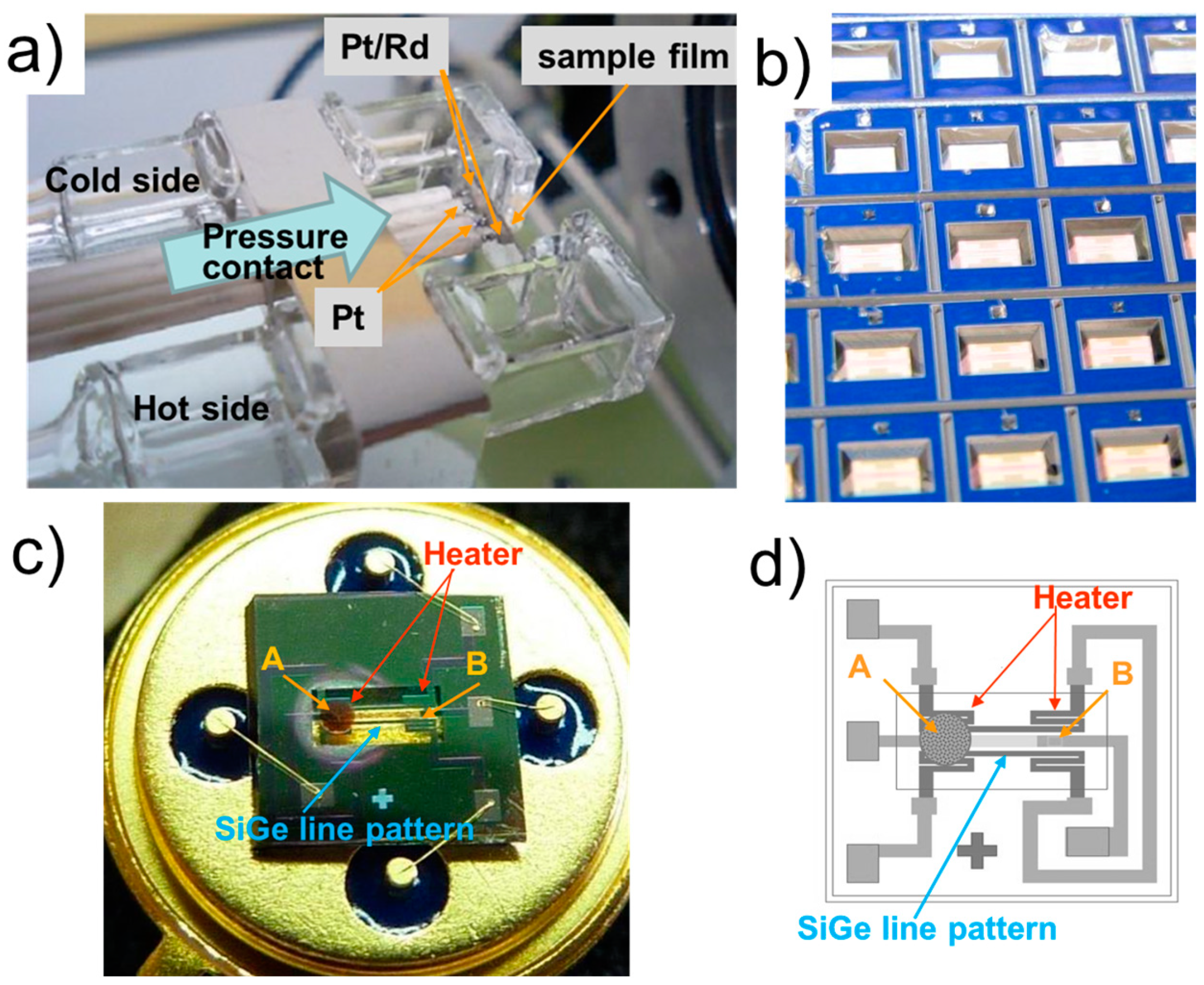
2.3. Micro-THGS Fabrication and Method for Testing Gas Detection
3. Results and Discussions
3.1. Analysis of Crystalline SiGe Thin Films Using XRD
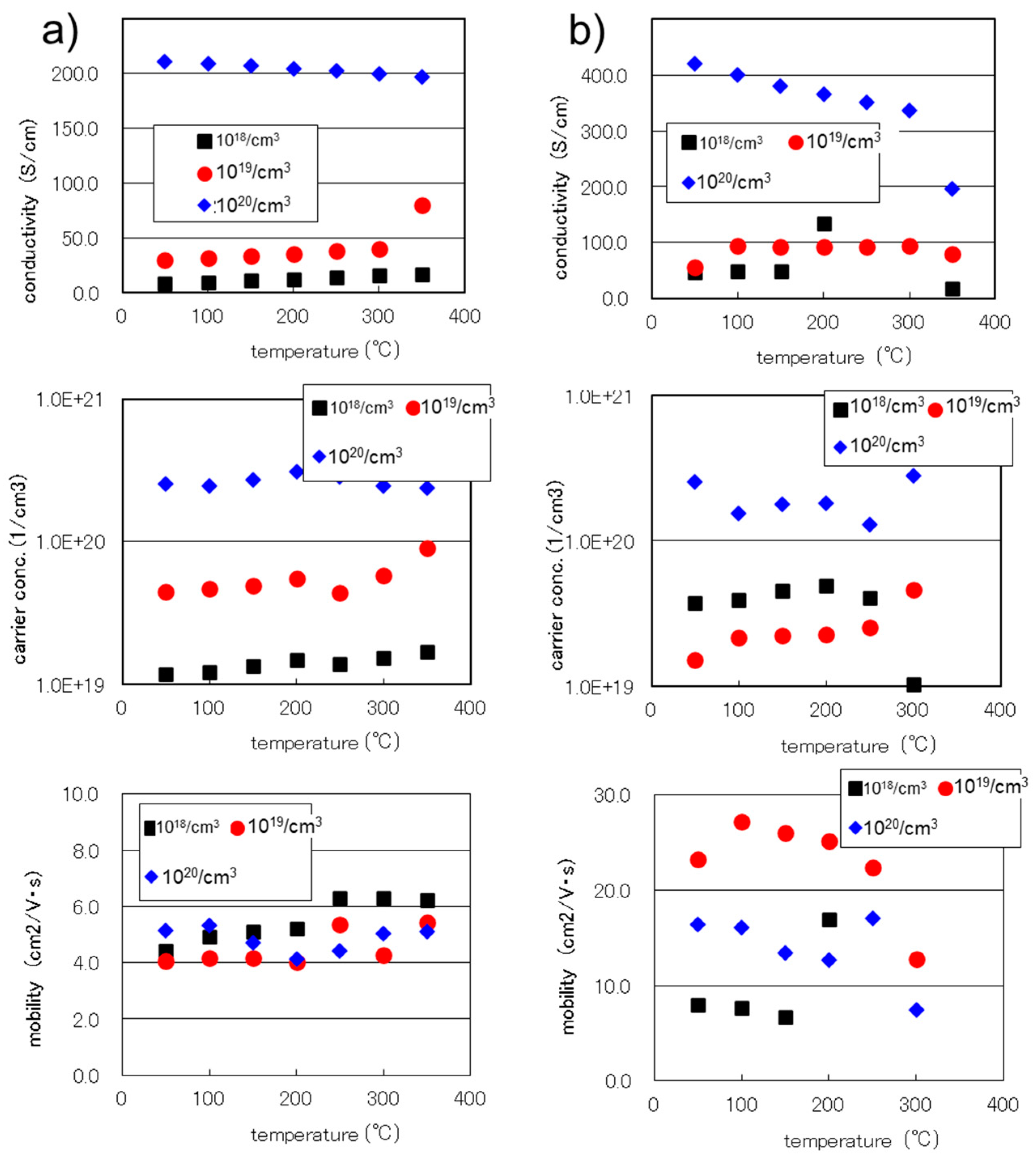
3.2. Carrier Concentration of SiGe Films Investigated Using Hall Measurements
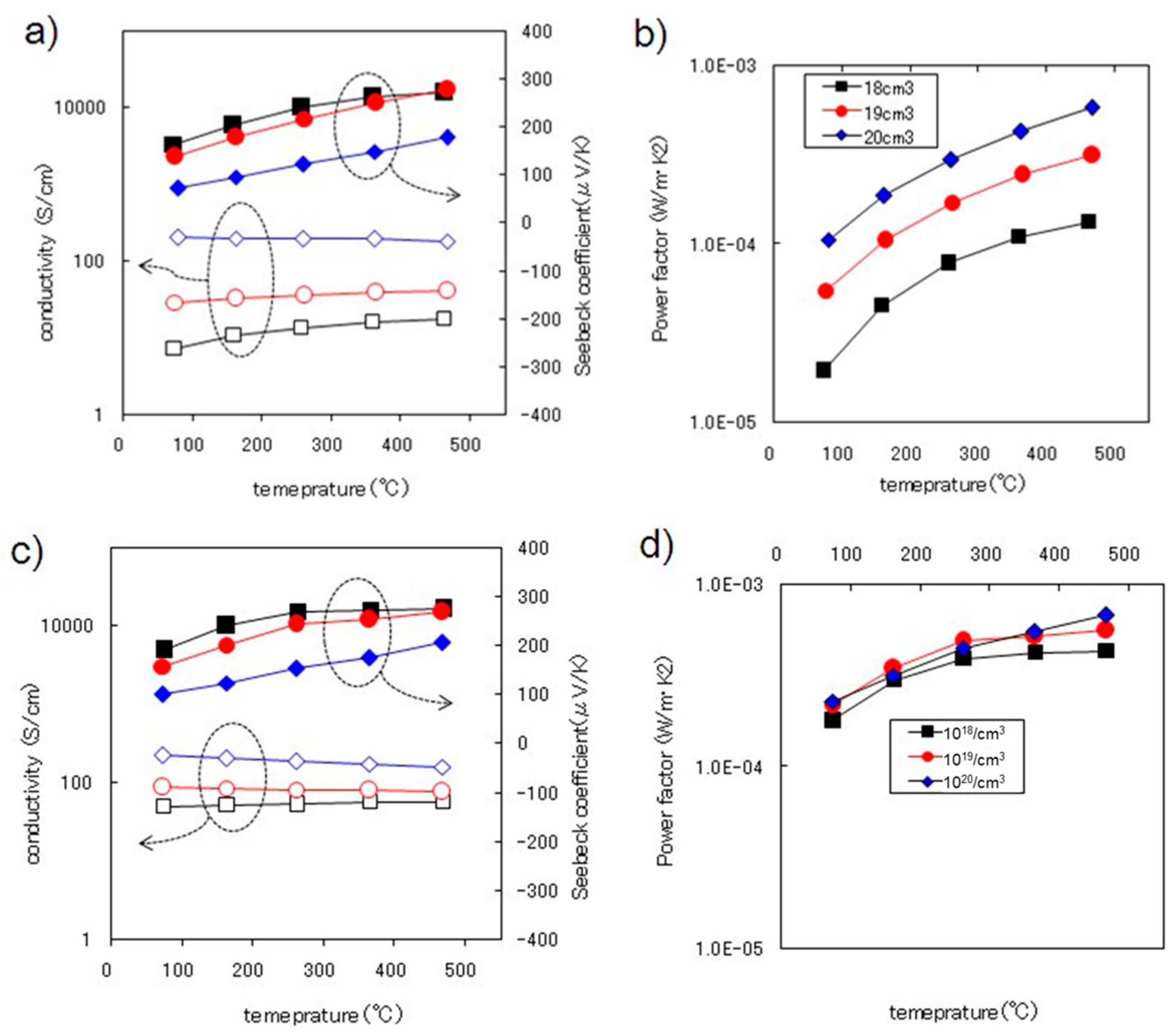
3.3. Thermoelectric Properties Enhanced through Thermal Annealing
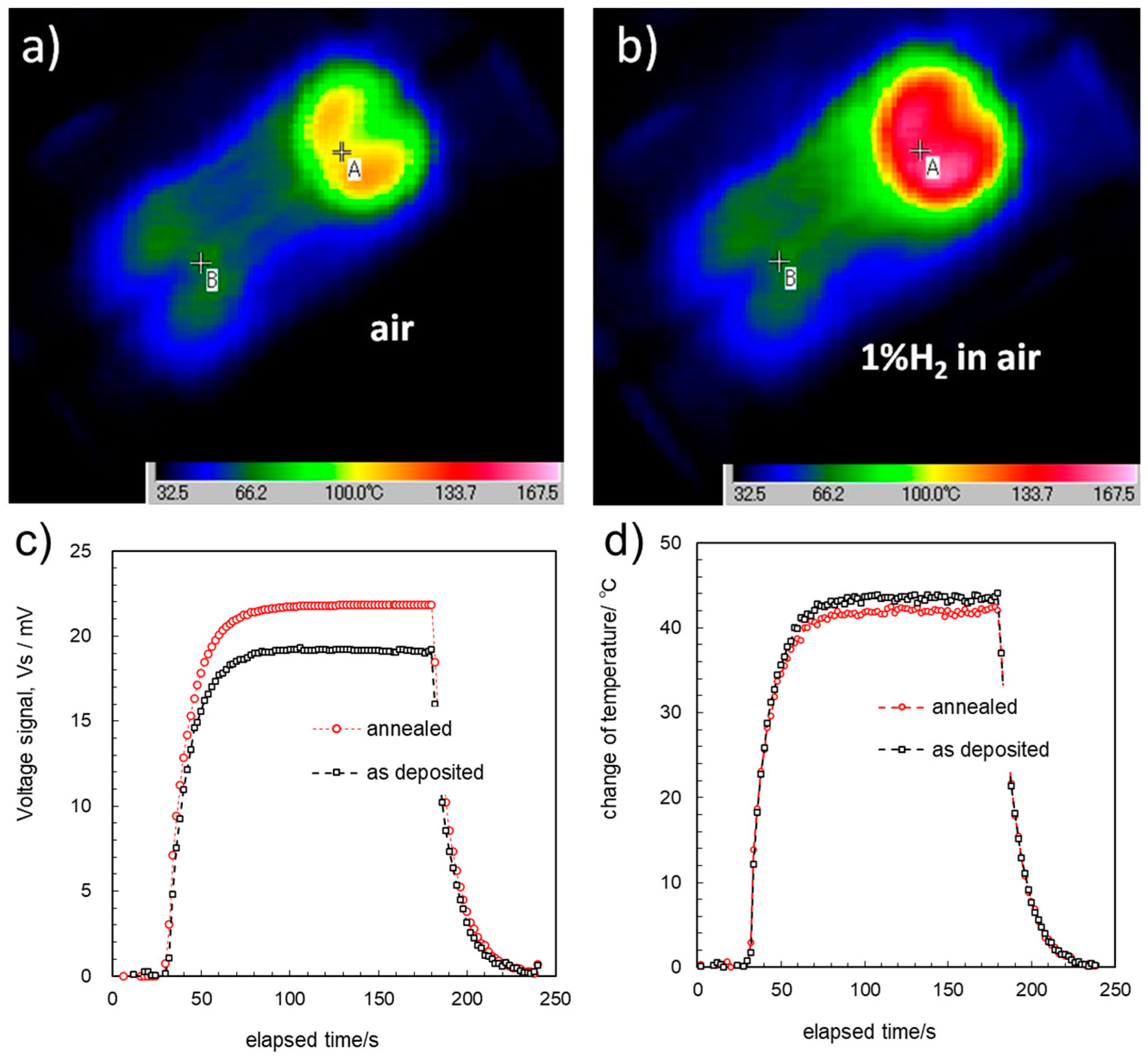
3.4. THGS Performance: Combustion and Thermal Transport
3.5. Discussion on the Relationship between the Thin Films and the Sensor’s Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, F.; Shin, W.; Matsumiya, M.; Izu, N.; Murayama, N. Hydrogen sensor based on RF-sputtered thermoelectric SiGe film. Jpn. J. Appl. Phys. 2003, 42, 1563–1567. [Google Scholar] [CrossRef]
- Tajima, K.; Qiu, F.; Shin, W.; Sawaguchi, N.; Izu, N.; Matsubara, I.; Murayama, N. Thermoelectric properties of RF-sputtered SiGe thin film for hydrogen gas sensor. Jpn. J. Appl. Phys. 2004, 43, 5978–5983. [Google Scholar] [CrossRef]
- Paul, O.M.; Korvink, J.; Baltes, H. Determination of the thermal conductivity of CMOS IC polysilicon. Sens. Actuators A 1994, 41–42, 161–164. [Google Scholar] [CrossRef]
- Wijngaards, D.D.L.; de Graf, G.; Wolffenbuttel, R.F. Design and fabrication of on-chip integrated polySiGe and polySi Peltier devices. Sens. Actuators A 2004, 110, 187–195. [Google Scholar] [CrossRef]
- Sedky, S.; Fiorini, P.; Caymax, M.; Loreti, S.; Baert, K.; Hermans, L.; Mertens, R. Characterization of bolometers based on polycrystalline silicon germanium alloys. IEEE Electron Device Lett. 1998, 19, 376–378. [Google Scholar] [CrossRef]
- Kusano, K.; Yamamoto, A.; Nakata, M.; Suemasu, T.; Toko, K. Thermoelectric Inorganic SiGe Film Synthesized on Flexible Plastic Substrate. ACS Appl. Energy Mater. 2018, 1, 5280–5285. [Google Scholar] [CrossRef]
- Ozawa, T.; Kusano, K.; Murata, M.; Yamamoto, A.; Suemasu, T.; Toko, K. Thickness-dependent thermoelectric properties of Si1−xGex films formed by Al-induced layer exchange. J. Appl. Phys. 2021, 129, 015303. [Google Scholar] [CrossRef]
- Kong, D.; Zhu, W.; Guo, Z.; Deng, Y. High-performance flexible Bi2Te3 films based wearable thermoelectric generator for energy harvesting. Energy 2019, 175, 292–299. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, Y.; Wang, W.; Hu, M.; Huang, X.; Mao, D.; Huang, S.; Xie, L.; Lin, P.; Jiang, B.; et al. Staggered-layer-boosted flexible Bi2Te3 films with high thermoelectric performance. Nat. Nanotechnol. 2023, 18, 1281–1288. [Google Scholar] [CrossRef]
- Su, Y.; Lu, J.; Villaroman, D.; Li, D.; Huang, B. Free-standing planar thermoelectric microrefrigerators based on nano-grained SiGe thin films for on-chip refrigeration. Nano Energy 2018, 48, 202. [Google Scholar] [CrossRef]
- Schaevitz, S.B.; Franz, A.J.; Jensen, K.F.; Schmidt, M.A. A combustion-based MEMS thermoelectric power generator. In Proceedings of the 11th International Conference on Solid-State Sensors and Actuators, Munich, Germany, 10–14 June 2001; pp. 30–33. [Google Scholar]
- Panel discussion of “Metrology and Standards for Thermoelectric Research” in the Book of abstract ICT2008. In Proceedings of the International Thermoelectric Conference 2008, Jacksonville, FL, USA, 10–14 August 2008.
- Shin, W.; Murayama, N.; Ikeda, K.; Sago, S. Fabrication of oxide thermoelectric generator element. Jpn. J. Appl. Phys. 2000, 39, 1254. [Google Scholar] [CrossRef]
- Martin, J.; Lu, Z.-Q.; Wong-Ng, W.; Krylyuk, S.; Wang, D.; Ren, Z. Development of a high-temperature (295–900 K) Seebeck coefficient Standard Reference Material. J. Mater. Res. 2021, 36, 3339–3352. [Google Scholar] [CrossRef]
- Uchida, S.; Lee, M.; Lee, C.H.; Suzuki, Y. High-temperature monolithic SiGe thermoelectric device directly heated by catalytic combustion. Appl. Phys. Lett. 2022, 120, 053901. [Google Scholar] [CrossRef]
- Shin, W.; Nakashima, T.; Nishibori, M.; Izu, N.; Itoh, T.; Matsubara, I. Planar-type thermoelectric micro devices using ceramic catalytic combustor. Curr. Appl. Phys. 2011, 11, S36–S40. [Google Scholar] [CrossRef]
- Nishibori, M.; Shin, W.; Houlet, L.F.; Tajima, K.; Izu, N.; Itoh, T.; Murayama, N.; Matsubara, I. New structural design of micro-thermoelectric sensor for wide range hydrogen detection. J. Ceram. Soc. Jpn. 2006, 114, 853–856. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, H.; Ni, Z.; Li, M.; Chen, Y.; Xu, P.; Li, X. 1ppm-detectable hydrogen gas sensors by using highly sensitive P+/N+ single-crystalline silicon thermopiles. Microsyst. Nanoeng. 2023, 9, 29. [Google Scholar] [CrossRef]
- Shin, W.; Ishikawa, M.; Nishibori, M.; Izu, N.; Itoh, T.; Matsubara, I. High-temperature thermoelectric measurement of B-doped SiGe and Si thin films. Mater. Trans. 2009, 50, 1596–1602. [Google Scholar] [CrossRef]
- Rosencwaig, A.; Opsal, J.L.; Smith, W.L.; Willenborg, D.L. Detection of thermal waves through optical reflectance. Appl. Phys. Lett. 1985, 46, 1013–1015. [Google Scholar] [CrossRef]
- Okuhata, R.; Watanabe, K.; Ikeuchi, S.; Ishida, A.; Nakamura, Y. Thermal Conductivity Measurement of Thermoelectric Thin Films by a Versatility-Enhanced 2ω Method. J. Electron. Mater. 2017, 46, 3089–3096. [Google Scholar] [CrossRef]
- Lu, J.; Guo, R.; Dai, W.; Huang, B. Enhanced in-plane thermoelectric figure of merit in p-type SiGe thin films by nanograin boundaries. Nanoscale 2015, 7, 7331–7339. [Google Scholar] [CrossRef]
- Jelenkovic, E.V.; Tong, K.Y.; Sun, Z.; Mak, C.L.; Cheung, W.Y. Properties of crystallized Si1−xGex thin films deposited by sputtering. J. Vac. Sci. Technol. A 1997, 15, 2836–2841. [Google Scholar] [CrossRef]
- Jelenkovic, E.V.; Jevtic, M.M.; Tong, K.Y.; Pang, G.K.H.; Cheung, W.Y.; Jha, S.K. On temperature coefficient of resistance of boron-doped SiGe films deposited by sputtering. Mater. Sci. Semicond. Process. 2007, 10, 143–149. [Google Scholar] [CrossRef]
- Vining, C.B. Silicon Germanium, CRC Handbook of Thermoelectrics; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 329–337. [Google Scholar]
- Dismukes, J.P.; Ekstrom, L.; Steigmeier, E.F.; Kudman, L.; Beers, D.S. Thermal and Electrical Properties of Heavily Doped Ge-Si Alloys up to 1300K. J. Appl. Phys. 1964, 62, 2899–2907. [Google Scholar] [CrossRef]
- Strasser, M.; Aigner, R.; Lauterbach, C.; Sturm, T.; Franosch, M.; Wachutka, G. Micromachined CMOS thermoelectric generators as on-chip power supply. Sens. Actuators A 2004, 114, 362–370. [Google Scholar] [CrossRef]
- Chang, Y.M.; Dai, C.L.; Cheng, T.C.; Hsu, C.W. Effect of annealing temperature for Si0.8Ge0.2 epitaxial thin films. Appl. Surf. Sci. 2008, 254, 3105–3109. [Google Scholar] [CrossRef]
- Nagai, D.; Akamatsu, T.; Itoh, T.; Izu, N.; Shin, W. Thermal Balance Analysis of a Micro-Thermoelectric Gas Sensor Using Catalytic Combustion of Hydrogen. Sensors 2014, 14, 1822–1834. [Google Scholar] [CrossRef]
- Goto, T.; Itoh, T.; Akamatsu, T.; Sasaki, Y.; Sato, K.; Shin, W. Heat transfer control of micro-thermoelectric gas sensor for breath gas monitoring. Sen. Actuators B 2017, 249, 571–580. [Google Scholar] [CrossRef]
- Gu, Y.; Tang, X.; Xu, Y.; Hatta, I. Ingenious Method for Eliminating Effects of Heat Loss in Measurements of Thermal Diffusivity by ac Calorimetric Method. Jpn. J Appl. Phys. 1993, 32, L1365–L1367. [Google Scholar] [CrossRef]
- Llin, L.F.; Samarelli, A.; Zhang, Y.; Weaver, J.M.; Dobson, P.; Cecchi, S.; Chrastina, D.; Isella, G.; Etzelstorfer, T.; Stangl, J.; et al. Thermal Conductivity Measurement Methods for SiGe Thermoelectric Materials. J. Electron. Mater. 2013, 42, 2376–2380. [Google Scholar] [CrossRef]
- Johnson, J.B. Thermal agitation of electricity in conductors. Phys. Rev. 1928, 32, 97–109. [Google Scholar] [CrossRef]


| B Doping Level | α | σ | PF | κ | ZT | |
|---|---|---|---|---|---|---|
| cm3 | μV/K | S/cm | 10−4 W/mK2 | W/m K | ||
| 1 × 1018 | as-deposited | 165 | 7.21 | 0.196 | 2.6 ± 0.4 | 0.025 |
| annealed | 191 | 49.0 | 1.79 | 0.019 * | ||
| 1 × 1019 | as-deposited | 137 | 29.3 | 0.552 | 2.4 ± 0.6 | 0.0075 |
| annealed | 159 | 87.0 | 2.20 | 0.024 * | ||
| 1 × 1020 | as-deposited | 72.0 | 205 | 1.06 | 2.5 ± 0.4 | 0.014 |
| annealed | 102 | 222 | 2.31 | 3.0 ± 0.5 | 0.025 | |
| B Doping Level | α | 1% H2 | 0.1% H2 | 0.01% H2 | ||||
|---|---|---|---|---|---|---|---|---|
| cm3 | μV/K | Vs/mV | ΔT/K | Vs/mV | ΔT/K | Vs/mV | ΔT/K | |
| 1 × 1018 | as-deposited | 165 | 19.16 | 43.6 | 2.81 | 6.6 | 0.01 | 1 |
| annealed | 191 | 21.8 | 42.3 | 3.17 | 6.2 | 0.41 | 1 | |
| 1 × 1019 | as-deposited | 137 | 13.35 | 32.8 | 1.39 | 3.8 | -- | -- |
| annealed | 159 | 20.67 | 40 | 2.14 | 3.6 | -- | -- | |
| 1 × 1020 | as-deposited | 72.0 | 9.03 | 38.1 | 1.04 | 6.3 | 0.10 | 1 |
| annealed | 102 | 16.1 | 42.1 | 2.39 | 6.5 | 0.37 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, W.; Nishibori, M.; Itoh, T.; Izu, N.; Matsubara, I. Enhancing the Responsiveness of Thermoelectric Gas Sensors with Boron-Doped and Thermally Annealed SiGe Thin Films via Low-Pressure Chemical Vapor Deposition. Sensors 2024, 24, 3058. https://doi.org/10.3390/s24103058
Shin W, Nishibori M, Itoh T, Izu N, Matsubara I. Enhancing the Responsiveness of Thermoelectric Gas Sensors with Boron-Doped and Thermally Annealed SiGe Thin Films via Low-Pressure Chemical Vapor Deposition. Sensors. 2024; 24(10):3058. https://doi.org/10.3390/s24103058
Chicago/Turabian StyleShin, Woosuck, Maiko Nishibori, Toshio Itoh, Noriya Izu, and Ichiro Matsubara. 2024. "Enhancing the Responsiveness of Thermoelectric Gas Sensors with Boron-Doped and Thermally Annealed SiGe Thin Films via Low-Pressure Chemical Vapor Deposition" Sensors 24, no. 10: 3058. https://doi.org/10.3390/s24103058
APA StyleShin, W., Nishibori, M., Itoh, T., Izu, N., & Matsubara, I. (2024). Enhancing the Responsiveness of Thermoelectric Gas Sensors with Boron-Doped and Thermally Annealed SiGe Thin Films via Low-Pressure Chemical Vapor Deposition. Sensors, 24(10), 3058. https://doi.org/10.3390/s24103058







