Advancements in Chemical and Biosensors for Point-of-Care Detection of Acrylamide
Abstract
:1. Introduction
2. Principles of Bio/Chemical Sensors for AA Detection
2.1. Optical Sensor for AA Detection
2.1.1. Colorimetric Sensing
2.1.2. Fluorescence Sensing
2.1.3. SERS Sensing
2.2. Electrochemical (EC) Sensor for AA Detection
| Sensor Type | Test Material | Linear Range | LoD | Time | Average Recovery (%) | Food Sample | Year, Refs |
|---|---|---|---|---|---|---|---|
| Colorimetric | AuNPs modified with GSH | 0.1–80 μmol/L | 28.6 nmol/L | 1 min | - | Potato chips | 2016, [32] |
| AuNPs modified with PEG | - | 0.2 nM | - | 98.8–109.1 | Potato chips, baked cookies, and non-fried cookies | 2018, [33] | |
| e-AgNPs modified with TU | 0.1–1000 μM | 0.024 μM | - | 82–90 | Biscuits | 2021, [34] | |
| THMS structure of DNA strands | 0.038 nmol/L | 0.05–200 nmol/L | - | 92–102 | Chips, coffee, and bread | 2022, [35] | |
| MGzyme-csDNA | 0.01–100 μM | 1.53 nM | 50 min | 99.00–104.4 | Artificial meat, biscuits, and potato chips | 2023, [65] | |
| Fe-PHS nanozyme | 0.75–36.00 μM | 0.27 μM | 1 h | 87.72–112.87 | Chips, coffee, and bread | 2023, [66] | |
| Fluorescent | CSUCNPs modified with csDNA of AA aptamers | 0.001–10 μM | 1.00 nM | 30 min | - | Potato chips | 2024, [67] |
| FAM-ssDNA and PCN-224 | 10–0.5 mM | 1.9 nM | - | 94.7–104.3 | Potato chips and biscuits | 2022, [48] | |
| CuNCs | 5–300 μM | 1.48 μM | 5 min | 90.29–101.30 | Toast | 2023, [49] | |
| ALP-based ELISA platform | 0.21~6.48 μg/L | 0.16 μg/L | - | 81.0–105.6 | Drinking water, cookies, and potato chips | 2021, [45] | |
| FAM-csDNA | 0.67–16.7 μM | 0.16 μM | - | 95–110 | Potato chips | 2022, [36] | |
| CQD-Au nanoprobe | 0–200 nM | 0.1 pM | 10 min | 98.6–102.6 | Fried bread sticks and potato chips | 2023, [42] | |
| SERS | Biodegradable zein/gold SERS platform | - | - | - | - | - | 2016, [54] |
| PDA@AgNPs | 0.1–1000 g/L | 0.04 g/L | - | - | Tap water | 2017, [60] | |
| rGo/AuNPs composite | 5–100 μg/kg | 2 μg/kg | 9.5 min | 73.4–92.8 | Three kinds of thirty fried foods collected from 6 provinces in China | 2019, [61] | |
| Strawberry-like SiO2/Ag nanocomposites (F-SANC) | 0.1–50 μM | 0.02 nM | - | 80.5–105.6 | Cookies, chips, and bread | 2020, [13] | |
| AgNPs substrate | 10–500 μg/L | 2.5 μg/L | - | 94.67–117.50 | Potato chips | 2023, [62] | |
| Core-shell structured Au@Ag NPs | 10−8–10−3 mol/L | 1.27 × 10−9 mol/L | - | 85.68–102.50 | Potato chips, fried dough twist, and instant coffee | 2024, [68] |
| Sensor Type | Type | Modifier_Electrode | Linear Range | LoD | Detection Time | Average Recovery (%) | Food Sample | Year, Refs |
|---|---|---|---|---|---|---|---|---|
| Voltammetric | Hb | Hb_Carbon-paste electrode | - | 1.2 × 10−10 M | - | - | Potato chips | 2007, [69] |
| Hb/SWCNTs_GCE | 1.0 × 10−11–1.0 × 10−3 M | 1.0 × 10−9 M | - | - | Potato chips | 2008, [70] | ||
| Coulometric | Hb | Hb/AuNPs_ITO glass | - | 0.1 μM | - | - | - | 2011, [71] |
| c-MWCNT/CuNPS/PANI_PGE | 5–75 mM | 0.2 nM | 2 s | 95.40–97.56 | Potato chips | 2012, [72] | ||
| MWCNTs/Fe3O4NPs/PANI_PGE | 3–90 nM | 0.02 nM | 8 s | 95.40–97.56 | Potato chips | 2013, [73] | ||
| Voltammetric | Cell | PC-12 cells/ERGO_GCE | 0.1–5 mM | 0.04 mM | - | - | - | 2013, [74] |
| DNA | DNA/GO_GCE | 5.0 × 10−8–1.0 × 10−3 mol/L | - | - | - | - | 2014, [75] | |
| MIT | P-ATP/AuNPs/PMA_MIP_GCE | 0.5 × 10−12 mol/L | 5 × 10−13 mol/L | - | above 95 | Potatoes | 2014, [76] | |
| MWCNTs/AuNPs/Ch_MIP_GCE | 0.028 μg m/L | 0.05–5 μg mL/L | - | 84.7–94.8 | Potato chips | 2016, [77] | ||
| DNA | ssDNA_AuE | 8.1 nM | 0.4–200 M | - | 93.8–109.3 | Tap water and potato chips | 2016, [78] | |
| Hb | MWCNTS-IL/Ch-IL/PtAuPd NPs/Hb-DDAB_GCE | 0.01 nM | 0.03–150.0 nM | 8 s | 99.36–101.4 | Potato chips | 2018, [79] | |
| dsDNA(ssDNA1-Hb/ssDNA2-SPE)/Hb _SPGE | 2.0 × 10−6–5.0 × 10−2 M | 1.58 × 10−7 M | - | 91–120 | Potato fries | 2019, [80] | ||
| MIT | PPy/ZnO/AA(MIP)_FTO electrode | 10−1–2.5× 10−9 M | 2.147 × 10−9 M | - | 92.64–106.0 | Potato chips and cookies | 2020, [81] | |
| Hb | Au@Ag CS-Hb/MXene/AuE | 1–150 μM | 3.46 μM | - | above 96 | Sunflower oil | 2022, [82] | |
| MIT | NOMG/3-TAA@AuNPs/PMA(MIP)_QCM chip | 0.08–100 ng/mL | 5.1 pg/mL | - | 88.3–97.2 | Bread, potato chips, and cookies | 2022, [83] | |
| Nbs | XAA Nbs_SPCE | 0.39–50.0 μg/mL | 0.033 μg/mL | 30 min | 88.29–111.76 | Piked baked biscuits and potato crisps | 2022, [84] | |
| DNA | Adenine _BDD electrode | 0.14–1.00 μM | 0.10 μM | - | - | - | 2023, [85] | |
| Hybrid | MIP-Apt-Au@rGO-MWCNTs_GCE | 1–600 nM | 0.104 nM | - | 98.7–103.4 | Potato fries | 2023, [86] | |
| ECL | @ZnO-Au(MIP)_GCE | 1– nM | 0.123 nM | - | 93.3–104.7 | Potato chips, cookies, and instant coffee | 2024, [87] | |
| _Pt electrode | 5 μM–10 mM | 1.2 μM | - | - | - | 2019, [88] |
2.2.1. Hb Label-Based EC Sensor
2.2.2. Immunosensors
2.2.3. MIP-Based EC Sensors
2.2.4. Electrochemiluminescence (ECL)-Based Sensors
2.2.5. Label-Free DNA-Based EC Sensors
2.2.6. Label-Free Cell-Based EC Sensors
3. Recent Development
3.1. Nanozyme-Based Colorimetric Sensor
3.2. UCNPs-Based Aptasensor
3.3. Hybrid Multirecognition-Controlled Sensors
4. Conclusions and Outlook
- Improving sensitivity: Employing more amiable receptors/ligands and nanomaterials with high conductivity, biocompatibility, and large surface areas such as nanozyme, ZnO, CNTs, MXene, and alloy nanoparticles significantly enhance the interaction between AA and the probe surface. Alternatively, various signal-amplification techniques, such as enzyme labels, ECL, SPR, microfluidic systems, and ratiometric fluorescence strategies, and even multiplexing technology, could be utilized.
- Strengthening anti-interference: Use highly selective recognition elements, such as antibodies, aptamers, or MIPs, that bind specifically to AA while discriminating against other substances. Blocking agents, such as BSA, could be applied to prevent non-specific binding. Implement advanced sample preparation techniques such as SPE, dSPE, and QuEChERS and calibration techniques such as matrix-matched calibration and the standard addition method to eliminate matrix effects.
- Enhancing reproducibility: Using reliable coating technologies, such as graphene-based substrates based on the GERS effect, to achieve a uniform response. Employing composite materials could integrate the advantages of various components to enhance stability and reproducibility.
- Advancing sustainability: Utilizing biodegradable or recyclable materials for sensor components and choosing eco-friendly, non-toxic, or less toxic alternatives to avoid hazardous substances. Implementing waste reduction strategies in the production process, such as recycling byproducts. Establishing modular components that are easy to replace or upgrade could extend the lifespan of the sensor.
- Easy to use: Establishing direct sampling or integrated sample handling steps to minimize sample preparation. Integrating portable bio/chemical sensing arrays with smartphone sensing techniques and other mobile devices.
Author Contributions
Funding
Conflicts of Interest
References
- van Boekel, M.; Fogliano, V.; Pellegrini, N.; Stanton, C.; Scholz, G.; Lalljie, S.; Somoza, V.; Knorr, D.; Jasti, P.R.; Eisenbrand, G. A review on the beneficial aspects of food processing. Mol. Nutr. Food Res. 2010, 54, 1215–1247. [Google Scholar] [CrossRef]
- Fan, M.; Xu, X.; Lang, W.; Wang, W.; Wang, X.; Xin, A.; Zhou, F.; Ding, Z.; Ye, X.; Zhu, B. Toxicity, formation, contamination, determination and mitigation of acrylamide in thermally processed plant-based foods and herbal medicines: A review. Ecotoxicol. Environ. Saf. 2023, 260, 115059. [Google Scholar] [CrossRef]
- Pan, M.; Liu, K.; Yang, J.; Hong, L.; Xie, X.; Wang, S. Review of Research into the Determination of Acrylamide in Foods. Foods 2020, 4, 524. [Google Scholar] [CrossRef]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, P.; Chen, F.; Yuan, Y.; Zhu, Y.; Yan, H.; Hu, X. Role of plant polyphenols in acrylamide formation and elimination. Food Chem. 2015, 186, 46–53. [Google Scholar] [CrossRef]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef]
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Robert, M.C.; Riediker, S. Acrylamide from Maillard reaction products. Nature 2002, 419, 449–450. [Google Scholar] [CrossRef]
- Yaylayan, V.A.; Stadler, R.H. Acrylamide formation in food: A mechanistic perspective. J. Aoac Int. 2005, 88, 262–267. [Google Scholar] [CrossRef]
- Friedman, M.; Mottram, D. Chemistry and Safety of Acrylamide in Food; Springer Science & Business Media: New York, NY, USA, 2006; Volume 561. [Google Scholar]
- Pérez-Nevado, F.; Cabrera-Bañegil, M.; Repilado, E.; Martillanes, S.; Martín-Vertedor, D. Effect of different baking treatments on the acrylamide formation and phenolic compounds in Californian-style black olives. Food Control 2018, 94, 22–29. [Google Scholar] [CrossRef]
- Commission Regulation (EU). Establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. J. Eur. Union. 2017, 60, 24–44. [Google Scholar]
- European Commission. Commission recommendation (EU) 2019/1888 of 7 November 2019 on the monitoring of the presence of acrylamide in certain foods. Off. J. Eur. Union 2019, 62, 31–33. [Google Scholar]
- Wu, L.; Zhang, W.; Liu, C.; Foda, M.F.; Zhu, Y. Strawberry-like SiO2/Ag nanocomposites immersed filter paper as SERS substrate for acrylamide detection. Food Chem. 2020, 328, 127106. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, Y.; Lin, L.; Zhou, C.; Li, S.; Zhang, L.; Li, J. Low-cost fabrication of paper-based microfluidic devices by one-step plotting. Anal. Chem. 2012, 84, 6331–6335. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; She, X.; Chen, Y.; Wang, Y.; Zou, C.; Zhou, Y. Black phosphorus nanosheets-sensitized Zn-doped α-Fe2O3 nanoclusters for trace acetone detection. Sens. Actuators Chem. 2023, 395, 134496. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; She, X.; Chen, Y.; Wang, M.; Wang, Y.; Du, A.; Tang, C.; Zou, C.; Zhou, Y. Oxygen Vacancy-Rich Bimetallic Au@ Pt Core–Shell Nanosphere-Functionalized Electrospun ZnFe2O4 Nanofibers for Chemiresistive Breath Acetone Detection. ACS Sens. 2024, 9, 2183–2193. [Google Scholar] [CrossRef]
- Yogeswaran, U.; Chen, S.M. A review on the electrochemical sensors and biosensors composed of nanowires as sensing material. Sensors 2008, 8, 290–313. [Google Scholar] [CrossRef]
- Herrmann, M. Sensor Models for the Development and Validation of Automated Driving Functions. ATZ Electron. Worldw. 2019, 14, 50–53. [Google Scholar] [CrossRef]
- Yang, T.; Huang, H.; Zhu, F.; Lin, Q.; Zhang, L.; Liu, J. Recent progresses in nanobiosensing for food safety analysis. Sensors 2016, 16, 1118. [Google Scholar] [CrossRef]
- Girma, K.; Lorenz, V.; Blaurock, S.; Edelmann, F.T. Coordination chemistry of acrylamide. Coord. Chem. Rev. 2005, 249, 1283–1293. [Google Scholar] [CrossRef]
- Fernandes, G.M.; Silva, W.R.; Barreto, D.N.; Lamarca, R.S.; Gomes, P.C.F.L.; da S Petruci, J.F.; Batista, A.D. Novel approaches for colorimetric measurements in analytical chemistry—A review. Anal. Chim. Acta 2020, 1135, 187–203. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar]
- Ren, J.; Wang, J.; Wang, J.; Wang, E. Colorimetric enantiorecognition of oligopeptide and logic gate construction based on DNA aptamer–ligand–gold nanoparticle interactions. Chem.-Eur. J. 2013, 2, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wang, Z.G.; Ding, B. Programmed colorimetric logic devices based on DNA-gold nanoparticle interactions. Small 2013, 9, 1016–1020. [Google Scholar] [CrossRef]
- Guo, L.; Xu, Y.; Ferhan, A.R.; Chen, G.; Kim, D.H. Oriented gold nanoparticle aggregation for colorimetric sensors with surprisingly high analytical figures of merit. J. Am. Chem. Soc. 2013, 135, 12338–12345. [Google Scholar] [CrossRef] [PubMed]
- Lesniewski, A.; Los, M.; Jonsson-Niedziółka, M.; Krajewska, A.; Szot, K.; Los, J.M.; Niedziolka-Jonsson, J. Antibody modified gold nanoparticles for fast and selective, colorimetric T7 bacteriophage detection. Bioconj. Chem. 2014, 25, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.J.; Suk, H.J.; Sung, H.Y.; Li, T.; Poo, H.; Kim, M.G. Novel antibody/gold nanoparticle/magnetic nanoparticle nanocomposites for immunomagnetic separation and rapid colorimetric detection of Staphylococcus aureus in milk. Biosens. Bioelectron. 2013, 43, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, Z.; Jiang, X. Gold nanoparticles for the colorimetric and fluorescent detection of ions and small organic molecules. Nanoscale 2011, 3, 1421–1433. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, J.; Yang, F.; Yang, X. Colorimetric logic gates for small molecules using split/integrated aptamers and unmodified gold nanoparticles. Chem. Commun. 2011, 47, 9435–9437. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Zuo, X.; Yang, R.; Xiao, Y.; Kang, D.; Vallée-Bélisle, A.; Gong, X.; Yuen, J.D.; Hsu, B.B.; Heeger, A.J.; et al. Colorimetric detection of DNA, small molecules, proteins, and ions using unmodified gold nanoparticles and conjugated polyelectrolytes. Proc. Natl. Acad. Sci. USA 2010, 107, 10837–10841. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol–ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Hu, Q.; Fu, Y.; Xu, X.; Qiao, Z.; Wang, R.; Zhang, Y.; Li, Y. A colorimetric detection of acrylamide in potato chips based on nucleophile-initiated thiol–ene Michael addition. Analyst 2016, 141, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Lu, D.; Wang, Z.; Zhang, D.; Gao, W.; Zhang, C.; Deng, J.; Guo, S. Colorimetric and visual determination of acrylamide via acrylamide-mediated polymerization of acrylamide-functionalized gold nanoparticles. Microchim. Acta 2018, 185, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.T.; Ngo, X.D.; Trang, N.L.N.; Nga, D.T.N.; Khi, N.T.; Trang, V.T.; Lam, V.D.; Le, A.T. Highly selective recognition of acrylamide in food samples using colorimetric sensor based on electrochemically synthesized colloidal silver nanoparticles: Role of supporting agent on cross-linking aggregation. Colloids Surfaces Physicochem. Eng. Asp. 2022, 636, 128165. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Moeenfard, M.; Abnous, K.; Taghdisi, S.M. Nano-gold mediated aptasensor for colourimetric monitoring of acrylamide: Smartphone readout strategy for on-site food control. Food Chem. 2023, 399, 133983. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meng, S.; Qin, J.; Zhang, R.; He, N.; Jiang, Y.; Chen, H.; Li, N.; Zhao, Y. A fluorescence biosensor based on double-stranded DNA and a cationic conjugated polymer coupled with exonuclease III for acrylamide detection. Int. J. Biol. Macromol. 2022, 219, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ding, J.; Zhang, K.; Ding, L. Construction of biomass carbon dots based fluorescence sensors and their applications in chemical and biological analysis. TrAC Trends Anal. Chem. 2019, 118, 315–337. [Google Scholar] [CrossRef]
- Liu, C.; Luo, F.; Chen, D.; Qiu, B.; Tang, X.; Ke, H.; Chen, X. Fluorescence determination of acrylamide in heat-processed foods. Talanta 2014, 123, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Xu, X.; Li, Z.; Zhang, Y.; Wang, J.; Fu, Y.; Li, Y. Detection of acrylamide in potato chips using a fluorescent sensing method based on acrylamide polymerization-induced distance increase between quantum dots. Biosens. Bioelectron. 2014, 54, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Liu, T.; Pu, H.; Sun, D.W. Determination of acrylamide in food products based on the fluorescence enhancement induced by distance increase between functionalized carbon quantum dots. Talanta 2020, 218, 121152. [Google Scholar] [CrossRef]
- Nag, A.; Pawar, S.; Bhattacharya, A. Gold nanoparticle induced enhancement of molecular fluorescence for Zn2+ detection in aqueous niosome solution. In Proceedings of the International Conference on Fibre Optics and Photonics, Chandigarh, India, 30 October 2016; Optica Publishing Group: Washington, DC, USA, 2016; p. W2D-1. [Google Scholar]
- Pattnayak, B.C.; Mohapatra, S. A smartphone-assisted ultrasensitive detection of acrylamide in thermally processed snacks using CQD@ Au NP integrated FRET sensor. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2023, 286, 122009. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Y.L.; Wang, J.H. Inner filter effect-based fluorescent sensing systems: A review. Anal. Chim. Acta 2018, 999, 13–26. [Google Scholar] [CrossRef]
- Li, H.; Wen, K.; Dong, B.; Zhang, J.; Bai, Y.; Liu, M.; Li, P.; Mujtaba, M.G.; Yu, X.; Yu, W.; et al. Novel inner filter effect-based fluorescence immunoassay with gold nanoclusters for bromadiolone detection in human serum. Sens. Actuators Chem. 2019, 297, 126787. [Google Scholar] [CrossRef]
- Luo, L.; Jia, B.Z.; Wei, X.Q.; Xiao, Z.L.; Wang, H.; Sun, Y.M.; Shen, Y.D.; Lei, H.T.; Xu, Z.L. Development of an inner filter effect-based fluorescence immunoassay for the detection of acrylamide using 9-xanthydrol derivatization. Sens. Actuators Chem. 2021, 332, 129561. [Google Scholar] [CrossRef]
- Asnaashari, M.; Kenari, R.E.; Farahmandfar, R.; Taghdisi, S.M.; Abnous, K. Fluorescence quenching biosensor for acrylamide detection in food products based on double-stranded DNA and gold nanoparticles. Sens. Actuators Chem. 2018, 265, 339–345. [Google Scholar] [CrossRef]
- Liu, S.; Bai, J.; Huo, Y.; Ning, B.; Peng, Y.; Li, S.; Han, D.; Kang, W.; Gao, Z. A zirconium-porphyrin MOF-based ratiometric fluorescent biosensor for rapid and ultrasensitive detection of chloramphenicol. Biosens. Bioelectron. 2020, 149, 111801. [Google Scholar] [CrossRef]
- Gan, Z.; Zhang, W.; Arslan, M.; Hu, X.; Zhang, X.; Li, Z.; Shi, J.; Zou, X. Ratiometric Fluorescent Metal–Organic Framework Biosensor for Ultrasensitive Detection of Acrylamide. J. Agric. Food Chem. 2022, 70, 10065–10074. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Xia, X.; Han, Z.; Yu, H.; Xie, Y.; Guo, Y.; Yao, W.; Qian, H.; Cheng, Y. A ratiometric fluorescent “off-on” sensor for acrylamide detection in toast based on red-emitting copper nanoclusters stabilized by bovine serum albumin. Food Chem. 2024, 437, 137878. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef]
- Howes, P.D.; Rana, S.; Stevens, M.M. Plasmonic nanomaterials for biodiagnostics. Chem. Soc. Rev. 2014, 43, 3835–3853. [Google Scholar] [CrossRef]
- Pahlow, S.; März, A.; Seise, B.; Hartmann, K.; Freitag, I.; Kämmer, E.; Böhme, R.; Deckert, V.; Weber, K.; Cialla, D.; et al. Bioanalytical application of surface-and tip-enhanced R aman spectroscopy. Eng. Life Sci. 2012, 12, 131–143. [Google Scholar] [CrossRef]
- Cialla, D.; März, A.; Böhme, R.; Theil, F.; Weber, K.; Schmitt, M.; Popp, J. Surface-enhanced Raman spectroscopy (SERS): Progress and trends. Anal. Bioanal. Chem. 2012, 403, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Gezer, P.G.; Liu, G.L.; Kokini, J.L. Detection of acrylamide using a biodegradable zein-based sensor with surface enhanced Raman spectroscopy. Food Control 2016, 68, 7–13. [Google Scholar] [CrossRef]
- Creighton, J.A. Surface Raman electromagnetic enhancement factors for molecules at the surface of small isolated metal spheres: The determination of adsorbate orientation from SERS relative intensities. Surf. Sci. 1983, 124, 209–219. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, Z.; Zhang, X.; Zhu, Y. Quantitative SERS by electromagnetic enhancement normalization with carbon nanotube as an internal standard. Opt. Express 2018, 26, 23534–23539. [Google Scholar]
- Pang, R.; Zhang, X.G.; Zhou, J.Z.; Wu, D.Y.; Tian, Z.Q. SERS chemical enhancement of water molecules from halide ion coadsorption and photoinduced charge transfer on silver electrodes. J. Phys. Chem. 2017, 121, 10445–10454. [Google Scholar] [CrossRef]
- Zhang, D.; Liang, P.; Yu, Z.; Huang, J.; Ni, D.; Shu, H.; Dong, Q.m. The effect of solvent environment toward optimization of SERS sensors for pesticides detection from chemical enhancement aspects. Sens. Actuators Chem. 2018, 256, 721–728. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, Y.; Zhou, Y.; Wen, Y.; Wang, F.; Yang, H. In-situ growth of raspberry-like silver composites for Raman detection of acrylamide. Sens. Actuators Chem. 2017, 243, 856–862. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, S.; Wang, S.; Wang, P.; Su, X.O.; Xie, J. Rapid and sensitive detection of acrylamide in fried food using dispersive solid-phase extraction combined with surface-enhanced Raman spectroscopy. Food Chem. 2019, 276, 157–163. [Google Scholar] [CrossRef]
- Ye, Z.H.; Chen, X.T.; Zhu, H.Y.; Liu, X.Q.; Deng, W.H.; Song, W.; Li, D.X.; Hou, R.Y.; Cai, H.M.; Peng, C.Y. Aggregating-agent-assisted surface-enhanced Raman spectroscopy–based detection of acrylamide in fried foods: A case study with potato chips. Food Chem. 2023, 403, 134377. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Li, X.; Lin, Y.; Luo, N.; Long, M.; Zhao, N.; Xu, J.B. Flexible piezoelectric-induced pressure sensors for static measurements based on nanowires/graphene heterostructures. ACS Nano 2017, 11, 4507–4513. [Google Scholar] [CrossRef]
- Fabry, P.; Siebert, E. Electrochemical sensors. In Handbook of Solid State Electrochemistry; CRC Press: Boca Raton, FL, USA, 2019; pp. 329–369. [Google Scholar]
- Guo, K.; Lin, X.; Duan, N.; Lu, C.; Wang, Z.; Wu, S. Detection of acrylamide in food based on MIL-glucose oxidase cascade colorimetric aptasensor. Anal. Chim. Acta 2024, 1288, 342150. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, J.; Liu, Y.; Fan, J.; Ning, F.; Peng, H. Tunable assembly of Ferric ion-dopamine molecules into hedgehog-like nanozyme for colorimetric and sensitive detection of acrylamide in thermally processed foods. Microchem. J. 2023, 194, 109287. [Google Scholar] [CrossRef]
- Rong, Y.; Hassan, M.M.; Wu, J.; Chen, S.; Yang, W.; Li, Y.; Zhu, J.; Huang, J.; Chen, Q. Enhanced detection of acrylamide using a versatile solid-state upconversion sensor through spectral and visual analysis. J. Hazard. Mater. 2024, 466, 133369. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Chen, C.; Waterhouse, G.I.; Sun, Y.; Xu, Z. SERS Sensor Based on Core–Shell Au@ Ag Nanoparticles for the Sensitive Detection of Acrylamide in Foods. Food Anal. Methods 2024, 17, 585–593. [Google Scholar] [CrossRef]
- Stobiecka, A.; Radecka, H.; Radecki, J. Novel voltammetric biosensor for determining acrylamide in food samples. Biosens. Bioelectron. 2007, 22, 2165–2170. [Google Scholar] [CrossRef]
- Krajewska, A.; Radecki, J.; Radecka, H. A voltammetric biosensor based on glassy carbon electrodes modified with single-walled carbon nanotubes/hemoglobin for detection of acrylamide in water extracts from potato crisps. Sensors 2008, 8, 5832–5844. [Google Scholar] [CrossRef]
- Garabagiu, S.; Mihailescu, G. Simple hemoglobin–gold nanoparticles modified electrode for the amperometric detection of acrylamide. J. Electroanal. Chem. 2011, 659, 196–200. [Google Scholar] [CrossRef]
- Batra, B.; Lata, S.; Sharma, M.; Pundir, C. An acrylamide biosensor based on immobilization of hemoglobin onto multiwalled carbon nanotube/copper nanoparticles/polyaniline hybrid film. Anal. Biochem. 2013, 433, 210–217. [Google Scholar] [CrossRef]
- Batra, B.; Lata, S.; Pundir, C. Construction of an improved amperometric acrylamide biosensor based on hemoglobin immobilized onto carboxylated multi-walled carbon nanotubes/iron oxide nanoparticles/chitosan composite film. Bioprocess Biosyst. Eng. 2013, 36, 1591–1599. [Google Scholar] [CrossRef]
- Sun, X.; Ji, J.; Jiang, D.; Li, X.; Zhang, Y.; Li, Z.; Wu, Y. Development of a novel electrochemical sensor using pheochromocytoma cells and its assessment of acrylamide cytotoxicity. Biosens. Bioelectron. 2013, 44, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, Y.; Zhang, L.; Tong, H. A label-free electrochemical biosensor for acrylamide based on DNA immobilized on graphene oxide-modified glassy carbon electrode. Int. J. Electrochem. Sci. 2014, 9, 7217–7227. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, J.; Jiang, D.; Wang, Y.; Zhang, Y.; Sun, X. An electrochemical sensor based on molecularly imprinted membranes on a P-ATP–AuNP modified electrode for the determination of acrylamide. Anal. Methods 2014, 6, 6452–6458. [Google Scholar] [CrossRef]
- Liu, X.; Mao, L.G.; Wang, Y.L.; Shi, X.B.; Liu, Y.; Yang, Y.; He, Z. Electrochemical sensor based on imprinted sol-gel polymer on Au NPs-MWCNTs-CS modified electrode for the determination of acrylamide. Food Anal. Methods 2016, 9, 114–121. [Google Scholar] [CrossRef]
- Huang, S.; Lu, S.; Huang, C.; Sheng, J.; Zhang, L.; Su, W.; Xiao, Q. An electrochemical biosensor based on single-stranded DNA modified gold electrode for acrylamide determination. Sens. Actuators Chem. 2016, 224, 22–30. [Google Scholar] [CrossRef]
- Varmira, K.; Abdi, O.; Gholivand, M.B.; Goicoechea, H.C.; Jalalvand, A.R. Intellectual modifying a bare glassy carbon electrode to fabricate a novel and ultrasensitive electrochemical biosensor: Application to determination of acrylamide in food samples. Talanta 2018, 176, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Asnaashari, M.; Kenari, R.E.; Farahmandfar, R.; Abnous, K.; Taghdisi, S.M. An electrochemical biosensor based on hemoglobin-oligonucleotides-modified electrode for detection of acrylamide in potato fries. Food Chem. 2019, 271, 54–61. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Y.; Ji, S.; Lu, Y.; Bai, X.; Yin, M.; Huang, C.; Jia, N. Molecularly imprinted photoelectrochemical sensing based on ZnO/polypyrrole nanocomposites for acrylamide detection. Biosens. Bioelectron. 2021, 173, 112816. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.P.; Keerthana, S.; Viswanathan, C.; Ponpandian, N. Bimetallic Coreshell-Hemoglobin Complex Immobilized MXene Based Voltammetric Biosensor for the Electrochemical Detection of Acrylamide. J. Electrochem. Soc. 2022, 169, 127510. [Google Scholar] [CrossRef]
- Chi, H.; Liu, G. Determination of acrylamide by a quartz crystal microbalance sensor based on nitrogen-doped ordered mesoporous carbon composite and molecularly imprinted poly (3-thiophene acetic acid) with gold nanoparticles. Food Control 2022, 141, 109166. [Google Scholar] [CrossRef]
- Liang, Y.; Zeng, Y.; Luo, L.; Xu, Z.; Shen, Y.; Wang, H.; Hammock, B.D. Detection of acrylamide in foodstuffs by nanobody-based immunoassays. J. Agric. Food Chem. 2022, 70, 9179–9186. [Google Scholar] [CrossRef] [PubMed]
- Anggraini, L.E.; Rahmawati, I.; Nasution, M.A.F.; Jiwanti, P.K.; Einaga, Y.; Ivandini, T.A. Development of an acrylamide biosensor using guanine and adenine as biomarkers at boron-doped diamond electrodes: Integrated molecular docking and experimental studies. Bull. Chem. Soc. Jpn. 2023, 96, 420–428. [Google Scholar] [CrossRef]
- Ali, R.; El-Wekil, M.M. A dual-recognition-controlled electrochemical biosensor for selective and ultrasensitive detection of acrylamide in heat-treated carbohydrate-rich food. Food Chem. 2023, 413, 135666. [Google Scholar] [CrossRef] [PubMed]
- Kuang, K.; Li, Y.; Ji, Y.; Liu, Y.; Jia, N. Molecular imprinting-electrochemiluminescence sensor based on Ru (bpy) 32+@ ZnO-Au composite for sensitive detection of acrylamide. Microchem. J. 2024, 196, 109558. [Google Scholar] [CrossRef]
- Yang, X.; Pan, P.; Tu, L.; Liao, Z.; Niu, H.; Zang, C.; Li, M.; Liu, J.; Yang, Z.; Qi, Y.; et al. Novel detection of acrylamide by electrochemiluminescence sensor and optical imaging analysis. Int. J. Electrochem. Sci. 2019, 14, 7380–7388. [Google Scholar] [CrossRef]
- Rudén, C. Acrylamide and cancer risk—Expert risk assessments and the public debate. Food Chem. Toxicol. 2004, 42, 335–349. [Google Scholar] [CrossRef]
- Buzzeo, M.C.; Evans, R.G.; Compton, R.G. Non-haloaluminate room-temperature ionic liquids in electrochemistry—A review. ChemPhysChem 2004, 5, 1106–1120. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shen, Y.D.; Lei, H.T.; Sun, Y.M.; Yang, J.Y.; Xiao, Z.L.; Wang, H.; Xu, Z.L. Hapten synthesis and development of a competitive indirect enzyme-linked immunosorbent assay for acrylamide in food samples. J. Agric. Food Chem. 2014, 62, 7078–7084. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, C.; Wang, D.; Zhao, M. Antigen synthetic strategy and immunoassay development for detection of acrylamide in foods. Analyst 2008, 133, 903–909. [Google Scholar] [CrossRef]
- Wu, M.F.; Wang, Y.; Li, S.; Dong, X.X.; Yang, J.Y.; Shen, Y.D.; Wang, H.; Sun, Y.M.; Lei, H.T.; Xu, Z.L. Ultrasensitive immunosensor for acrylamide based on chitosan/SnO2-SiC hollow sphere nanochains/gold nanomaterial as signal amplification. Anal. Chim. Acta 2019, 1049, 188–195. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Xu, Z.L.; Wang, F.; Cai, J.; Dong, J.X.; Zhang, J.R.; Si, R.; Wang, C.L.; Wang, Y.; Shen, Y.D.; et al. Isolation of bactrian camel single domain antibody for parathion and development of one-step dc-FEIA method using VHH-alkaline phosphatase fusion protein. Anal. Chem. 2018, 90, 12886–12892. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, R.; Frenken, L.; De Geus, B.; Harmsen, M.; Ruuls, R.; Stok, W.; De Ron, L.; Wilson, S.; Davis, P.; Verrips, C. Comparison of physical chemical properties of llama VHH antibody fragments and mouse monoclonal antibodies. Biochim. Biophys. Acta-(Bba)-Protein Struct. Mol. Enzymol. 1999, 1431, 37–46. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, H. Expression of single-domain antibody in different systems. Appl. Microbiol. Biotechnol. 2018, 102, 539–551. [Google Scholar] [CrossRef] [PubMed]
- González-Fuentes, F.J.; Manríquez, J.; Godínez, L.A.; Escarpa, A.; Mendoza, S. Electrochemical Analysis of Acrylamide Using Screen-Printed Carboxylated Single-Walled Carbon Nanotube Electrodes. Electroanalysis 2014, 26, 1039–1044. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, H.; Zhong, M.; Zhang, T.; Lu, X.; Kan, X. Imprinted sol–gel electrochemical sensor for melamine direct recognition and detection. J. Electroanal. Chem. 2014, 713, 112–118. [Google Scholar] [CrossRef]
- Soleimani, M.; Afshar, M.G.; Shafaat, A.; Crespo, G.A. High-Selective Tramadol Sensor Based on Modified Molecularly Imprinted Polymer—Carbon Paste Electrode with Multiwalled Carbon Nanotubes. Electroanalysis 2013, 25, 1159–1168. [Google Scholar] [CrossRef]
- Hong, S.; Lee, L.Y.S.; So, M.H.; Wong, K.Y. A dopamine electrochemical sensor based on molecularly imprinted poly (acrylamidophenylboronic acid) film. Electroanalysis 2013, 25, 1085–1094. [Google Scholar] [CrossRef]
- Marx, S.; Zaltsman, A.; Turyan, I.; Mandler, D. Parathion sensor based on molecularly imprinted sol- gel films. Anal. Chem. 2004, 76, 120–126. [Google Scholar] [CrossRef]
- Liu, L.; Tan, X.; Fang, X.; Sun, Y.; Lei, F.; Huang, Z. Electrochemical Sensor Based on Molecularly Imprinted Polymer Film Prepared with Functional Abietic-Type Acids as Cross-Linker for the Determination of Quinine. Electroanalysis 2012, 24, 1647–1654. [Google Scholar] [CrossRef]
- Farabullini, F.; Lucarelli, F.; Palchetti, I.; Marrazza, G.; Mascini, M. Disposable electrochemical genosensor for the simultaneous analysis of different bacterial food contaminants. Biosens. Bioelectron. 2007, 22, 1544–1549. [Google Scholar] [CrossRef]
- Ni, S.; Han, F.; Wang, W.; Han, D.; Bao, Y.; Han, D.; Wang, H.; Niu, L. Innovations upon antioxidant capacity evaluation for cosmetics: A photoelectrochemical sensor exploitation based on N-doped graphene/TiO2 nanocomposite. Sens. Actuators Chem. 2018, 259, 963–971. [Google Scholar] [CrossRef]
- Shu, J.; Tang, D. Recent advances in photoelectrochemical sensing: From engineered photoactive materials to sensing devices and detection modes. Anal. Chem. 2019, 92, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Battal, D.; Akgönüllü, S.; Yalcin, M.S.; Yavuz, H.; Denizli, A. Molecularly imprinted polymer based quartz crystal microbalance sensor system for sensitive and label-free detection of synthetic cannabinoids in urine. Biosens. Bioelectron. 2018, 111, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Plausinaitis, D.; Sinkevicius, L.; Samukaite-Bubniene, U.; Ratautaite, V.; Ramanavicius, A. Evaluation of electrochemical quartz crystal microbalance based sensor modified by uric acid-imprinted polypyrrole. Talanta 2020, 220, 121414. [Google Scholar] [CrossRef] [PubMed]
- Ratautaite, V.; Plausinaitis, D.; Baleviciute, I.; Mikoliunaite, L.; Ramanaviciene, A.; Ramanavicius, A. Characterization of caffeine-imprinted polypyrrole by a quartz crystal microbalance and electrochemical impedance spectroscopy. Sens. Actuators Chem. 2015, 212, 63–71. [Google Scholar] [CrossRef]
- Ke, H.; Zhang, X.; Guo, W.; Zhang, A.; Wang, Z.; Huang, C.; Jia, N. A MWCNTs-Pt nanohybrids-based highly sensitive electrochemiluminescence sensor for flavonoids assay. Talanta 2017, 171, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Ye, H.; Yuan, Z.; Liu, X.; Chen, X.; Yang, W. Recent advances in electrochemiluminescence-based simultaneous detection of multiple targets. TrAC Trends Anal. Chem. 2020, 123, 115767. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Fu, C.; Li, N.; Du, M.; Zhang, L.; Ge, S.; Yu, J. based bipolar electrode electrochemiluminescence platform for detection of multiple miRNAs. Anal. Chem. 2020, 93, 1702–1708. [Google Scholar] [CrossRef]
- McCoy, G.R.; Touzet, N.; Fleming, G.T.; Raine, R. An evaluation of the applicability of microarrays for monitoring toxic algae in Irish coastal waters. Environ. Sci. Pollut. Res. 2013, 20, 6751–6764. [Google Scholar] [CrossRef]
- Besaratinia, A.; Pfeifer, G.P. DNA adduction and mutagenic properties of acrylamide. Mutat. Res. Toxicol. Environ. Mutagen. 2005, 580, 31–40. [Google Scholar] [CrossRef]
- Atay, N.Z.; Çalgan, D.; Özakat, E.; Varnali, T. Acrylamide and glycidamide adducts of Guanine. J. Mol. Struct. THEOCHEM 2005, 728, 249–251. [Google Scholar] [CrossRef]
- Qiu, Y.; Qu, X.; Dong, J.; Ai, S.; Han, R. Electrochemical detection of DNA damage induced by acrylamide and its metabolite at the graphene-ionic liquid-Nafion modified pyrolytic graphite electrode. J. Hazard. Mater. 2011, 190, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Stenger, D.A.; Gross, G.W.; Keefer, E.W.; Shaffer, K.M.; Andreadis, J.D.; Ma, W.; Pancrazio, J.J. Detection of physiologically active compounds using cell-based biosensors. TRENDS Biotechnol. 2001, 19, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Karmali, A.; Serralheiro, M. Kinetic properties of wild-type and altered recombinant amidases by the use of ion-selective electrode assay method. Anal. Biochem. 2006, 355, 232–239. [Google Scholar] [CrossRef]
- Andrade, J.; Karmali, A.; Carrondo, M.A.; Frazao, C. Structure of amidase from Pseudomonas aeruginosa showing a trapped acyl transfer reaction intermediate state. J. Biol. Chem. 2007, 282, 19598–19605. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Gil, D.; Karmali, A.; Matos, M. Biosensor for acrylamide based on an ion-selective electrode using whole cells of Pseudomonas aeruginosa containing amidase activity. Biocatal. Biotransforma. 2009, 27, 143–151. [Google Scholar] [CrossRef]
- Silva, N.; Matos, M.J.; Karmali, A.; Rocha, M.M. An electrochemical biosensor for acrylamide determination: Merits and limitations. Port. Electrochim. Acta 2011, 29, 361–373. [Google Scholar] [CrossRef]
- Li, H.; Song, P.; Wu, T.; Zhao, H.; Liu, Q.; Zhu, X. In situ decorating of montmorillonite with ZnMn2O4 nanoparticles with enhanced oxidase-like activity and its application in constructing GSH colorimetric platform. Appl. Clay Sci. 2022, 229, 106656. [Google Scholar] [CrossRef]
- Liu, M.; Wei, J.; Wang, Y.; Ouyang, H.; Fu, Z. Dopamine-functionalized upconversion nanoparticles as fluorescent sensors for organophosphorus pesticide analysis. Talanta 2019, 195, 706–712. [Google Scholar] [CrossRef]
- Wang, P.; Li, H.; Hassan, M.M.; Guo, Z.; Zhang, Z.Z.; Chen, Q. Fabricating an acetylcholinesterase modulated UCNPs-Cu2+ fluorescence biosensor for ultrasensitive detection of organophosphorus pesticides-diazinon in food. J. Agric. Food Chem. 2019, 67, 4071–4079. [Google Scholar] [CrossRef]
- Pan, W.; Zhao, J.; Chen, Q. Fabricating upconversion fluorescent probes for rapidly sensing foodborne pathogens. J. Agric. Food Chem. 2015, 63, 8068–8074. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Hassan, M.M.; Ouyang, Q.; Wang, L.; Jiao, T.; Chen, Q. Ratiometric upconversion fluorometric turn-off nanosensor for quantification of furfural in foods. Sens. Actuators Chem. 2022, 350, 130843. [Google Scholar] [CrossRef]
- Rong, Y.; Hassan, M.M.; Ouyang, Q.; Zhang, Y.; Wang, L.; Chen, Q. An upconversion biosensor based on DNA hybridization and DNA-templated silver nanoclusters for the determination of acrylamide. Biosens. Bioelectron. 2022, 215, 114581. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ahmad, W.; Ouyang, Q.; Zhang, J.; Zhang, M.; Chen, Q. Regenerative flexible upconversion-luminescence biosensor for visual detection of diethylstilbestrol based on smartphone imaging. Anal. Chem. 2021, 93, 15667–15676. [Google Scholar] [CrossRef]
- Wu, J.; Ali, S.; Ouyang, Q.; Wang, L.; Rong, Y.; Chen, Q. Highly specific and sensitive detection of aflatoxin B1 in food based on upconversion nanoparticles-black phosphorus nanosheets aptasensor. Microchem. J. 2021, 171, 106847. [Google Scholar] [CrossRef]


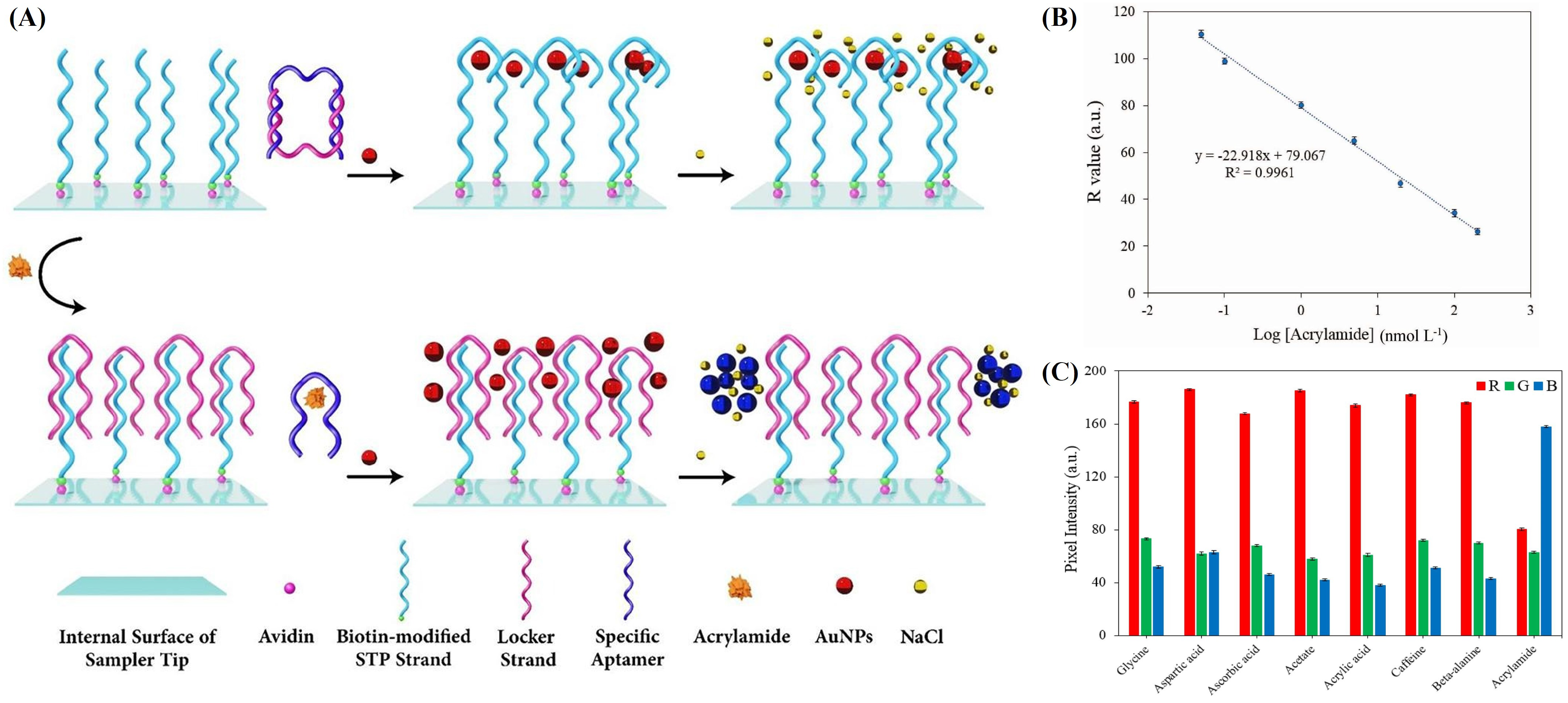
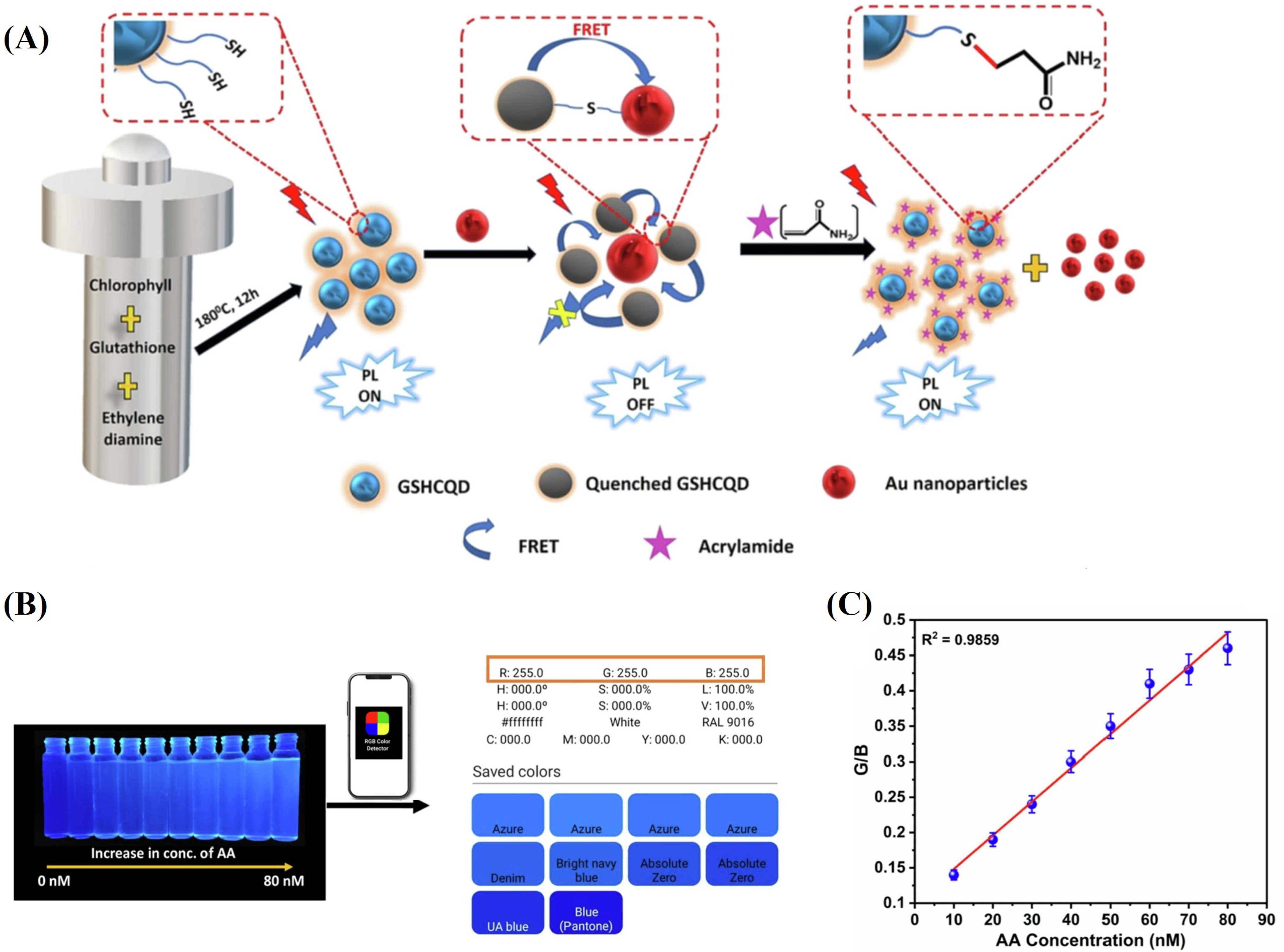
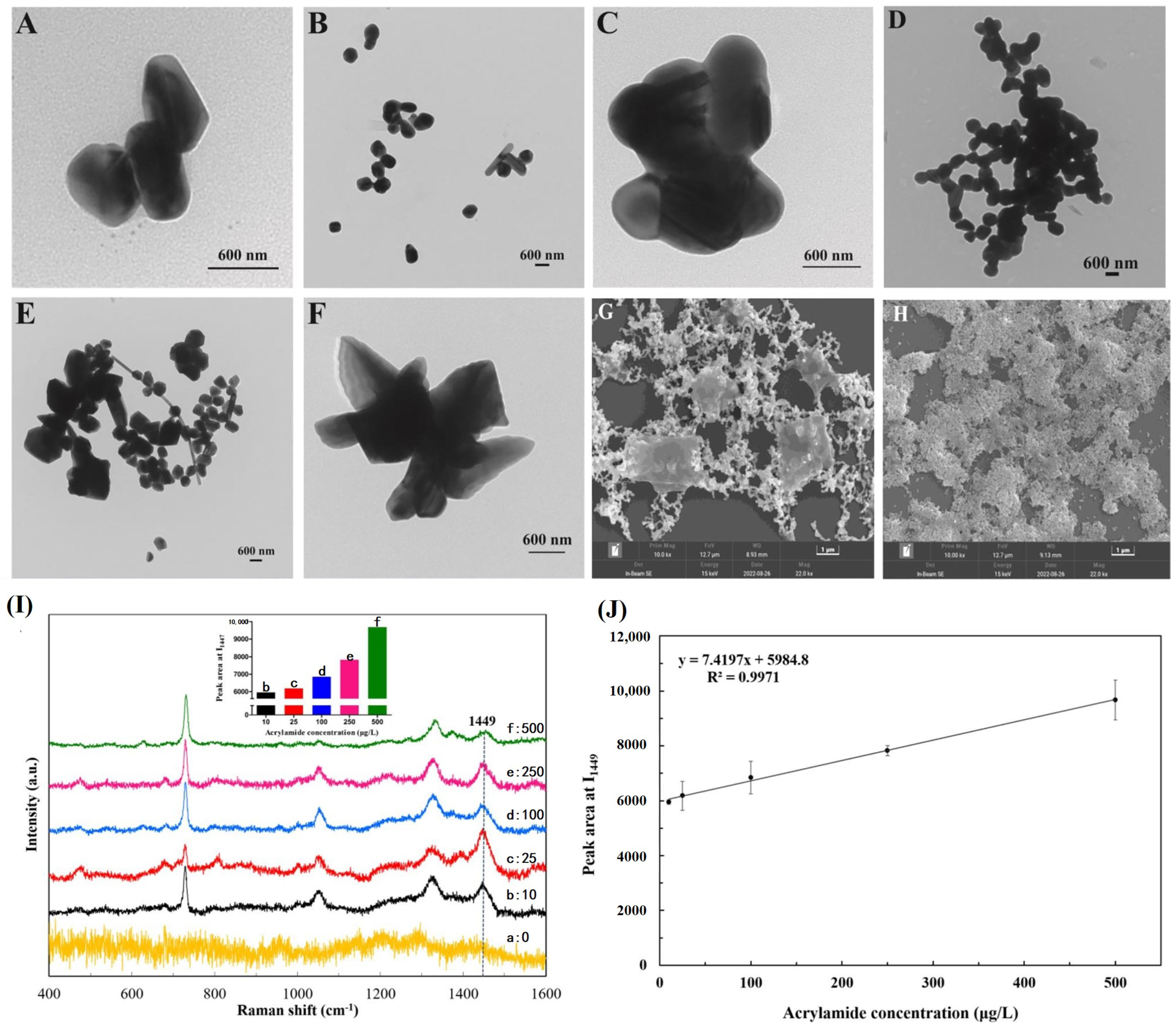

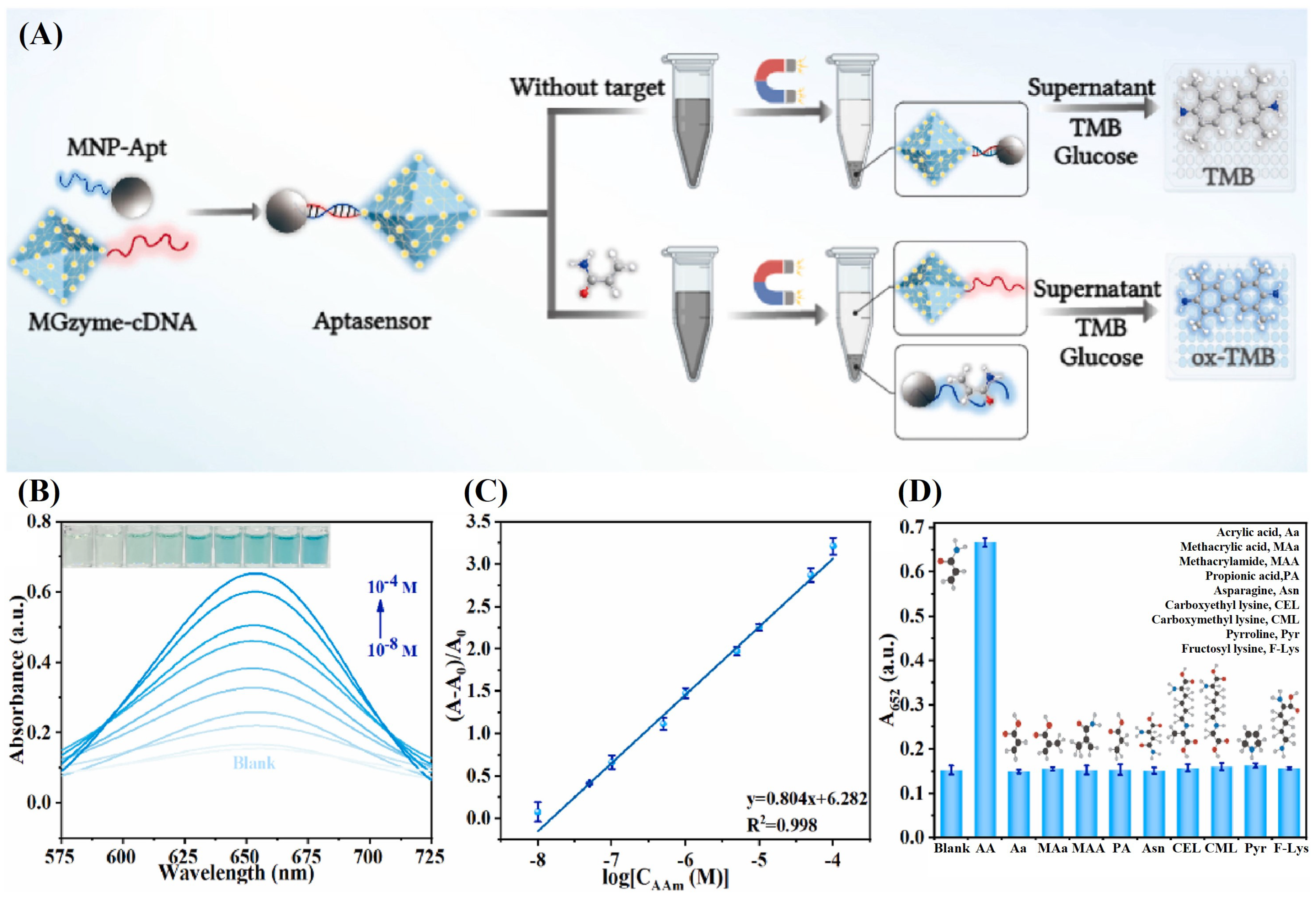


| Ligand | Receptor | Adduct and Complex |
|---|---|---|
| Acrylamide | Cysteine | Cysteine-acrylamide adduct |
| Glutathione | Glutathione-acrylamide adduct | |
| Thiol group functionalized oligonucleotide | Thiol group functionalized oligonucleotide-acrylamide adduct | |
| Guanine | Guanine-Acrylamide adduct | |
| Fluorescein | Fluorescein-Acrylamide derivative | |
| Fluorescamine | Fluorescamine-Acrylamide derivative | |
| Acrylic acid | Acrylic acid-Acrylamide complex via hydrogen bonding | |
| Carboxylic acid radical | Carboxylic acid radical mediated acrylamide complex | |
| Hemoglobin (in Fe2+ state) | Hemoglobin valine mono- and bis-adducts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, M.; Lv, X.; Wang, K.; Zhou, Y.; Lin, X. Advancements in Chemical and Biosensors for Point-of-Care Detection of Acrylamide. Sensors 2024, 24, 3501. https://doi.org/10.3390/s24113501
Xie M, Lv X, Wang K, Zhou Y, Lin X. Advancements in Chemical and Biosensors for Point-of-Care Detection of Acrylamide. Sensors. 2024; 24(11):3501. https://doi.org/10.3390/s24113501
Chicago/Turabian StyleXie, Mingna, Xiao Lv, Ke Wang, Yong Zhou, and Xiaogang Lin. 2024. "Advancements in Chemical and Biosensors for Point-of-Care Detection of Acrylamide" Sensors 24, no. 11: 3501. https://doi.org/10.3390/s24113501






