An Efficient Pulse Circuit Design for Magnetic Stimulation with Diversified Waveforms and Adjustable Parameters
Abstract
:1. Introduction
2. Methods
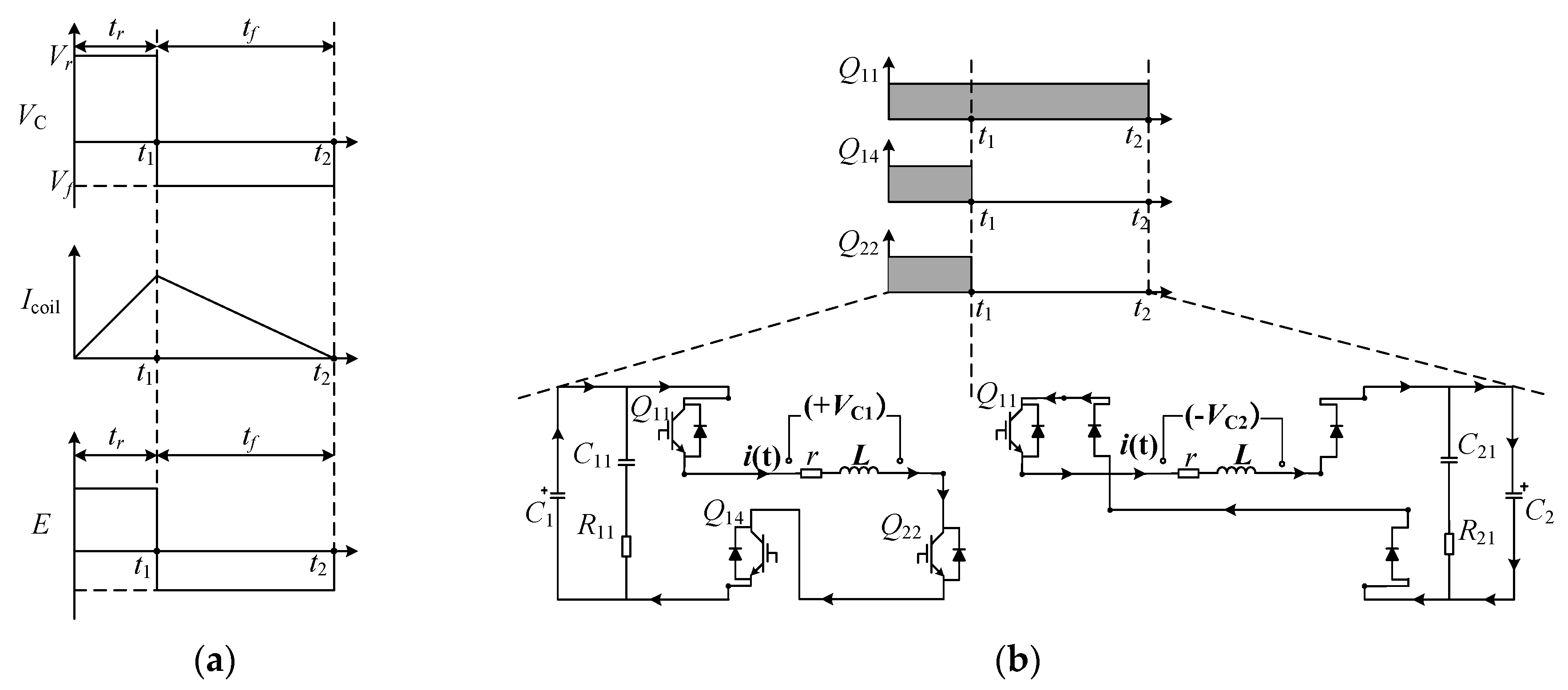
2.1. Transient Analysis of the Discharge Circuit
2.2. Working Principles of the EPMS Circuit
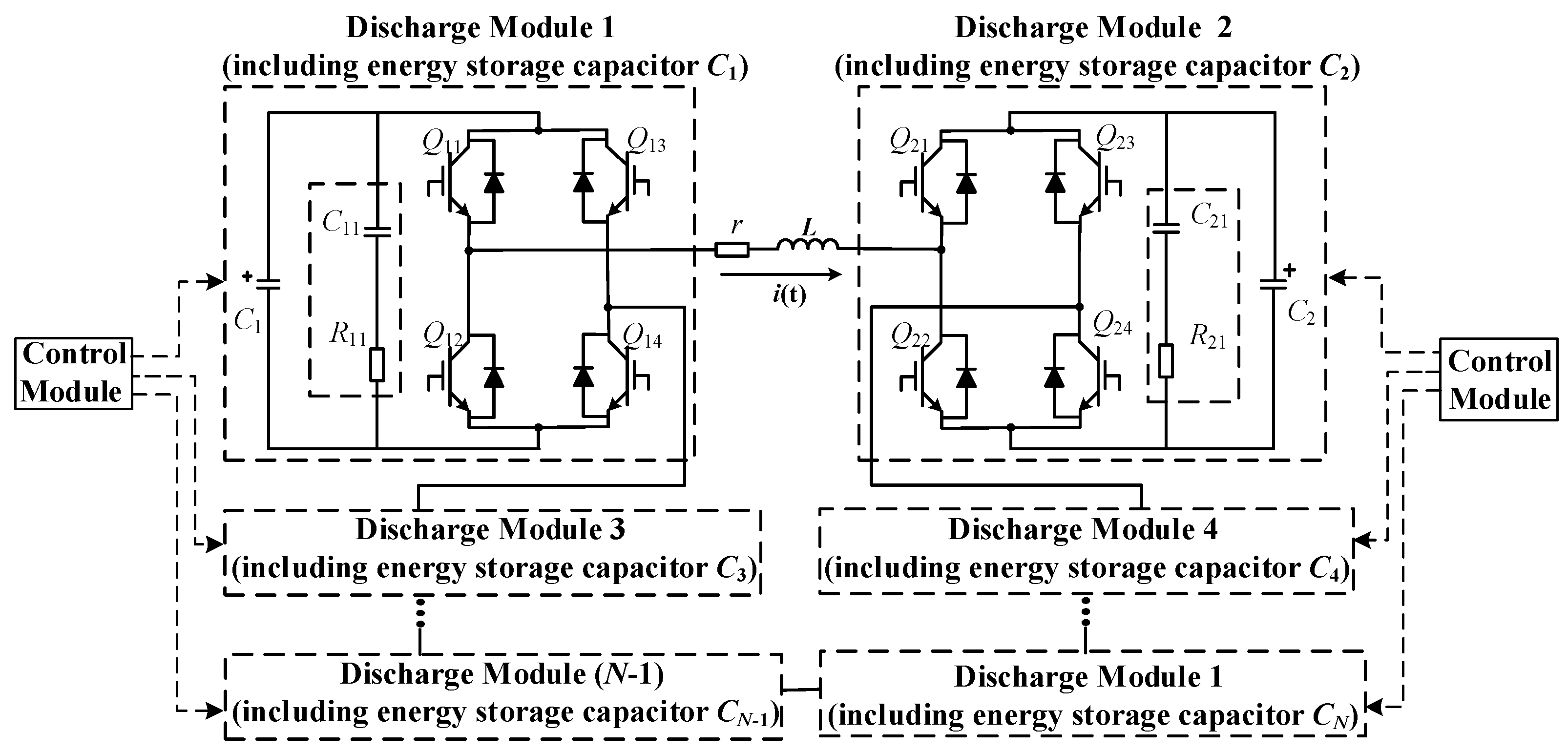
2.2.1. Topology of the EPMS Circuit
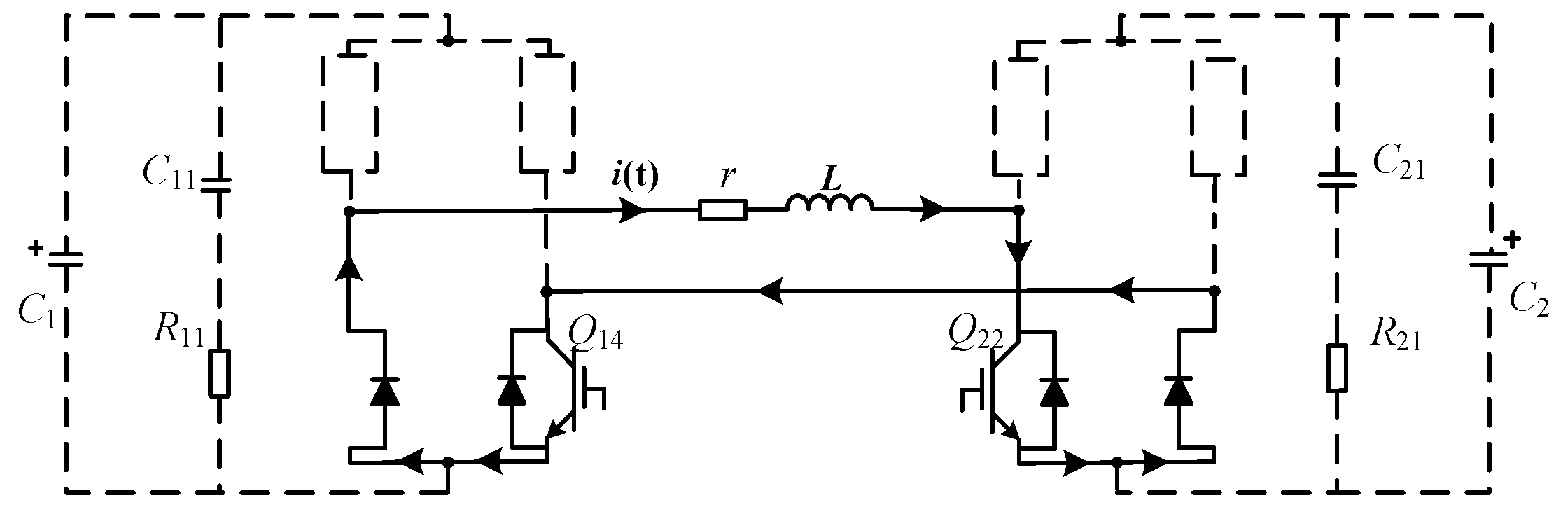
2.2.2. Different Working Modes of the EPMS Circuit
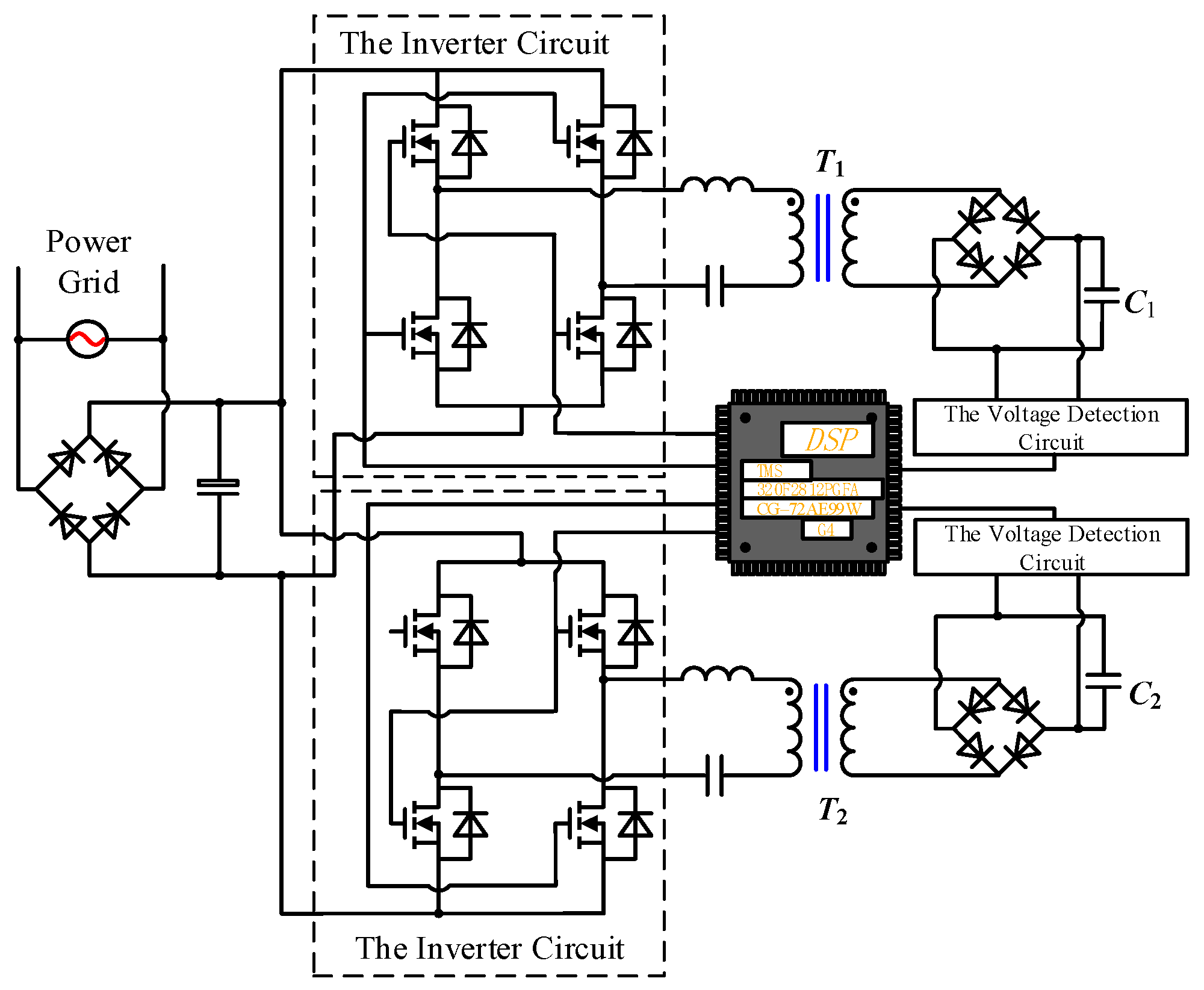
2.3. Implementation Scheme of the EPMS Circuit
2.3.1. Charging Method for the EPMS Circuit
2.3.2. Components Selection of the EPMS Circuit
3. Results
3.1. Three Types of New E-Field Waveform Generated by the EPMS Circuit
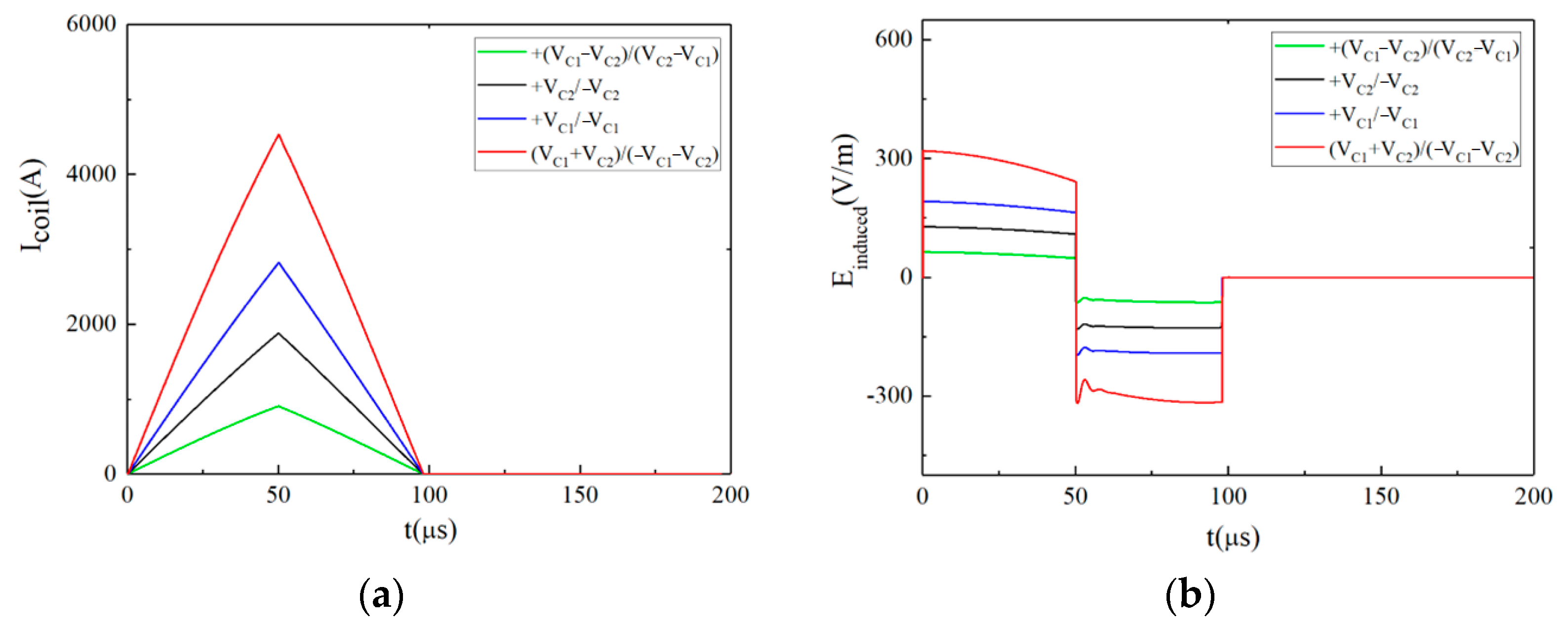
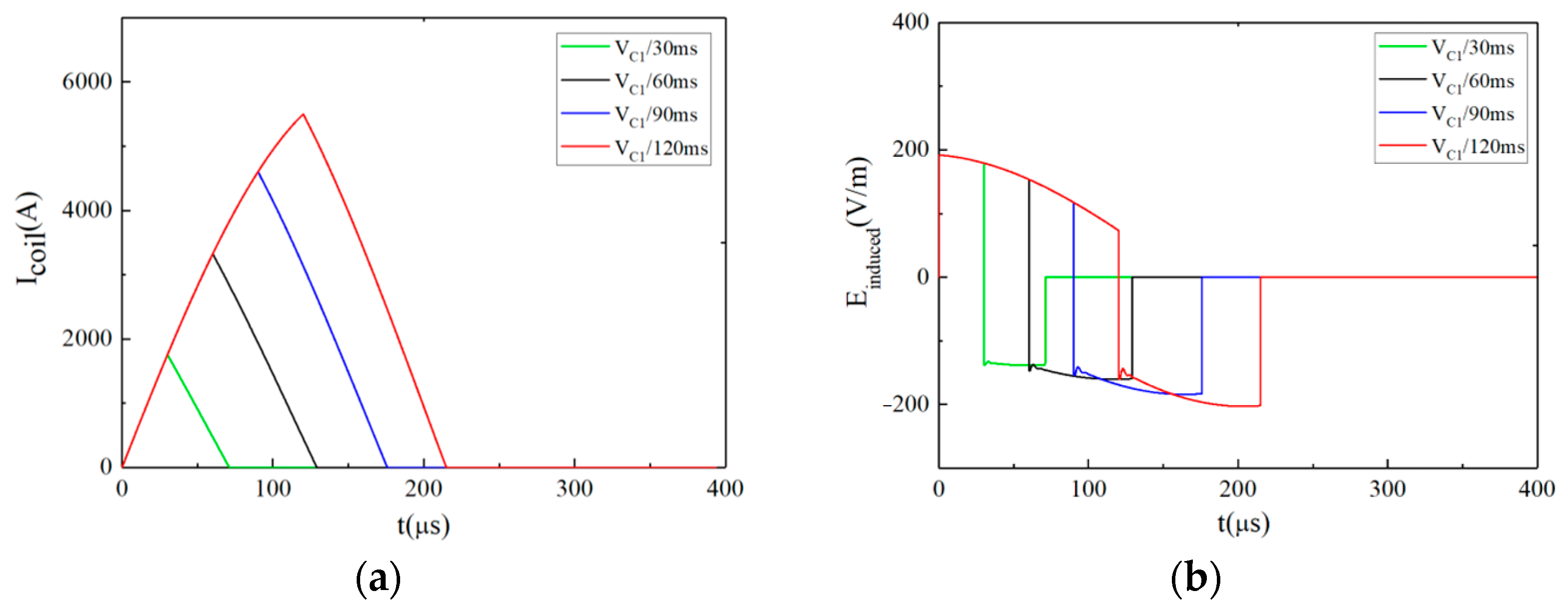
3.1.1. Type 1: The Monophasic Approximate Rectangular Pulsed E-Field
3.1.2. Type 2: The Biphasic Approximately Rectangular Pulsed E-Field
3.1.3. Type 3: The Ladder-Shaped Pulsed E-Field
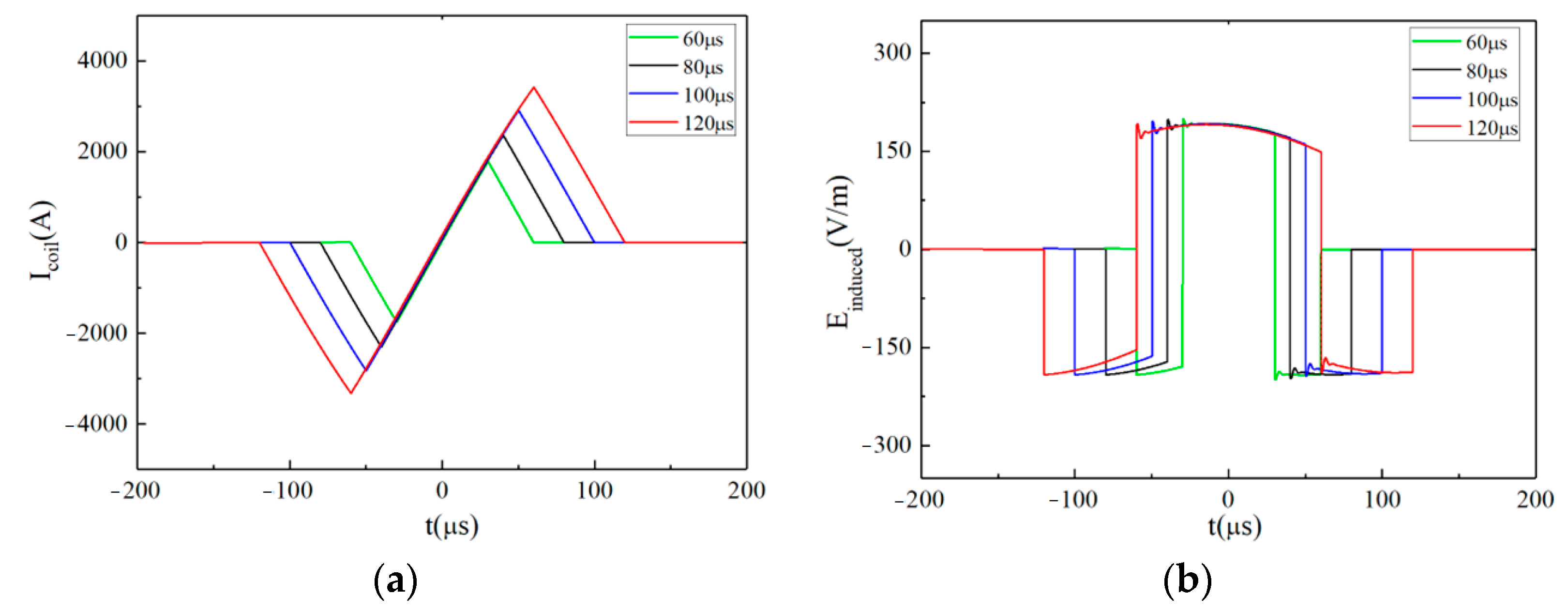
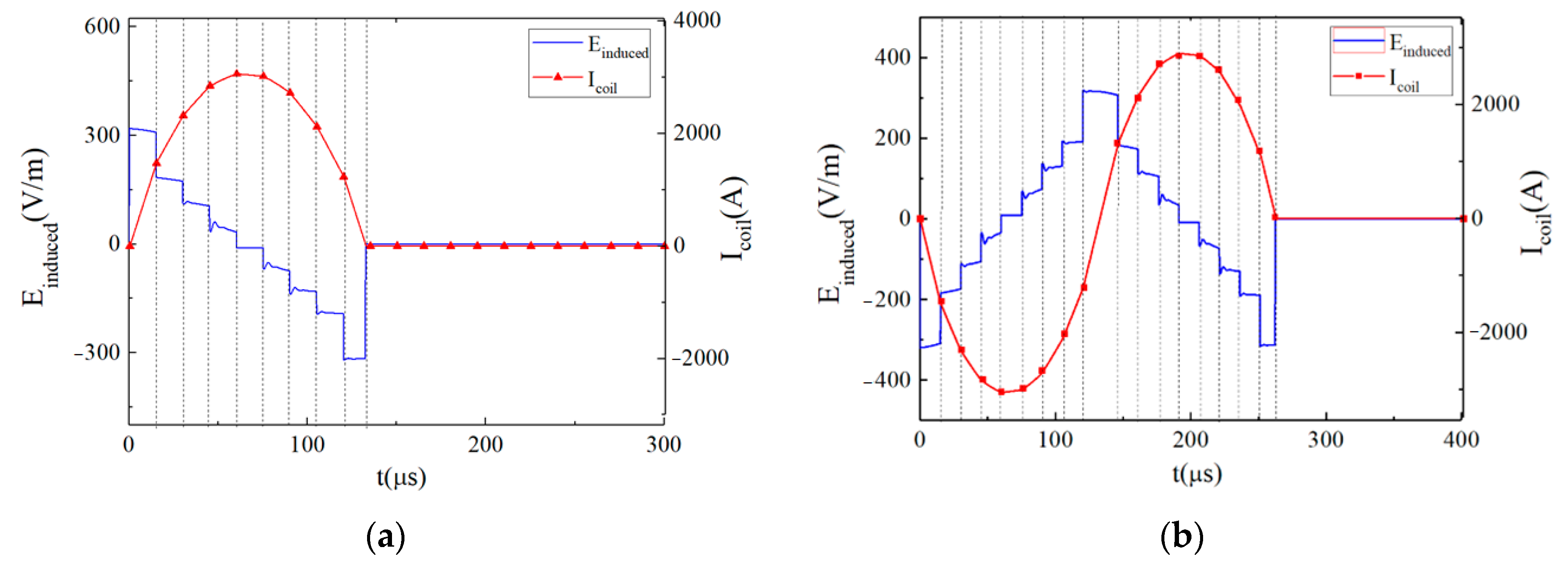
3.2. Further Analysis: A Special Waveform Generated by the EPMS Circuit—The BFL Waveform
3.2.1. Characteristics of the BFL Waveform
3.2.2. Neuron Dynamic Responses under the Stimulation of the BFL Waveform
- (1)
- Neuron Membrane Potential Change ΔVm
- (2)
- Neuronal Polarizability Ratio ξP
3.2.3. Comparison of BFL Waveform and Traditional TMS Waveform
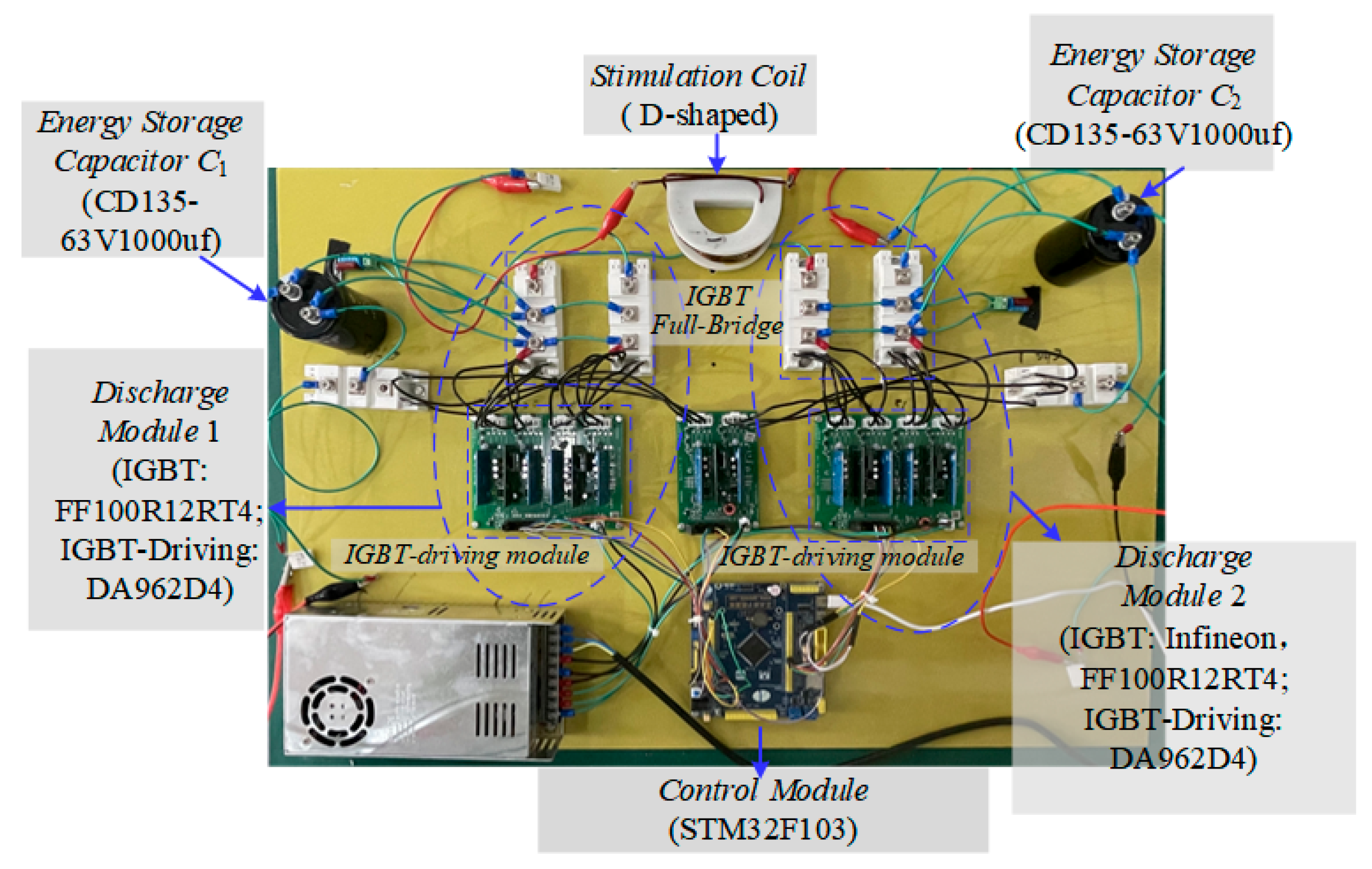
3.3. Experimental Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- José, C.; Fernando, B.; Iván, C. TMS for Major Depressive Disorder in naïve patients. Brain Stimul. 2019, 12, 133. [Google Scholar] [CrossRef]
- Rao, M.; Nomura, S. TMS in treatment of Cocaine Use Disorder. Brain Stimul. 2019, 12, 540. [Google Scholar] [CrossRef]
- Bhedita, J.S.; Sarah, J.E.; Jennifer, R. Transcranial Magnetic Stimulation; American Cancer Society: Atlanta, GA, USA, 2019. [Google Scholar]
- Fagan, M.J.; Faulkner, G.; Duncan, M.; Yun, L.; Blumberger, D.; Daskalakis, Z.J.; Downar, J.; Vila-Rodriguez, F. Moving Away from Depression: Physical Activity Changes in Patients Undergoing rTMS for Major Depressive Disorder. Ment. Health Phys. Act. 2019, 16, 50–53. [Google Scholar] [CrossRef]
- Perera, T.; George, M.S.; Grammer, G.; Janicak, P.G.; Pascual-Leone, A.; Wirecki, T.S. The Clinical TMS Society Consensus Review and Treatment Recommendations for TMS Therapy for Major Depressive Disorder. Brain Stimul. 2016, 9, 336–346. [Google Scholar] [CrossRef]
- Canali, P. A Role for TMS/EEG in neuropsychiatric disorders. Neurol. Psychiatry Brain Res. 2014, 20, 37–40. [Google Scholar] [CrossRef]
- Zheng, W.; Yu, H.; Ding, W.; Guo, L.; Xu, G.; Yin, N. Changes in Brain Functional Networks of Insomniacs Induced by Magnetic Stimulation at Acupoints. IEEE Trans. Appl. Supercond. 2018, 29, 0500104. [Google Scholar] [CrossRef]
- Peterchev, A.V.; Jalinous, R.; Lisanby, S.H. A Transcranial Magnetic Stimulator Inducing Near-Rectangular Pulses with Controllable Pulse Width (cTMS). IEEE Trans. Biomed. Eng. 2008, 55, 257–266. [Google Scholar] [CrossRef]
- Zuo, J. Research and Optimal Design of Transcranial Magnetic Stimulator Power System. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2018. [Google Scholar]
- Gattinger, N.; Mossnang, G.; Gleich, B. flexTMS—A Novel Repetitive Transcranial Magnetic Stimulation Device with Freely Programmable Stimulation Currents. IEEE Trans. Biomed. Eng. 2012, 59, 1962–1970. [Google Scholar] [CrossRef]
- Peterchev, A.V.; D’Ostilio, K.; Rothwell, J.C.; Murphy, D.L. Controllable pulse parameter transcranial magnetic stimulator with enhanced circuit topology and pulse shaping. J. Neural Eng. 2014, 11, 056023. [Google Scholar] [CrossRef]
- Peterchev, A.V.; Murphy, D.L.; Lisanby, S.H. Repetitive Transcranial Magnetic Stimulator with Controllable Pulse Parameters (cTMS). In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine & Biology, Buenos Aires, Argentina, 31 August–4 September 2010. [Google Scholar]
- Peterchev, A.V.; Murphy, D.L.; Lisanby, S.H. Repetitive Transcranial Magnetic Stimulator with Controllable Pulse Parameters (cTMS). J. Neural Eng. 2011, 8, 036. [Google Scholar] [CrossRef]
- Barker, A.T.; Jalinous, R.I.; Freeston, I.L. Noninvasive Magnetic Stimulation of the Human Motor Cortex. Lancet 1985, 1, 1106–1107. [Google Scholar] [CrossRef] [PubMed]
- Ueno, S.; Tashiro, T.; Harada, K. Localized Stimulation of Neural Tissues in the Brain by Means of a Paired Configuration of Time-varying Magnetic Fields. J. Appl. Phys. 1988, 64, 5862. [Google Scholar] [CrossRef]
- Hui, X.; Yuling, W.; Hao, F. An Energy Efficient Excitation Source for Transcranial Magnetic Stimulation with Controllable Pulse Width. Trans. China Electrotech. Soc. 2020, 35, 679–686. [Google Scholar]
- Cole, J. Handbook of Transcranial Magnetic Stimulation and Magnetic Stimulation of the Human Nervous System. Spinal Cord 2002, 40, 258. [Google Scholar] [CrossRef]
- Taylor, J.L.; Loo, C.K. Stimulus Waveform Influences the Efficacy of Repetitive Transcranial Magnetic Stimulation. J. Affect. Disord. 2007, 97, 271–276. [Google Scholar] [CrossRef]
- Rothkegel, H.; Sommer, M.; Paulus, W.; Lang, N. Impact of Pulse Duration in Single Pulse TMS. Clin. Neurophysiol. 2009, 121, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Kincses, T.Z.; Nitsche, M.A.; Bartfai, O.; Demmer, I.; Sommer, M.; Paulus, W. Pulse Configuration-dependent Effects of Repetitive Transcranial Magnetic Stimulation on Visual Perception. NeuroReport 2002, 13, 2229–2233. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.; Lang, N.; Tergau, F.; Paulus, W. Neuronal Tissue Polarization Induced by Repetitive Transcranial Magnetic Stimulation? Neuroreport 2002, 13, 809–811. [Google Scholar] [CrossRef]
- Tings, T.; Lang, N.; Tergau, F.; Paulus, W.; Sommer, M. Orientation-specific Fast rTMS Maximizes Corticospinal Inhibition and Facilitation. Exp. Brain Res. 2005, 164, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Arai, N.; Okabe, S.; Furubayashi, T.; Terao, Y.; Yuasa, K.; Ugawa, Y. Comparison between Short Train, Monophasic and Biphasic Repetitive Transcranial Magnetic Stimulation (rTMS) of the Human Motor Cortex. Clin. Neurophysiol. 2005, 116, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Arai, N.; Okabe, S.; Furubayashi, T.; Mochizuki, H.; Iwata, N.K.; Hanajima, R.; Terao, Y.; Ugawa, Y. Differences in After-effect Between Monophasic and Biphasic High Frequency rTMS of the Human Motor Cortex. Clin. Neurophysiol. 2007, 118, 2227–2233. [Google Scholar] [CrossRef] [PubMed]
- Novickij, V.; Grainys, A.; Novickij, J.; Lucinskis, A. Programmable Pulsed Magnetic Field System for Biological Applications. IEEE Trans. Magn. 2014, 50, 5101004. [Google Scholar] [CrossRef]
- Barker, A.T.; Garnham, C.W.; Freeston, I.L. Magnetic Nerve Stimulation: The Effect of Waveform on Efficiency, Determination of Neural Membrane Time Constants and the Measurement of Stimulator Output. Electroencephalogr. Clin. Neurophysiol. 1991, 43, 227–237. [Google Scholar]
- Panizza, M.; Nilsson, J.; Roth, B.J.; Basser, P.J.; Hallett, M. Relevance of Stimulus Duration for Activation of Motor and Sensory Fibers: Implications for the Study of H-reflexes and Magnetic Stimulation. Electroencephalogr. Clin. Neurophysiol. 1992, 85, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Pauri, F. Central Condution Time Studies. In Handbook of Transcranial Magnetic Stimulation; Hodder Education Publishers: London, UK, 2002. [Google Scholar]
- Petterteig, A.; Lode, J.; Undeland, T. IGBT turn-off Losses for Hard Switching and with Capacitive snubbers. In Proceedings of the IEEE Industry Applications Society Meeting, Dearborn, MI, USA, 28 September–4 October 1991; pp. 1501–1507. [Google Scholar]
- Trivedi, M.; Shenai, K. Modeling the Turn-off of IGBT’s in Hardand Soft-switching Applications. IEEE Trans Electron. Device 1997, 44, 887–893. [Google Scholar] [CrossRef]
- Giesselmann, M.; Palmer, B.; Neuber, A.; Donlon, J. High Voltage Impulse Generator Using HV-IGBTs. In Proceedings of the IEEE Pulsed Power Conference 2005, Monterey, CA, USA, 13–15 June 2005; pp. 763–766. [Google Scholar]
- Severns, R. Snubber Circuits for Power Electronics SMPS Technology [E-book]. 2008. Available online: http://www.snubberdesign.com/snubber-book.html (accessed on 10 December 2007).
- Han, B.H.; Park, T.S.; Lee, S.H.; Yang, J.S.; Cho, M.H.; Lee, S.Y. Development of four-channel magnetic nerve stimulator. In Proceedings of the 2001 Conference Proceedings of the 23rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Istanbul, Turkey, 25–28 October 2001; Volume 2, pp. 1325–1327.
- Walsh, V.; Cowey, A. Transcranial Magnetic Stimulation and Cognitive Neuroscience. Nat. Rev. Neurosci. 2000, 1, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Hanajima, R.; Ugawa, Y.; Terao, Y.; Sakai, K.; Furubayashi, T.; Machii, K.; Kanazawa, I. Paired-pulse Magnetic Stimulation of the Human Motor Cortex: Differences among I waves. J. Physiol. 1998, 509, 607–618. [Google Scholar] [CrossRef]
- Hanajima, R.; Furubayashi, T.; Iwata, N.K.; Shiio, Y.; Okabe, S.; Kanazawa, I.; Ugawa, Y. Further Evidence to Support Different Mechanisms Underlyning Intracortical Inhibition of the Motor Cortex. Exp. Brain Res. 2003, 151, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Wang, J.; Wei, X.; Deng, B.; Li, H.; Han, C. Dynamic Analysis of Hodgkin’s Three Classes of Neurons Exposed to Extremely Low-frequency Sinusoidal Induced E-field. Appl. Math. Comput. 2014, 231, 100–110. [Google Scholar] [CrossRef]
- Peterchev, A.V.; Wagner, T.A.; Miranda, P.C.; Nitsche, M.A.; Paulus, W.; Lisanby, S.H.; Pascual-Leone, A.; Bikson, M. Fundamentals of Transcranial Electric and Magnetic Stimulation Dose: Definition, Selection, and Reporting Practices. Brain Stimul. 2012, 5, 435–453. [Google Scholar] [CrossRef]
- Corthout, E.; Barker, A.T.; Cowey, A. Transcranial Magnetic Stimulation. Which Part of the Current Waveform Causes the Stimulation? Exp. Brain Res. 2001, 141, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Hosono, Y.; Urushihara, R.; Harada, M.; Morita, N.; Murase, N.; Kunikane, Y.; Shimazu, H.; Asanuma, K.; Uguisu, H.; Kaji, R. Comparison of Monophasic Versus Biphasic Stimulation in rTMS Over Premotor Cortex: SEP and SPECT studies. Clin. Neurophysiol. 2008, 119, 2538–2545. [Google Scholar] [CrossRef] [PubMed]



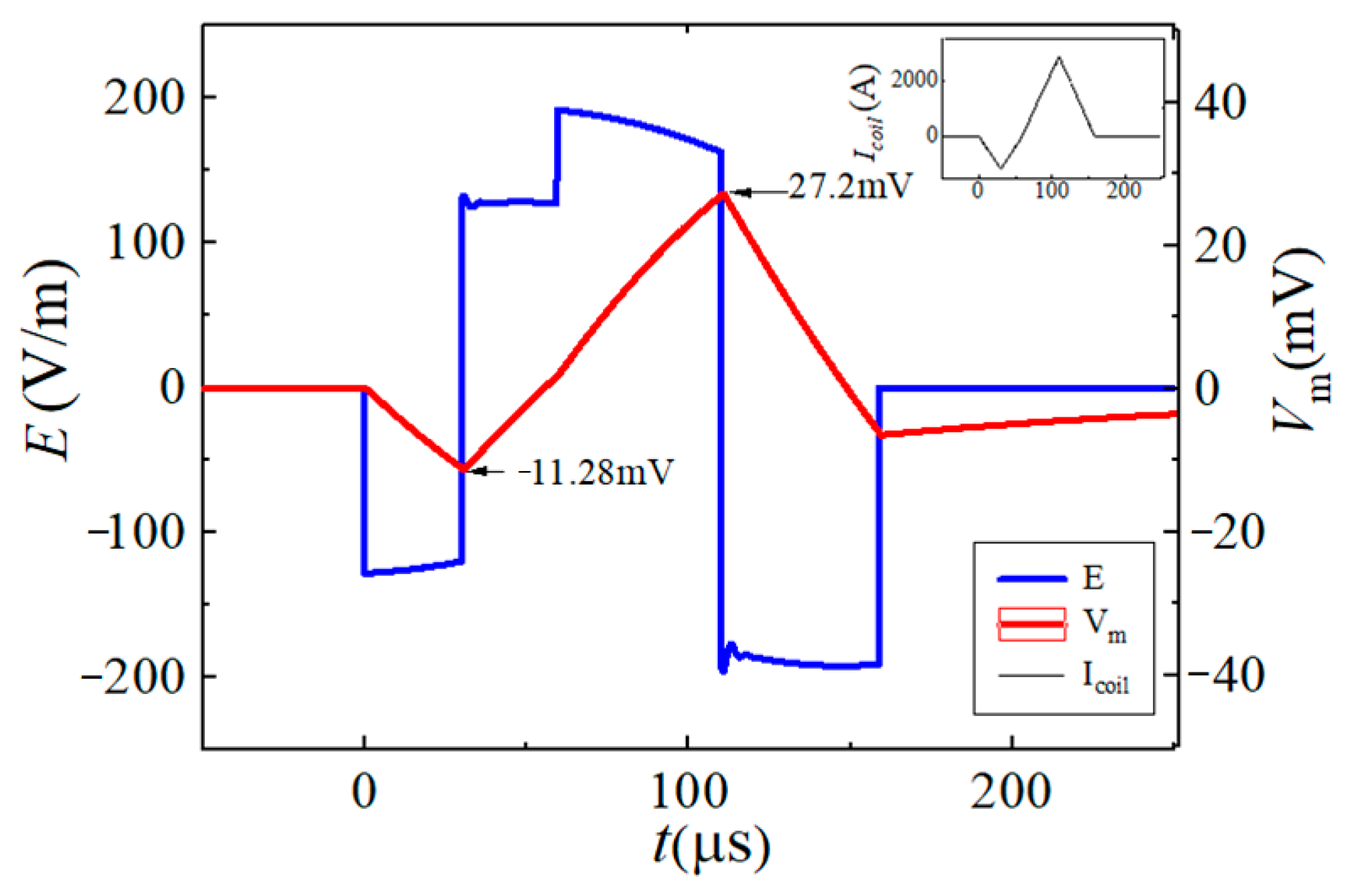
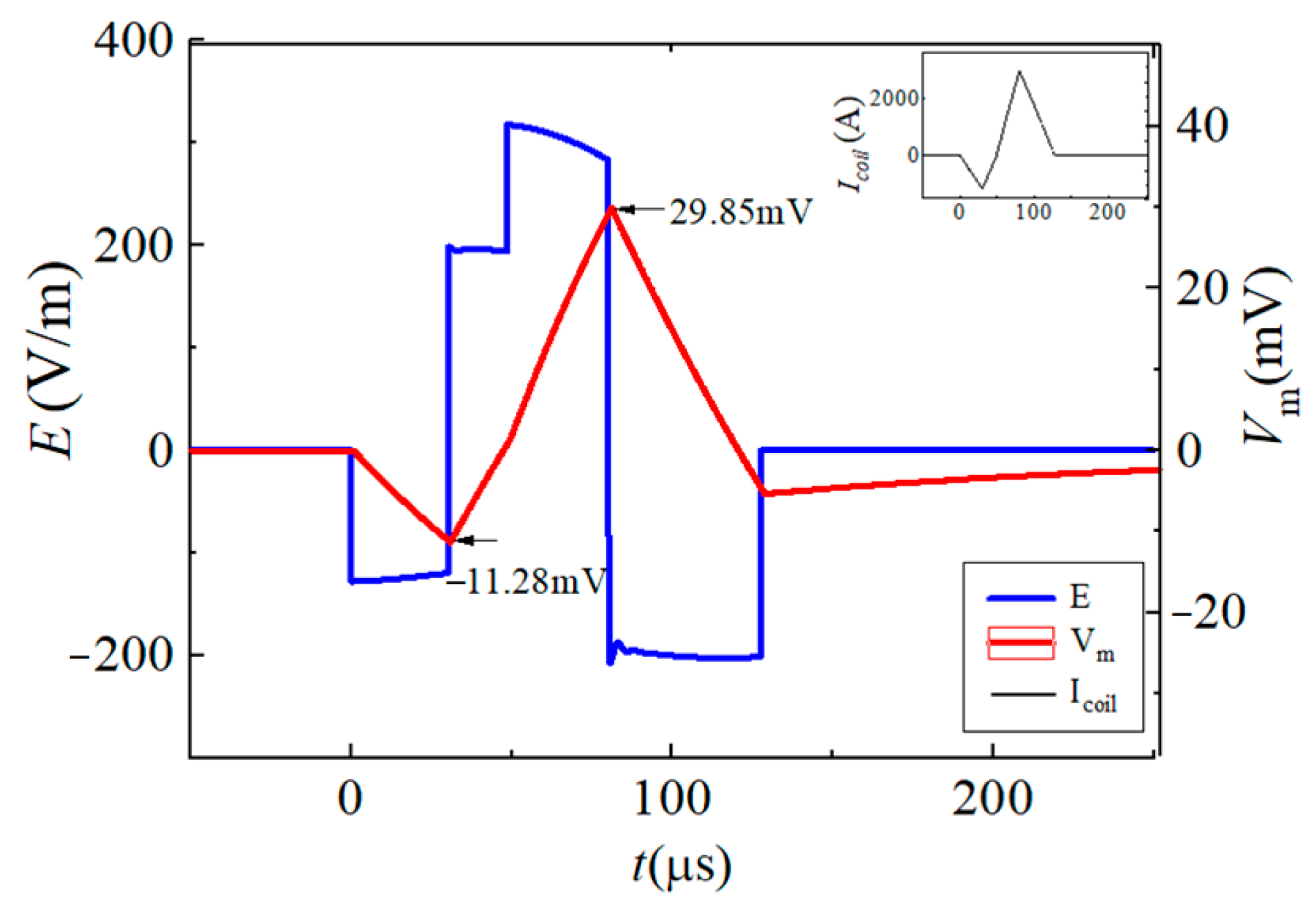
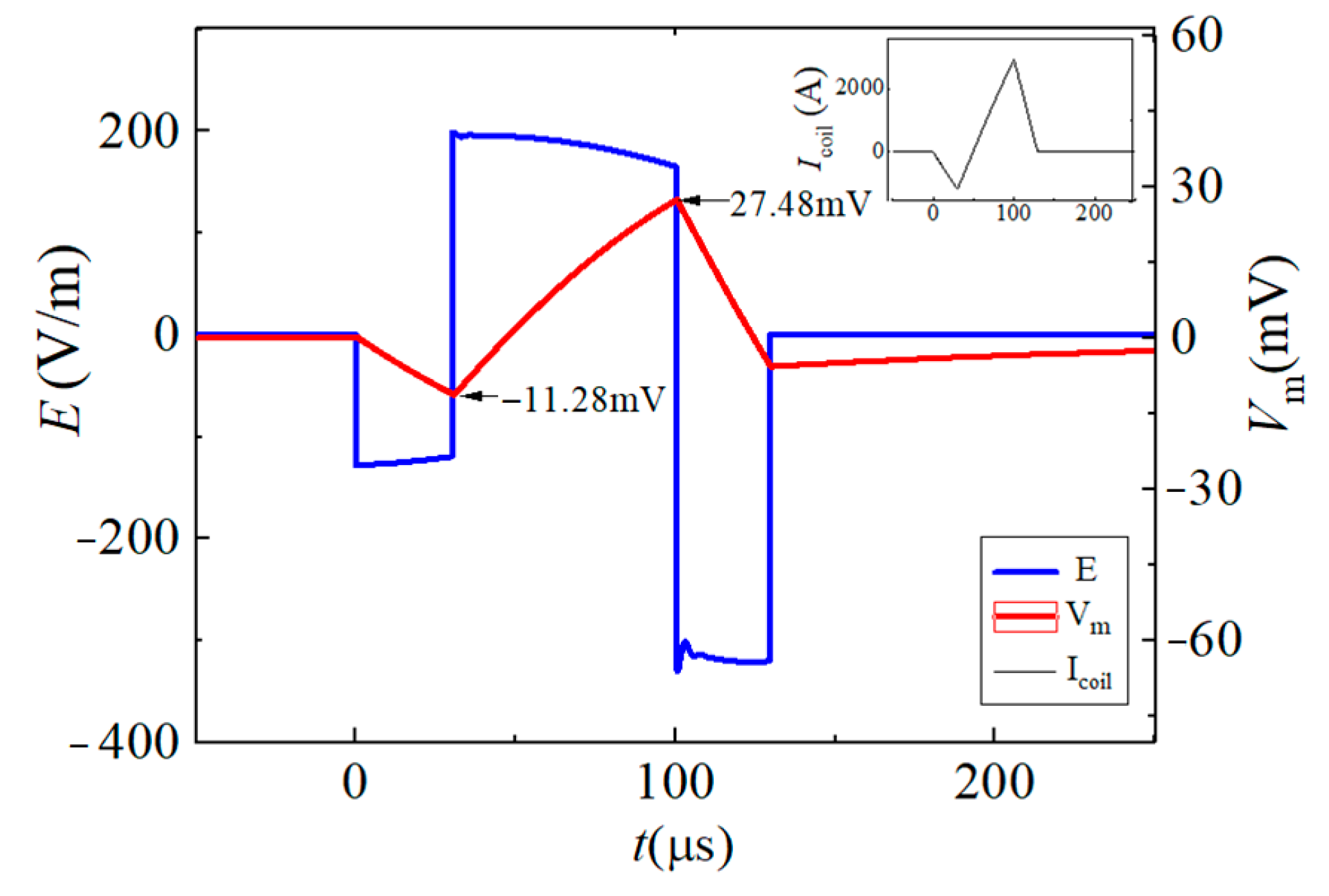


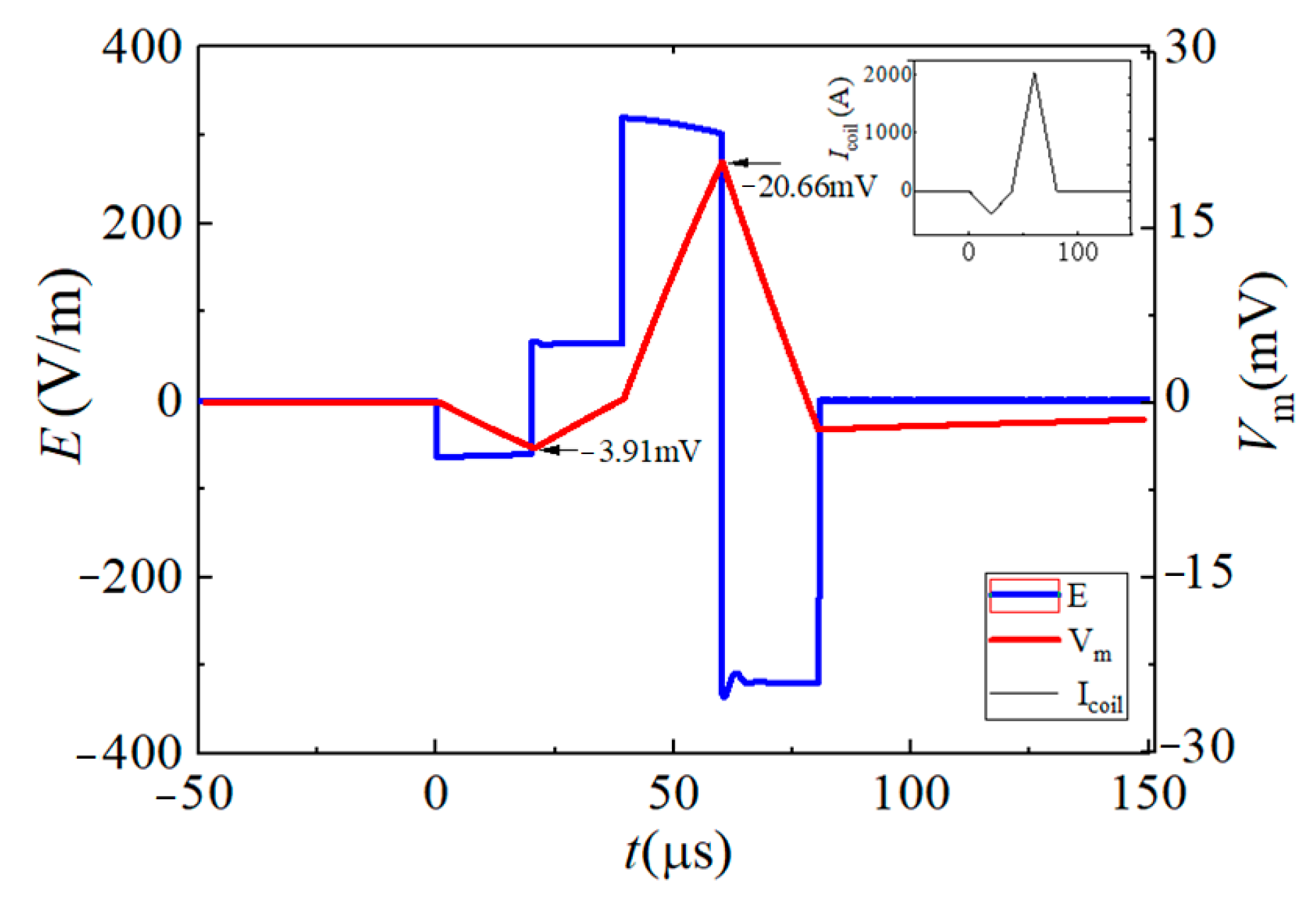

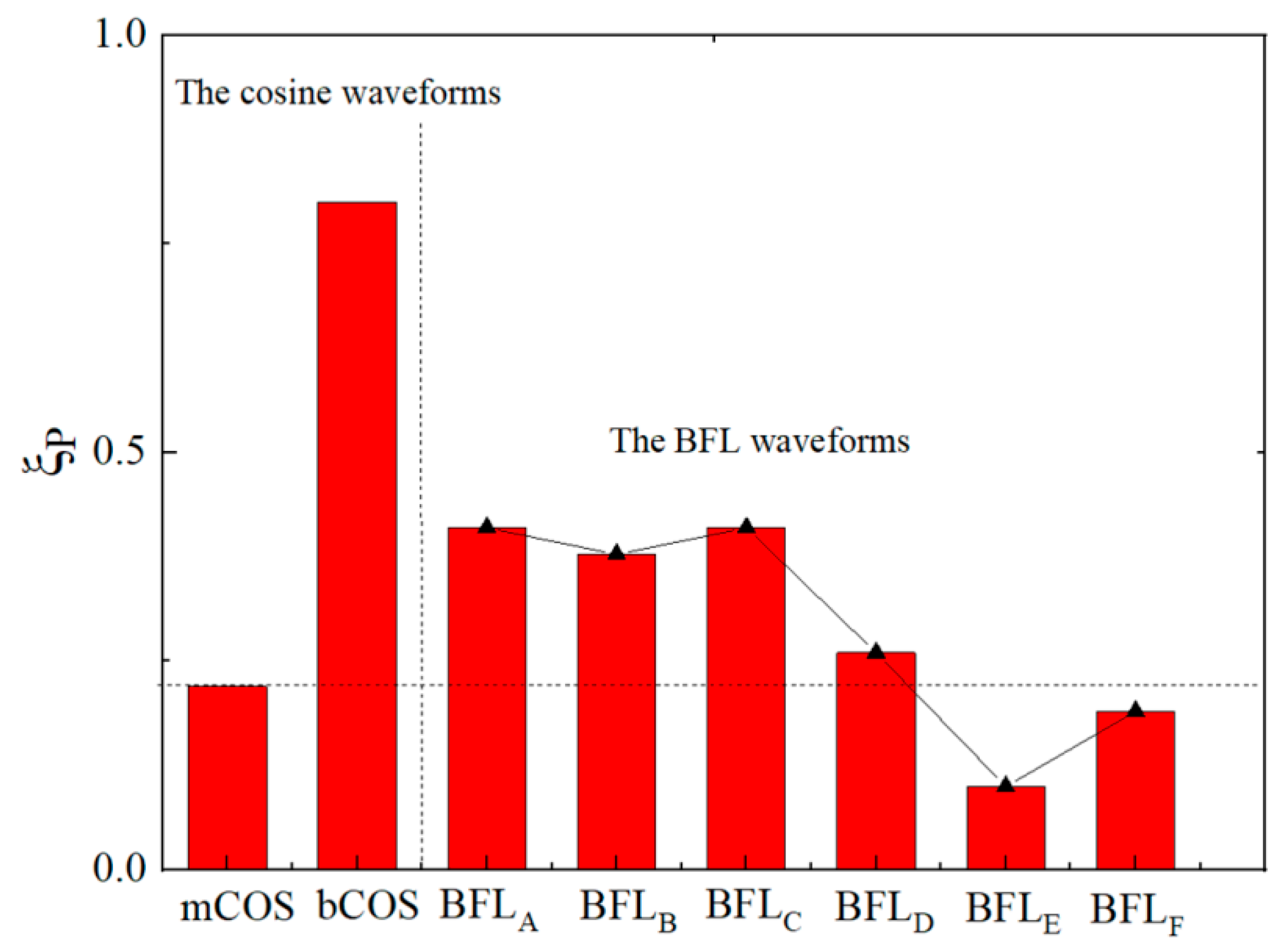




| Vf1/tf1 | Vr1/tr1 | Vr2/tr2 | Vf2/tf2 | |
|---|---|---|---|---|
| BFLA | −VC2/30 μs | +VC2/30 μs | +VC1/50 μs | −VC1/50 μs |
| BFLB | −VC2/30 μs | +VC1/20 μs | (VC1 + VC2)/30 μs | −VC1/50 μs |
| BFLC | −VC2/30 μs | +VC1/20 μs | +VC1/50 μs | (VC1 + VC2)/30 μs |
| BFLD | −VC2/30 μs | +VC2/30 μs | (VC1 + VC2)/50 μs | (−VC1 − VC2)/50 μs |
| BFLE | −(VC1 − VC2)/10 μs | +(VC1 − VC2)/10 μs | (VC1 + VC2)/20 μs | −(VC1 + VC2)/20 μs |
| BFLF | −(VC1 − VC2)/20 μs | +(VC1 − VC2)/20 μs | (VC1 + VC2)/20 μs | −(VC1 + VC2)/20 μs |
| BFLA | BFLB | BFLC | BFLD | BFLE | BFLF | |
|---|---|---|---|---|---|---|
| 0.400 | 0.400 | 0.400 | 0.240 | 0.100 | 0.200 | |
| ξP | 0.415 | 0.378 | 0.410 | 0.263 | 0.104 | 0.189 |
| Δ | 3.6% | 5.8% | 2.4% | 8.7% | 3.8% | 5.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Zhang, T.; Luo, Y.; Wang, S. An Efficient Pulse Circuit Design for Magnetic Stimulation with Diversified Waveforms and Adjustable Parameters. Sensors 2024, 24, 3839. https://doi.org/10.3390/s24123839
Fang X, Zhang T, Luo Y, Wang S. An Efficient Pulse Circuit Design for Magnetic Stimulation with Diversified Waveforms and Adjustable Parameters. Sensors. 2024; 24(12):3839. https://doi.org/10.3390/s24123839
Chicago/Turabian StyleFang, Xiao, Tao Zhang, Yaoyao Luo, and Shaolong Wang. 2024. "An Efficient Pulse Circuit Design for Magnetic Stimulation with Diversified Waveforms and Adjustable Parameters" Sensors 24, no. 12: 3839. https://doi.org/10.3390/s24123839





