Amphibious Multifunctional Hydrogel Flexible Haptic Sensor with Self-Compensation Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of DN-SPEZ Hydrogels
2.3. Formation of Hydrogels
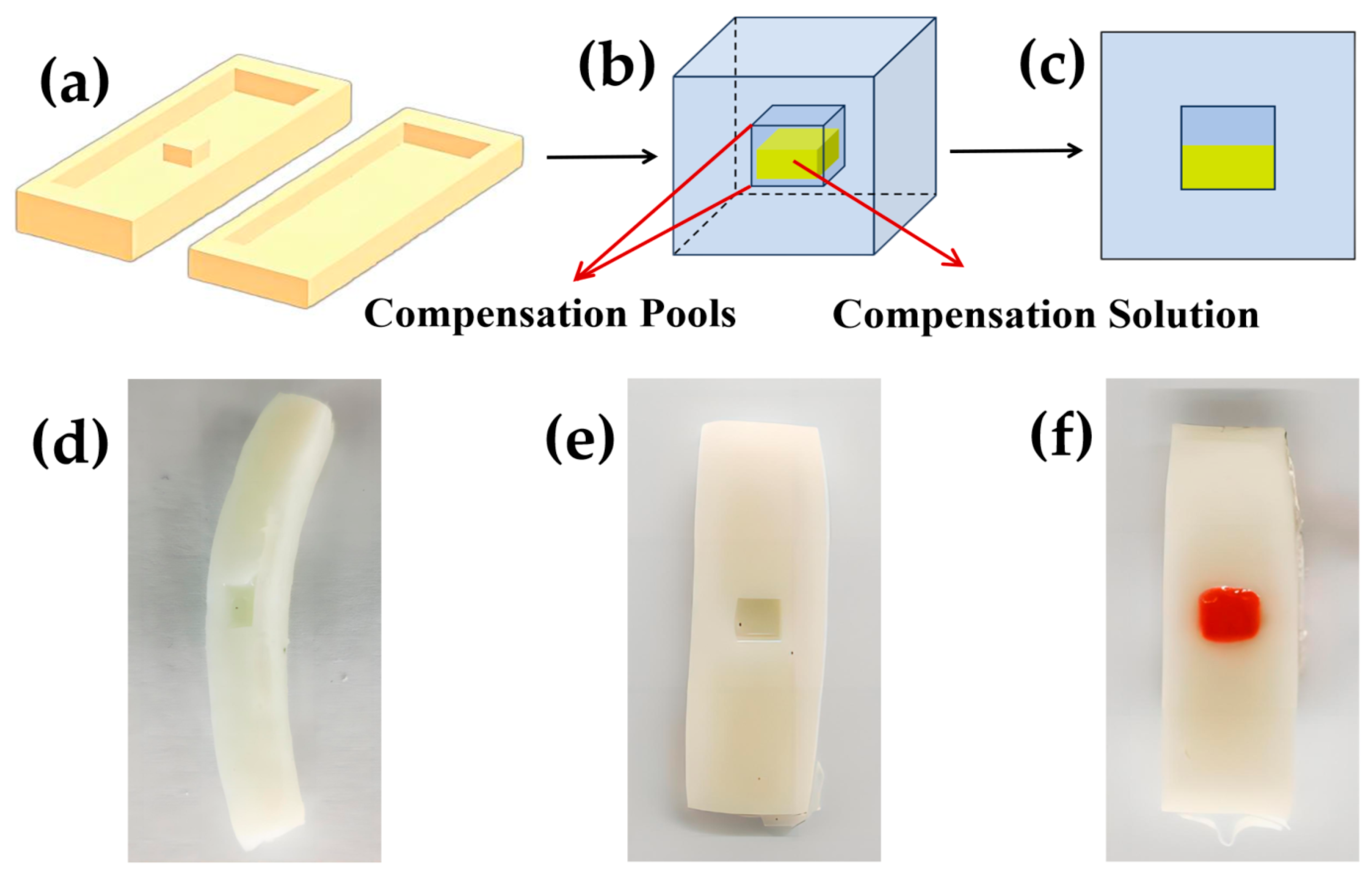
2.4. Implementation of a Self-Calibrating Compensation Strategy
3. Results
3.1. Mechanical Properties of DN-SPEZ Hydrogels
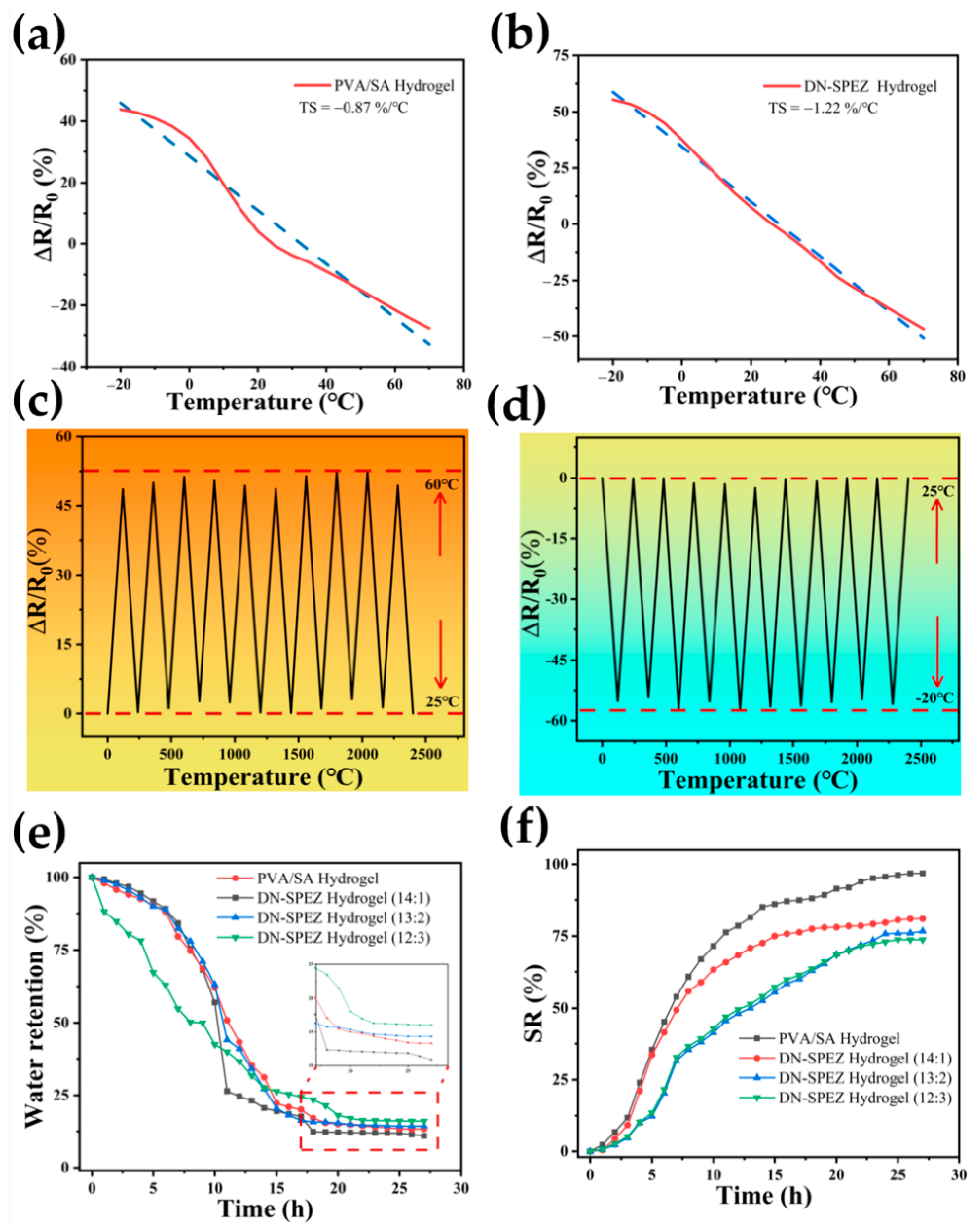
3.2. Physical Properties of DN-SPEZ Hydrogels
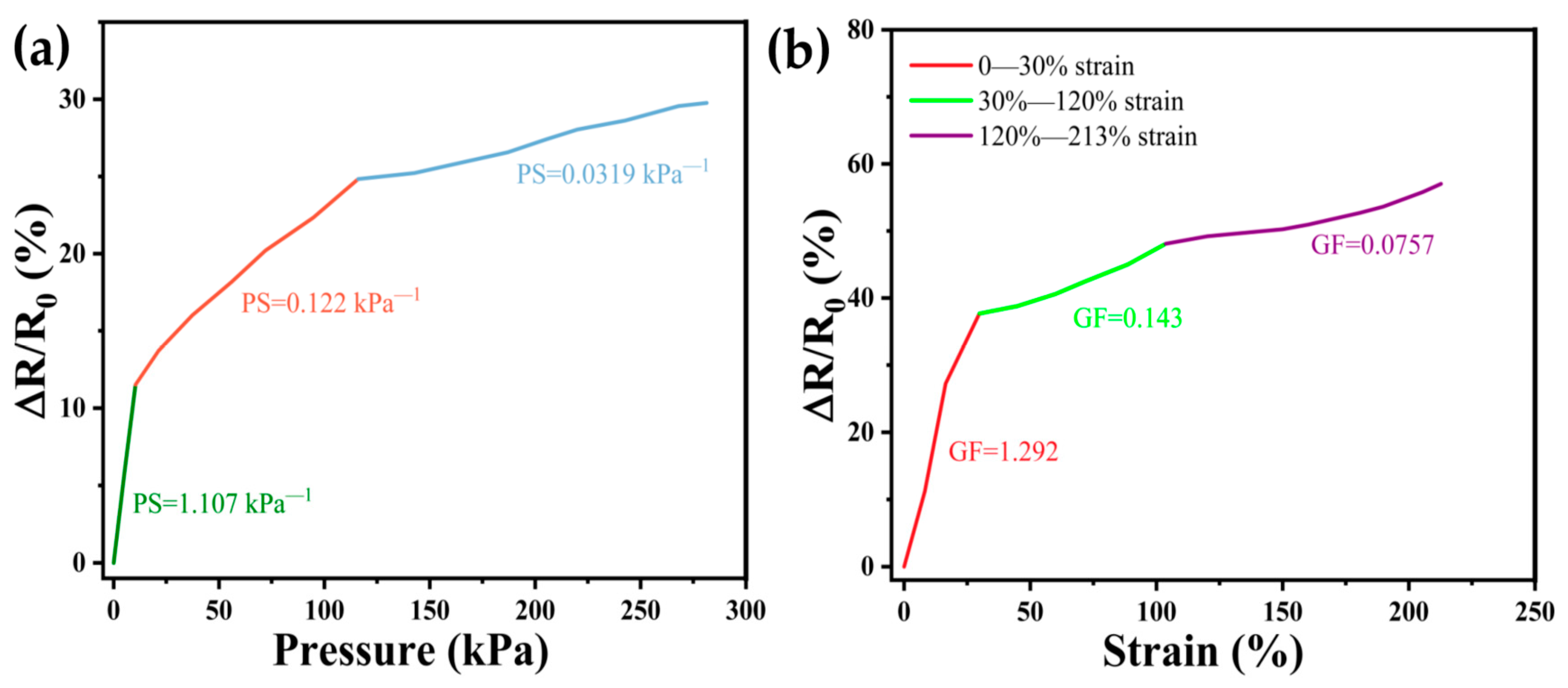
3.3. Pressure Sensitivity and Strain Sensitivity
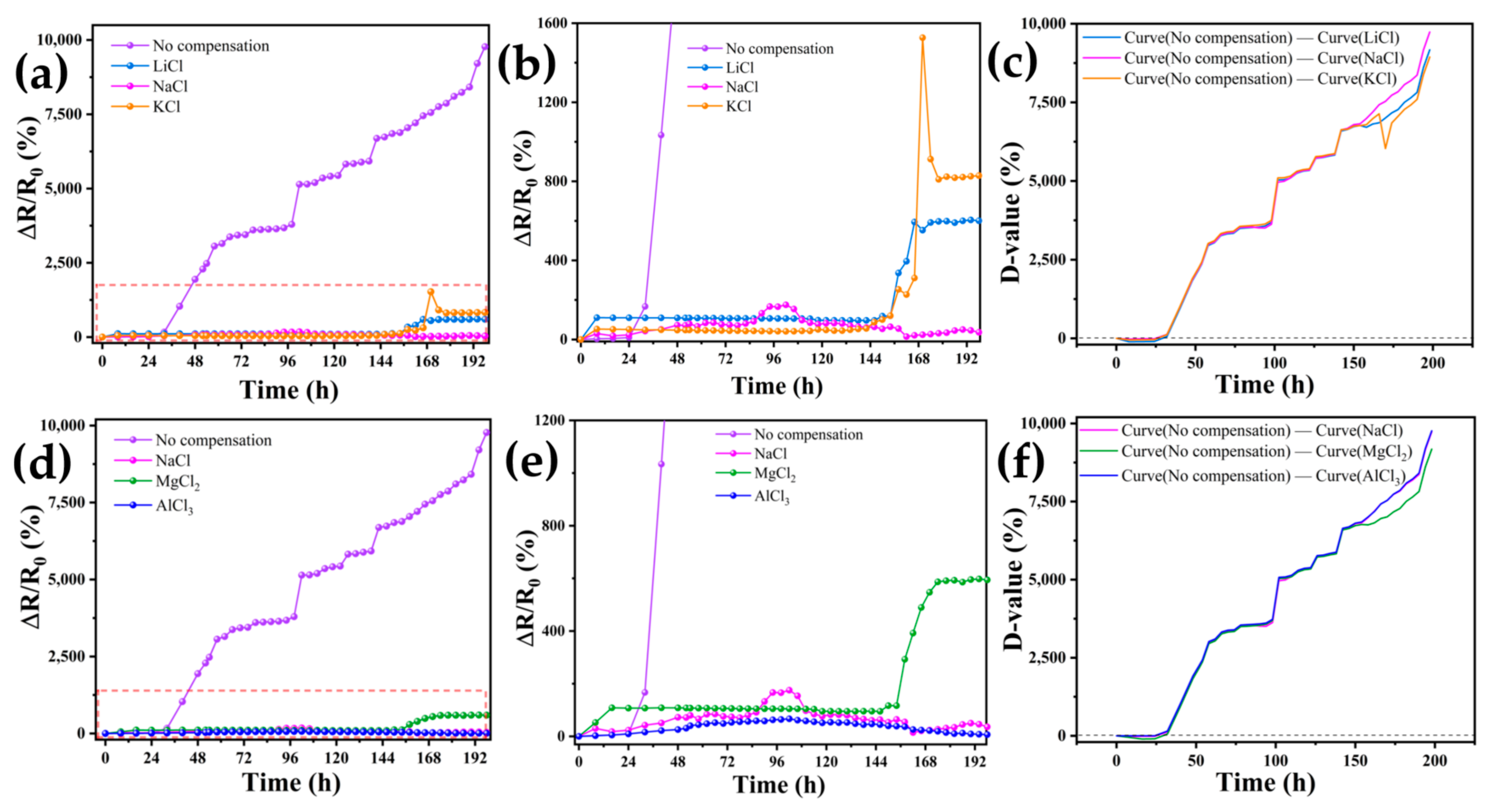
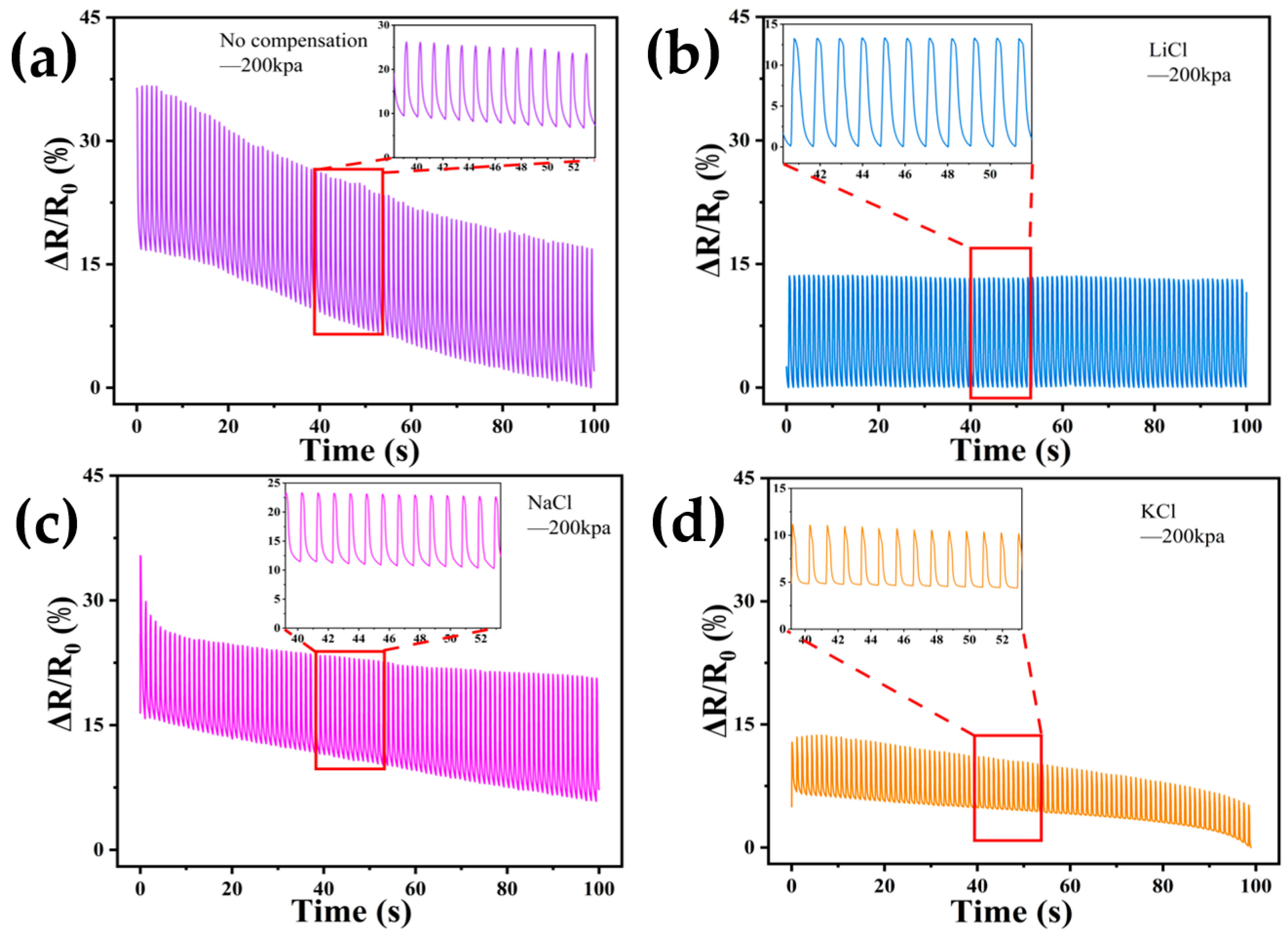
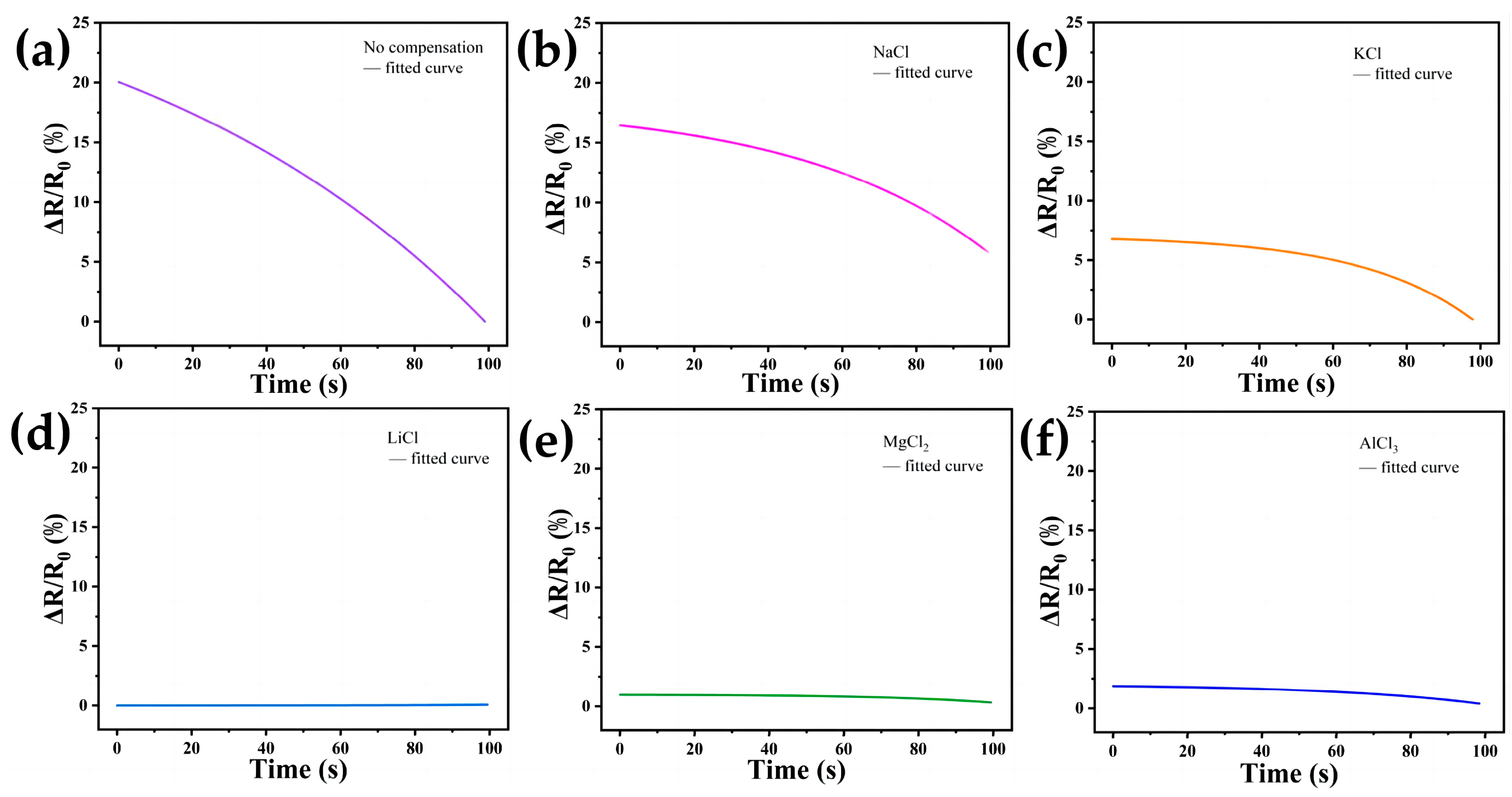
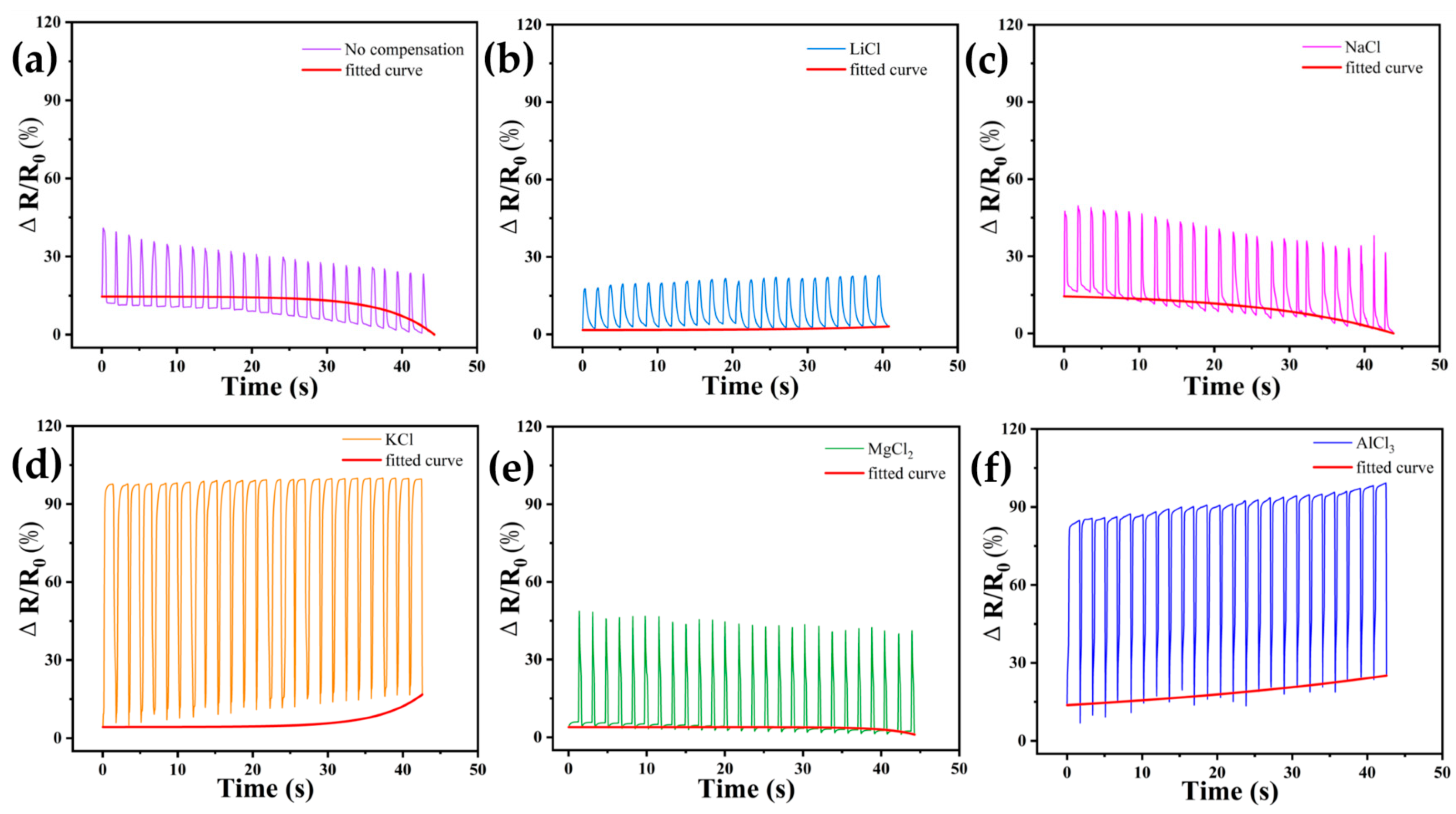
3.4. Compensation Effects of Performing Self-Calibrating Compensation Strategies Using Different Solutions
3.5. Application Effect of DN-SPEZ Hydrogel Self-Calibrating Compensation Sensor
3.6. Multi-Scenario Application of DN-SPEZ Hydrogel Self-Calibrating Compensation Sensor
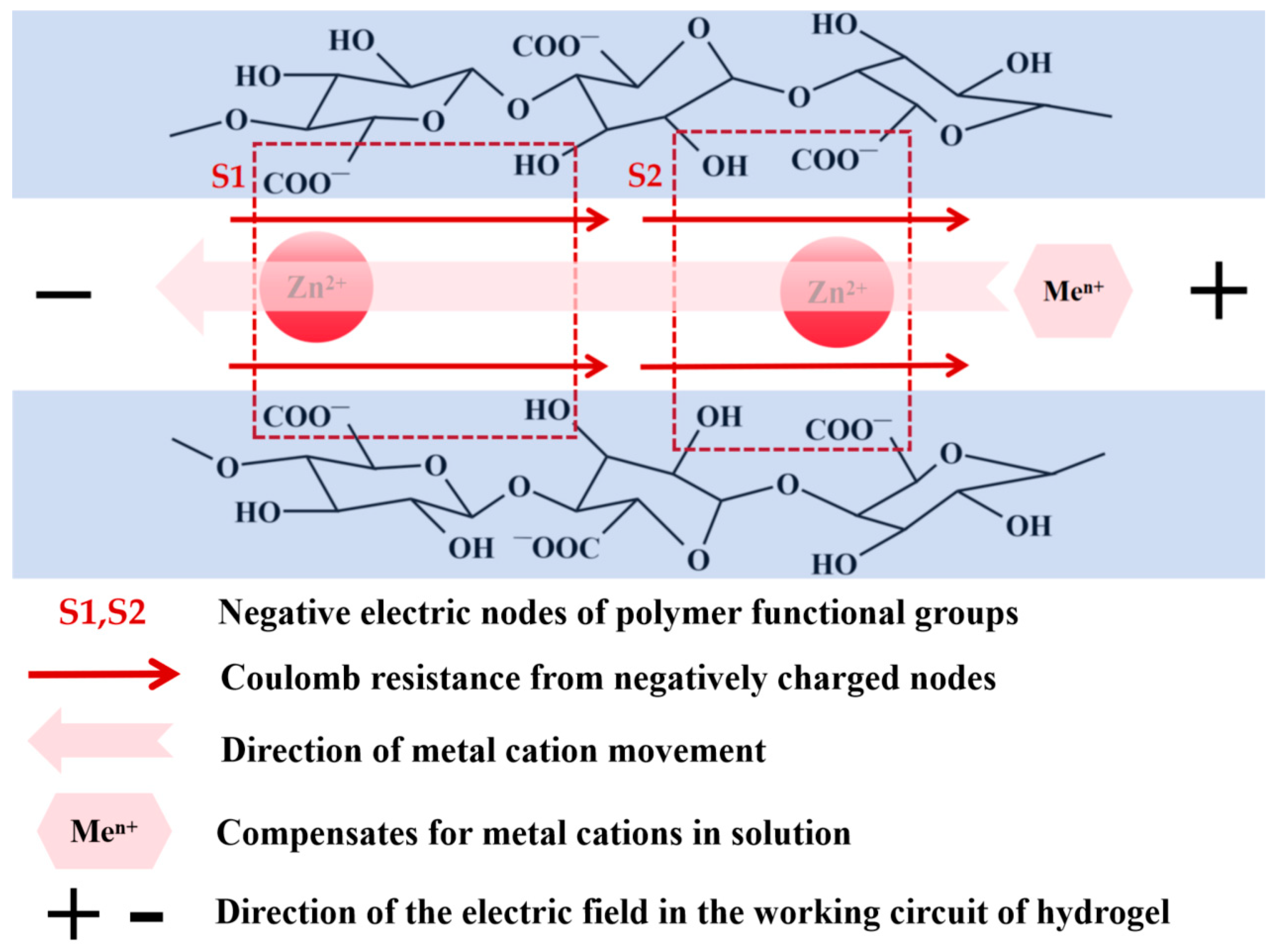
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Song, H.; Chen, L.; Li, W.; Yang, D.; Cheng, P.; Duan, H. 3D Printed Ultrasensitive Graphene Hydrogel Self-Adhesive Wearable Devices. ACS Appl. Electron. Mater. 2022, 4, 5199–5207. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.; Luo, J.; Ren, G.; Wang, J.; Chen, Y.; Jia, P. Ultrastretchable, Adhesive, Anti-Freezing, Conductive, and Self-Healing Hydrogel for Wearable Devices. ACS Appl. Polym. Mater. 2022, 4, 1784–1793. [Google Scholar] [CrossRef]
- An, R.; Zhang, B.; Han, L.; Wang, X.; Zhang, Y.; Shi, L.; Ran, R. Strain-Sensitivity Conductive MWCNTs Composite Hydrogel for Wearable Device and near-Infrared Photosensor. J. Mater. Sci. 2019, 54, 8515–8530. [Google Scholar] [CrossRef]
- Ying, B.; Liu, X. Skin-like Hydrogel Devices for Wearable Sensing, Soft Robotics and Beyond. iScience 2021, 24, 103174. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, J.; Chen, Y.; Zheng, X.; Liu, H.; Li, H. Multiple-Stimuli-Responsive and Cellulose Conductive Ionic Hydrogel for Smart Wearable Devices and Thermal Actuators. ACS Appl. Mater. Interfaces 2021, 13, 1353–1366. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, Y.; Hedenqvist, M.S.; Chen, C.; Cai, C.; Li, H.; Liu, H.; Fu, J. Multifunctional Conductive Hydrogels and Their Applications as Smart Wearable Devices. J. Mater. Chem. B 2021, 9, 2561–2583. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, T.; Zhang, X. Multifunctional Conductive Hydrogel-Based Flexible Wearable Sensors. TrAC Trends Anal. Chem. 2021, 134, 116130. [Google Scholar] [CrossRef]
- Guo, Y.; Yin, F.; Li, Y.; Shen, G.; Lee, J. Incorporating Wireless Strategies to Wearable Devices Enabled by a Photocurable Hydrogel for Monitoring Pressure Information. Adv. Mater. 2023, 35, 2300855. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Liu, Z.; Xiang, X. Graphene Oxide Composite Hydrogels for Wearable Devices. Carbon Lett. 2022, 32, 1395–1410. [Google Scholar] [CrossRef]
- Garg, V.; Gupta, T.; Rani, S.; Bandyopadhyay-Ghosh, S.; Ghosh, S.B.; Qiao, L.; Liu, G. A Hierarchically Designed Nanocomposite Hydrogel with Multisensory Capabilities towards Wearable Devices for Human-Body Motion and Glucose Concentration Detection. Compos. Sci. Technol. 2021, 213, 108894. [Google Scholar] [CrossRef]
- Han, F.; Xie, X.; Wang, T.; Cao, C.; Li, J.; Sun, T.; Liu, H.; Geng, S.; Wei, Z.; Li, J.; et al. Wearable Hydrogel-Based Epidermal Sensor with Thermal Compatibility and Long Term Stability for Smart Colorimetric Multi-Signals Monitoring. Adv. Healthc. Mater. 2023, 12, 2201730. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Ke, T.; Liu, W.; Ren, Z.; Zhao, L.; Gu, H. Tough, Repeatedly Adhesive, Cyclic Compression-Stable, and Conductive Dual-Network Hydrogel Sensors for Human Health Monitoring. Ind. Eng. Chem. Res. 2021, 60, 18373–18383. [Google Scholar] [CrossRef]
- Xue, G.; Shi, Y.; Wang, S.; Zhou, H.; Chen, Z.; Guo, W.; Yang, Y.; Ye, M. Merkel Cell-Inspired Skin-like Hybrid Hydrogels for Wearable Health Monitoring. Chem. Eng. J. 2023, 456, 140976. [Google Scholar] [CrossRef]
- Hua, J.; Su, M.; Sun, X.; Li, J.; Sun, Y.; Qiu, H.; Shi, Y.; Pan, L. Hydrogel-Based Bioelectronics and Their Applications in Health Monitoring. Biosensors 2023, 13, 696. [Google Scholar] [CrossRef] [PubMed]
- Tejavibulya, N.; Colburn, D.A.M.; Marcogliese, F.A.; Yang, K.-A.; Guo, V.; Chowdhury, S.; Stojanovic, M.N.; Sia, S.K. Hydrogel Microfilaments toward Intradermal Health Monitoring. iScience 2019, 21, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Hu, Y.; Shi, S.; Wang, S.; Li, H.; Wang, Y.; Zhou, K. Hydrogel Bioelectronics for Health Monitoring. Biosensors 2023, 13, 815. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Hong, B.; Yan, X.; Wu, H.; Zhong, Q.; Qi, D.; Wang, W.; Lei, L.; Fan, H.; Müller-Buschbaum, P. Hybrid Hydrogel-Based Skin Health Monitor for Tracing Solar UV Radiation in Aqueous Environments. ACS Appl. Polym. Mater. 2023, 5, 7528–7538. [Google Scholar] [CrossRef]
- Xu, J.; Huang, H.; Sun, C.; Yu, J.; Wang, M.; Dong, T.; Wang, S.; Chen, X.; Cui, T.; Li, J. Flexible Accelerated-Wound-Healing Antibacterial Hydrogel-Nanofiber Scaffold for Intelligent Wearable Health Monitoring. ACS Appl. Mater. Interfaces 2024, 16, 5438–5450. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ding, Q.; Wang, H.; Wu, Z.; Gui, X.; Li, C.; Hu, N.; Tao, K.; Wu, J. Engineering Smart Composite Hydrogels for Wearable Disease Monitoring. Nano-Micro Lett. 2023, 15, 105. [Google Scholar] [CrossRef]
- Lei, H.; Zhao, J.; Ma, X.; Li, H.; Fan, D. Antibacterial Dual Network Hydrogels for Sensing and Human Health Monitoring. Adv. Healthc. Mater. 2021, 10, 2101089. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, Y.-L.; Tang, W.-J.; Wu, T.-J.; Huang, P.; Yu, Q.; Xu, L.; Zhang, X.-S. An Ultra-Thin Transparent Multi-Functional Sensor Based on Silk Hydrogel for Health Monitoring. J. Micromech. Microeng. 2022, 32, 084003. [Google Scholar] [CrossRef]
- Shen, B.; Li, J.; Tang, Y.; Xu, H.; Li, F. An Ultra-Stretchable Sensitive Hydrogel Sensor for Human Motion and Pulse Monitoring. Micromachines 2021, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Sheng, F.; Yi, J.; Shen, S.; Cheng, R.; Ning, C.; Ma, L.; Peng, X.; Deng, W.; Dong, K.; Wang, Z.L. Self-Powered Smart Arm Training Band Sensor Based on Extremely Stretchable Hydrogel Conductors. ACS Appl. Mater. Interfaces 2021, 13, 44868–44877. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Li, Y.; Chen, B.; Chen, X.; Han, Y.; Guo, M.; Xia, H.; Song, R.; Zhang, X.; Zhou, J. In Situ Forming Epidermal Bioelectronics for Daily Monitoring and Comprehensive Exercise. ACS Nano 2022, 16, 17931–17947. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, T.; Zhang, Z.; Xu, P.; Ji, X.; Xu, M. Flexible and Wearable Hydrogel-Based Triboelectric Nanogenerator for Self-Power Sensing in Boxing Match. In Proceedings of the 2021 China Automation Congress (CAC), Beijing, China, 22–24 October 2021; IEEE: Beijing, China, 2021; pp. 3623–3628. [Google Scholar] [CrossRef]
- Zou, J.; Chen, Z.; Wang, S.-J.; Mi, H.-Y.; Hu, X.-S.; Zhang, Z.; Shang, Y.-H.; Jing, X. A Stretchable and Zigzag Structured Hydrogel for Highly Sensitive Strain Sensors. Mater. Lett. 2022, 325, 132835. [Google Scholar] [CrossRef]
- Sun, F.; Zhu, Y.; Jia, C.; Ouyang, B.; Zhao, T.; Li, C.; Ba, N.; Li, X.; Chen, S.; Che, T.; et al. A Flexible Lightweight Triboelectric Nanogenerator for Protector and Scoring System in Taekwondo Competition Monitoring. Electronics 2022, 11, 1306. [Google Scholar] [CrossRef]
- Cho, H.-J.; Kim, I.-D.; Jung, S.M. Multifunctional Inorganic Nanomaterial Aerogel Assembled into fSWNT Hydrogel Platform for Ultraselective NO2 Sensing. ACS Appl. Mater. Interfaces 2020, 12, 10637–10647. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, S.; Yoon, I.; Kook, G.; Jung, Y.; Bawazir, S.; Stefanini, C.; Lee, H. Polypyrrole/Agarose Hydrogel-Based Bladder Volume Sensor with a Resistor Ladder Structure. Sensors 2018, 18, 2288. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, J.; Li, L.; Xu, L.; Feng, N.; Wang, Y.; Fei, X.; Tian, J.; Li, Y. Synthesis of a Novel Anti-Freezing, Non-Drying Antibacterial Hydrogel Dressing by One-Pot Method. Chem. Eng. J. 2019, 372, 216–225. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, X.; Lin, C.; Lin, D.; Ni, Y.; Chen, L.; Huang, L.; Cao, S.; Ma, X. Biocompatible, Self-Wrinkled, Antifreezing and Stretchable Hydrogel-Based Wearable Sensor with PEDOT: Sulfonated Lignin as Conductive Materials. Chem. Eng. J. 2019, 370, 1039–1047. [Google Scholar] [CrossRef]
- Bao, D.; Wen, Z.; Shi, J.; Xie, L.; Jiang, H.; Jiang, J.; Yang, Y.; Liao, W.; Sun, X. An Anti-Freezing Hydrogel Based Stretchable Triboelectric Nanogenerator for Biomechanical Energy Harvesting at Sub-Zero Temperature. J. Mater. Chem. A 2020, 8, 13787–13794. [Google Scholar] [CrossRef]
- Yu, B.; Wang, C.; Ju, Y.M.; West, L.; Harmon, J.; Moussy, Y.; Moussy, F. Use of Hydrogel Coating to Improve the Performance of Implanted Glucose Sensors. Biosens. Bioelectron. 2008, 23, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-Y.; Moon, S.E.; Kim, J.H.; Kang, S.M. Ultrasensitive and Highly Stretchable Multiple-Crosslinked Ionic Hydrogel Sensors with Long-Term Stability. Nano-Micro Lett. 2023, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ye, L.; Sun, X.; Lv, Q.; Liang, H. Tough and Stretchable Dual Ionically Cross-Linked Hydrogel with High Conductivity and Fast Recovery Property for High-Performance Flexible Sensors. ACS Appl. Mater. Interfaces 2020, 12, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yao, F.; Li, J. Nanocomposite Hydrogel-Based Strain and Pressure Sensors: A Review. J. Mater. Chem. A 2020, 8, 18605–18623. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Lee, K.H.; Anjum, D.H.; Sougrat, R.; Jiang, Q.; Kim, H.; Alshareef, H.N. MXenes Stretch Hydrogel Sensor Performance to New Limits. Sci. Adv. 2018, 4, eaat0098. [Google Scholar] [CrossRef] [PubMed]
- Mitala, J.J.; Michael, A.C. Improving the Performance of Electrochemical Microsensors Based on Enzymes Entrapped in a Redox Hydrogel. Anal. Chim. Acta 2006, 556, 326–332. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Han, S.; Yang, B.-R.; Gui, X.; Tao, K.; Liu, C.; Miao, J.; Norford, L.K. Extremely Deformable, Transparent, and High-Performance Gas Sensor Based on Ionic Conductive Hydrogel. ACS Appl. Mater. Interfaces 2019, 11, 2364–2373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xue, W.; Dai, Y.; Wu, L.; Liao, B.; Zeng, W.; Tao, X. Double Network Hydrogel Sensors with High Sensitivity in Large Strain Range. Macromol. Mater. Eng. 2021, 306, 2100486. [Google Scholar] [CrossRef]
- Ma, Z.; Shi, W.; Yan, K.; Pan, L.; Yu, G. Doping Engineering of Conductive Polymer Hydrogels and Their Application in Advanced Sensor Technologies. Chem. Sci. 2019, 10, 6232–6244. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, X.; Wu, J. Conductive Hydrogel- and Organohydrogel-Based Stretchable Sensors. ACS Appl. Mater. Interfaces 2021, 13, 2128–2144. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tao, K.; Zhang, J.; Guo, Y.; Miao, J.; Norford, L.K. Chemically Functionalized 3D Graphene Hydrogel for High Performance Gas Sensing. J. Mater. Chem. A 2016, 4, 8130–8140. [Google Scholar] [CrossRef]
- Rahmani, P.; Shojaei, A. A Review on the Features, Performance and Potential Applications of Hydrogel-Based Wearable Strain/Pressure Sensors. Adv. Colloid Interface Sci. 2021, 298, 102553. [Google Scholar] [CrossRef] [PubMed]
- Binder, S.; Gerlach, G. Intramolecular Force-Compensated Hydrogel-Based Sensors with Reduced Response Times. TM—Tech. Mess. 2019, 86, 227–236. [Google Scholar] [CrossRef]
- Binder, S.; Gerlach, G. Performance of Force-Compensated Chemical Sensors Based on Bisensitive Hydrogels. Sens. Actuators B Chem. 2021, 342, 129420. [Google Scholar] [CrossRef]
- Binder, S.; Gerlach, G. Funktionsprinzip und Anwendung der Kraftkompensationsmessmethode für miniaturisierte hydrogelbasierte Sensoren. TM—Tech. Mess. 2022, 89, 465–477. [Google Scholar] [CrossRef]
- Lee, J.; Le, Q.T.; Lee, D.; Nam, S.; Nguyen, T.H.; Song, Y.; Sung, J.; Son, S.-W.; Kim, J. Micropyramidal Flexible Ion Gel Sensor for Multianalyte Discrimination and Strain Compensation. ACS Appl. Mater. Interfaces 2023, 15, 26138–26147. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, W.; Ding, H.; Zhong, B.; Huang, W.; Zhou, Y.; Gui, X.; Xie, X.; Wu, J. Ultrastable, Stretchable, Highly Conductive and Transparent Hydrogels Enabled by Salt-Percolation for High-Performance Temperature and Strain Sensing. J. Mater. Chem. C 2021, 9, 13668–13679. [Google Scholar] [CrossRef]
- Mo, F.; Chen, Z.; Liang, G.; Wang, D.; Zhao, Y.; Li, H.; Dong, B.; Zhi, C. Zwitterionic Sulfobetaine Hydrogel Electrolyte Building Separated Positive/Negative Ion Migration Channels for Aqueous Zn-MnO 2 Batteries with Superior Rate Capabilities. Adv. Energy Mater. 2020, 10, 2000035. [Google Scholar] [CrossRef]
- Zhu, K.; Luo, J.; Zhang, D.; Wang, N.; Pan, S.; Zhou, S.; Zhang, Z.; Guo, G.; Yang, P.; Fan, Y.; et al. Molecular Engineering Enables Hydrogel Electrolyte with Ionic Hopping Migration and Self-Healability toward Dendrite-Free Zinc-Metal Anodes. Adv. Mater. 2024, 36, 2311082. [Google Scholar] [CrossRef]
- Tian, C.; Wang, J.; Sun, R.; Ali, T.; Wang, H.; Xie, B.; Zhong, Y.; Hu, Y. Improved Interfacial Ion Migration and Deposition through the Chain-Liquid Synergistic Effect by a Carboxylated Hydrogel Electrolyte for Stable Zinc Metal Anodes. Angew. Chem. Int. Ed. 2023, 62, e202310970. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Sun, X.; Li, Q.; Wu, J.; Lin, J. Fabrication of a High-Strength Hydrogel with an Interpenetrating Network Structure. Colloids Surf. Physicochem. Eng. Asp. 2009, 346, 91–98. [Google Scholar] [CrossRef]
- Butylina, S.; Geng, S.; Oksman, K. Properties of As-Prepared and Freeze-Dried Hydrogels Made from Poly(Vinyl Alcohol) and Cellulose Nanocrystals Using Freeze-Thaw Technique. Eur. Polym. J. 2016, 81, 386–396. [Google Scholar] [CrossRef]
- Liang, Y.; Xue, J.; Du, B.; Nie, J. Ultrastiff, Tough, and Healable Ionic–Hydrogen Bond Cross-Linked Hydrogels and Their Uses as Building Blocks To Construct Complex Hydrogel Structures. ACS Appl. Mater. Interfaces 2019, 11, 5441–5454. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xie, Y.; Xue, C.; Chen, K.; Yang, X.; Xu, L.; Qi, J.; Zhang, D. Preparation of PVA-GO Composite Hydrogel and Effect of Ionic Coordination on Its Properties. Mater. Res. Express 2019, 6, 075306. [Google Scholar] [CrossRef]
- Li, Y.; Hu, C.; Lan, J.; Yan, B.; Zhang, Y.; Shi, L.; Ran, R. Hydrogel-Based Temperature Sensor with Water Retention, Frost Resistance and Remoldability. Polymer 2020, 186, 122027. [Google Scholar] [CrossRef]
- Nam, C.; Zimudzi, T.J.; Geise, G.M.; Hickner, M.A. Increased Hydrogel Swelling Induced by Absorption of Small Molecules. ACS Appl. Mater. Interfaces 2016, 8, 14263–14270. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Jing, X.; Chen, Z.; Wang, S.; Hu, X.; Feng, P.; Liu, Y. Multifunctional Organohydrogel with Ultralow-Hysteresis, Ultrafast-Response, and Whole-Strain-Range Linearity for Self-Powered Sensors. Adv. Funct. Mater. 2023, 33, 2213895. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, J.; Zu, G.; Huang, J. Transparent, Flexible, and Multifunctional Starch-Based Double-Network Hydrogels as High-Performance Wearable Electronics. Carbohydr. Polym. 2021, 267, 118198. [Google Scholar] [CrossRef]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A Stretchable Carbon Nanotube Strain Sensor for Human-Motion Detection. Nat. Nanotechnol. 2011, 6, 296–301. [Google Scholar] [CrossRef]
- Zou, Q.; Zhang, S.; Su, Q.; Xue, T.; Lan, K. Flexible Multimodal Sensor Based on Double-network Hydrogel for Human and Robotic Applications. ChemistrySelect 2023, 8, e202204319. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Guo, Z.; Tang, Z.; Luo, Y.; Xie, S.; Li, N.; Xu, J. Flexible Li+/Agar/pHEAA Double-Network Conductive Hydrogels with Self-Adhesive and Self-Repairing Properties as Strain Sensors for Human Motion Monitoring. React. Funct. Polym. 2021, 168, 105054. [Google Scholar] [CrossRef]
- Sui, X.; Guo, H.; Cai, C.; Li, Q.; Wen, C.; Zhang, X.; Wang, X.; Yang, J.; Zhang, L. Ionic Conductive Hydrogels with Long-Lasting Antifreezing, Water Retention and Self-Regeneration Abilities. Chem. Eng. J. 2021, 419, 129478. [Google Scholar] [CrossRef]
- Tang, L.; Wu, S.; Li, Y.; Jiang, K.; Xu, Y.; Dai, B.; Wang, W.; Tang, J.; Gong, L. A Super-Tough Ionic Conductive Hydrogel with Anti-Freezing, Water Retention, and Self-Regenerated Properties for Self-Powered Flexible Sensor. Appl. Mater. Today 2023, 32, 101820. [Google Scholar] [CrossRef]
- Zhang, S.D.; Zhai, Y.C.; Zhang, Z.F. Preparation and Properties of Polyvinyl Alcohol (PVA)/Polyvinyl Pyrrolidone (PVP) Hydrogel. Appl. Mech. Mater. 2011, 84–85, 485–488. [Google Scholar] [CrossRef]
- Hou, K.; Nie, Y.; Tendo Mugaanire, I.; Guo, Y.; Zhu, M. A Novel Leaf Inspired Hydrogel Film Based on Fiber Reinforcement as Rapid Steam Sensor. Chem. Eng. J. 2020, 382, 122948. [Google Scholar] [CrossRef]
- Qin, Z.; Sun, X.; Yu, Q.; Zhang, H.; Wu, X.; Yao, M.; Liu, W.; Yao, F.; Li, J. Carbon Nanotubes/Hydrophobically Associated Hydrogels as Ultrastretchable, Highly Sensitive, Stable Strain, and Pressure Sensors. ACS Appl. Mater. Interfaces 2020, 12, 4944–4953. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Cao, Y.; Yang, Y.; Yang, Y.; Gao, Y.; Ma, Z.; Wang, J.; Wang, W.; Wu, D. Freezing-Tolerant, Highly Sensitive Strain and Pressure Sensors Assembled from Ionic Conductive Hydrogels with Dynamic Cross-Links. ACS Appl. Mater. Interfaces 2020, 12, 25334–25344. [Google Scholar] [CrossRef] [PubMed]
- Agmon, N. Isoelectronic Theory for Cationic Radii. J. Am. Chem. Soc. 2017, 139, 15068–15073. [Google Scholar] [CrossRef]
- Bouchaala, A.; Jaber, N.; Yassine, O.; Shekhah, O.; Chernikova, V.; Eddaoudi, M.; Younis, M.I. Nonlinear-Based MEMS Sensors and Active Switches for Gas Detection. Sensors 2016, 16, 758. [Google Scholar] [CrossRef]
- Bandari, N.; Dargahi, J.; Packirisamy, M. Miniaturized Optical Force Sensor for Minimally Invasive Surgery with Learning-Based Nonlinear Calibration. IEEE Sens. J. 2020, 20, 3579–3592. [Google Scholar] [CrossRef]
- Lewis, G.; Merken, P.; Vandewal, M. Enhanced Accuracy of CMOS Smart Temperature Sensors by Nonlinear Curvature Correction. Sensors 2018, 18, 4087. [Google Scholar] [CrossRef] [PubMed]
- Berges, J.A.; Franklin, D.J.; Harrison, P.J. Evolution of an artificial seawater medium: Improvements in enriched seawater, artificial water over the last two decades. J. Phycol. 2001, 37, 1138–1145. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Yin, Y.; Liu, B.; Xue, T.; Zou, Q. Amphibious Multifunctional Hydrogel Flexible Haptic Sensor with Self-Compensation Mechanism. Sensors 2024, 24, 3232. https://doi.org/10.3390/s24103232
Sun Z, Yin Y, Liu B, Xue T, Zou Q. Amphibious Multifunctional Hydrogel Flexible Haptic Sensor with Self-Compensation Mechanism. Sensors. 2024; 24(10):3232. https://doi.org/10.3390/s24103232
Chicago/Turabian StyleSun, Zhenhao, Yunjiang Yin, Baoguo Liu, Tao Xue, and Qiang Zou. 2024. "Amphibious Multifunctional Hydrogel Flexible Haptic Sensor with Self-Compensation Mechanism" Sensors 24, no. 10: 3232. https://doi.org/10.3390/s24103232
APA StyleSun, Z., Yin, Y., Liu, B., Xue, T., & Zou, Q. (2024). Amphibious Multifunctional Hydrogel Flexible Haptic Sensor with Self-Compensation Mechanism. Sensors, 24(10), 3232. https://doi.org/10.3390/s24103232







