Novel Metric for Non-Invasive Beat-to-Beat Blood Pressure Measurements Demonstrates Physiological Blood Pressure Fluctuations during Pregnancy
Abstract
:1. Introduction
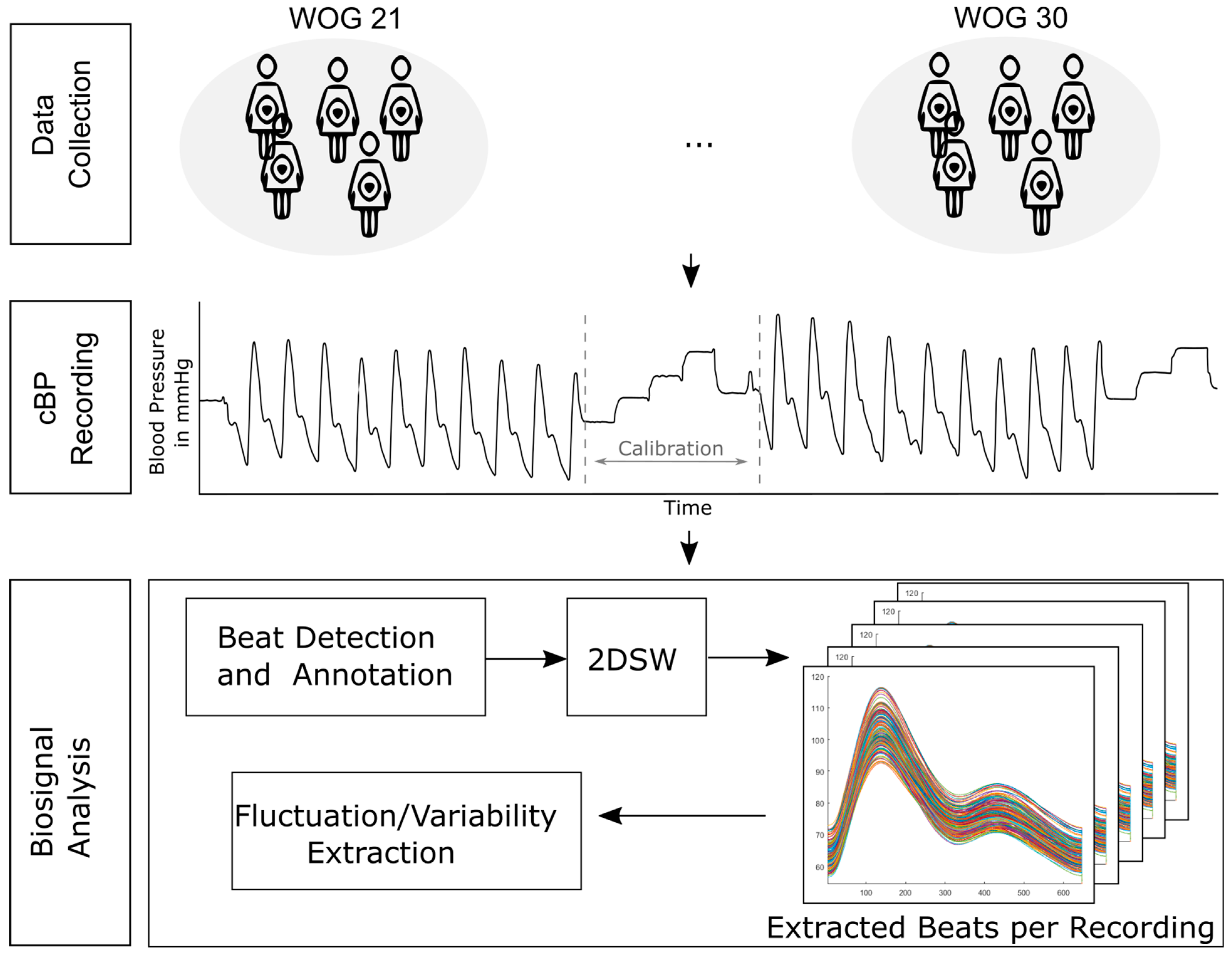
2. Materials and Methods
2.1. BP Beat Annotation with ELZA Algorithm
2.2. Functionality and Adaptation of i2DSW Algorithm
2.3. Parametrization
2.4. Calculation of Fluctuation Parameters
2.5. Validation
2.5.1. Simulated Data
2.5.2. Validation Results
2.6. Study Design
2.7. Non-Invasive B2B-BP Signal Measurement
2.8. Signal Processing and B2B-BP Assessment
2.9. Statistics and Linear Models
3. Results
3.1. Relation between Pregnancy Progression and B2B-BP Fluctuation
3.2. Impact of Exercises on B2B-BP Fluctuations with Regard to WOG
3.3. Comparison of B2B-BP Fluctuations and Conventional Variability Measures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tachmatzidis, D.; Filos, D.; Chouvarda, I.; Tsarouchas, A.; Mouselimis, D.; Bakogiannis, C.; Lazaridis, C.; Triantafyllou, K.; Antoniadis, A.P.; Fragakis, N.; et al. Beat-to-Beat P-Wave Analysis Outperforms Conventional P-Wave Indices in Identifying Patients with a History of Paroxysmal Atrial Fibrillation during Sinus Rhythm. Diagnostics 2021, 11, 1694. [Google Scholar] [CrossRef] [PubMed]
- Parati, G.; Ongaro, G.; Bilo, G.; Glavina, F.; Castiglioni, P.; Di Rienzo, M.; Mancia, G. Non-invasive beat-to-beat blood pressure monitoring: New developments. Blood Press. Monit. 2003, 8, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lakhal, K.; Martin, M.; Faiz, S.; Ehrmann, S.; Blanloeil, Y.; Asehnoune, K.; Rozec, B.; Boulain, T. The CNAP™ Finger Cuff for Noninvasive Beat-To-Beat Monitoring of Arterial Blood Pressure: An Evaluation in Intensive Care Unit Patients and a Comparison with 2 Intermittent Devices. Obstet. Anesthesia Dig. 2016, 123, 1126–1135. [Google Scholar] [CrossRef]
- Schutte, A.E.; Kollias, A.; Stergiou, G.S. Blood pressure and its variability: Classic and novel measurement techniques. Nat. Rev. Cardiol. 2022, 19, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, K.H.; Settels, J.J.; de Wit, B. The Measurement of Continuous Finger Arterial Pressure Noninvasively in Sta-tionary Subjects. In Biological and Psychological Factors in Cardiovascular Disease; Springer: Berlin/Heidelberg, Germany, 1986; pp. 355–375. [Google Scholar] [CrossRef]
- Bi, R.; Du, Y.; Singh, G.; Ho, J.-H.; Zhang, S.; Attia, A.B.E.; Li, X.; Olivo, M.C. Fast pulsatile blood flow measurement in deep tissue through a multimode detection fiber. J. Biomed. Opt. 2020, 25, 055003. [Google Scholar] [CrossRef]
- Teng, Z.; Gao, F.; Xia, H.; Chen, W.; Li, C. In Vivo Pulse Wave Measurement Through a Multimode Fiber Diffuse Speckle Analysis System. Front. Phys. 2021, 8, 613342. [Google Scholar] [CrossRef]
- Bakkar, N.-M.Z.; El-Yazbi, A.F.; Zouein, F.A.; Fares, S.A. Beat-to-beat blood pressure variability: An early predictor of disease and cardiovascular risk. J. Hypertens. 2021, 39, 830–845. [Google Scholar] [CrossRef]
- Goh, C.-H.B.; Ng, S.-C.; Kamaruzzaman, S.B.; Chin, A.-V.; Tan, M.P. Standing beat-to-beat blood pressure variability is reduced among fallers in the Malaysian Elders Longitudinal Study. Medicine 2017, 96, e8193. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wu, D.; Gao, Z.; Liu, X.; Chen, Q.; Ren, L.; Wu, W. Association between beat-to-beat blood pressure variability and vascular elasticity in normal young adults during the cold pressor test. Medicine 2017, 96, e6000. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Martinez, F.; Akey, M.A.; Aysola, R.S.; Henderson, L.A.; Malhotra, A.; Macey, P.M. Breathing rate variability in obstructive sleep apnea during wakefulness. Sleep. Med. 2022, 18, 825–833. [Google Scholar] [CrossRef]
- Schmidt, M.; Dunker, R.; Malberg, H.; Zaunseder, S. Quantification of Ventricular Repolarization Fluctuations in Patients With Myocardial Infarction. In Proceedings of the 2020 Computing in Cardiology Conference, Rimini, Italy, 13–16 September 2020. [Google Scholar] [CrossRef]
- Schmidt, M.; Baumert, M.; Porta, A.; Malberg, H.; Zaunseder, S. Two-Dimensional Warping for One-Dimensional Signals—Conceptual Framework and Application to ECG Processing. IEEE Trans. Signal Process. 2014, 62, 5577–5588. [Google Scholar] [CrossRef]
- Schmidt, M.; Baumert, M.; Malberg, H.; Zaunseder, S. Iterative two-dimensional signal warping—Towards a generalized approach for adaption of one-dimensional signals. Biomed. Signal Process. Control 2018, 43, 311–319. [Google Scholar] [CrossRef]
- Wessel, N.; Gapelyuk, A.; Weiß, J.; Schmidt, M.; Kraemer, J.F.; Berg, K.; Malberg, H.; Stepan, H.; Kurths, J. Instantaneous Cardiac Baroreflex Sensitivity: xBRS Method Quantifies Heart Rate Blood Pressure Variability Ratio at Rest and During Slow Breathing. Front. Neurosci. 2020, 14, 547433. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Tan, H.; Zhou, S.; Smith, G.N.; Walker, M.C.; Wen, S.W. Trajectory of blood pressure change during pregnancy and the role of pre-gravid blood pressure: A functional data analysis approach. Sci. Rep. 2017, 7, 1–6. [Google Scholar] [CrossRef]
- August, P.; Mueller, F.; Sealey, J.; Edersheim, T. Role of renin-angiotensin system in blood pressure regulation in pregnancy. Lancet 1995, 345, 896–897. [Google Scholar] [CrossRef] [PubMed]
- Laurin, A. BP_Annotate—File Exchange—MATLAB Central. Available online: https://de.mathworks.com/matlabcentral/fileexchange/60172-bp_annotate (accessed on 7 February 2024).
- Zong, W.; Heldt, T.; Moody, G.B.; Mark, R.G. An open-source algorithm to detect onset of arterial blood pressure pulses. Comput. Cardiol. 2003, 30, 259–262. [Google Scholar] [CrossRef]
- Wesseling, K.H.; de Wit, B.; Hoeven van der GM, A.; van Goudoever, J.; Settels, J.J. Physiocal, calibrating finger vascular physiology for finapres. Homeost. Health Dis. 1995, 36, 67–82. Available online: https://research.tue.nl/en/publications/physiocal-calibrating-finger-vascular-physiology-for-finapres (accessed on 30 April 2024).
- Vermunt, J.V.; Kennedy, S.H.; Garovic, V.D. Blood Pressure Variability in Pregnancy: An Opportunity to Develop Improved Prognostic and Risk Assessment Tools. Curr. Hypertens. Rep. 2020, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- McClure, J.H.; Cooper, G.M.; Clutton-Brock, T.H. Saving mothers’ lives: Reviewing maternal deaths to make motherhood safer: 20068: A review. Br. J. Anaesth. 2011, 107, 127–132. [Google Scholar] [CrossRef]
- Magee, L.A.; Pels, A.; Helewa, M.; Rey, E.; von Dadelszen, P.; Audibert, F.; Bujold, E.; Côté, A.-M.; Douglas, M.J.; Eastabrook, G.; et al. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: Executive summary. J. Obstet. Gynaecol. Can. 2014, 36, 416–438. [Google Scholar] [CrossRef] [PubMed]




| (a) Women Meta Data. Mean ± Standard Deviation | ||||
| WOG | n | Height in cm | Age in years | Weight in kg |
| 21 | 13 | 166.5 ± 6.6 | 32.0 ± 5.9 | 79.2 ± 11.4 |
| 22 | 19 | 166.3 ± 7.0 | 30.8 ± 5.1 | 71.6 ± 11.6 |
| 23 | 33 | 166.8 ± 7.5 | 32.5 ± 5.9 | 72.9 ± 12.8 |
| 24 | 27 | 167.0 ± 7.8 | 31.2 ± 5.8 | 73.4 ± 14.2 |
| 25 | 32 | 166.3 ± 8.0 | 31.8 ± 5.8 | 73.5 ± 13.6 |
| 26 | 28 | 166.4 ± 6.9 | 32.1 ± 5.2 | 74.9 ± 11.7 |
| 27 | 36 | 166.5 ± 7.3 | 31.3 ± 5.6 | 75.0 ± 14.9 |
| 28 | 27 | 167.5 ± 6.9 | 31.8 ± 5.8 | 74.6 ± 16.7 |
| 29 | 24 | 166.2 ± 7.5 | 30.4 ± 5.7 | 73.0 ± 12.3 |
| 30 | 2 | 162.5 ± 2.1 | 25.0 ± 5.7 | 78.5 ± 14.8 |
| 241 | 166.2 ± 6.8 | 30.9 ± 5.7 | 74.7 ± 13.4 | |
| (b) Protocol Details | ||||
| Intervention | Task | Duration in min | Breathing Rate in BPM | |
| I1 | Resting | 10 | ||
| I2 | Paced Breathing | 5 | 8 | |
| I3 | Resting | 5 | ||
| I4 | Paced Breathing | 5 | 20 | |
| I5 | Resting | 5 | ||
| I6 | Stand-Up | 1 | ||
| I7 | Resting | 5 | ||
| Parameter | Intercept | WOG 24–25 | WOG 26–27 | WOG 28–30 | Age | Weight | Height | ||
|---|---|---|---|---|---|---|---|---|---|
| B2B-BPV | SBP-SD | Coeff | −0.751 | 0.595 | 0.7 | 1.02 | 0.004 | −0.001 | 0.001 |
| p-val | 0.78 | 0.058 | 0.021 | 0.002 | 0.832 | 0.937 | 0.967 | ||
| DBP-SD | Coeff | −0.468 | 0.266 | 0.536 | 0.619 | 0.002 | −0.001 | 0.001 | |
| p-val | 0.808 | 0.23 | 0.014 | 0.007 | 0.864 | 0.913 | 0.959 | ||
| SBP-ARV | Coeff | −0.033 | 0.007 | 0.003 | 0.069 | 0.000 | −0.000 | 0.000 | |
| p-val | 0.953 | 0.909 | 0.961 | 0.302 | 0.923 | 0.997 | 0.996 | ||
| DBP-ARV | Coeff | −0.073 | −0.005 | 0.07 | 0.124 | 0.001 | −0.000 | 0.000 | |
| p-val | 0.885 | 0.939 | 0.222 | 0.04 | 0.872 | 0.936 | 0.97 | ||
| B2B-BPF | Γx | Coeff | −0.444 | 0.151 | 0.278 | 0.774 | 0.004 | −0.000 | 0.000 |
| p-val | 0.928 | 0.793 | 0.622 | 0.189 | 0.914 | 0.981 | 0.989 | ||
| Γy | Coeff | −1.125 | 0.852 | 1.163 | 1.491 | 0.006 | −0.001 | 0.001 | |
| p-val | 0.773 | 0.058 | 0.008 | 0.001 | 0.834 | 0.924 | 0.964 | ||
| Γ | Coeff | −1.041 | 0.568 | 0.877 | 1.592 | 0.007 | −0.001 | 0.001 | |
| p-val | 0.852 | 0.384 | 0.165 | 0.019 | 0.876 | 0.965 | 0.977 | ||
| Parameter | WOG 21–23 | WOG 24–25 | WOG 26–27 | WOG 28–30 | Slope | R2 | p-Value | |
|---|---|---|---|---|---|---|---|---|
| B2B-BPV | SBP-SD | −0.554 | 0.04 | 0.145 | 0.461 | 0.319 | 0.199 | 0.002 |
| DBP-SD | −0.342 | −0.077 | 0.192 | 0.273 | 0.215 | 0.191 | 0.003 | |
| SBP-ARV | −0.018 | −0.01 | −0.015 | 0.051 | 0.019 | 0.059 | 0.362 | |
| DBP-ARV | −0.045 | −0.049 | 0.025 | 0.079 | 0.044 | 0.148 | 0.021 | |
| B2B-BPF | Γy | −0.843 | 0.008 | 0.318 | 0.641 | 0.485 | 0.211 | 0.001 |
| Γx | −0.279 | −0.13 | −0.002 | 0.489 | 0.237 | 0.084 | 0.196 | |
| Γ | −0.719 | −0.153 | 0.156 | 0.864 | 0.502 | 0.156 | 0.016 | |
| Parameter | WOG 21–23 | WOG 24–25 | WOG 26–27 | WOG 28–30 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coeff | p-Value | Coeff | p-Value | Coeff | p-Value | Coeff | p-Value | |||
| B2B-BPV | SBP-SD | Intercept | 14.78 | <0.05 | 7.19 | 0.39 | 4.83 | 0.36 | 8.47 | 0.21 |
| I3 | 0.86 | <0.05 | 0.09 | 0.81 | −0.34 | 0.28 | −0.34 | 0.31 | ||
| I5 | 0.68 | 0.03 | −0.34 | 0.37 | −0.54 | 0.08 | −0.34 | 0.3 | ||
| I7 | 0.84 | 0.01 | 1.27 | <0.05 | 0.05 | 0.87 | −0.11 | 0.76 | ||
| DBP-SD | Intercept | 8.98 | 0.03 | 3.07 | 0.52 | 0.4 | 0.92 | 6.07 | 0.21 | |
| I3 | 0.63 | 0.01 | 0.25 | 0.34 | −0.31 | 0.17 | −0.21 | 0.38 | ||
| I5 | 0.83 | <0.05 | 0.2 | 0.44 | −0.23 | 0.29 | −0.04 | 0.88 | ||
| I7 | 1.02 | <0.05 | 1.27 | <0.05 | 0.14 | 0.56 | 0.14 | 0.56 | ||
| SBP-ARV | Intercept | 3.6 | 0.09 | 1.04 | 0.59 | −0.17 | 0.93 | 3.11 | 0.2 | |
| I3 | 0.04 | 0.58 | 0.03 | 0.71 | −0.1 | 0.11 | −0.05 | 0.63 | ||
| I5 | 0.11 | 0.09 | 0.08 | 0.23 | −0.08 | 0.23 | −0.04 | 0.71 | ||
| I7 | 0.37 | <0.05 | 0.44 | <0.05 | 0.22 | <0.05 | 0.45 | <0.05 | ||
| DBP-ARV | Intercept | 1.91 | 0.22 | −0.46 | 0.73 | −0.32 | 0.84 | 1.87 | 0.33 | |
| I3 | 0.1 | 0.09 | 0.09 | 0.13 | −0.08 | 0.22 | −0.04 | 0.66 | ||
| I5 | 0.17 | <0.05 | 0.1 | 0.11 | −0.01 | 0.88 | <0.05 | 0.99 | ||
| I7 | 0.39 | <0.05 | 0.46 | <0.05 | 0.25 | <0.05 | 0.34 | 0 | ||
| B2B-BPF | Γx | Intercept | 17.69 | 0.02 | 4.55 | 0.69 | 11.11 | 0.59 | 12.47 | 0.28 |
| I3 | 1.93 | 0.01 | 3.15 | <0.05 | 1.58 | 0.29 | 2.3 | <0.05 | ||
| I5 | 2.43 | <0.05 | 3.37 | <0.05 | 3.84 | 0.01 | 4.08 | <0.05 | ||
| I7 | 5.66 | <0.05 | 6.79 | <0.05 | 6.58 | <0.05 | 5.16 | <0.05 | ||
| Γy | Intercept | 26.62 | 0.03 | 15.28 | 0.25 | 4.43 | 0.6 | 4.24 | 0.75 | |
| I3 | 1.93 | <0.05 | 1.18 | 0.07 | 0.17 | 0.7 | 0.4 | 0.48 | ||
| I5 | 2.11 | <0.05 | 0.66 | 0.31 | 0.03 | 0.96 | 0.91 | 0.11 | ||
| I7 | 1.19 | 0.02 | 2.14 | <0.05 | 0.04 | 0.93 | −0.13 | 0.83 | ||
| Γ | Intercept | 30.73 | 0.01 | 14.29 | 0.36 | 11.74 | 0.59 | 12.87 | 0.34 | |
| I3 | 2.76 | <0.05 | 3.38 | <0.05 | 1.37 | 0.35 | 2.09 | 0.02 | ||
| I5 | 3.3 | <0.05 | 3.2 | <0.05 | 3.36 | 0.02 | 4.07 | <0.05 | ||
| I7 | 5.69 | <0.05 | 6.86 | <0.05 | 5.93 | <0.05 | 4.41 | <0.05 | ||
| WOG 21–23 | WOG 24–25 | WOG 26–27 | WOG 28–30 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coeff | p-Value | Coeff | p-Value | Coeff | p-Value | Coeff | p-Value | |||
| B2B-BPV | SBP-SD | Intercept | 12.1 | 0.03 | 3.98 | 0.54 | 10.41 | 0.04 | 9.32 | 0.13 |
| I4 | −1.6 | <0.05 | −1.39 | <0.05 | −0.91 | <0.05 | −1.05 | <0.05 | ||
| I5 | −0.18 | 0.48 | −0.43 | 0.2 | −0.2 | 0.35 | −0.01 | 0.98 | ||
| BDP-SD | Intercept | 8.36 | 0.06 | 0.91 | 0.77 | 3.98 | 0.15 | 5.15 | 0.24 | |
| I4 | −1.35 | <0.05 | −1.24 | <0.05 | −1.05 | <0.05 | −0.98 | <0.05 | ||
| I5 | 0.21 | 0.29 | −0.05 | 0.79 | 0.08 | 0.58 | 0.17 | 0.47 | ||
| SBP-ARV | Intercept | 2.4 | 0.26 | 0.23 | 0.9 | 0.09 | 0.96 | 2.68 | 0.21 | |
| I4 | 0.27 | <0.05 | 0.28 | <0.05 | 0.28 | <0.05 | 0.19 | 0.02 | ||
| I5 | 0.07 | 0.26 | 0.06 | 0.46 | 0.03 | 0.66 | 0.01 | 0.88 | ||
| DBP-ARV | Intercept | 1.35 | 0.37 | −0.37 | 0.77 | 0.44 | 0.75 | 1.62 | 0.36 | |
| I4 | −0.04 | 0.48 | −0.08 | 0.22 | −0.02 | 0.71 | −0.05 | 0.51 | ||
| I5 | 0.07 | 0.19 | 0 | 0.96 | 0.07 | 0.25 | 0.04 | 0.65 | ||
| B2B-BPF | Γx | Intercept | 26.78 | <0.05 | 7.57 | 0.56 | 16.51 | 0.25 | −0.51 | 0.97 |
| I4 | −1.55 | <0.05 | −1.99 | <0.05 | −1.82 | 0.1 | −1.56 | 0.04 | ||
| I5 | 0.5 | 0.27 | 0.22 | 0.73 | 2.26 | 0.04 | 1.77 | 0.02 | ||
| Γy | Intercept | 25.01 | 0.05 | 10.15 | 0.24 | 13.36 | 0.08 | 4.97 | 0.7 | |
| I4 | −2.91 | <0.05 | −2.84 | <0.05 | −2.19 | <0.05 | −1.96 | <0.05 | ||
| I5 | 0.18 | 0.71 | −0.51 | 0.22 | −0.15 | 0.64 | 0.5 | 0.39 | ||
| Γ | Intercept | 37.93 | <0.05 | 11.49 | 0.4 | 20.73 | 0.19 | 0.52 | 0.98 | |
| I4 | −2.94 | <0.05 | −3.22 | <0.05 | −2.67 | 0.01 | −2.29 | 0.01 | ||
| I5 | 0.54 | 0.34 | −0.18 | 0.78 | 1.99 | 0.06 | 1.97 | 0.02 | ||
| Parameter (Unit) | Our Results (Intervention 1) | [10] | ||||
|---|---|---|---|---|---|---|
| WOG 21–23 | WOG 24–25 | WOG 26–27 | WOG 28–30 | Baseline | ||
| B2B-BPV | SBP-SD (mmHg) | 5.7 ± 1.91 | 6.3 ± 1.77 | 6.38 ± 2.23 | 6.78 ± 2.17 | 5.35 ± 1.28 |
| DBP-SD (mmHg) | 3.68 ± 1.36 | 3.96 ± 1.17 | 4.24 ± 1.82 | 4.38 ± 1.36 | 3.78 ± 0.85 | |
| SBP-ARV (mmHg) | 2.11 ± 0.64 | 2.13 ± 0.51 | 2.1 ± 0.65 | 2.15 ± 0.47 | 1.68 ± 0.38 | |
| DBP-ARV (mmHg) | 1.5 ± 0.47 | 1.51 ± 0.38 | 1.58 ± 0.48 | 1.65 ± 0.38 | 1.39 ± 0.45 | |
| B2B-BPV | Γx (n.u.) | 13.23 ± 3.25 | 13.4 ± 3.39 | 13.61 ± 4.08 | 14.03 ± 2.97 | - |
| Γy (n.u.) | 8.01 ± 2.89 | 8.77 ± 2.79 | 9.15 ± 3.73 | 9.76 ± 3.42 | - | |
| Γ (n.u.) | 15.59 ± 3.85 | 16.17 ± 3.78 | 16.56 ± 5.00 | 17.34 ± 3.45 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmermann, D.; Malberg, H.; Schmidt, M. Novel Metric for Non-Invasive Beat-to-Beat Blood Pressure Measurements Demonstrates Physiological Blood Pressure Fluctuations during Pregnancy. Sensors 2024, 24, 3151. https://doi.org/10.3390/s24103151
Zimmermann D, Malberg H, Schmidt M. Novel Metric for Non-Invasive Beat-to-Beat Blood Pressure Measurements Demonstrates Physiological Blood Pressure Fluctuations during Pregnancy. Sensors. 2024; 24(10):3151. https://doi.org/10.3390/s24103151
Chicago/Turabian StyleZimmermann, David, Hagen Malberg, and Martin Schmidt. 2024. "Novel Metric for Non-Invasive Beat-to-Beat Blood Pressure Measurements Demonstrates Physiological Blood Pressure Fluctuations during Pregnancy" Sensors 24, no. 10: 3151. https://doi.org/10.3390/s24103151







