NMR in Battery Anode Slurries with a V-Shaped Sensor
Abstract
:1. Introduction
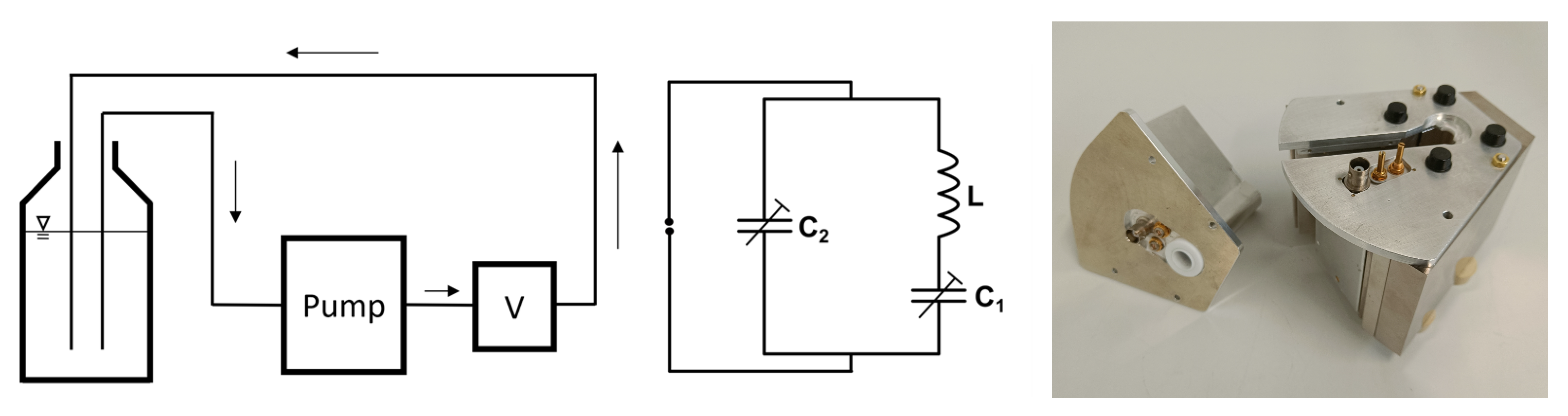
2. Materials and Methods
3. Results
3.1. Determination of the Chemical Composition of Anode Slurries
3.1.1. Measurements on Dry Anode Mixtures
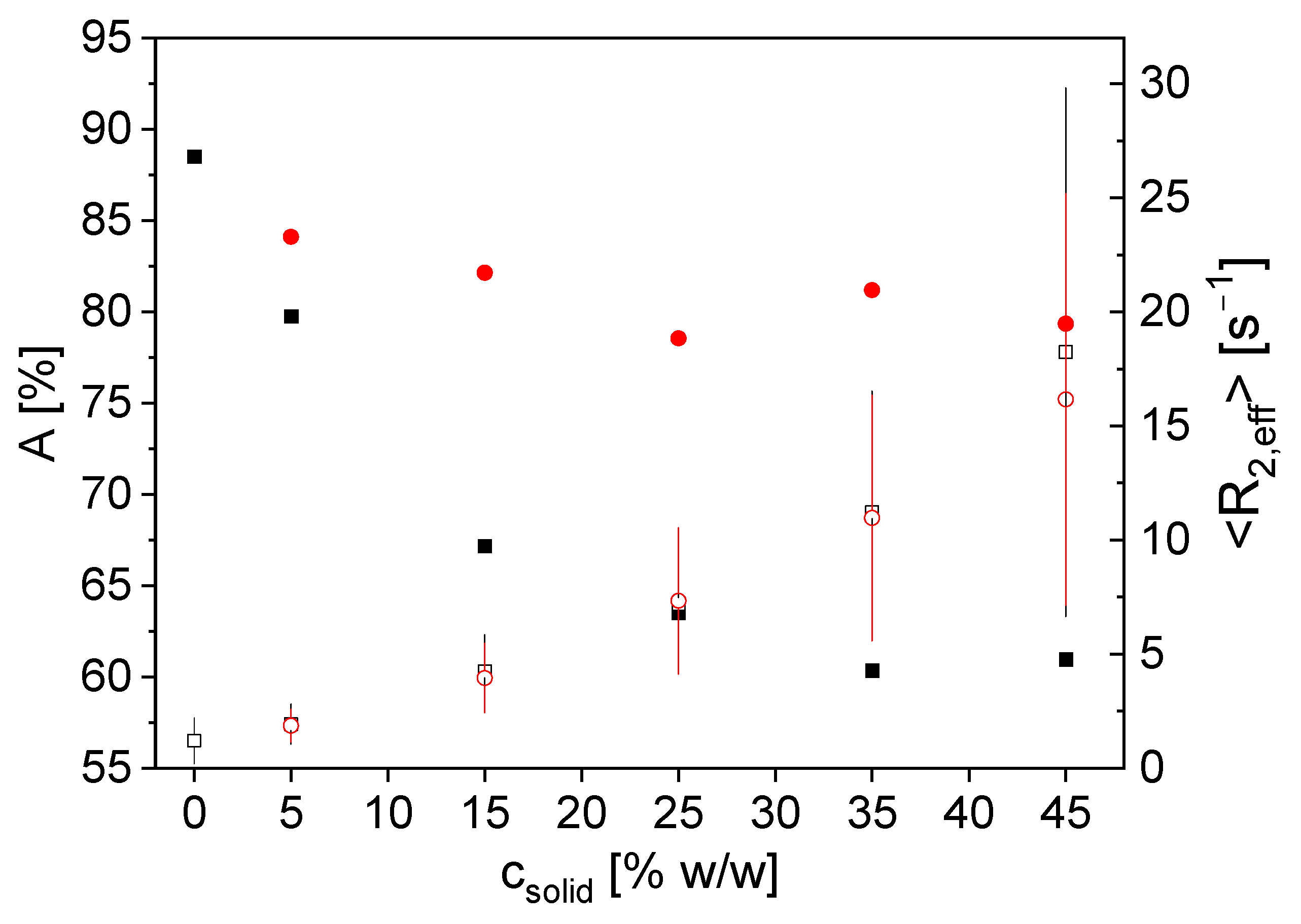
3.1.2. Measurements on Aqueous Anode Slurries
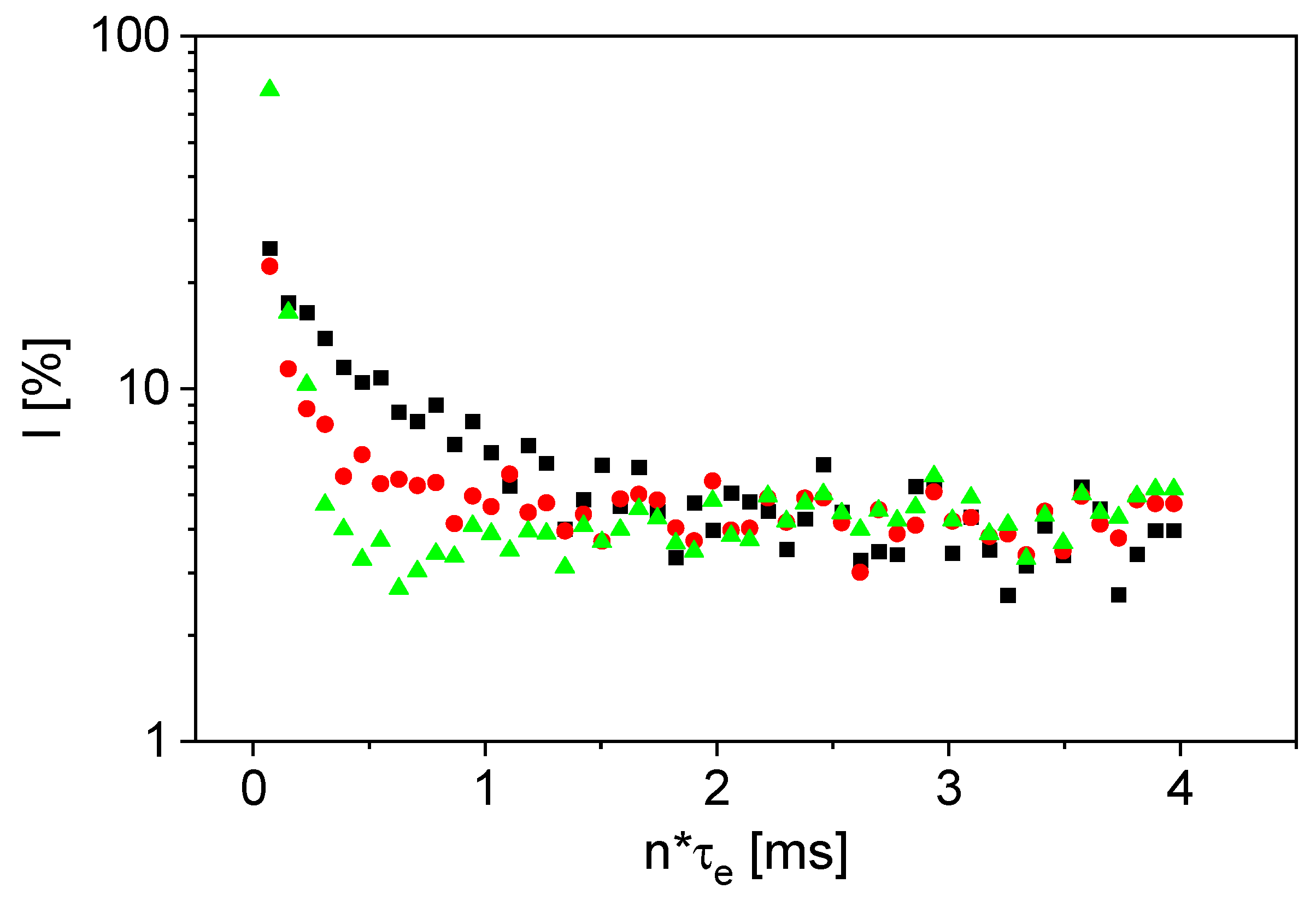
3.2. Inline Detection of Gas Inclusions
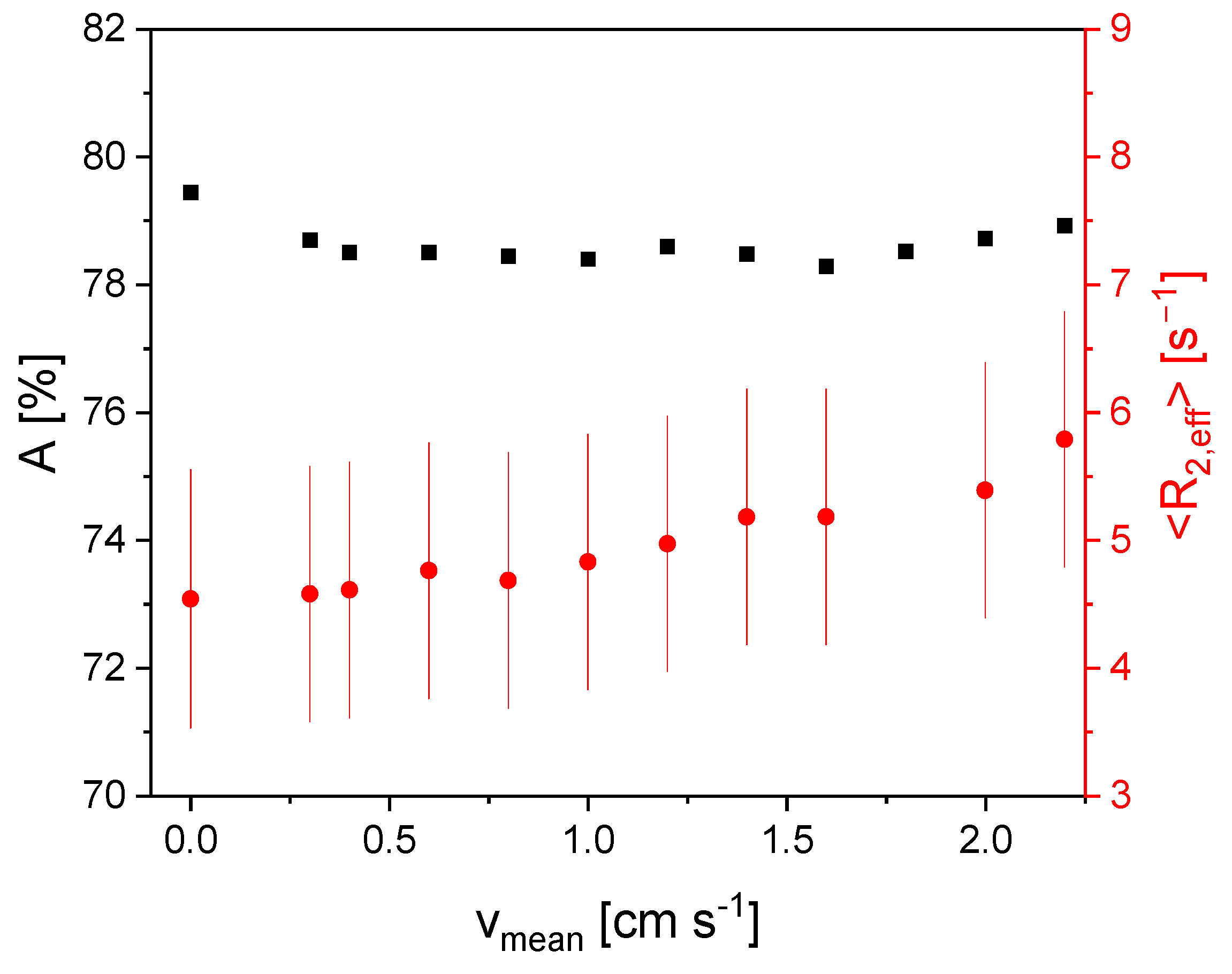
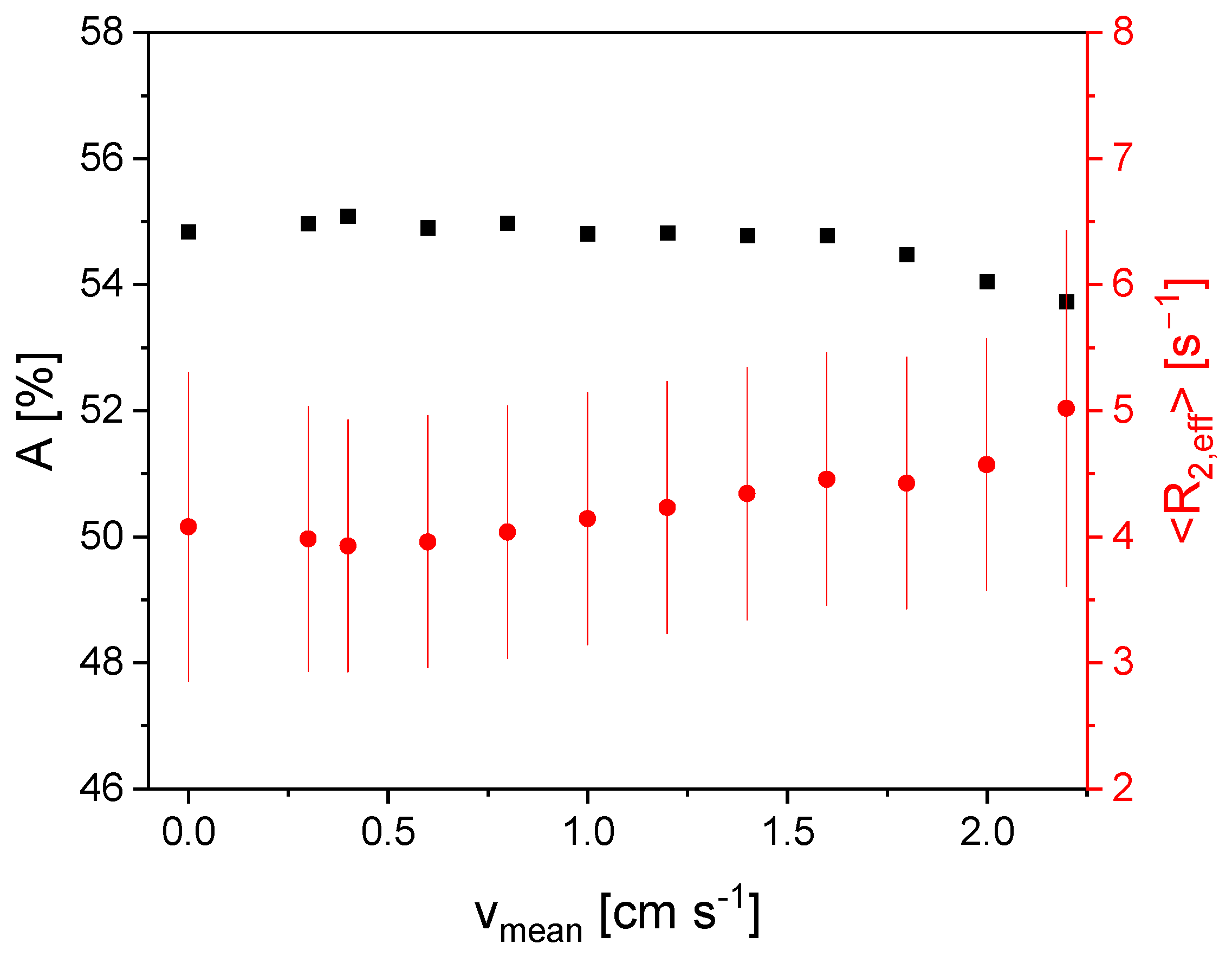
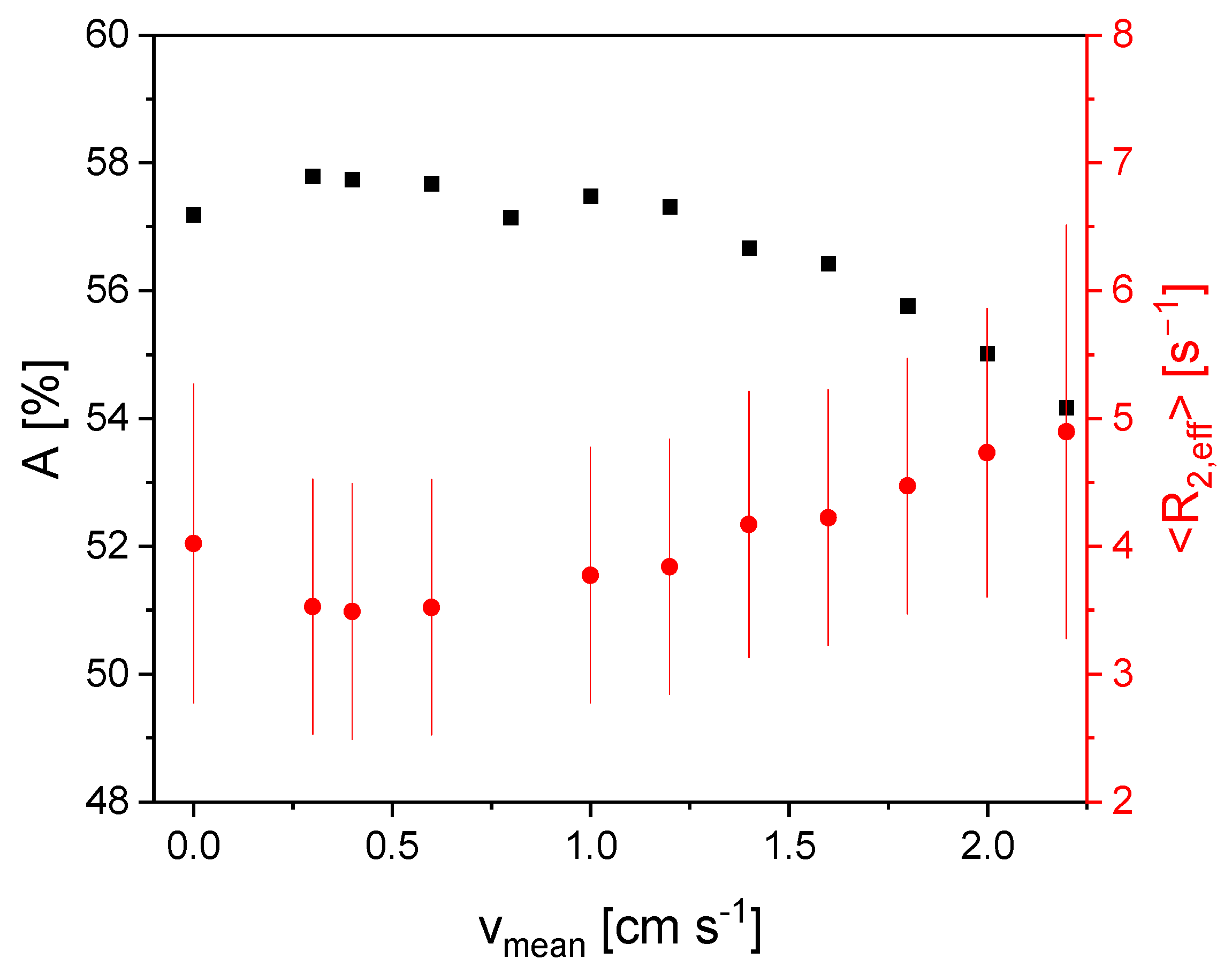
3.3. Measurement of Flow Velocity Distributions Exploring the In- and Outflow Effects
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kern, S.; Wander, L.; Meyer, K.; Guhl, S.; Mukkula, A.R.G.; Holtkamp, M.; Salge, M.; Fleischer, C.; Weber, N.; King, R.; et al. Flexible automation with compact NMR spectroscopy for continuous production of pharmaceuticals. Anal. Bioanal. Chem. 2019, 411, 3037–3046. [Google Scholar] [CrossRef] [PubMed]
- Cobo, M.F.; Deublein, E.J.; Haber, A.; Kwamen, R.; Nimbalkar, M.; Decker, F. TD-NMR in quality control: Standard applications. Mod. Magn. Reson. 2017, 1–18. [Google Scholar] [CrossRef]
- Chinn, S.C.; Cook-Tendulkar, A.; Maxwell, R.; Wheeler, H.; Wilson, M.; Xie, Z.H. Qualification of Automated Low-Field NMR Relaxometry for Quality Control of Polymers in a Production Setting. Polym. Test. 2007, 26, 1015–1024. [Google Scholar] [CrossRef]
- Corver, J.; Guthausen, G.; Kamlowski, A. In-line non-contact check weighing (NCCW) with nuclear magnetic resonance (NMR) presents new opportunities and challenges in process control. Pharm. Eng. 2005, 25, 18–30. [Google Scholar]
- Colnago, L.A.; Andrade, F.D.; Souza, A.A.; Azeredo, R.B.V.; Lima, A.A.; Cerioni, L.M.; Osan, T.M.; Pusiol, D.J. Why is Inline NMR Rarely Used as Industrial Sensor? Challenges and Opportunities. Chem. Eng. Technol. 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Foley, D.A.; Bez, E.; Codina, A.; Colson, K.L.; Fey, M.; Krull, R.; Piroli, D.; Zell, M.T.; Marquez, B.L. NMR flow tube for online NMR reaction monitoring. Anal. Chem. 2014, 86, 12008–12013. [Google Scholar] [CrossRef] [PubMed]
- Keifer, P.A. Flow techniques in NMR spectroscopy. Annu. Rep. NMR Spectrosc. 2007, 62, 1–47. [Google Scholar] [CrossRef]
- Dalitz, F.; Cudaj, M.; Maiwald, M.; Guthausen, G. Process and reaction monitoring by low-field NMR spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 60, 52–70. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.B.; Simões-Moreira, J.R.; Costa, H.K.M.; Santos, M.M.; Moutinho dos Santos, E. Energy storage in the energy transition context: A technology review. Renew. Sustain. Energy Rev. 2016, 65, 800–822. [Google Scholar] [CrossRef]
- Dreger, H.; Huelsebrock, M.; Froboese, L.; Kwade, A. Method Development for Quality Control of Suspensions for Lithium-Ion Battery Electrodes. Ind. Eng. Chem. Res. 2017, 56, 2466–2474. [Google Scholar] [CrossRef]
- Matzkanin, G.; Gardner, C. Nuclear magnetic resonance sensors for moisture measurement in roadways. Trans. Res. Rev. 1975, 532, 77–86. [Google Scholar]
- Blümich, B.; Blümler, P.; Eidmann, G.; Guthausen, A.; Haken, R.; Schmitz, U.; Saito, K.; Zimmer, G. The NMR-MOUSE: Construction, excitation, and applications. Magn. Reson. Imaging 1998, 16, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Veliyulin, E.; Mastikhin, I.V.; Marble, A.E.; Balcom, B.J. Rapid determination of the fat content in packaged dairy products by unilateral NMR. J. Sci. Food Agric. 2008, 88, 2563–2567. [Google Scholar] [CrossRef]
- McDonald, P.; Aptaker, P.; Mitchell, J.; Mulheron, M. A unilateral NMR magnet for sub-structure analysis in the built environment: The Surface GARField. J. Magn. Reson. 2007, 185, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.M.B.; Wilbur, G.R.; Cano Barrita, P.F.d.J.; Balcom, B.J. A portable, submersible, MR sensor—The Proteus magnet. J. Magn. Reson. 2021, 326, 106964. [Google Scholar] [CrossRef] [PubMed]
- Polishchuk, D.; Gardeniers, H. A compact permanent magnet for microflow NMR relaxometry. J. Magn. Reson. 2023, 347, 107364. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ross, M.M.B.; Newling, B.; Balcom, B.J. Non-Newtonian Fluid Velocity Profiles Determined with Simple Magnetic Resonance Spin Echoes. Phys. Rev. Appl. 2021, 16, L021001. [Google Scholar] [CrossRef]
- Aydin, E.; Makinwa, K.A.A. A Low-Field Portable Nuclear Magnetic Resonance (NMR) Microfluidic Flowmeter. In Proceedings of the 2021 21st International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), Orlando, FL, USA, 20–24 June 2021; 2021; pp. 1020–1023. [Google Scholar]
- Schmid, E.; Rondeau, S.; Rudszuck, T.; Nirschl, H.; Guthausen, G. Inline NMR via a Dedicated V-Shaped Sensor. Sensors 2023, 23, 2388. [Google Scholar] [CrossRef] [PubMed]
- Carr, H.Y.; Purcell, E.M. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954, 94, 630–638. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified spin-echo method for measuring nuclear relaxation times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Förster, E.; Nirschl, H.; Guthausen, G. NMR Diffusion and Relaxation for Monitoring of Degradation in Motor Oils. Appl. Magn. Reson. 2017, 48, 51–65. [Google Scholar] [CrossRef]
- Röding, M.; Bernin, D.; Jonasson, J.; Sarkka, A.; Topgaard, D.; Rudemo, M.; Nyden, M. The gamma distribution model for pulsed-field gradient NMR studies of molecular-weight distributions of polymers. J. Magn. Reson. 2012, 222, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Stieß, M. Mechanische Verfahrenstechnik-Partikeltechnologie 1; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Pope, J.M. Quantitative NMR Imaging of Flow. Concept Magn. Res 1993, 5, 281–302. [Google Scholar] [CrossRef]

| Parameter | Graphite Powder | CMC Powder | Powder Mixture |
|---|---|---|---|
| AGraphite [%] | 22 | - | 3 |
| ACMC [%] | - | 100 | 29 |
| R2,eff,Graphite [ms−1] | 2.38 | - | 2.38 |
| R2,eff,CMC [ms−1] | - | 9.71 | 9.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmid, E.; Kontschak, L.; Nirschl, H.; Guthausen, G. NMR in Battery Anode Slurries with a V-Shaped Sensor. Sensors 2024, 24, 3353. https://doi.org/10.3390/s24113353
Schmid E, Kontschak L, Nirschl H, Guthausen G. NMR in Battery Anode Slurries with a V-Shaped Sensor. Sensors. 2024; 24(11):3353. https://doi.org/10.3390/s24113353
Chicago/Turabian StyleSchmid, Eric, Louis Kontschak, Hermann Nirschl, and Gisela Guthausen. 2024. "NMR in Battery Anode Slurries with a V-Shaped Sensor" Sensors 24, no. 11: 3353. https://doi.org/10.3390/s24113353






