Synchronization of Neurophysiological and Biomechanical Data in a Real-Time Virtual Gait Analysis System (GRAIL): A Proof-of-Principle Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reasons for the Design of the Measurement System
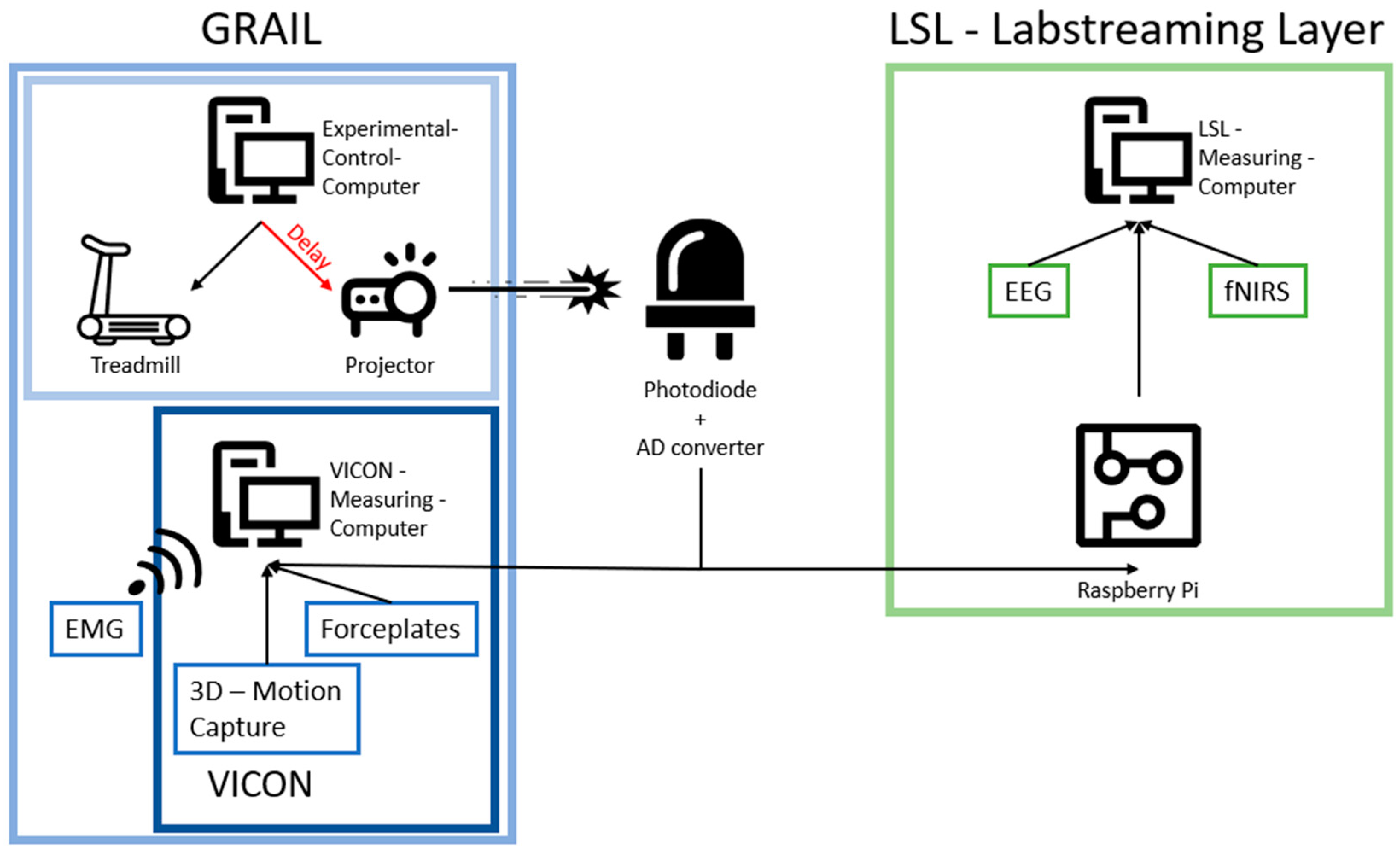
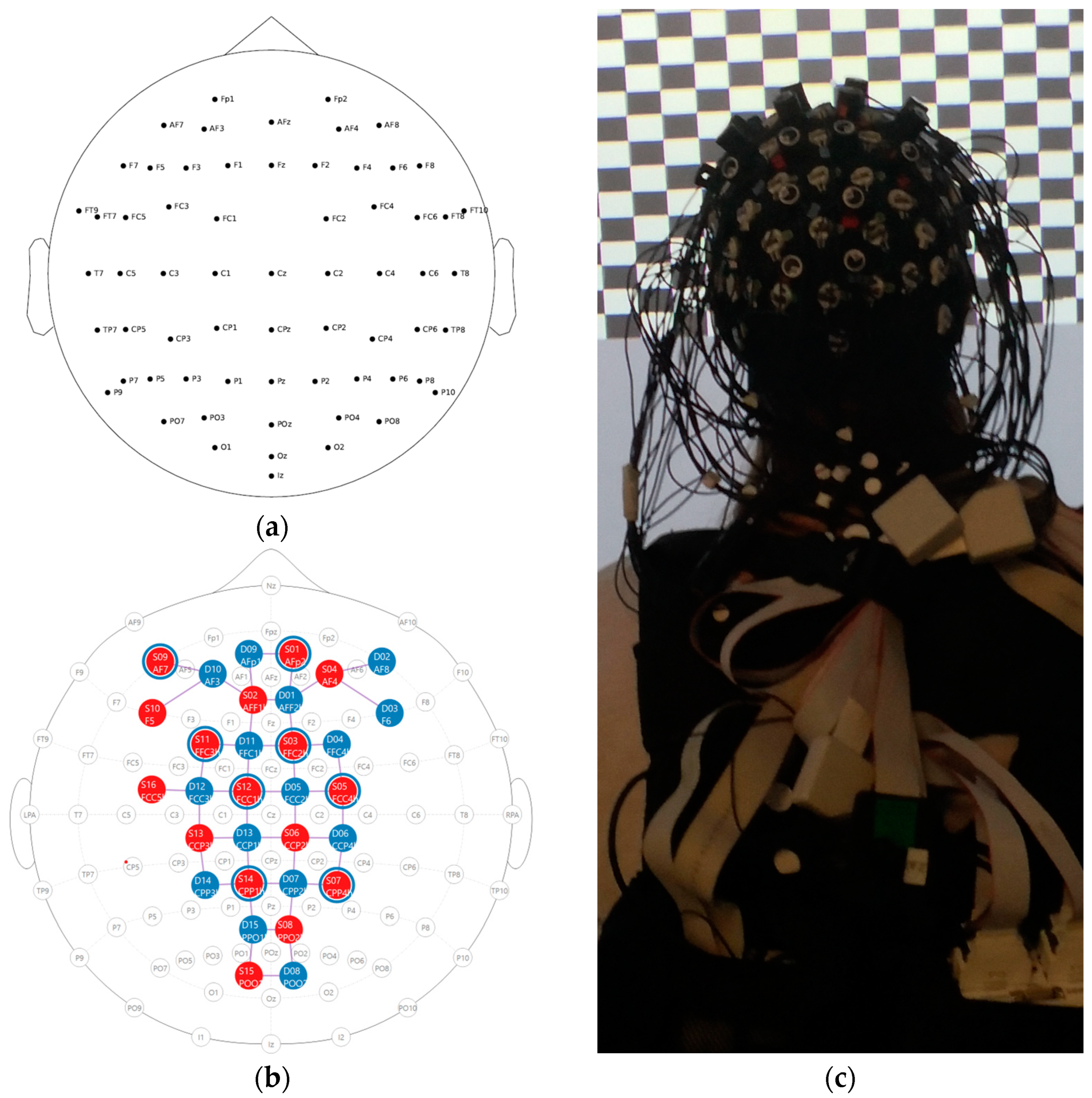
2.2. Measurement Systems and Synchronization—The Components of the System
2.3. Participants
2.4. Task and Procedure of the Piloting
2.4.1. Task and Experimental Setup
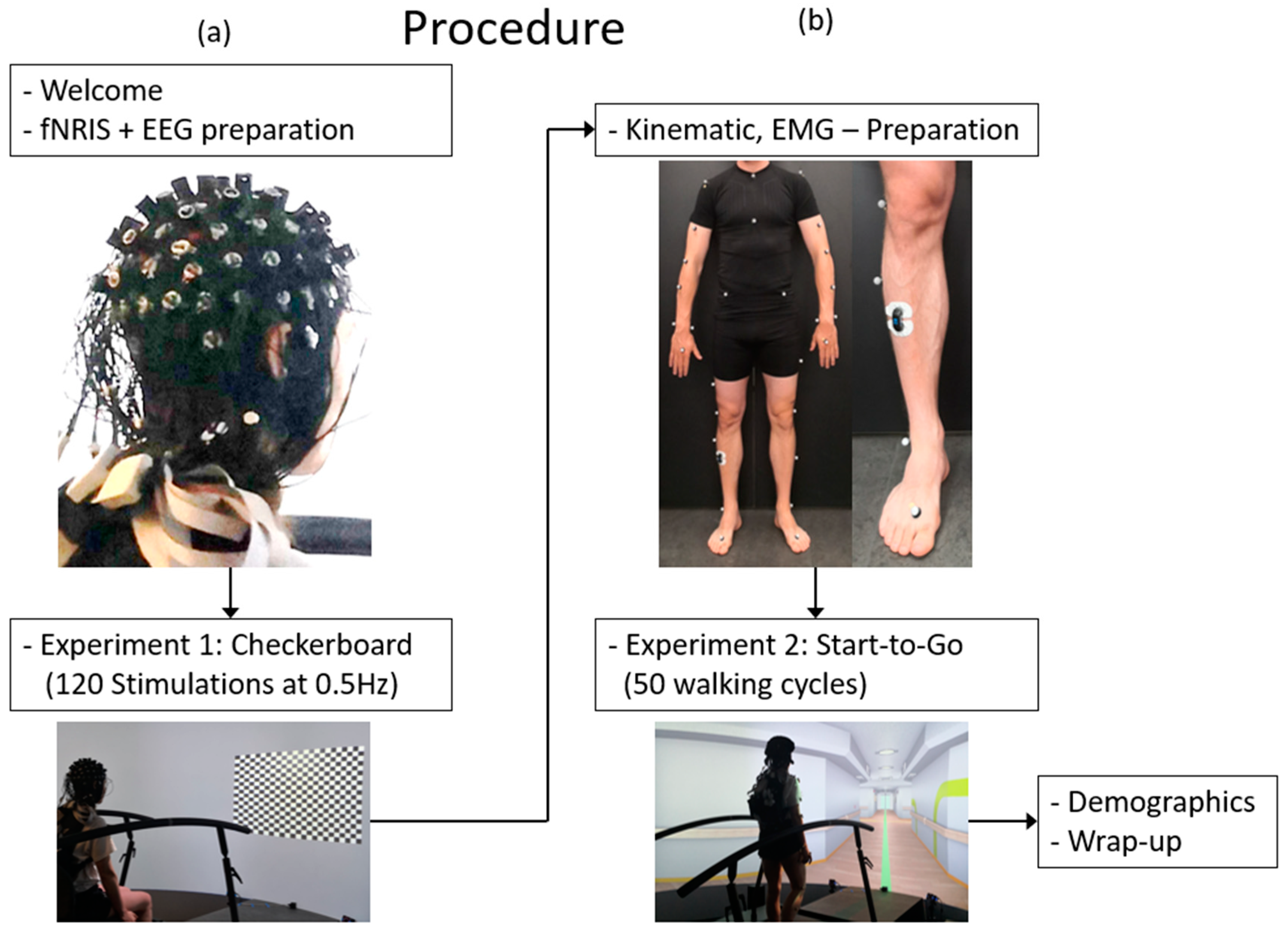
2.4.2. Procedure
2.5. Preprocessing and Data Analysis
2.5.1. Preprocessing and Data Analysis Experiment 1—Checkerboard
2.5.2. Preprocessing and Data Analysis Experiment 2—Start-to-Go
3. Results
3.1. Experiment 1—Checkerboard—Synchronization between GRAIL and EEG
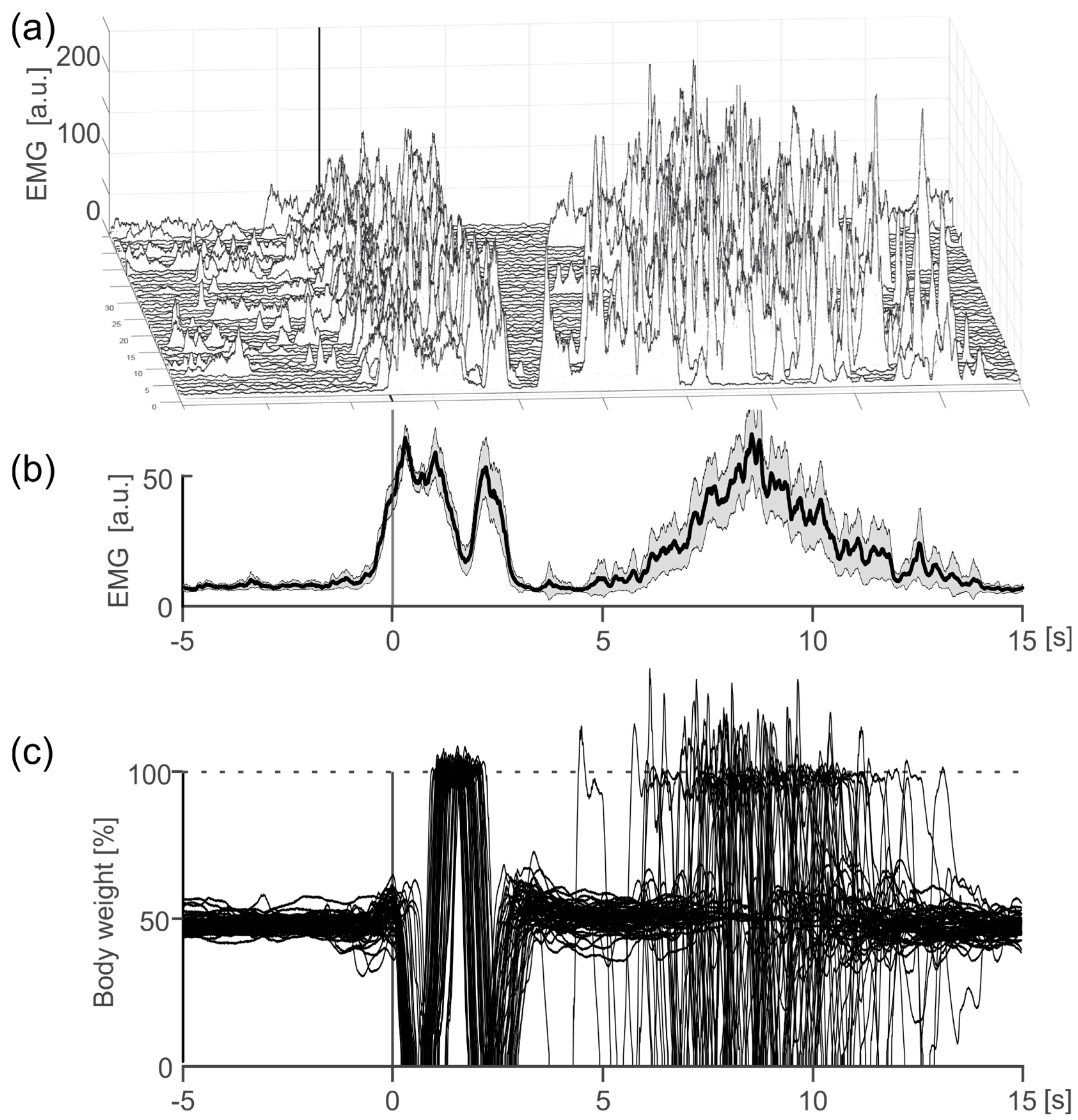
3.2. Experiment 2—Start-to-Go—Synchronization between GRAIL and fNIRS
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirelman, A.; Shema, S.; Maidan, I.; Hausdorff, J.M. Chapter 7—Gait. In Handbook of Clinical Neurology; Day, B.L., Lord, S.R., Eds.; Balance, Gait, and Falls; Elsevier: Amsterdam, The Netherlands, 2018; Volume 159, pp. 119–134. [Google Scholar] [CrossRef]
- Potter, J.M.; Evans, A.L.; Duncan, G. Gait speed and activities of daily living function in geriatric patients. Arch. Phys. Med. Rehabil. 1995, 76, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S.; El Manira, A. Current Principles of Motor Control, with Special Reference to Vertebrate Locomotion. Physiol. Rev. 2020, 100, 271–320. [Google Scholar] [CrossRef] [PubMed]
- Kimijanová, J.; Hirjaková, Z.; Bzdúšková, D.; Hlavačka, F. Influence of Vision on Gait Initiation and First Step Kinematics in Young and Older Adults. Physiol. Res. 2021, 70, S409. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K.; Tomita, N.; Yano, M. Substrates for normal gait and pathophysiology of gait disturbances with respect to the basal ganglia dysfunction. J. Neurol. 2008, 255, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Snijders, A.H.; van de Warrenburg, B.P.; Giladi, N.; Bloem, B.R. Neurological gait disorders in elderly people: Clinical approach and classification. Lancet Neurol. 2007, 6, 63–74. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Costa, R.M.; Dauer, W.T.; Factor, S.A.; Giladi, N.; Hallett, M.; Lewis, S.J.G.; Nieuwboer, A.; Nutt, J.G.; Takakusaki, K.; et al. Discussion of Research Priorities for Gait Disorders in Parkinson’s Disease. Mov. Disord. 2022, 37, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Schmuckler, M.A. What Is Ecological Validity? A Dimensional Analysis. Infancy 2001, 2, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Bock, O.; Beurskens, R. Changes of locomotion in old age depend on task setting. Gait Posture 2010, 32, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Benson, L.C.; Räisänen, A.M.; Clermont, C.A.; Ferber, R. Is This the Real Life, or Is This Just Laboratory? A Scoping Review of IMU-Based Running Gait Analysis. Sensors 2022, 22, 1722. [Google Scholar] [CrossRef]
- Canning, C.G.; Allen, N.E.; Nackaerts, E.; Paul, S.S.; Nieuwboer, A.; Gilat, M. Virtual reality in research and rehabilitation of gait and balance in Parkinson disease. Nat. Rev. Neurol. 2020, 16, 409–425. [Google Scholar] [CrossRef]
- Bock, O.; Drescher, U.; Van Winsum, W.; Kesnerus, T.; Voelcker-Rehage, C. A Virtual-Reality Approach for the Assessment and Rehabilitation of Multitasking Deficits. Int. J. Virtual Augment. Real. 2018, 2, 48–58. [Google Scholar] [CrossRef]
- Bock, O.; Drescher, U.; Janouch, C.; Haeger, M.; van Winsum, W.; Voelcker-Rehage, C. An experimental paradigm for the assessment of realistic human multitasking. Virtual Real. 2019, 23, 61–70. [Google Scholar] [CrossRef]
- Nutakki, C.; Bodda, S.; Diwakar, S.; Nutakki, C.; Bodda, S.; Diwakar, S. Correlations of Gait Phase Kinematics and Cortical EEG: Modelling Human Gait with Data from Sensors. In Advances in Neural Signal Processing; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Stojan, R.; Mack, M.; Bock, O.; Voelcker-Rehage, C. Inefficient frontal and parietal brain activation during dual-task walking in a virtual environment in older adults. NeuroImage 2023, 273, 120070. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V.; Quintern, J.; Berger, W. Cerebral evoked potentials associated with the compensatory reactions following stance and gait perturbation. Neurosci. Lett. 1984, 50, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Plug-in Gait Reference Guide—Nexus 2.12 Documentation—Vicon Documentation. Available online: https://help.vicon.com/space/Nexus212/11247555/Plug-in+Gait+Reference+Guide (accessed on 1 May 2024).
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Nuwer, M.R.; Comi, G.; Emerson, R.; Fuglsang-Frederiksen, A.; Guérit, J.-M.; Hinrichs, H.; Ikeda, A.; Jose, C.; Luccas, F.; Rappelsburger, P. IFCN standards for digital recording of clinical EEG. Electroencephalogr. Clin. Neurophysiol. 1998, 106, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Oostenveld, R.; Praamstra, P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2001, 112, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Yücel, M.A.; Selb, J.; Aasted, C.M.; Petkov, M.P.; Becerra, L.; Borsook, D.; Boas, D.A. Short separati on regression improves statistical significance and better localizes the hemodynamic response obtained by near-infrared spectroscopy for tasks with differing autonomic responses. Neurophotonics 2015, 2, 035005. [Google Scholar] [CrossRef] [PubMed]
- Kothe, C.; Medine, D.; Boulay, C.; Grivich, M.; Stenner, T. Lab Streaming Layer [Software]. 2019. Available online: https://github.com/sccn/labstreaminglayer (accessed on 1 May 2024).
- Kothe, C.; Shirazi, S.Y.; Stenner, T.; Medine, D.; Boulay, C.; Grivich, M.I.; Mullen, T.; Delorme, A.; Makeig, S. The Lab Streaming Layer for Synchronized Multimodal Recording. To Be Published. [Online]. Available online: https://www.biorxiv.org/content/10.1101/2024.02.13.580071v1.full (accessed on 2 May 2024).
- Stöhr, M.; Dichgans, J.; Büttner, U.; Hess, C.W. Evozierte Potenziale: SEP-VEP-AEP-EKP-MEP; Springer: Heidelberg, Germany, 2005. [Google Scholar]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Goj, R.; Jas, M.; Brooks, T.; Parkkonen, L.; et al. MEG and EEG data analysis with MNE-Python. Front. Neurosci. 2013, 7. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Van Rossum, G. The Python Library Reference, Release 3.8.2; Python Software Foundation: Wilmington, DE, USA, 2020. [Google Scholar]
- McKinney, W. Others Data structures for statistical computing in python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28–30 June 2010; Volume 445, pp. 51–56. [Google Scholar]
- Konrad, P. EMG-FIBEL. Noraxon INC. USA. 2011. Available online: https://www.velamed.com/wp-content/uploads/EMG-FIBEL-V1.1.pdf (accessed on 1 May 2024).
- Seabold, S.; Perktold, J. Statsmodels: Econometric and statistical modeling with python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28–30 June 2010. [Google Scholar]
- Abraham, A.; Pedregosa, F.; Eickenberg, M.; Gervais, P.; Mueller, A.; Kossaifi, J.; Gramfort, A.; Thirion, B.; Varoquaux, G. Machine learning for neuroimaging with scikit-learn. Front. Neuroinform. 2014, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Delpy, D.T.; Cope, M.; van der Zee, P.; Arridge, S.; Wray, S.; Wyatt, J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys. Med. Biol. 1988, 33, 1433. [Google Scholar] [CrossRef] [PubMed]
- Krobot, A.; Kolarova, B.; Kolar, P.; Schusterova, B.; Tomsova, J. Gait Neurorehabilitation in Stroke Patients. CESKA Slov. Neurol. Neurochir. 2017, 80, 521–526. [Google Scholar] [CrossRef]
- Bluett, B.; Bayram, E.; Litvan, I. The virtual reality of Parkinson’s disease freezing of gait: A systematic review. Park. Relat. Disord. 2019, 61, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kutz, D.F.; Kaulich, T.; Föhre, W.; Gerwig, M.; Timmann, D.; Kolb, F.P. Comparison of the classically conditioned withdrawal reflex in cerebellar patients and healthy control subjects during stance: 2. Biomechanical characteristics. Neurobiol. Learn. Mem. 2014, 109, 178–192. [Google Scholar] [CrossRef]
- Šlosar, L.; Voelcker-Rehage, C.; Paravlić, A.H.; Abazovic, E.; de Bruin, E.D.; Marusic, U. Combining physical and virtual worlds for motor-cognitive training interventions: Position paper with guidelines on technology classification in movement-related research. Front. Psychol. 2022, 13, 1009052. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maas, S.A.; Göcking, T.; Stojan, R.; Voelcker-Rehage, C.; Kutz, D.F. Synchronization of Neurophysiological and Biomechanical Data in a Real-Time Virtual Gait Analysis System (GRAIL): A Proof-of-Principle Study. Sensors 2024, 24, 3779. https://doi.org/10.3390/s24123779
Maas SA, Göcking T, Stojan R, Voelcker-Rehage C, Kutz DF. Synchronization of Neurophysiological and Biomechanical Data in a Real-Time Virtual Gait Analysis System (GRAIL): A Proof-of-Principle Study. Sensors. 2024; 24(12):3779. https://doi.org/10.3390/s24123779
Chicago/Turabian StyleMaas, Stefan A., Tim Göcking, Robert Stojan, Claudia Voelcker-Rehage, and Dieter F. Kutz. 2024. "Synchronization of Neurophysiological and Biomechanical Data in a Real-Time Virtual Gait Analysis System (GRAIL): A Proof-of-Principle Study" Sensors 24, no. 12: 3779. https://doi.org/10.3390/s24123779






