Utilizing COVID-19 as a Model for Diagnostics Using an Electrochemical Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical and Sensing Interface Preparation
2.3. Electrochemical Measurements and Optimization
2.4. Clinical Application
3. Results and Discussion
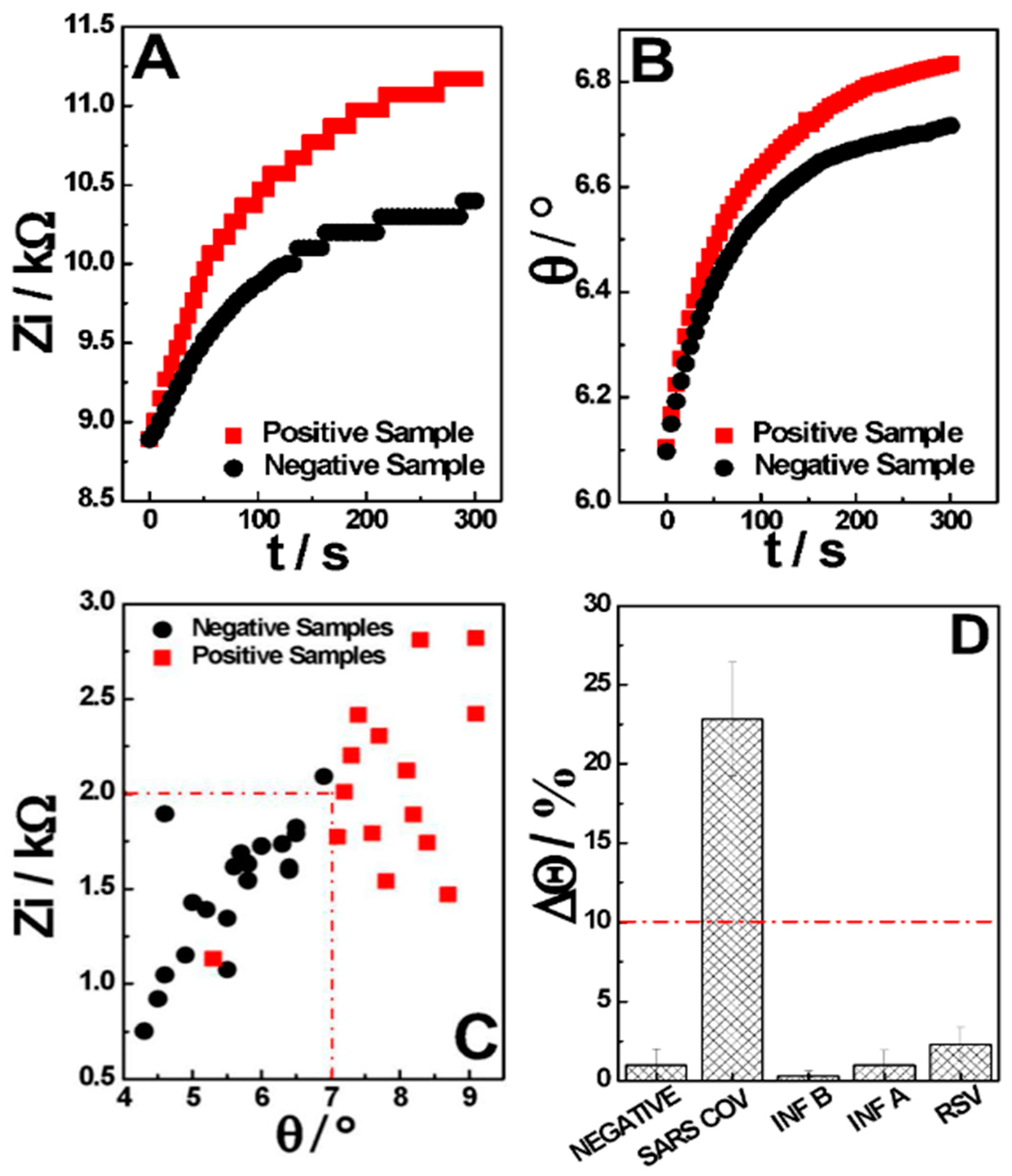
3.1. Impedimetric Measurement
3.2. Chemical Optimization and Analytical Performance
3.3. AI-Based Classification and Clinical Performance
| Sensor | Sample | Electrochemical Feature | LOD/ng mL−1 | Time Required | Ref |
|---|---|---|---|---|---|
| Aptasensing nucleocapsid protein on nanodiamond-assembled gold-interdigitated | Nasopharyngeal | Redox probe solution using EIS | 1.82 × 10−5 | 5 min 1 + Measurement | [33] |
| Anti-N on MUA-AuNPs-modified SPE | Nasopharyngeal | Redox probe detection using SWV | 0.0004 | 15 min 1 + Measurement | [34] |
| N-protein on carbon nanofiber-modified SPE | Nasopharyngeal | Competitive redox probe detection using SWV | 0.0008 | 20 min 1 + Measurement | [35] |
| Magnetic bead-based immunosensor combined with carbon black-modified screen-printed electrode | Saliva | Magnetic beads and alkaline phosphatase labeled using DPV | 8.0 | 30 min 1 + Measurement | [36] |
| Anti-N on screen-printed gold electrodes assisted by labeled magnetic beads | Serum | Redox probe amperometric detection | 0.23 | 20 min 1 + Measurement | [37] |
| N-protein molecularly imprinted polymer | Nasopharyngeal | Redox probe detection using DPV | 0.0007 | 15 min 1 + Measurement | [38] |
| Anti-N conjugated with AuNPs/Sac/Tre/SPE (CovAg-SPE) | Nasopharyngeal | Capacitive measurement using EIS | 1.0 | 5 min | * |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qazi, S.; Raza, K. Smart Biosensors for an Efficient Point of Care (PoC) Health Management. In Smart Biosensors in Medical Care; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 65–85. ISBN 9780128207819. [Google Scholar]
- Kelly-Cirino, C.D.; Nkengasong, J.; Kettler, H.; Tongio, I.; Gay-Andrieu, F.; Escadafal, C.; Piot, P.; Peeling, R.W.; Gadde, R.; Boehme, C. Importance of Diagnostics in Epidemic and Pandemic Preparedness. BMJ Glob. Health 2019, 4, e001179. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yang, D.; Yuan, Z.; Zhang, Y.; Li, H.; Gao, P.; Liu, X.; Zhao, W.; Xiao, T.; Guan, Y.; et al. Improving the Early Diagnosis of Suspected Patients with COVID-19: A Retrospective Study of 106 Patients. J. Infect. Dev. Ctries. 2020, 14, 547. [Google Scholar] [CrossRef] [PubMed]
- Tertis, M.; Hosu, O.; Florea, A.; Cristea, C. Biosensors for Clinical Samples: Consideration and Approaches. In Immunodiagnostic Technologies from Laboratory to Point-of-Care Testing; Springer: Singapore, 2021; pp. 1–32. [Google Scholar]
- Wang, Y.; Xu, H.; Zhang, J.; Li, G. Electrochemical Sensors for Clinic Analysis. Sensors 2008, 8, 2043. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; He, B.; Gao, B. Emerging electrochemical sensors for life healthcare. Eng. Regen. 2021, 2, 175–181. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Kumar, N.; Shetti, N.P.; Jagannath, S.; Aminabhavi, T.M. Electrochemical Sensors for the Detection of SARS-CoV-2 Virus. J. Chem. Eng. 2022, 430, 132966. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.C.; Soares, A.C.; Angelim, M.K.S.; Proença-Modena, J.L.; Moraes-Vieira, P.M.; Mattoso, L.H.; Oliveira, O.N., Jr. Diagnostics of SARS-CoV-2 Infection Using Electrical Impedance Spectroscopy with an Immunosensor to Detect the Spike Protein. Talanta 2022, 239, 123076. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, A.; Wang, X. Paper-Based Electrochemical Biosensor for Diagnosing COVID-19: Detection of SARS-CoV-2 Antibodies and Antigen. Biosens. Bioelectron. 2020, 176, 112912. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.S.; Pourmand, N. Label-Free Impedance Biosensors: Opportunities and Challenges. Electroanalysis 2007, 19, 1239. [Google Scholar] [CrossRef]
- Bertok, T.; Lorencova, L.; Chocholova, E.; Jane, E.; Vikartovska, A.; Kasak, P.; Tkac, J. Electrochemical Impedance Spectroscopy Based Biosensors: Mechanistic Principles, Analytical Examples and Challenges towards Commercialization for Assays of Protein Cancer Biomarkers. ChemElectroChem 2019, 6, 989. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. Electrochemical Impedance Spectroscopy: An Overview of Bioanalytical Applications. Anal. Methods 2013, 5, 1098. [Google Scholar] [CrossRef]
- Stevenson, H.; Shanmugam, N.R.; Selvam, A.P.; Prasad, S. The Anatomy of a Nonfaradaic Electrochemical Biosensor. SLAS Technol. 2018, 23, 5. [Google Scholar] [CrossRef] [PubMed]
- Kirchhain, A.; Bonini, A.; Vivaldi, F.; Poma, N.; Di Francesco, F. Latest Developments in Non-Faradic Impedimetric Biosensors: Towards Clinical Applications. TrAC Trends Anal. Chem. 2020, 133, 116073. [Google Scholar] [CrossRef]
- Nicollete, D.R.P.; Benedetti, R.; Valença, B.A.; Kuniyoshi, K.K.; de Jesus, T.C.S.; Gevaerd, A.; Santiago, E.B.; de Almeida, B.M.M.; Júnior, S.R.R.; Figueredo, M.V.M. Cost Aware Strategies for Sensitivity Enhancement in a SARS-CoV-2 Antigen Test Prototype: Insertion of a Cotton Intermembrane Doubles Analytical Sensitivity. Sci. Rep. 2023, 13, 4690. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C. Conjugation of Colloidal Gold to Proteins. In Immunocytochemical Methods and Protocols; Methods in Molecular Biology; Oliver, C., Jamur, M., Eds.; Humana Press: Totowa, NJ, USA, 2010; Volume 588. [Google Scholar] [CrossRef]
- Rayavarapu, R.G.; Petersen, W.; Ungureanu, C.; Post, J.N.; van Leeuwen, T.G.; Manohar, S. Synthesis and bioconjugation of gold nanoparticles as potential molecular probes for light-based imaging techniques. Int. J. Biomed. Imaging 2007, 2007, 29817. [Google Scholar] [CrossRef] [PubMed]
- Faria, R.A.D.; Heneine, L.G.D.; Matencio, T.; Messaddeq, Y. Faradaic and Non-Faradaic Electrochemical Impedance Spectroscopy as Transduction Techniques for Sensing Applications. Int. J. Biosens. Bioelectron. 2019, 5, 29–31. [Google Scholar] [CrossRef]
- Tanak, A.S.; Jagannath, B.; Tamrakar, Y.; Muthukumar, S.; Prasad, S. Non-faradaic electrochemical impedimetric profiling of procalcitonin and C-reactive protein as a dual marker biosensor for early sepsis detection. Anal. Chim. Acta X 2019, 3, 100029. [Google Scholar] [CrossRef] [PubMed]
- Shoute, L.C.T.; Abdelrasoul, G.N.; Ma, Y.; Duarte, P.A.; Edwards, C.; Zhuo, R.; Zeng, J.; Feng, Y.; Charlton, C.L.; Kanji, J.N.; et al. Label-Free Impedimetric Immunosensor for Point-of-Care Detection of COVID-19 Antibodies. Microsyst. Nanoeng. 2023, 9, 3. [Google Scholar] [CrossRef]
- Arakawa, T.; Timasheff, S.N. Stabilization of protein structure by sugars. Biochemistry 1982, 21, 6536–6544. [Google Scholar] [CrossRef]
- Chang, L.L.; Pikal, M.J. Mechanisms of protein stabilization in the solid state. J. Pharm. Sci. 2009, 98, 2886–2908. [Google Scholar] [CrossRef]
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L. How sugars protect proteins in the solid state and during drying (review): Mechanisms of stabilization in relation to stress conditions. Eur. J. Pharm. Biopharm. 2017, 114, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Jeyachandran, Y.L.; Mielczarski, J.A.; Mielczarski, E.; Rai, B. Efficiency of blocking of non-specific interaction of different proteins by BSA adsorbed on hydrophobic and hydrophilic surfaces. J. Colloid Interface Sci. 2010, 341, 136–142. [Google Scholar] [CrossRef]
- Lichtenberg, J.Y.; Ling, Y.; Kim, S. Non-Specific Adsorption Reduction Methods in Biosensing. Sensors 2019, 19, 2488. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chisti, M.M.; Zeng, X. General Signal Amplification Strategy for Nonfaradic Impedimetric Sensing: Trastuzumab Detection Employing a Peptide Immunosensor. Anal. Chem. 2017, 89, 4013. [Google Scholar] [CrossRef] [PubMed]
- Poghossian, A.; Schöning, M.J. Capacitive Field-Effect EIS Chemical Sensors and Biosensors: A Status Report. Sensors 2020, 20, 5639. [Google Scholar] [CrossRef]
- Li, X.; Xiong, M.; Deng, Q.; Guo, X.; Li, Y. The utility of SARS-CoV-2 nucleocapsid protein in laboratory diagnosis. J. Clin. Lab. Anal. 2022, 36, 24534. [Google Scholar] [CrossRef]
- Linares, M.; Pérez-Tanoira, R.; Carrero, A.; Romanyk, J.; Pérez-García, F.; Gómez-Herruz, P.; Arroyo, T.; Cuadros, J. Panbio antigen rapid test is reliable to diagnose SARS-CoV-2 infection in the first 7 days after the onset of symptoms. J. Clin. Microbiol. 2020, 58, 00977-20. [Google Scholar] [CrossRef]
- Prince-Guerra, J.L. Evaluation of Abbott BinaxNOW Rapid Antigen Test for SARS-CoV-2 Infection at Two Community-Based Testing Sites—Pima County, Arizona, November 3–17, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 100. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater Reliability: The Kappa Statistic. Biochem. Med. (Zagreb) 2012, 22, 276. [Google Scholar] [CrossRef]
- Ramanathan, S.; Gopinath, S.C.B.; Ismail, Z.H.; Md Arshad, M.K.; Poopalan, P. Aptasensing Nucleocapsid Protein on Nanodiamond Assembled Gold Interdigitated Electrodes for Impedimetric SARS-CoV-2 Infectious Disease Assessment. Biosens. Bioelectron. 2022, 197, 113735. [Google Scholar] [CrossRef]
- Eissa, S.; Alhadrami, H.A.; Al-Mozaini, M.; Hassan, A.M.; Zourob, M. Voltammetric-Based Immunosensor for the Detection of SARS-CoV-2 Nucleocapsid Antigen. Microchim. Acta 2021, 188, 199. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Zourob, M. Development of a Low-Cost Cotton-Tipped Electrochemical Immunosensor for the Detection of SARS-CoV-2. Anal. Chem. 2021, 93, 1826. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, L.; Saroglia, M.; Galatà, G.; De Santis, R.; Fillo, S.; Luca, V.; Faggioni, G.; D’amore, N.; Regalbuto, E.; Salvatori, P.; et al. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021, 171, 112686. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lillehoj, P.B. Microfluidic Magneto Immunosensor for Rapid, High Sensitivity Measurements of SARS-CoV-2 Nucleocapsid Protein in Serum. ACS Sens. 2021, 6, 1270. [Google Scholar] [CrossRef]
- Raziq, A.; Kidakova, A.; Boroznjak, R.; Reut, J.; Öpik, A.; Syritski, V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021, 178, 113029. [Google Scholar] [CrossRef]



| Gold Standard | Total | |||
|---|---|---|---|---|
| + | − | |||
| Hilab | + | 78 | 6 | 84 |
| − | 16 | 152 | 168 | |
| Total | 94 | 158 | 252 | |
| CI/% | ||
|---|---|---|
| Sensitivity | 83.0% | 74.0—89.0% |
| Specificity | 96.2% | 92.0—98.0% |
| Accuracy | 91.3% | 86.4—100.0% |
| PPV 1 | 92.9% | - |
| NPV 2 | 90.5% | - |
| p0 1 | 0.91 |
| pe 2 | 0.54 |
| SE(k) | 0.04 |
| k | 0.81 |
| CI | 0.73 to 0.89 |
| Strong Agreement | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gevaerd, A.; Carneiro, E.A.; Gogola, J.L.; Nicollete, D.R.P.; Santiago, E.B.; Riedi, H.P.; Timm, A.; Predebon, J.V.; Hartmann, L.F.; Ribeiro, V.H.A.; et al. Utilizing COVID-19 as a Model for Diagnostics Using an Electrochemical Sensor. Sensors 2024, 24, 3772. https://doi.org/10.3390/s24123772
Gevaerd A, Carneiro EA, Gogola JL, Nicollete DRP, Santiago EB, Riedi HP, Timm A, Predebon JV, Hartmann LF, Ribeiro VHA, et al. Utilizing COVID-19 as a Model for Diagnostics Using an Electrochemical Sensor. Sensors. 2024; 24(12):3772. https://doi.org/10.3390/s24123772
Chicago/Turabian StyleGevaerd, Ava, Emmanuelle A. Carneiro, Jeferson L. Gogola, Diego R. P. Nicollete, Erika B. Santiago, Halanna P. Riedi, Adriano Timm, João V. Predebon, Luis F. Hartmann, Victor H. A. Ribeiro, and et al. 2024. "Utilizing COVID-19 as a Model for Diagnostics Using an Electrochemical Sensor" Sensors 24, no. 12: 3772. https://doi.org/10.3390/s24123772







