Ultrasound and Photoacoustic Imaging for the Guidance of Laser Ablation Procedures
Abstract
:1. Introduction
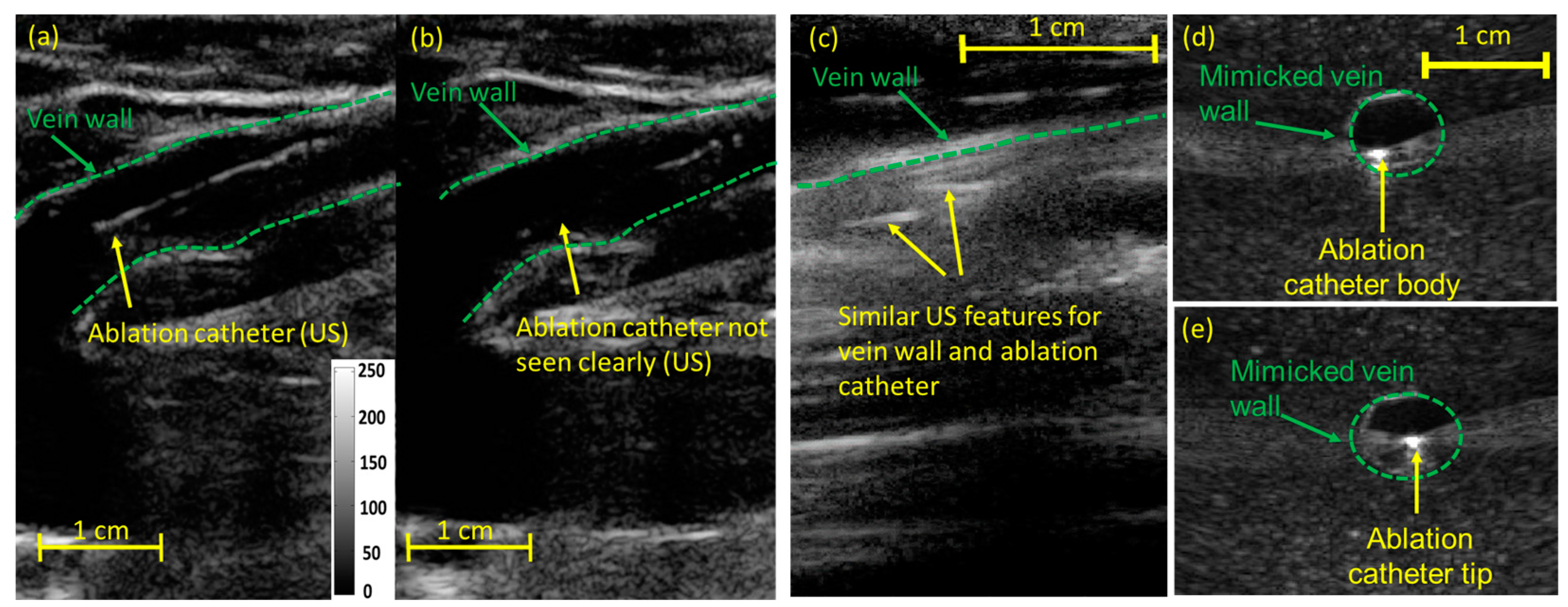
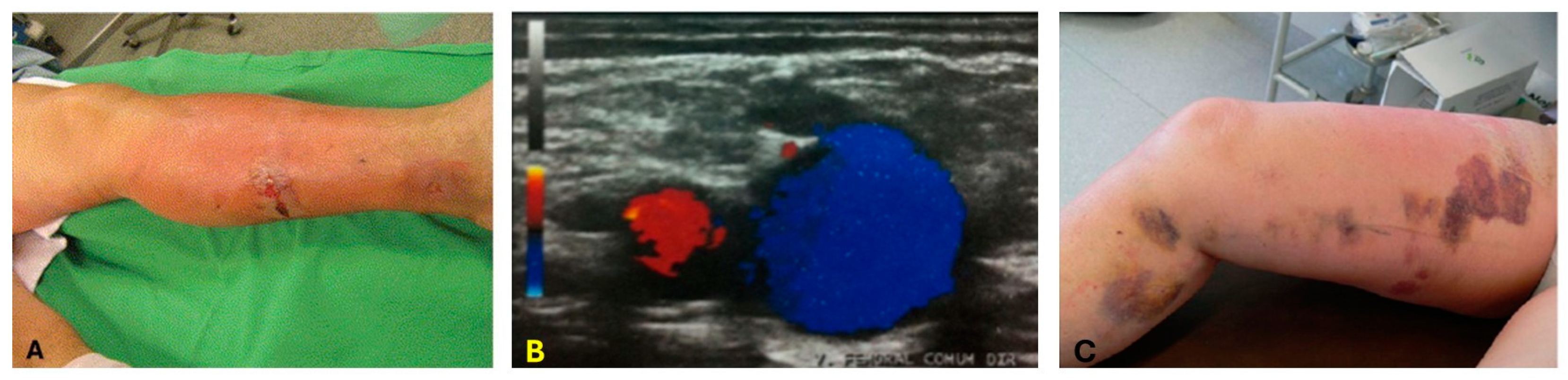
2. Ultrasound and Photoacoustic Imaging for Guidance of Laser Ablation Catheters
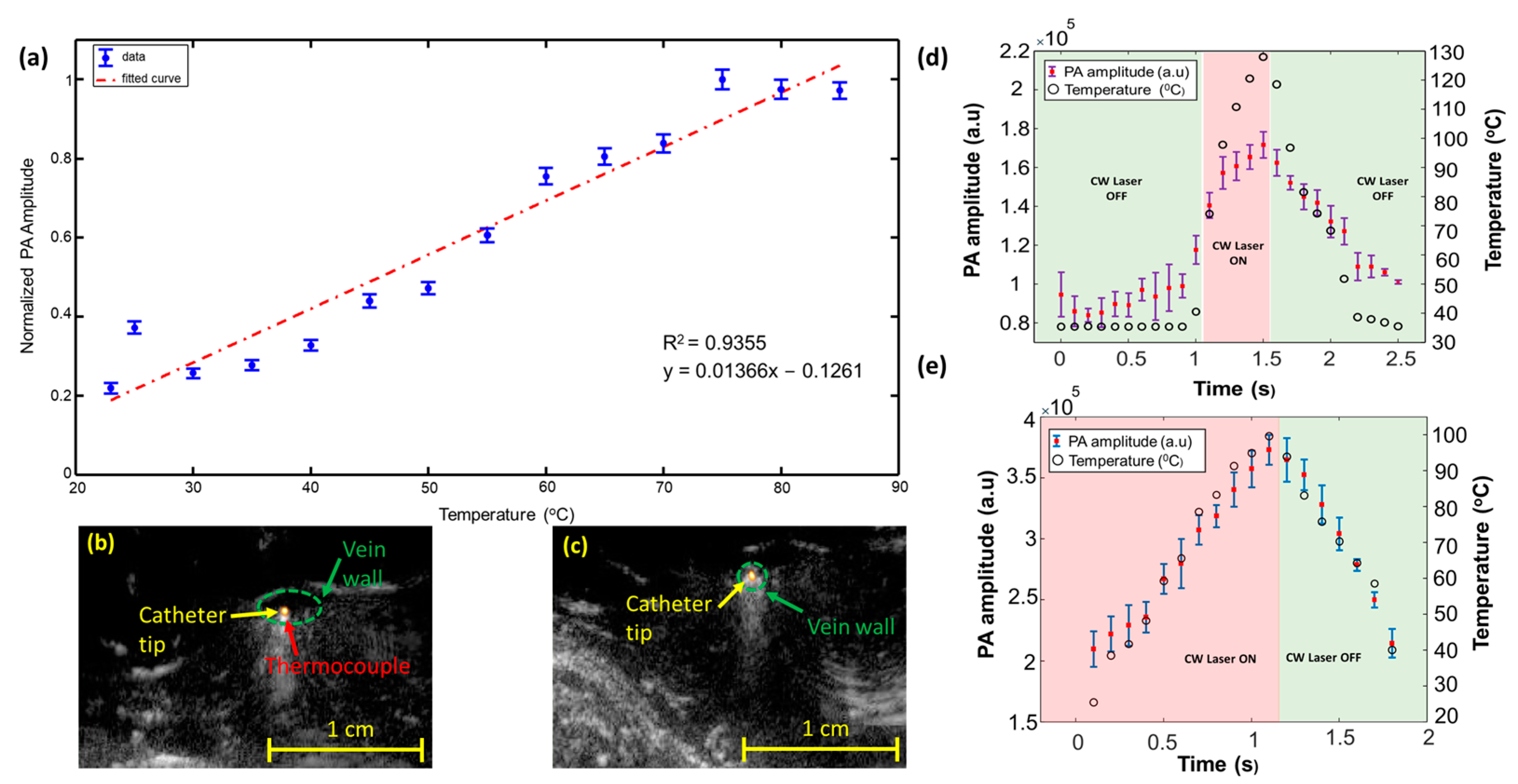
3. Photoacoustic Thermometry during Laser Ablation
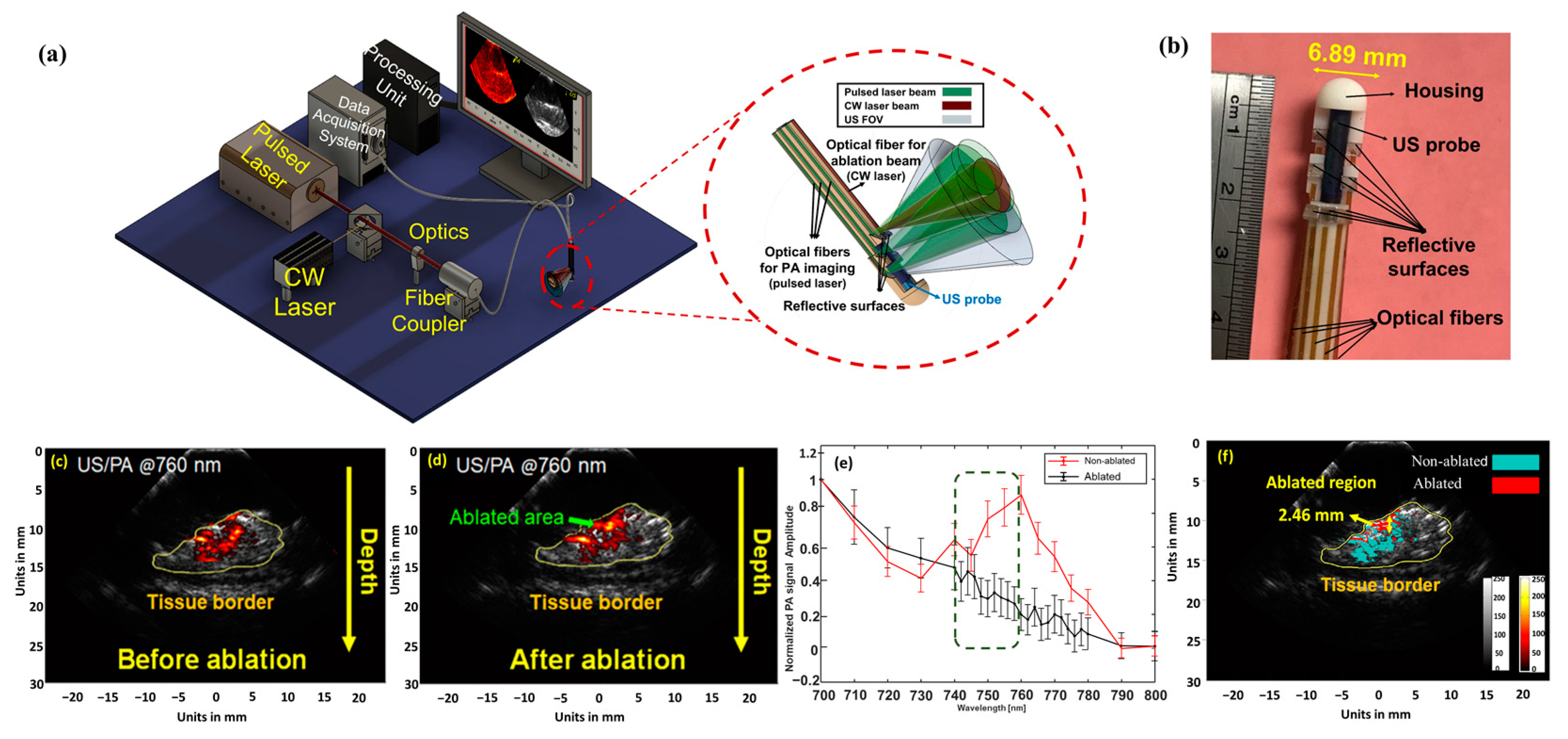
4. Integration of Photoacoustic Imaging and Laser Ablation Devices
5. Endoscopic Photoacoustic Imaging for Detecting the Formation and Extension of Thermal Lesions
6. Importance of High Temporal Resolution in PA-Guided Ablation Procedures
7. Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peters, T.M. Image-guidance for surgical procedures. Phys. Med. Biol. 2006, 51, R505. [Google Scholar] [CrossRef]
- Beigi, P.; Salcudean, S.E.; Ng, G.C.; Rohling, R. Enhancement of needle visualization and localization in ultrasound. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Chapman, G.; Johnson, D.; Bodenham, A.R. Visualisation of needle position using ultrasonography. Anaesthesia 2006, 61, 148–158. [Google Scholar] [CrossRef]
- Mudra, H.; Klauss, V.; Blasini, R.; Kroetz, M.; Rieber, J.; Regar, E.; Theisen, K. Ultrasound guidance of Palmaz-Schatz intracoronary stenting with a combined intravascular ultrasound balloon catheter. Circulation 1994, 90, 1252–1261. [Google Scholar] [CrossRef]
- Russo, R.J. Ultrasound-guided stent placement. Cardiol. Clin. 1997, 15, 49–61. [Google Scholar] [CrossRef]
- Yaniv, Z.; Cleary, K.; Robotics, M. Image-guided procedures: A review. Comput. Aided Interv. Med. Robot. 2006, 3, 7. [Google Scholar]
- Choy, D.S.J. History of lasers in medicine. Thorac. Cardiovasc. Surg. 1988, 36, 114–117. [Google Scholar] [CrossRef]
- Parker, S. Introduction, history of lasers and laser light production. Br. Dent. J. 2007, 202, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, N.; Okamoto, K.; Kimura, T.; Kishi, K.; Okahisa, T.; Okamura, S.; Takayama, T. Endoscopic ablation therapy for gastrointestinal superficial neoplasia. Dig. Endosc. 2012, 24, 139–149. [Google Scholar] [CrossRef]
- Smith, J.A.; Stein, B.S.; Benson, R.C. Lasers in Urologic Surgery; Mosby Inc.: London, UK, 1985. [Google Scholar]
- Adams, D.H. Holmium: YAG laser and pulsed dye laser: A cost comparison. Lasers Surg. Med. Off. J. Am. Soc. Laser Med. Surg. 1997, 21, 29–31. [Google Scholar]
- Chan, K.F.; Vassar, G.J.; Pfefer, T.J.; Teichman, J.M.; Glickman, R.D.; Weintraub, S.T.; Welch, A.J. Holmium: YAG laser lithotripsy: A dominant photothermal ablative mechanism with chemical decomposition of urinary calculi. Lasers Surg. Med. Off. J. Am. Soc. Laser Med. Surg. 1999, 25, 22–37. [Google Scholar]
- Schwarz, T.; von Hodenberg, E.; Furtwängler, C.; Rastan, A.; Zeller, T.; Neumann, F.-J. Endovenous laser ablation of varicose veins with the 1470-nm diode laser. J. Vasc. Surg. 2010, 51, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Hellebust, T. Place of modern imaging in brachytherapy planning. Cancer Radiother. 2018, 22, 326–333. [Google Scholar] [CrossRef] [PubMed]
- van Dyk, S.; Khaw, P.; Lin, M.-Y.; Chang, D.; Bernshaw, D. Ultrasound-guided brachytherapy for cervix cancer. Clin. Oncol. 2021, 33, e403–e411. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Yan, V.; Kabbani, L.; Kennedy, N.A.; Mehrmohammadi, M. Integration of Endovenous Laser Ablation and Photoacoustic Imaging Systems for Enhanced Treatment of Venous Insufficiency. In Proceedings of the 2018 IEEE International Ultrasonics Symposium (IUS), Kobe, Japan, 22–25 October 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–4. [Google Scholar]
- John, S.; Yan, Y.; Forta, S.Y.; Kabbani, L.; Mehrmohammadi, M. Integrated ultrasound and photoacoustic imaging for effective endovenous laser ablation: A characterization study. In Proceedings of the 2019 IEEE International Ultrasonics Symposium (IUS), Glasgow, UK, 6–9 October 2019; pp. 122–125. [Google Scholar]
- Haines, D.E.; Wright, M.; Harks, E.; Deladi, S.; Fokkenrood, S.; Brink, R.; Belt, H.; Kolen, A.F.; Mihajlovic, N.; Zuo, F.; et al. Near-field ultrasound imaging during radiofrequency catheter ablation: Tissue thickness and epicardial wall visualization and assessment of radiofrequency ablation lesion formation and depth. Circ. Arrhythmia Electrophysiol. 2017, 10, e005295. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.J.; Perlas, A.; Chan, V.W.; Brull, R.; Medicine, P. Needle visualization in ultrasound-guided regional anesthesia: Challenges and solutions. Reg. Anesth. Pain Med. 2008, 33, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Natarajan, S.; Lee, M.; Dann, A.E.; Cox, B.P.; Bennett, D.B.; Brown, E.R.; Lee, H.; Grundfest, W.S.; Culjat, M.O. Development of an ultrasound imaging system for needle guidance. In Proceedings of the 2009 IEEE International Ultrasonics Symposium, Rome, Italy, 20–23 September 2009; pp. 1852–1855. [Google Scholar]
- Belavy, D.; Ruitenberg, M.; Brijball, R.B. Feasibility study of real-time three-/four-dimensional ultrasound for epidural catheter insertion. Br. J. Anaesth. 2011, 107, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Rafii-Tari, H.; Abolmaesumi, P.; Rohling, R. Panorama ultrasound for guiding epidural anesthesia: A feasibility study. In Proceedings of the Information Processing in Computer-Assisted Interventions: Second International Conference, IPCAI 2011, Berlin, Germany, 22 June 2011; pp. 179–189. [Google Scholar]
- Zaroff, J.G.; Picard, M.H. Transesophageal echocardiographic (TEE) evaluation of the mitral and tricuspid valves. Cardiol. Clin. 2000, 18, 731–750. [Google Scholar] [CrossRef]
- Aly, I.; Rizvi, A.; Roberts, W.; Khalid, S.; Kassem, M.W.; Salandy, S.; du Plessis, M.; Tubbs, R.S.; Loukas, M. Cardiac ultrasound: An anatomical and clinical review. Transl. Res. Anat. 2021, 22, 100083. [Google Scholar] [CrossRef]
- Prabhakar, C.; Uppal, V.; Sondekoppam, R.V. Effect of beam steering on echogenic and nonechogenic needle visibility at 40, 50, and 60 needle insertion angles. Obstet. Anesth. Dig. 2018, 126, 1926–1929. [Google Scholar] [CrossRef]
- Uppal, V.; Sondekoppam, R.V.; Ganapathy, S. Effect of beam steering on the visibility of echogenic and non-echogenic needles: A laboratory study. Can. J. Anaesth 2014, 61, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.M.; Fronheiser, M.P.; Reach, J.; Nielsen, K.C.; Smith, S.W. Piezoelectric vibrating needle and catheter for enhancing ultrasound-guided peripheral nerve blocks. Obstet. Anesth. Dig. 2007, 105, 1858–1860. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; John, S.; Ghalehnovi, M.; Kabbani, L.; Kennedy, N.A.; Mehrmohammadi, M. Photoacoustic Imaging for Image-guided endovenous Laser Ablation procedures. Sci. Rep. 2019, 9, 2933. [Google Scholar] [CrossRef] [PubMed]
- Mehrmohammadi, M.; Alizad, A.; Kinnick, R.R.; Davis, B.J.; Fatemi, M. Vibro-acoustography with 1.75 D ultrasound array transducer for detection and localization of permanent prostate brachytherapy seeds: Ex vivo study. In Proceedings of the Medical Imaging 2013: Ultrasonic Imaging, Tomography, and Therapy, Lake Buena Vista, FL, USA, 12–14 February 2013; pp. 252–258. [Google Scholar]
- Wei, C.-W.; Nguyen, T.-M.; Xia, J.; Arnal, B.; Pelivanov, I.; O’Donnell, M. Clinically translatable ultrasound/photoacoustic imaging for real-time needle biopsy guidance. In Proceedings of the 2014 IEEE International Ultrasonics Symposium, Chicago, IL, USA, 3–6 September 2014; pp. 839–842. [Google Scholar]
- Reusz, G.; Sarkany, P.; Gal, J.; Csomos, A. Needle-related ultrasound artifacts and their importance in anaesthetic practice. Br. J. Anaesth. 2014, 112, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Ziskin, M.; Thickman, D.; Goldenberg, N.; Lapayowker, M.; Becker, J.M. The comet tail artifact. J. Ultrasound Med. 1982, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Varghese, T.; Zagzebski, J.; Chen, Q.; Techavipoo, U.; Frank, G.; Johnson, C.; Wright, A.; Lee, F.T., Jr. Ultrasound monitoring of temperature change during radiofrequency ablation: Preliminary in-vivo results. Ultrasound Med. Biol. 2002, 28, 321–329. [Google Scholar] [CrossRef] [PubMed]
- REN, J.F.; Marchlinski, F.E.; Callans, D.J.; Zado, E.S. Echocardiographic lesion characteristics associated with successful ablation of inappropriate sinus tachycardia. J. Cardiovasc. Electrophysiol. 2001, 12, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.A.; Staruch, R.M.; Chopra, R. Thermometry and ablation monitoring with ultrasound. Int. J. Hyperth. 2015, 31, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Iskander-Rizk, S.; Kruizinga, P.; Van Der Steen, A.F.; Van Soest, G. Spectroscopic photoacoustic imaging of radiofrequency ablation in the left atrium. Biomed. Opt. Express 2018, 9, 1309–1322. [Google Scholar] [CrossRef]
- Malskat, W.; Stokbroekx, M.; van der Geld, C.; Nijsten, T.; Van den Bos, R. Temperature profiles of 980-and 1,470-nm endovenous laser ablation, endovenous radiofrequency ablation and endovenous steam ablation. Lasers Med. Sci. 2014, 29, 423–429. [Google Scholar] [CrossRef]
- van den Bos, R.R.; Kockaert, M.A.; Neumann, H.M.; Bremmer, R.H.; Nijsten, T.; van Gemert, M.J. Heat conduction from the exceedingly hot fiber tip contributes to the endovenous laser ablation of varicose veins. Lasers Med. Sci. 2009, 24, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Elias, S.; Peden, E. Ultrasound-guided percutaneous ablation for the treatment of perforating vein incompetence. Vascular 2007, 15, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.; Taylor, M.; Watkins, N.; Franke, V.; Parker, K.; Fisk, N. Characterisation of deep arterio-venous anastomoses within monochorionic placentae by vascular casting. Placenta 2005, 26, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Dunst, K.M.; Huemer, G.M.; Wayand, W.; Shamiyeh, A. Diffuse phlegmonous phlebitis after endovenous laser treatment of the greater saphenous vein. J. Vasc. Surg. 2006, 43, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Araujo WJ, B.D.; Timi JR, R.; Erzinger, F.L.; Caron, F.C. Endothermal heat-induced thrombosis (EHIT): Reports on two case treated with rivaroxaban and literature review. J. Vasc. Bras. 2016, 15, 147–152. [Google Scholar] [PubMed]
- Vuylsteke, M.E.; Vandekerckhove, P.J.; De Bo, T.; Moons, P.; Mordon, S. Use of a new endovenous laser device: Results of the 1,500 nm laser. Ann. Vasc. Surgery 2010, 24, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Beard, P.C. Biomedical photoacoustic imaging. J. Acoust. Soc. Am. 2011, 1, 602–631. [Google Scholar] [CrossRef] [PubMed]
- Mirg, S.; Turner, K.L.; Chen, H.; Drew, P.J.; Kothapalli, S. Photoacoustic imaging for microcirculation. Microcirculation 2022, 29, e12776. [Google Scholar] [CrossRef]
- Yan, Y.; John, S.; Shaik, T.; Patel, B.; Lam, M.T.; Kabbani, L.; Mehrmohammadi, M. Photoacoustic-guided endovenous laser ablation: Characterization and in vivo canine study. Photoacoustics 2021, 24, 100298. [Google Scholar] [CrossRef]
- Li, M.; Tang, Y.; Yao, J. Photoacoustic tomography of blood oxygenation: A mini review. Photoacoustics 2018, 10, 65–73. [Google Scholar] [CrossRef]
- Turgay, E.; Salcudean, S.; Rohling, R. Identifying the mechanical properties of tissue by ultrasound strain imaging. Ultrasound Med. Biol. 2006, 32, 221–235. [Google Scholar] [CrossRef]
- Emelianov, S.Y.; Li, P.-C.; O’Donnell, M. Photoacoustics for molecular imaging and therapy. Phys. Today 2009, 62, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; John, S.; Ghalehnovi, M.; Kabbani, L.; Kennedy, N.A.; Mehrmohammadi, M. Ultrasound and photoacoustic imaging for enhanced image-guided endovenous laser ablation procedures. In Proceedings of the Medical Imaging 2018: Ultrasonic Imaging and Tomography, Houston, TX, USA, 14–15 February 2018; pp. 184–189. [Google Scholar]
- Prahl, S. Optical Properties Spectra of Hemoglobin and Water. 2001. Available online: http://omlc.ogi.edu/spectra/ (accessed on 10 April 2024).
- Tsai, C.-L.; Chen, J.-C.; Wang, W.-J. Near-infrared absorption property of biological soft tissue constituents. J. Med. Biol. Eng. 2001, 21, 7–14. [Google Scholar]
- Pannier, F.; Rabe, E.; Rits, J.; Kadiss, A.; Maurins, U. Endovenous laser ablation of great saphenous veins using a 1470 nm diode laser and the radial fibre–follow-up after six months. Phlebol. J. Venous Dis. 2011, 26, 35–39. [Google Scholar] [CrossRef]
- Peng, Q.; Juzeniene, A.; Chen, J.; Svaasand, L.O.; Warloe, T.; Giercksky, K.-E.; Moan, J. Lasers in medicine. Rep. Prog. Phys. 2008, 71, 056701. [Google Scholar] [CrossRef]
- Nilsson, A.M.; Lucassen, G.W.; Verkruysse, W.; Andersson-Engels, S.; van Gemert, M.J.C. Changes in optical properties of human whole blood in vitro due to slow heating. Photochem. Photobiol. 1997, 65, 366–373. [Google Scholar] [CrossRef]
- Landa, F.J.O.; Deán-Ben, X.L.; Sroka, R.; Razansky, D. Volumetric optoacoustic temperature mapping in photothermal therapy. Sci. Rep. 2017, 7, 9695. [Google Scholar] [CrossRef]
- Wang, L.V.; Wu, H.I. Biomedical Optics: Principles and Imaging; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Xia, J.; Yao, J.; Wang, L.V. Photoacoustic tomography: Principles and advances. Electromagn. Waves 2014, 147, 1–22. [Google Scholar] [CrossRef]
- Malskat, W.S.; Poluektova, A.A.; van der Geld, C.W.; Neumann, H.M.; Weiss, R.A.; Bruijninckx, C.M.; van Gemert, M.J.C. Endovenous laser ablation (EVLA): A review of mechanisms, modeling outcomes, and issues for debate. Lasers Med. Sci. 2014, 29, 393–403. [Google Scholar] [CrossRef]
- Mordon, S.R.; Wassmer, B.; Zemmouri, J. Mathematical modeling of endovenous laser treatment (ELT). BioMed. Eng. OnLine 2006, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Van Ruijven, P.W.; Poluektova, A.A.; van Gemert, M.J.; Neumann, H.M.; Nijsten, T.; van der Geld, C.W. Optical-thermal mathematical model for endovenous laser ablation of varicose veins. Lasers Med. Sci. 2014, 29, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Van den Bos, R.; Kockaert, M.; Neumann, H.; Nijsten, T. Technical review of endovenous laser therapy for varicose veins. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Vuylsteke, M.E.; Mordon, S.R. Endovenous laser ablation: A review of mechanisms of action. Ann. Vasc. Surg. 2012, 26, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L. Heat transfer applications in biological systems. In Biomedical Engineering & Design Handbook; McGraw-Hill Professional: New York, NY, USA, 2009; Volume 1, pp. 2–33. [Google Scholar]
- Ochsner, T.E.; Horton, R.; Ren, T. A new perspective on soil thermal properties. Soil Sci. Soc. Am. J. 2001, 65, 1641–1647. [Google Scholar] [CrossRef]
- Bigg, P.H. Density of water in SI units over the range 0–40 C. Br. J. Appl. Phys. 1967, 18, 521. [Google Scholar] [CrossRef]
- Huber, M.L.; Perkins, R.A.; Friend, D.G.; Sengers, J.V.; Assael, M.J.; Metaxa, I.N.; Miyagawa, K.; Hellmann, R.; Vogel, E. New international formulation for the thermal conductivity of H2O. J. Phys. Chem. Ref. Data 2012, 41, 033102. [Google Scholar] [CrossRef]
- Kosky, P.; Balmer, R.T.; Keat, W.D.; Wise, G. Exploring Engineering: An Introduction to Engineering and Design; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- John, S.; Maghsoodi, N.; Ghag, A.; Kabbani, L.; Yan, Y.; Mehrmohammadi, M. Ultrasound and Photoacoustic Guided Tissue Temperature Mapping during Ablation Therapies. In Proceedings of the 2022 IEEE International Ultrasonics Symposium (IUS), Venice, Italy, 10–13 October 2022; pp. 1–4. [Google Scholar]
- Fehm, T.F.; Deán-Ben, X.L.; Schaur, P.; Sroka, R.; Razansky, D. Volumetric optoacoustic imaging feedback during endovenous laser therapy–an ex vivo investigation. J. Biophotonics 2016, 9, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Dana, N.; Di Biase, L.; Natale, A.; Emelianov, S.; Bouchard, R. In vitro photoacoustic visualization of myocardial ablation lesions. Heart Rhythm. 2014, 11, 150–157. [Google Scholar] [CrossRef]
- Basij, M.; John, S.; Bustamante, D.; Kabbani, L.; Maskoun, W.; Mehrmohammadi, M. Integrated Ultrasound and Photoacoustic-guided Laser Ablation Theranostic Endoscopic System. IEEE Trans. Biomed. Eng. 2022, 70, 67–75. [Google Scholar] [CrossRef]
- Yan, Y.; John, S.; Meiliute, J.; Kabbani, L.; Mehrmohammadi, M. Efficacy of High Temporal Frequency Photoacoustic Guidance of Laser Ablation Procedures. Ultrasonic Imaging 2021, 43, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Ashikaga, H.; Mansi, T.; Halperin, H.R.; Zhang, H.K. Photoacoustic necrotic region mapping for radiofrequency ablation guidance. In Proceedings of the 2021 IEEE International Ultrasonics Symposium (IUS), Virtual, 11–16 September 2021; pp. 1–4. [Google Scholar]
- Gao, S.; Ashikaga, H.; Suzuki, M.; Mansi, T.; Kim, Y.-H.; Ghesu, F.-C.; Kang, J.; Boctor, E.M.; Halperin, H.R.; Zhang, H.K. Cardiac-Gated Spectroscopic Photoacoustic Imaging for Ablation-Induced Necrotic Lesion Visualization: In Vivo Demonstration in a Beating Heart. bioRxiv 2022. [Google Scholar] [CrossRef]
- Gao, S.; Rahaman, A.; Ashikaga, H.; Halperin, H.R.; Zhang, H.K. Miniaturized catheter-integrated photoacoustic ablation monitoring system: A feasibility study. In Proceedings of the 2022 IEEE International Ultrasonics Symposium (IUS), Venice, Italy, 10–13 October 2022; pp. 1–4. [Google Scholar]



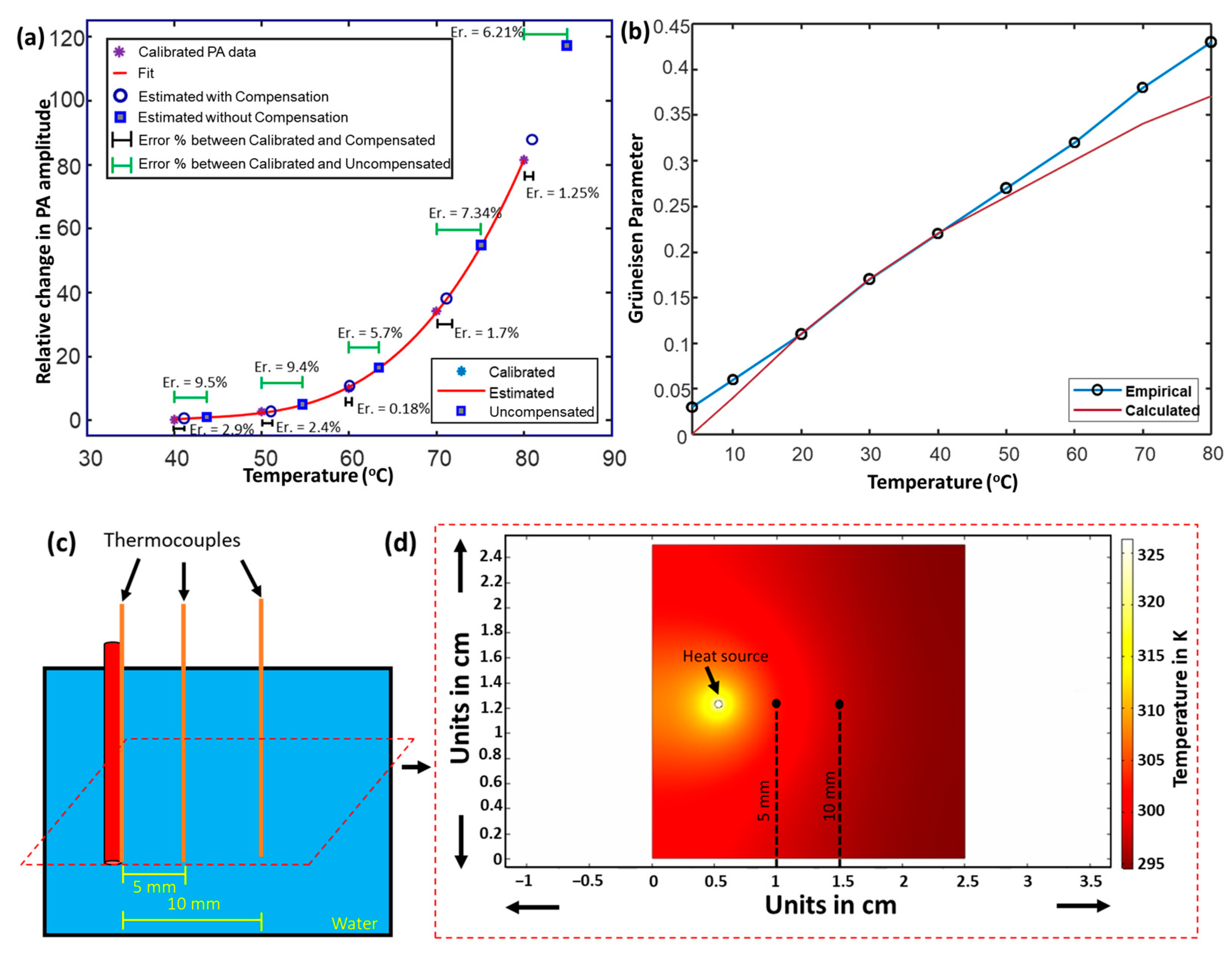
| Expected Temperature (°C) | Estimated Temperature (Compensated) (°C) | Estimated Temperature (Un-Compensated) (°C) | Error% Expected Temperature vs. Compensated | Error% Expected Temperature vs. Uncompensated |
|---|---|---|---|---|
| 40 | 41.19 | 43.79 | 2.97% | 9.5% |
| 50 | 51.12 | 54.71 | 2.24% | 9.42% |
| 60 | 60.11 | 63.44 | 0.18% | 5.73% |
| 70 | 71.19 | 75.14 | 1.7% | 7.3% |
| 80 | 81.00 | 84.97 | 1.25% | 6.2% |
| Temperature @ 5mm (°C) | Error % | Temperature @ 10 mm (°C) | Error % | ||
|---|---|---|---|---|---|
| Approx. | Experimental | 5.6% | Approx. | Experimental | 8.26% |
| 34.23 | 36.16 | 31.7 | 34.32 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
John, S.; Yan, Y.; Abbasi, S.; Mehrmohammadi, M. Ultrasound and Photoacoustic Imaging for the Guidance of Laser Ablation Procedures. Sensors 2024, 24, 3542. https://doi.org/10.3390/s24113542
John S, Yan Y, Abbasi S, Mehrmohammadi M. Ultrasound and Photoacoustic Imaging for the Guidance of Laser Ablation Procedures. Sensors. 2024; 24(11):3542. https://doi.org/10.3390/s24113542
Chicago/Turabian StyleJohn, Samuel, Yan Yan, Shirin Abbasi, and Mohammad Mehrmohammadi. 2024. "Ultrasound and Photoacoustic Imaging for the Guidance of Laser Ablation Procedures" Sensors 24, no. 11: 3542. https://doi.org/10.3390/s24113542





