II–VI Semiconductor-Based Conductometric Gas Sensors: Is There a Future for These Sensors?
Abstract
1. II–VI Semiconductor Compounds and Their Prospects for Gas Sensor Applications
2. Current Status
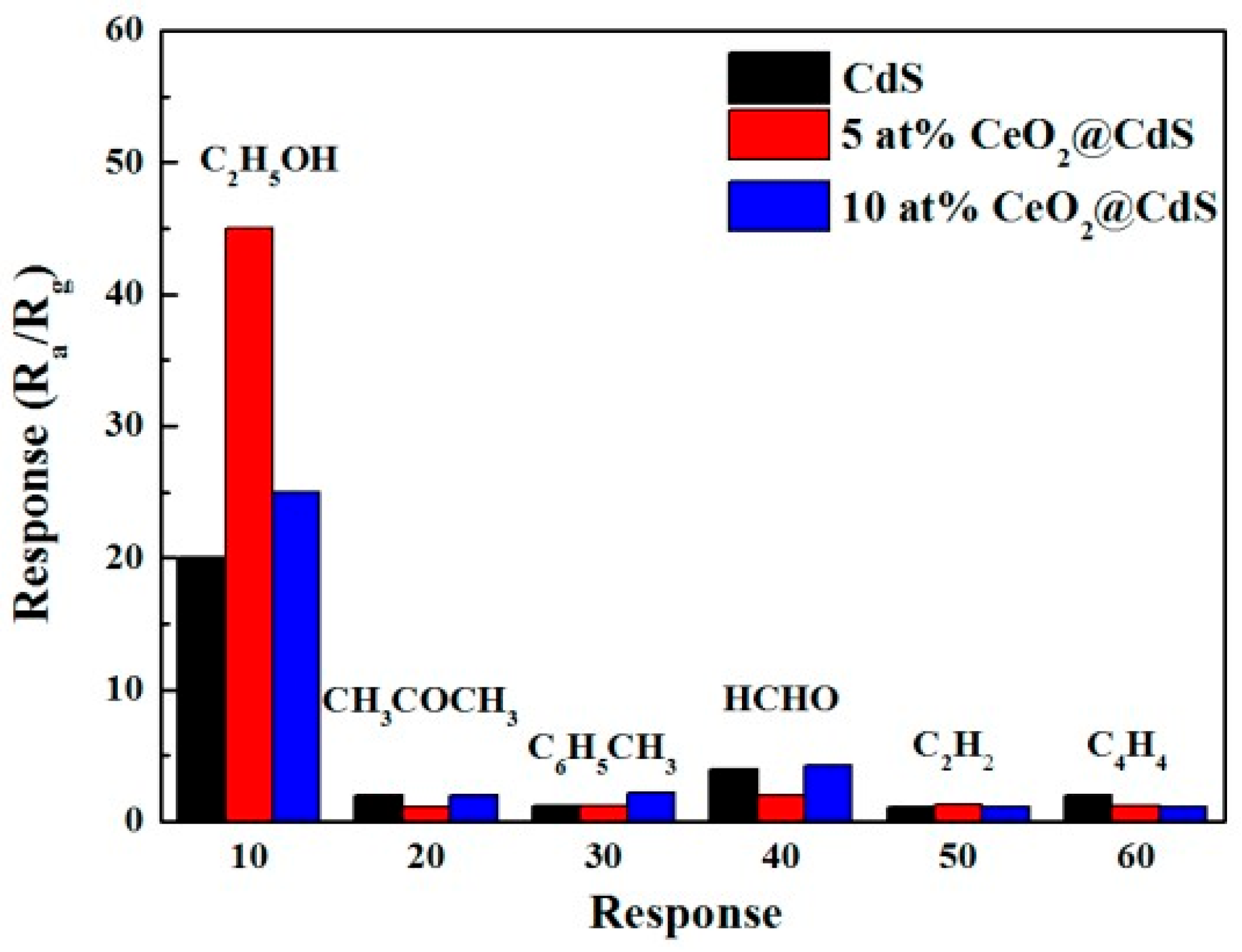
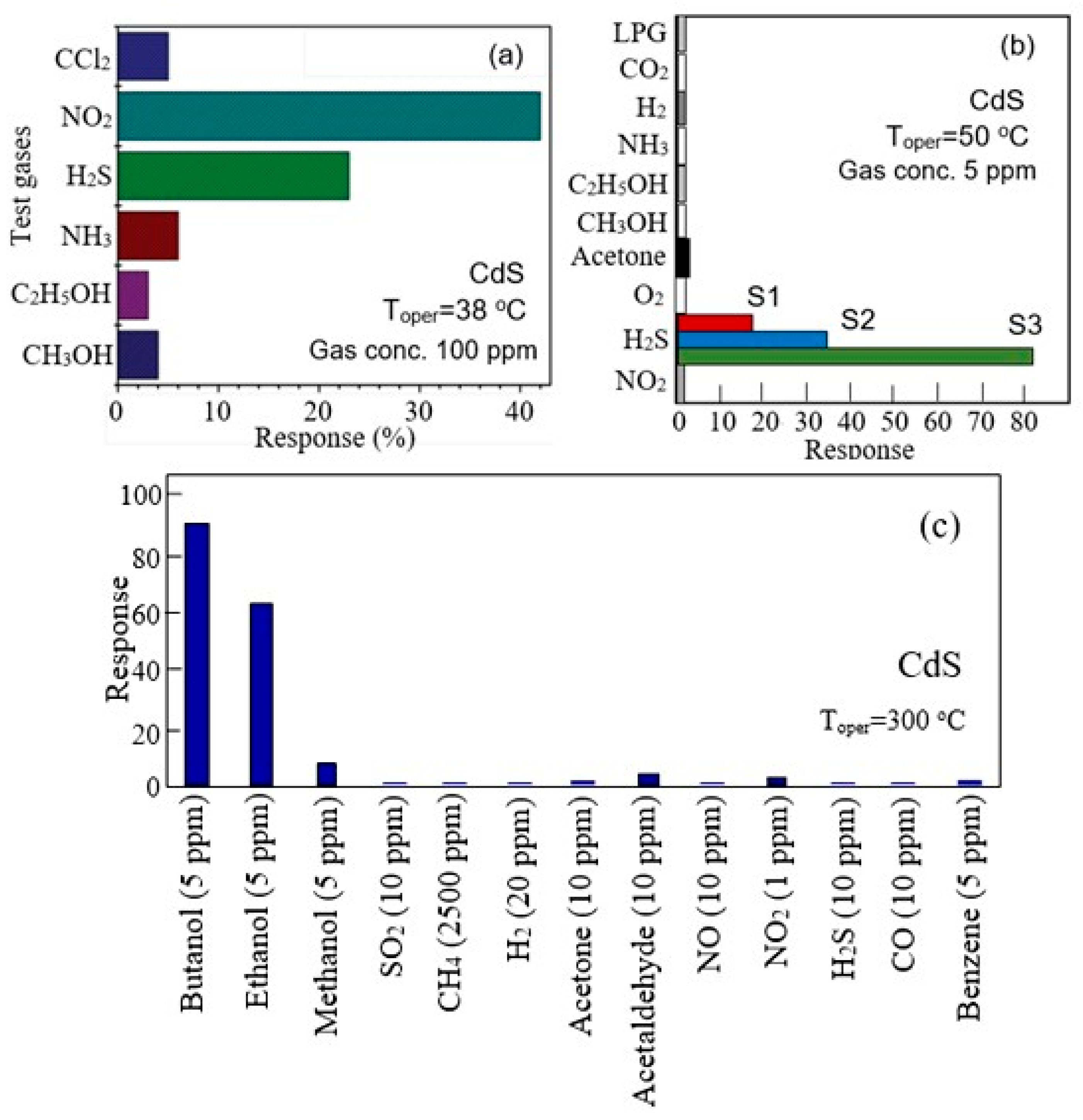
- First, gas sensors based on II–VI semiconductors are sensitive to toxic and flammable gases and vapors. There are currently reports of the development of II–VI compound-based gas sensors capable of detecting O2, O3, SO2, H2, H2S, NH3, NO2, Cl2, CCl4, CO2, ethanol, methanol, acetone, benzene, formaldehyde, isopropanol, gasoline, toluene, and liquefied petroleum gas (LPG) [37,41,50]. Moreover, these sensors demonstrated sensitivity to these gases, acceptable for real use. As a rule, among the most dangerous gases, in most cases, the sensors had a maximum response to H2S [51,52] or NO2 [53,54], and among organic solvents, sensitivity decreased when moving from ethanol to methanol and acetone (formaldehyde, benzene, toluene, and acetylene) [39,50,55]. One example illustrating this behavior of sensors is shown in Figure 5. Of course, sensors developed in different laboratories could demonstrate completely different characteristics. For example, ZnS sensors developed by Shinde et al. [56], among toxic gases, had the maximum sensitivity to Cl2, and in the CdS sensors developed by Fu et al. [57] and Liu et al. [58] among organic solvents, isopropanol had the highest sensitivity.
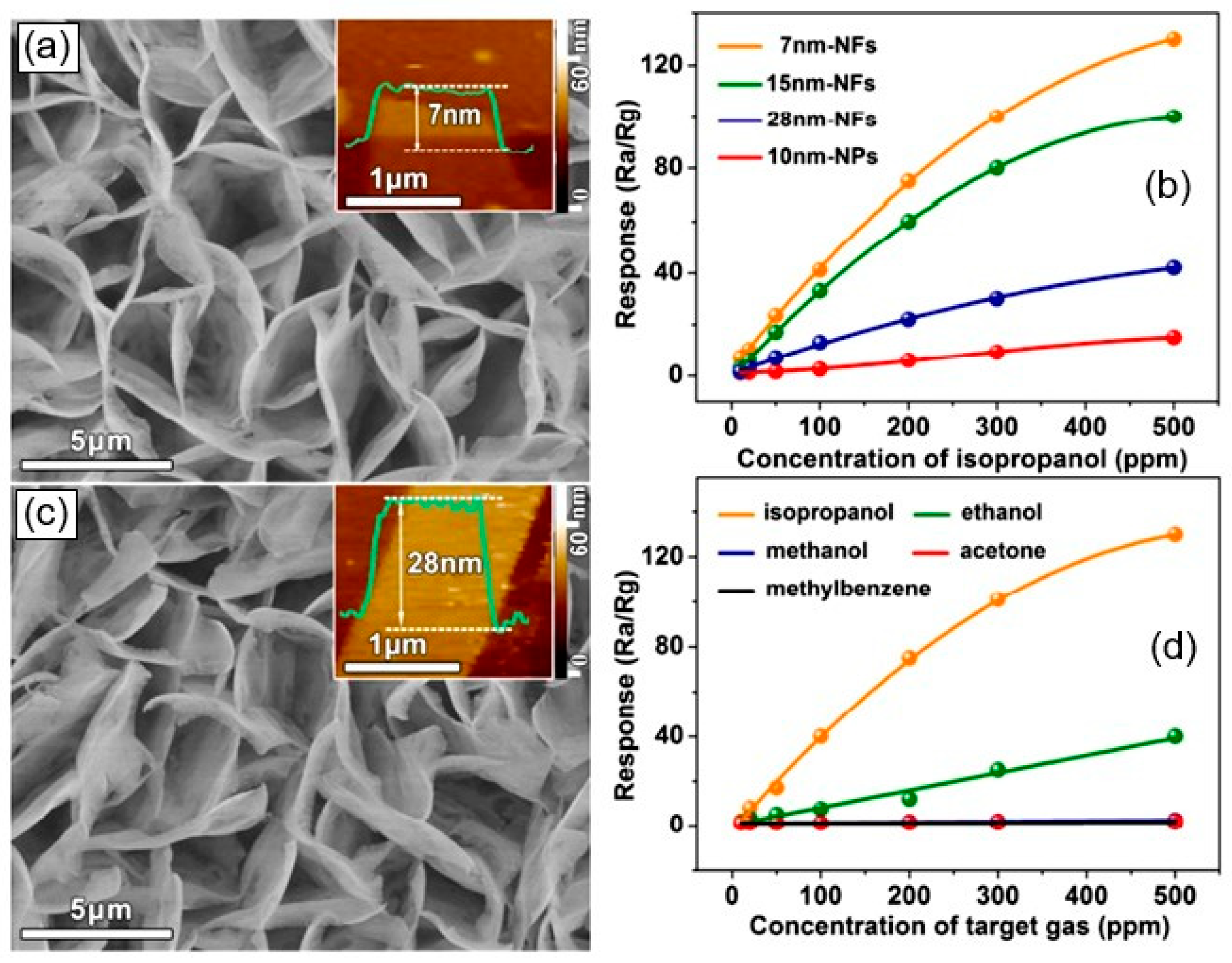
- Secondly, it was found that the gas-sensitive mechanism [16,17,19] and all the regularities established for metal oxides [59] also work in gas sensors based on II–VI compounds [39,43,60]. This means that (i) for better sensitivity, films based on II–VI semiconductors should be porous with small grain size and a high specific surface area [61], (ii) reducing the film thickness and increasing the pore size helps reduce response and recovery time [62,63], and (iii) increasing operating temperature reduces response and recovery time [62]. As for the effect of film thickness on the sensory response, it depends on the type of semiconductor, film structure, and the gas being detected. For example, studies of CdSe and CdS films have demonstrated an increase in sensitivity to oxygen and carbon monoxide as the film thickness of these materials increases [62,63,64]. At the same time, Dzhurkov et al. [65] reported that their thermal sputtered ZnSe sensors showed a decrease in sensitivity to ethanol as the thickness increased from 50 to 160 nm. It was also found that the gas sensitivity of II–VI semiconductors showed a dependence of the gas sensor response on the crystal shape [58], as was previously found for metal oxides [66].
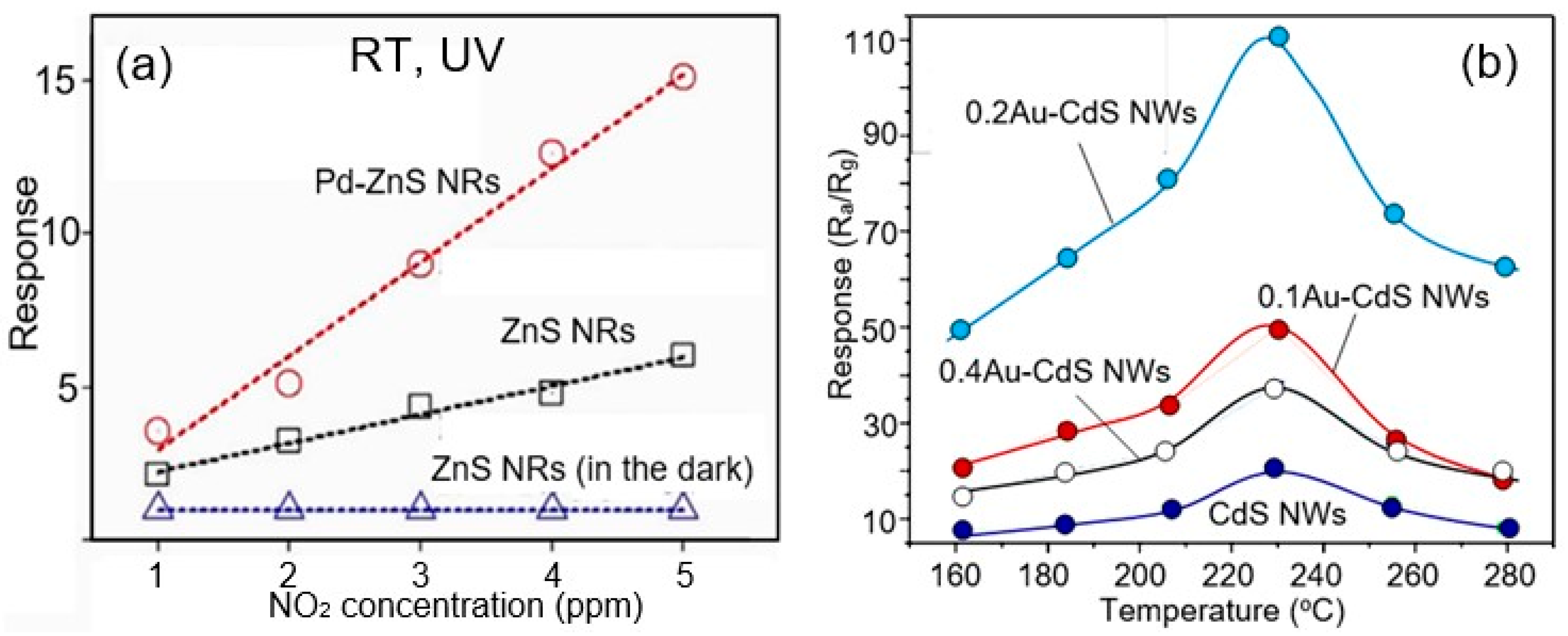
- Third, bulk doping and surface modification approaches developed for metal oxide gas sensors [67,68,69] can also be used to improve the selectivity and sensitivity of II–VI semiconductor-based sensors [43]. For example, doping CdS with zinc has been shown to increase the sensitivity of sensors to reducing gases such as methanol, ethanol, methylbenzene, formaldehyde, and acetone [70]. Something similar occurs when doping CdS with Ni2+ ions, which has been shown to improve the sensitivity of CdS to LPG [71]. Doping ZnS with Mn2+ ions also resulted in improved oxygen sensitivity [72]. Yue et al. [69] have shown that CdS doping with 1 mol%SnO2 strongly increases the sensitivity to ethanol. Decorating ZnS with Au nanoparticles enhanced the response to various gases (including nitrogen dioxide, ammonia, ethanol, carbon monoxide, and hydrogen sulfide) at room temperature compared to the equivalent system without nanoparticle decoration [73]. Decoration of CdS with Au nanoparticles increased the sensitivity to ethanol (Figure 6b). A similar study on palladium-decorated ZnS nanoparticles also showed a significant improvement in sensitivity to nitrogen dioxide compared to undecorated ZnS under UV illumination [74]. The results of this study are shown in Figure 6a.
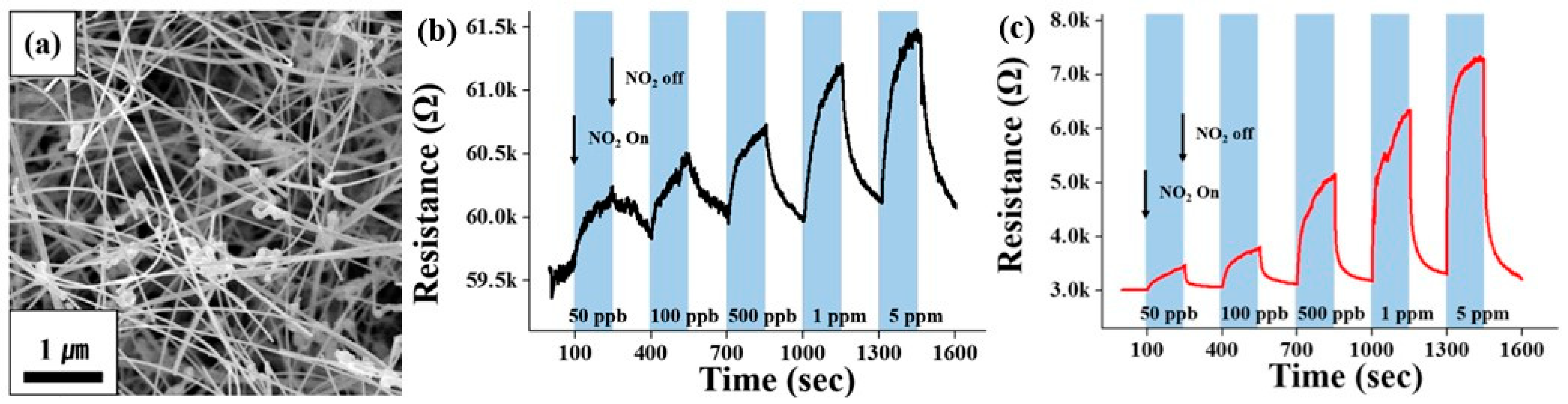
- Fourth, an approach based on the use of nanostructures such as 0D (quantum dots), 1D (nanowires, nanotubes, and nanofibers), 2D (nanosheets and nanobelts) and 3D (hierarchical structures such as nanoflowers and hollow nanospheres) for the optimization of sensor parameters is also suitable for the development of sensors based on II–VI semiconductors [41,49]. For example, CdS QDs with their large specific surface area and good charge transfer properties led to the sensitization of sensors based on Co3O4 microspheres to H2S [76]. The CdS QDs/Co3O4 composites also showed very short response and recovery times at room temperature. The CdSe QD/In2O3 structure showed a better sensor response to NO2 compared to other composites [77]. ZnSe nanowire-based sensors developed by Park et al. [49] were also sensitive to NO2. Due to the specific properties of ZnSe nanowires (see Figure 7a), the sensors were able to detect NO2 at room temperature (Figure 7b). Of course, the sensitivity to NO2 at room temperature was low but reproducible. In this case, UV irradiation (365 nm) increased the response of ZnSe nanowires to a NO2 concentration of 50 ppb–5 ppm by 1.1–2.3 times (Figure 7c). It is known that light at room temperature can activate chemoresistance in II–VI semiconductors. It is this effect that in many cases allows gas sensors based on metal oxides and II–VI semiconductors to operate effectively at room temperature. However, the influence of environmental conditions on the parameters of semiconductor QDs and II–VI nanowires, especially their stability, limits the use of semiconductor QDs and II–VI nanowires in gas sensors operating at elevated temperatures [49]. For example, as will be shown later, CdS QDs can easily oxidize and transform into CdO at temperatures above 25 °C [76]. The same effect is typical for nanowires [49].
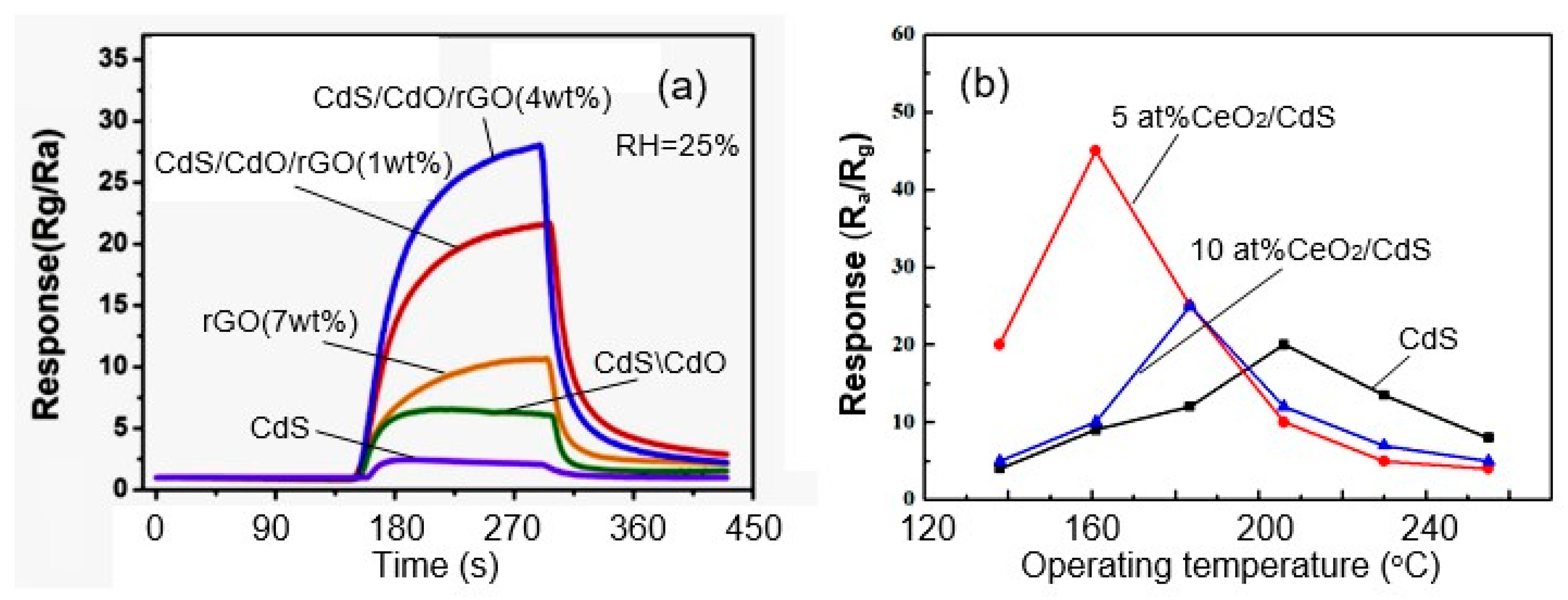
- Fifth, just as for metal oxides, the use of heterostructures and nanocomposites under certain conditions can optimize sensor parameters. The experiment showed that, as in the case of metal oxides [96], the creation of inorganic nanocomposites based on II–VI semiconductors is one of the approaches that can significantly improve sensor performance (primarily sensitivity and selectivity, but in some cases also reduce power consumption) compared to sensors made on the basis of individual components forming a composite. For example, Table 2 presents the results of testing gas sensors based on the Zn1−xCdxS nanocomposite. It can be seen that with the optimal composition (X = 0.4), there is an increase in the sensory response and a decrease in the response time and recovery compared to ZnS-based sensors. Zhu et al. [55] also showed that these sensors had less dependence on air humidity. Zhang et al. [97] synthesized CdS/WS2 composites by an environmentally friendly and ultra-low-cost hydrothermal method and found that, compared with pure WS2 and CdS, the CdS/WS2–40 wt% composite exhibits a superior sensor response of more than 4 orders of magnitude, very short recovery time (3 s), and ultra-high selectivity to NH3 at room temperature. Moreover, immunity tests showed that the CdS/WS2 sample remained stable under real-time NH3 monitoring at different ambient humidity levels.
3. Limitations
- ✓ These compounds, unlike group III-V semiconductors, exhibit a greater tendency to deviate from stoichiometry, which allows them to be considered as phases of variable composition. Because of this, the properties of type II–VI semiconductor compounds remain difficult to control [12].
- ✓ The use of these materials does not provide improved performance compared to gas sensors based on metal oxides. Sensors based on II–VI semiconductors are sensitive to the same gases and vapors as metal oxide sensors. In addition, they, like metal oxide sensors, are strongly influenced by humidity, which leads to unreliable sensory responses, especially at high concentrations of water vapor [113]. How significant the effect of humidity is on resistive II–VI semiconductor-based sensors can be shown in Figure 11. It can be seen that in the region of high humidity levels, the resistance of ZnS nanowires can change hundreds of times (Figure 13b). Sensors based on II–VI compounds also have low selectivity [43]. For example, ZnS-based sensors preferentially respond to Cl2 [56]. However, they may experience high interference from CO2, H2S, and H2 and moderate interference from LPG, NH3, and ethanol (see Figure 3, Figure 9b and Figure 10d). This means that the application of II–VI semiconductor compounds cannot solve the problems of low selectivity of conductometric gas sensors based on metal oxides. Moreover, as a rule, the parameters of gas sensors based on II–VI semiconductors are significantly worse than the parameters of sensors based on metal oxides.
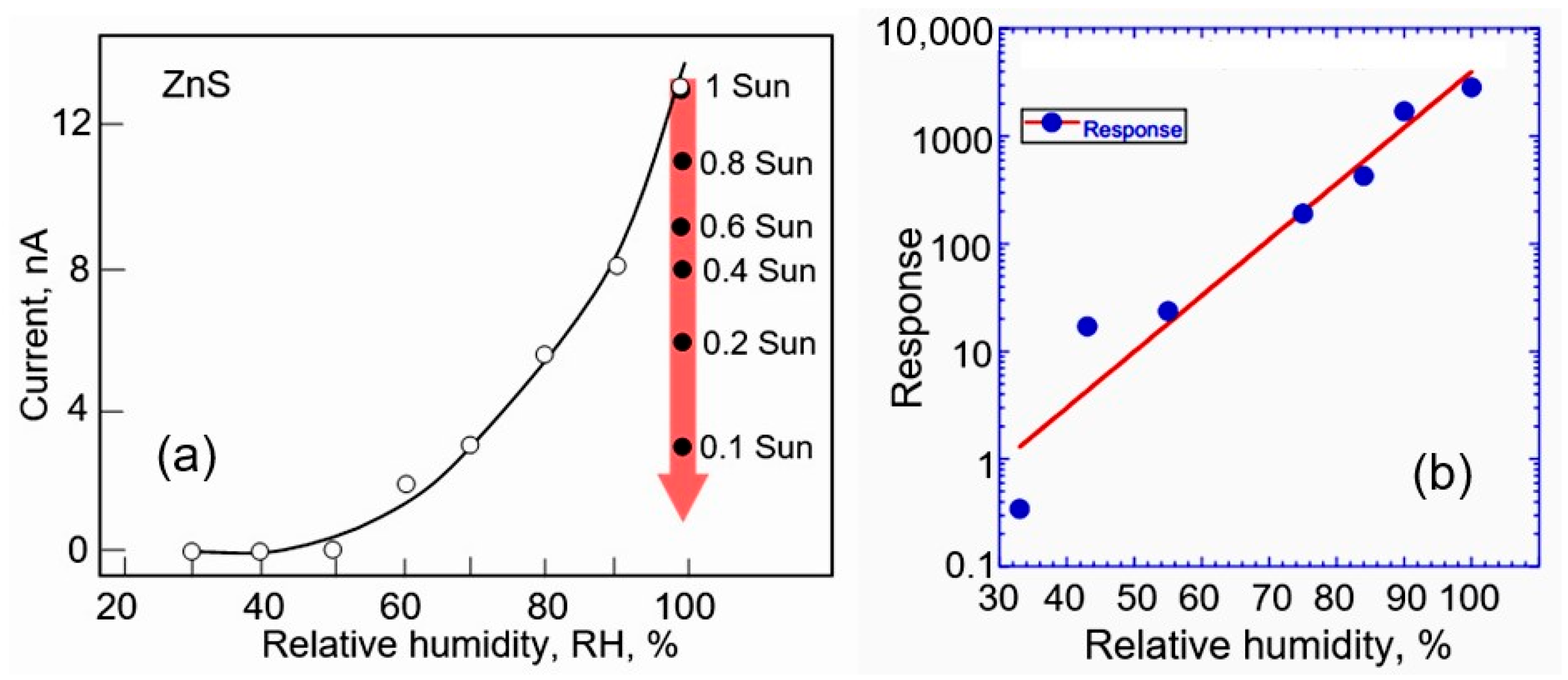
- ✓ The parameters of semiconductor compounds II–VI strongly depend on illumination (see, for example, Figure 13a) and temperature, which makes the choice of operating conditions for these sensors more stringent.
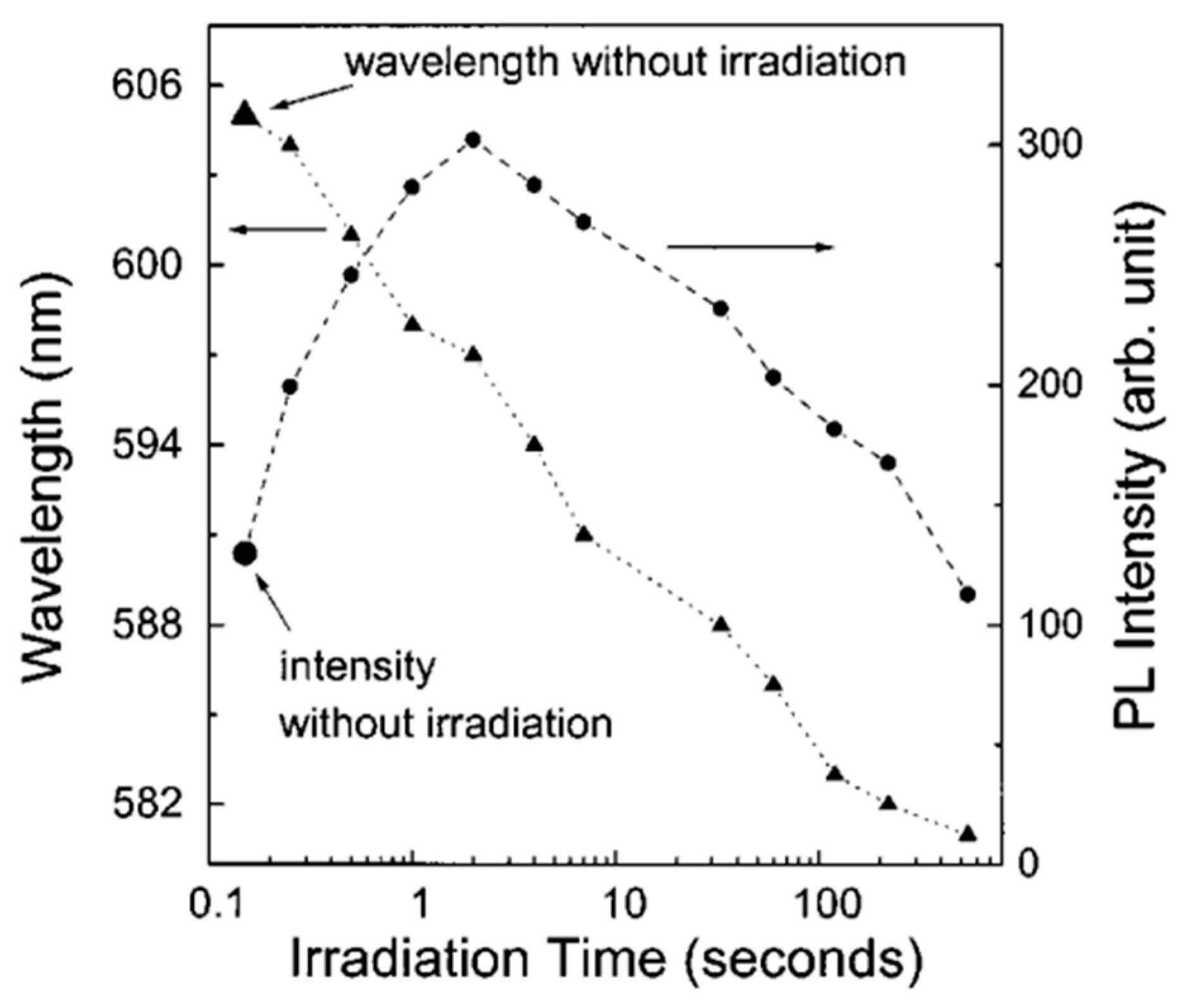
- ✓ But the most important limitation is still the lack of temporal stability of these sensors, especially at elevated temperatures, when there is a drift in the baseline resistance and a decrease in the sensory response over time [9]. As shown in Figure 8c, a temporary change in sensory response is observed even when operating at room temperature.
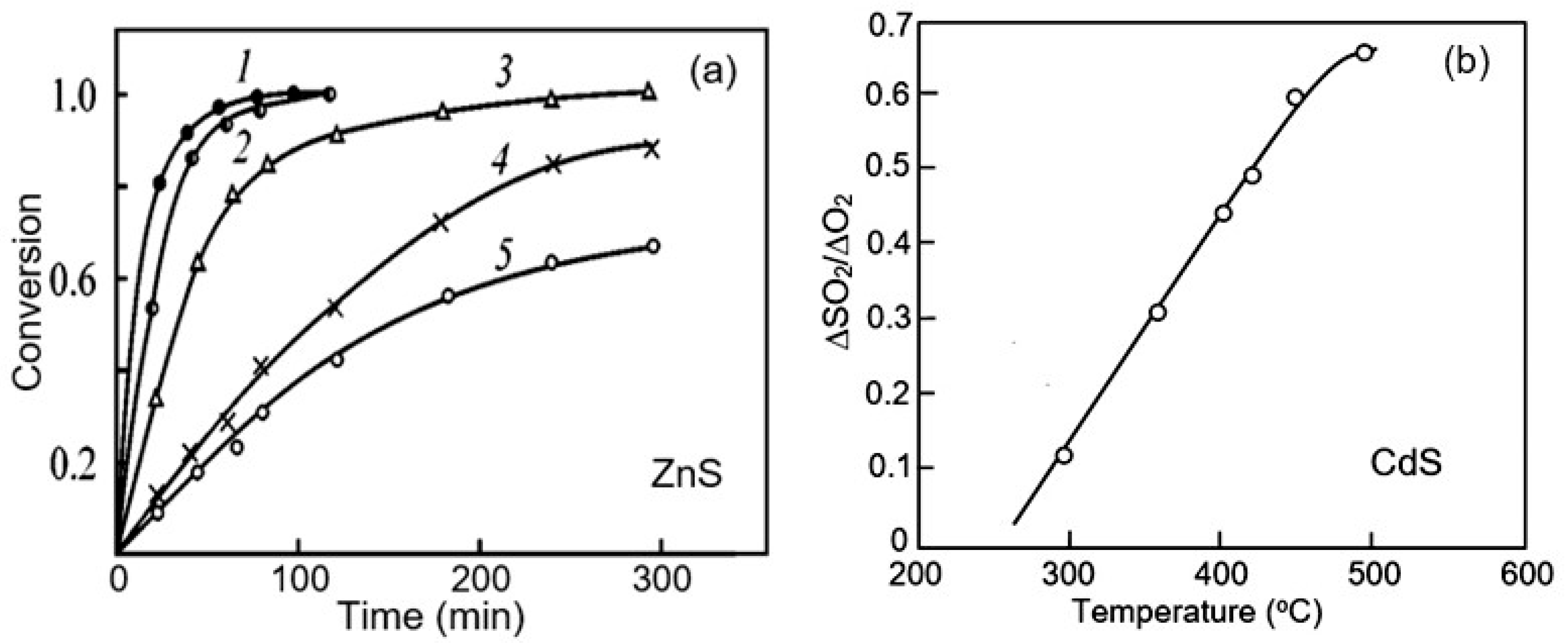
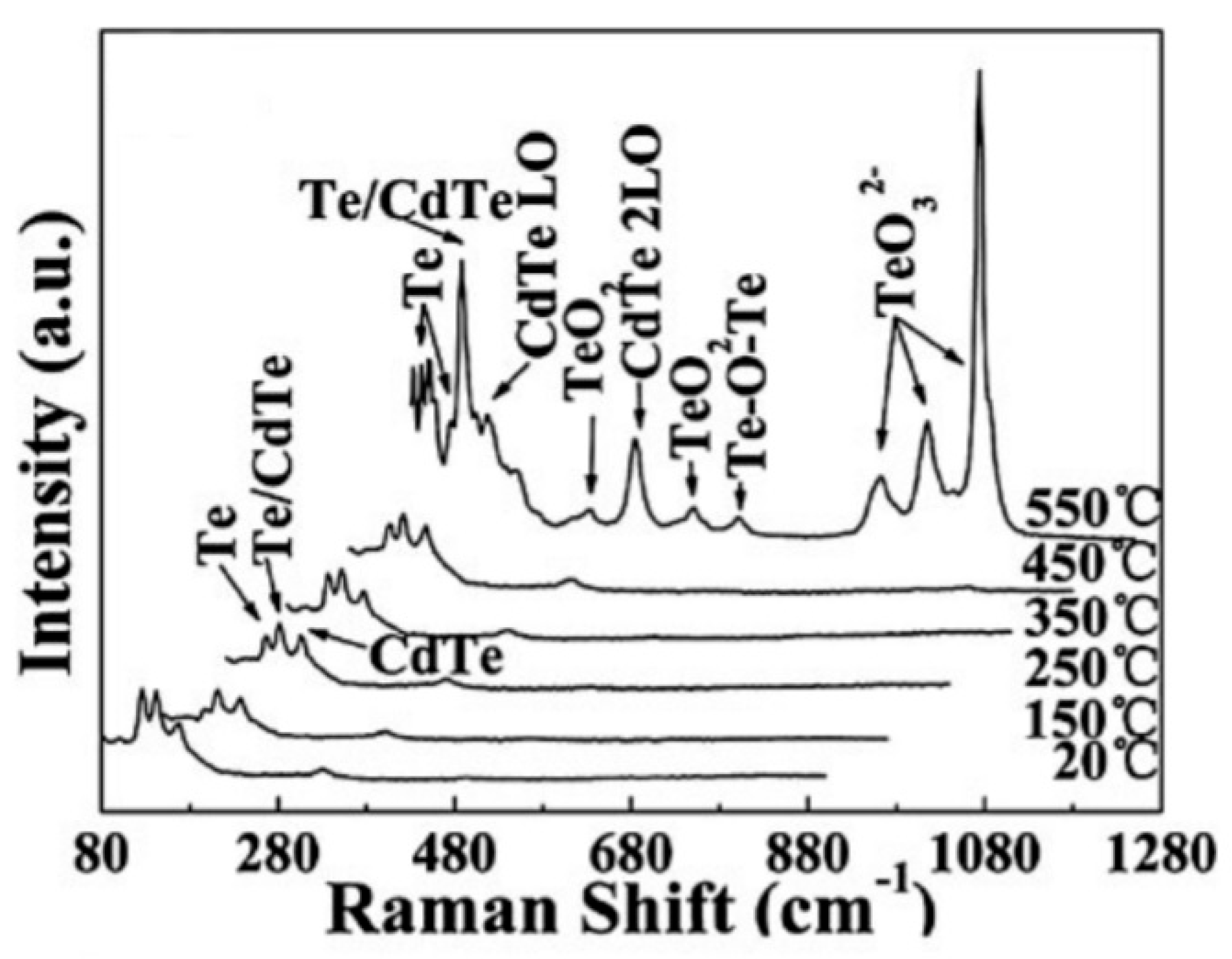
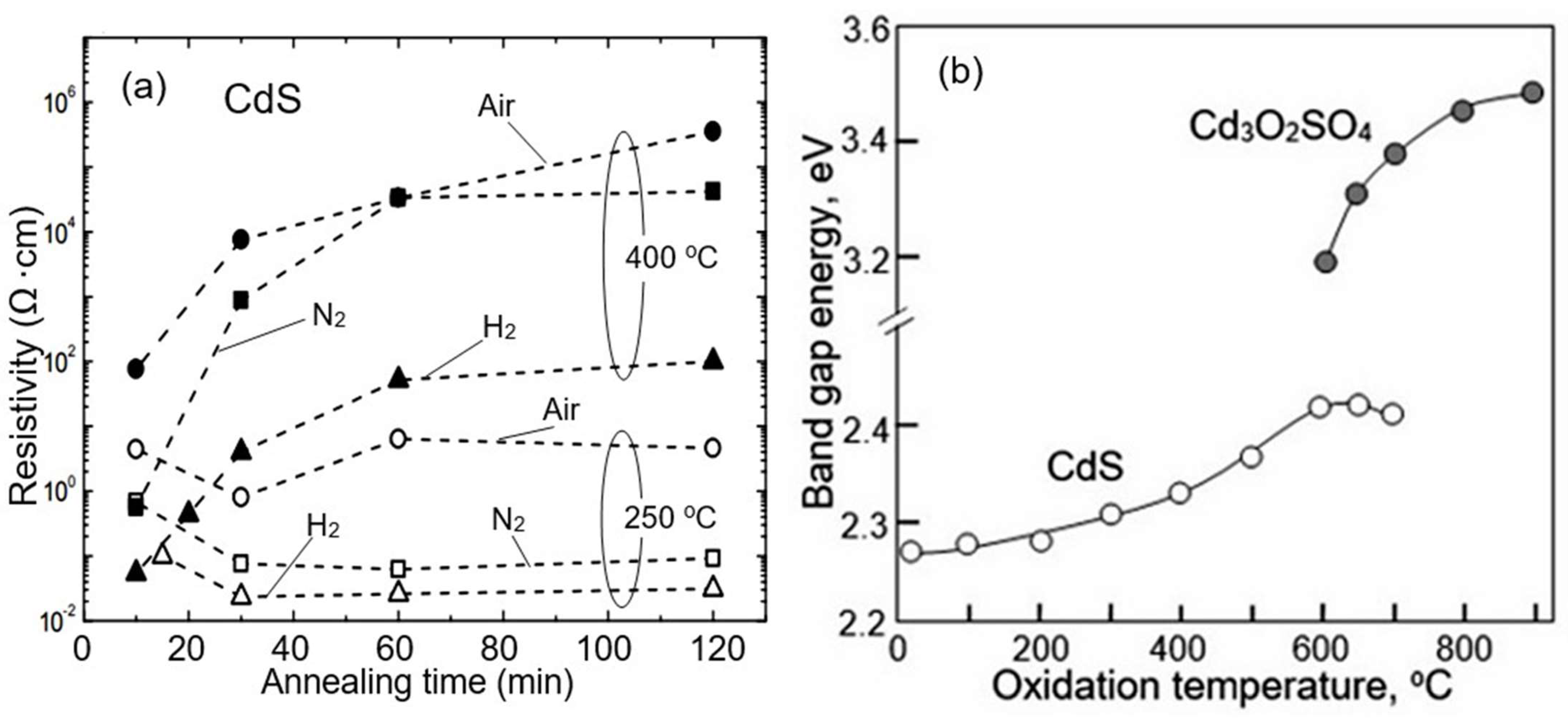
- ✓ It is important to note that studies have shown that II–VI compounds have more complex surface chemistry [157]. As is known, metal oxides do not change their phase state in an oxygen atmosphere. While only hydroxides can form on the surface of metal oxides in a humid atmosphere [158], metal hydroxides, oxides, sulfur dioxide, and sulfates can be observed on the surface of ZnS and CdS when heated in the atmosphere [124,128], the ratio of which will depend on temperature and air humidity. These CdS oxidation products and the corresponding reactions in a dry atmosphere that can lead to the formation of indicated compounds are listed in Table 3. The possibility of the formation of hydroxyls on the surface of II–VI compounds was shown by Hellgren et al. [159] using ZnSe as an example. They found that the oxide composition on the ZnSe surface is indeed very sensitive to the surface treatment method, and in the presence of water or water vapor, hydroxyls such as SeOH and ZnOH can be formed on the surface.
| No. | CdS Oxidation Product | Chemical Reaction Equation |
|---|---|---|
| 1 | CdSO4 | CdS + 2O2 → CdSO4 |
| 2 | Cd3SO6 + 2SO2 + O2 → 3CdSO4 | |
| 3 | Cd5S3O6 + 2SO2 + 5O2 → 5CdSO4 | |
| CdO | 3CdS + 5O2 → 2CdO⋅CdSO4 + 2SO2 | |
| 4 | 2CdS + 3O2 → 2CdO + 2SO2 | |
| 5 | CdS + CdSO4 → 2CdO + 2SO2 | |
| 6 | CdSO4 → CdO + SO2 + 1/2O2 | |
| 7 | Cd3SO6 | 6CdO + 2SO2 + O2 → 2Cd3SO6 |
| 8 | Cd5S3O6 | 5CdSO4 → Cd5S3O6 + 2SO2 + 5O2 |
| 9 | 5CdS + 5O2 → Cd5S3O6 + 2SO2 | |
| 10 | 5Cd3SO6 + 4SO2 → 3Cd5S3O6 + 10O2 |
- ✓ It is important to note that chemical processing also affects the surface stoichiometry of II–VI compounds, which further complicates the process of comparing sensors prepared in different laboratories [157]. This also makes it difficult to achieve the required reproducibility of sensor parameters. For example, fresh Br2/ethanol solutions have been reported to form a nearly stoichiometric CdSe surface slightly depleted in cadmium, as evidenced by a Cd:Se ratio of 0.91 as determined by X-ray photoelectron spectroscopy (XPS). In contrast, aged solutions (~5 h) resulted in the formation of a CdS surface rich in Cd (Cd:Se ≈ 1.6 by XPS) [45,164,165]. Jaseniak and Mulvaney [165] demonstrated that CdSe nanocrystals with Cd- or Se-rich surfaces have significant differences in chemical nature, adsorption properties, and stability. The results of the effect of chemical treatment on the surface stoichiometry of ZnSe are given in Table 4 [166].
| Etchant | Etchant Composition | ZnSe |
|---|---|---|
| Se/Zn | ||
| 1 | 1% Bromine in methanol | 1.84 |
| 2 | 1 g of K2Cr2O7 in 10 mL H2SO4 + 20 mL H2O | 1.21 |
| 3 | Ar+ ion bombarded for ZnSe (ref. surfaces) | 0.72 |
| 4 | 0.5 g of NaOH + 0.5 g of Na2S2O3 in 100 mL H2O | 0.69 |
| 5 | Etchant 2 followed by etchant 4 | 0.68 |
| 6 | Etchant 1 followed by a wash in hot NaOH solution (80–90 °C) | 0.58 |
| 7 | 1 g of NaOH in 20 mL H2O + 1 mL H2O2 | 0.57 |
| 8 | Etchant I followed by hydrazene-hydrate | - |
4. Summary
Funding
Data Availability Statement
Conflicts of Interest
References
- Korotcenkov, G. Handbook of II–VI Semiconductor-Based Sensors and Radiation Detectors. Vol. 1: Materials and Technology; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Afzaal, M.; O’Brien, P. Recent developments in II–VI and III–VI semiconductors and their applications in solar cells. J. Mater. Chem. 2006, 16, 1597–1602. [Google Scholar] [CrossRef]
- Ikhmayies, S.J. Advances in the II–VI Compounds Suitable for Solar Cell Applications; Signpost Publisher: New York, NY, USA, 2014. [Google Scholar]
- Korotcenkov, G. Handbook of II–VI Semiconductor-Based Sensors and Radiation Detectors. Vol. 2: Photodetectors; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Korotcenkov, G. Handbook of II–VI Semiconductor-Based Sensors and Radiation Detectors. Vol. 3: Sensors, Biosensors and Radiation Detectors; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Owens, A.; Peacock, A. Compound semiconductor radiation detectors. Nucl. Instrum. Methods Phys. Res. A 2004, 531, 18–37. [Google Scholar] [CrossRef]
- Takahashi, T.; Watanabe, S. Recent Progress in CdTe and CdZnTe detectors. IEEE Trans. Nucl. Sci. 2001, 48, 950–959. [Google Scholar] [CrossRef]
- Korotcenkov, G. Cd- and Zn-based wide band gap II–VI semiconductors. In Handbook of II–VI Semiconductor-Based Sensors and Radiation Detectors. Vol. 1: Materials and Technologies; Korotcenkov, G., Ed.; Springer: Cham, Switzerland, 2023; pp. 21–65. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Simonenko, N.P.; Fedorov, F.S.; Sysoev, V.V. II–VI wide-bandgap semiconductor device technology: Schottky barrier, ohmic contacts, and heterostructures. In Handbook of II–VI Semiconductor-Based Sensors and Radiation Detectors. Vol. 1: Materials and Technologies; Korotcenkov, G., Ed.; Springer: Cham, Switzerland, 2023; pp. 491–516. [Google Scholar] [CrossRef]
- Adachi, S. Properties of Group-IV, III–V and II–VI Semiconductors; Wiley: Chichester, UK, 2005. [Google Scholar] [CrossRef]
- Adachi, S. Handbook of Physical Properties of Semiconductors. Vol. 3. II–VI Compounds Semiconductors; Kluwer Academic Publishers: Boston, MA, USA, 2004. [Google Scholar] [CrossRef]
- Aven, J.S.; Prender, M. Physics and Chemistry of II–VI Compounds; Interscience (Wiley): New York, NY, USA, 1967. [Google Scholar]
- Bouroushian, M. Electrochemistry of Metal Chalcogenides: Monographs in Electrochemistry; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Mills, K.C. Thermodynamic Data for Inorganic Sulphides, Selenides and Tellurides; Butterworths: London, UK, 1974. [Google Scholar]
- Korotcenkov, G. Metal oxides for solid state gas sensors. What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Barsan, N.; Schierbaum, K. Gas Sensors Based on Conducting Metal Oxides: Basic Understanding, Technology and Applications; Metal Oxides Series; Korotcenkov, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Brinzari, V.; Korotcenkov, G. Kinetic approach to receptor function in chemiresistive gas sensor modeling of tin dioxide. Steady state consideration. Sens. Actuators B Chem. 2018, 259, 443–454. [Google Scholar] [CrossRef]
- Brynzari, V.; Korotchenkov, G.; Dmitriev, S. Theoretical study of semiconductor thin film gas sensitivity: Attempt to consistent approach. J. Electron. Technol. 2000, 33, 225–235. [Google Scholar]
- Korotcenkov, G. Chemical Sensors: Simulation and Modeling. Vol. 3: Solid State Sensors; Momentum Press: New York, NY, USA, 2012. [Google Scholar]
- Korotcenkov, G. Chemical Sensors: Simulation and Modeling. Vol. 2: Conductometric Gas Sensors; Momentum Press: New York, NY, USA, 2012. [Google Scholar]
- Korotcenkov, G. Chemical Sensors: Simulation and Modeling. Vol. 1: Microstructural Characterization and Modeling of Metal Oxides; Momentum Press: New York, NY, USA, 2012. [Google Scholar]
- Kurtin, S.; McGill, T.C.; Mead, C.A. Fundamental transition in the electronic nature of solids. Phys. Rev. Lett. 1969, 22, 1433–1436. [Google Scholar] [CrossRef]
- Nesheva, A.; Aneva, Z.; Reynolds, S.; Main, C.; Fitzgerald, A.G. Preparation of micro- and nanocrystalline CdSe and CdS thin films suitable for sensor applications. J. Optoelectron. Adv. Mater. 2006, 8, 2120–2125. [Google Scholar]
- Ebina, A.; Suda, Y.; Takahashi, T.T. Oxidation of ZnSe (110) and ZnTe (110). Int. J. Electron. 1982, 52, 77–88. [Google Scholar] [CrossRef]
- Noskar, R.W.; Mark, P.; Levine, J.D. Polar surfaces of wurtzite and zincblende lattices. Surf. Sci. 1970, 19, 291–317. [Google Scholar] [CrossRef]
- Alaarage, W.K.; Abo Nasria, A.H.; Hussein, T.A.; Abbood, H.I. Investigation of the electronic and optical properties of bilayer CdS as a gas sensor: First-principles calculations. RSC Adv. 2024, 14, 5994. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.; Yaqoob, J.; Khan, M.U.; Rizwan, H.A.; Jabbar, A.; Hussain, R.; Zafar, Z.; Darwish, H.W.; Abbas, F. Exploring the potential of ZnSe nanocage as a promising tool for CO2 and SO2 sensing: A computational study. Comp. Theor. Chem. 2024, 1231, 114428. [Google Scholar] [CrossRef]
- Sakr, M.A.S.; Saad, M.A.; Abdelsalam, H.; Abd Elkader, O.H.; Aleya, L.; Zhang, Q. Two-dimensional ZnS quantum dots for gas sensors: Electronic and adsorption properties. J. Electron. Mater. 2023, 52, 5227–5238. [Google Scholar] [CrossRef]
- Campbell, B.D.; Farnsworth, H.E. Studies of structure and oxygen adsorption of (0001(CdS surfaces by LEED. Surf. Sci. 1968, 10, 197–214. [Google Scholar] [CrossRef]
- Herl, W. Surface chemical properties of zinc sulfide. Langmuir 1988, 4, 595–598. [Google Scholar] [CrossRef]
- Bootsma, G.A. Gas adsorption on cadmium sulphide. Surf. Sci. 1968, 9, 396–406. [Google Scholar] [CrossRef]
- Gupta, S.S.; van Huis, M.A. Adsorption study of a water molecule on vacancy-defected nonpolar CdS surfaces. J. Phys. Chem. C 2017, 121, 9815–9824. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, Q.; Hao, W.; Sun, Z.-X. Surface stoichiometry of zinc sulfide and its effect on the adsorption behaviors of xanthate. Chem. Cent. J. 2011, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Nagao, M.; Tokuda, F. The relation between the amounts of chemisorbed and physisorbed water on metal oxides. J. Phys. Chem. 1969, 73, 243–248. [Google Scholar] [CrossRef]
- Kirovskaya, I.A.; Murashko, Y.A. IR-spectroscopic studies of the surface of the components of the ZnTe—CdTe system. Omsk Sci. Bull. 2004, 1, 66–67. (In Russian) [Google Scholar]
- Goodell, C.M.; Gilbert, B.; Weigand, S.J.; Banfield, J.F. Kinetics of water adsorption-driven structural transformation of ZnS nanoparticles. J. Phys. Chem. C 2008, 112, 4791–4796. [Google Scholar] [CrossRef]
- Zhang, H.; Rustad, J.R.; Banfield, J.F. Interaction between water molecules and zinc sulfide nanoparticles studied by temperature-programmed desorption and molecular dynamics simulations. J. Phys. Chem. A 2007, 111, 5008–5014. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Kawasaki, H.; Tamura, S.; Haramoto, Y.; Shigeri, Y. Recent advances in gas sensing technology using non-oxide II–VI semiconductors CdS, CdSe, and CdTe. Chemosensors 2022, 10, 482. [Google Scholar] [CrossRef]
- Gaiardo, A.; Fabbri, B.; Guidi, V.; Bellutti, P.; Giberti, A.; Gherardi, S.; Vanzetti, L.; Malagù, C.; Zonta, G. Metal sulfides as sensing materials for chemoresistive gas sensors. Sensors 2016, 16, 296. [Google Scholar] [CrossRef] [PubMed]
- Gaiardo, A.; Fabbri, B.; Valt, M. Nanomaterial-based electric and electronic gas sensors. In Handbook of II–VI Semiconductor-Based Sensors and Radiation Detectors, Vol. 3: Sensors, Biosensors and Radiation Detectors; Korotcenkov, G., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 253–280. [Google Scholar] [CrossRef]
- Kumar, A.; Jit, S.; Saxena, S.; Shukla, S. II–VI semiconductor-polymer nanocomposites and their gas-sensing properties. In Handbook of II–VI Semiconductor-Based Sensors and Radiation Detectors, Vol. 3, Sensors, Biosensors and Radiation Detectors; Korotcenkov, G., Ed.; Springer: Cham, Switzerland, 2023; pp. 233–251. [Google Scholar] [CrossRef]
- Vallejos, S.; Blackman, C. II–VI Semiconductor-based thin film electric and electronic gas sensor. In Handbook of II–VI Semiconductor-Based Sensors and Radiation Detectors, Vol. 3: Sensors, Biosensors and Radiation Detectors; Korotcenkov, G., Ed.; Springer: Cham, Switzerland; pp. 177–199. [CrossRef]
- Vasiliev, R.B.; Chizhov, A.S.; Rumyantseva, M.N. Nanocomposite and hybrid-based electric and electronic gas sensors. In Handbook of II–VI Semiconductor-Based Sensors and Radiation Detectors, Vol. 3: Sensors, Biosensors and Radiation Detectors; Korotcenkov, G., Ed.; Springer: Cham, Switzerland, 2023; pp. 201–231. [Google Scholar] [CrossRef]
- Seker, A.; Meeker, K.; Kuech, T.F.; Ellis, A.B. Surface chemistry of prototypical bulk II−VI and III−V semiconductors and implications for chemical sensing. Chem. Rev. 2000, 100, 2505–2536. [Google Scholar] [CrossRef]
- Chun, J.; Yang, W.; Kim, J.S. Thermal stability of CdSe/ZnS quantum dot-based optical fiber temperature sensor. Mol. Crystallogr. Liq. Crystallogr. 2011, 538, 333–340. [Google Scholar] [CrossRef]
- Korotcenkov, G. Chemical Sensors: Comprehensive Sensor Technologies. Vol. 4: Solid State Devices; Momentum Press: New York, NY, USA, 2011. [Google Scholar]
- Benkstein, K.D.; Semancik, S. Mesoporous nanoparticle TiO2 thin films for conductometric gas sensing on microhotplate platforms. Sens. Actuators B Chem. 2006, 113, 445–453. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Lee, W.I.; Kim, K.-K.; Lee, C. Room temperature, ppb-level NO2 gas sensing of multiple-networked ZnSe nanowire sensors under UV illumination. Beilstein J. Nanotechnol. 2014, 5, 1836–1841. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ren, W.; Wu, R.; Zhang, M. CeO2 enhanced ethanol sensing performance in a CdS gas sensor. Sensors 2017, 17, 1577. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.M.; Patil, D.S.; Pawar, P.H. Gas sensing properties of nanocrystalline fiber like structure of CdS thin films for H2S gas detection. Intern. J. Chem. Concepts 2016, 2, 96–105. [Google Scholar]
- Raut, B.T.; Godse, P.R.; Pawar, S.G.; Chougule, M.A.; Bandgar, D.K.; Patil, V.B. Novel method for fabrication of polyaniline–CdS sensor for H2S gas detection. Measurement 2012, 45, 94–100. [Google Scholar] [CrossRef]
- Giberti, A.; Casotti, D.; Cruciani, G.; Fabbri, B.; Gaiardo, A.; Guidi, V.; Malagù, C.; Zonta, G.; Gherardi, S. Electrical conductivity of CdS films for gas sensing: Selectivity properties to alcoholic chains. Sens. Actuators B Chem. 2015, 207, 504–510. [Google Scholar] [CrossRef]
- Navale, S.T.; Mane, A.T.; Chougule, M.A.; Shinde, N.M.; Kim, J.-H.; Patil, V.B. Highly selective and sensitive CdS thin film sensors for detection of NO2 gas. RSC Adv. 2014, 4, 44547–44554. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Zhang, D.; Li, C.; Sun, D.; Wen, S.; Chen, Y.; Ruan, S. Gas sensors based on metal sulfide Zn1−xCdxS nanowires with excellent performance. ACS Appl. Mater. Interfaces 2015, 7, 20793–20800. [Google Scholar] [CrossRef]
- Shinde, M.S.; Swapna Samanta, S.; Sonawane, M.S.; Ahirrao, P.B.; Patil, R.S. Gas sensing properties of nanostructured ZnS thin films. J. Nano Adv. Mater. 2015, 3, 99–106. [Google Scholar] [CrossRef]
- Fu, X.; Liu, J.; Wan, Y.; Zhang, X.; Meng, F.; Liu, J. Preparation of a leaf-like CdS micro-/nanostructure and its enhanced gas-sensing properties for detecting volatile organic compounds. J. Mater. Chem. 2012, 22, 17782–17791. [Google Scholar] [CrossRef]
- Liu, X.-H.; Yin, P.-F.; Kulinich, S.A.; Zhou, Y.-Z.; Mao, J.; Ling, T.; Du, X.-W. Arrays of ultrathin CdS nanoflakes with high-energy surface for efficient gas detection . ACS Appl. Mater. Interfaces 2017, 9, 602–609. [Google Scholar] [CrossRef]
- Korotcenkov, G. The role of morphology and crystallographic structure of metal oxides in response of conductometric-type gas sensors. Mater. Sci. Eng. R 2008, 61, 1–39. [Google Scholar] [CrossRef]
- Lantto, V.; Golovanov, V. A comparison of conductance behaviour between SnO2 and CdS gas-sensitive films. Sens. Actuators B Chem. 1995, 24–25, 614–618. [Google Scholar] [CrossRef]
- Xu, L.; Song, H.; Zhang, T.; Fan, H.; Fan, L.; Wang, Y.; Dong, B.; Bai, X. A novel ethanol gas sensor-ZnS/cyclohexylamine hybrid nanowires. J. Nanosci. Nanotechnol. 2011, 11, 2121–2125. [Google Scholar] [CrossRef] [PubMed]
- Afify, H.H.; Battisha, I.K. Oxygen interaction with CdS based gas sensors by varying different preparation parameters. J. Mater. Sci. Mater. Electron. 2000, 11, 373–377. [Google Scholar] [CrossRef]
- Al-Hilli, B.A. The effect of cadmium selenide thin film thickness on carbon monoxide gas sensing properties prepared by plasma DC-sputtering technique. Iraqi J. Sci. 2018, 59, 2234–2241. [Google Scholar]
- Smyntyna, V.A.; Gerasutenko, V.; Kashulis, S.; Mattogno, G.; Reghini, S. The causes of thickness dependence of CdSe and CdS gas-sensor sensitivity to oxygen. Sens. Actuators B Chem. 1994, 19, 464–465. [Google Scholar] [CrossRef]
- Dzhurkov, V.; Levi, Z.; Nesheva, D.; Hristova-Vasilev, T. Room temperature sensitivity of ZnSe nanolayers to ethanol vapours. IOP Conf. Ser. J. Phys. Conf. Ser. 2019, 1186, 012023. [Google Scholar] [CrossRef]
- Gurlo, A. Nanosensors: Towards morphological control of gas sensing activity. SnO2, In2O3, ZnO and WO3 case studies. Nanoscale 2011, 3, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G. Gas response control through structural and chemical modification of metal oxides: State of the art and approaches. Sens. Actuators B Chem. 2005, 107, 209–232. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Engineering approaches to improvement operating characteristics of conductometric gas sensors. Part 1: Improvement of sensor sensitivity and selectivity. Sens. Actuators B Chem. 2013, 188, 709–728. [Google Scholar] [CrossRef]
- Yue, C.; Zhang, Z.; Liu, Z.; Mu, Y.; Yang, Z.; Dastan, D.; Yin, X.-T.; Ma, X. High-performance ethanol gas sensor with fast response/recovery rate based on the construction of SnO2-CdS heterojunction. J. Alloys Compd. 2024, 981, 173742. [Google Scholar] [CrossRef]
- Huang, Z.; Wei, D.; Wang, T.; Jiang, W.; Liu, F.; Chuai, X.; Liang, X.; Gao, Y.; Sun, P.; Yan, X.; et al. Excellent gas sensing of hierarchical urchin-shaped Zn doped cadmium sulfide. J. Alloys Compd. 2019, 773, 299–304. [Google Scholar] [CrossRef]
- Mondal, S. LPG sensing property of nickel doped CdS thin film synthesised by silar method. Adv. Mater. Process. Technol. 2020, 8, 344. [Google Scholar] [CrossRef]
- Lin, F.; Lai, Z.; Zhang, L.; Huang, Y.; Li, F.; Chen, P.; Wang, Y.; Chen, X. Fluorometric sensing of oxygen using manganese(II)-doped zinc sulfide nanocrystals. Microchim. Acta 2020, 187, 66. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; An, S.; Ko, H.; Lee, S.; Lee, C. Synthesis, structure, and UV-enhanced gas sensing properties of Au-functionalized ZnS nanowires. Sens. Actuators B Chem. 2013, 188, 1270–1276. [Google Scholar] [CrossRef]
- Park, S.; An, S.; Mun, Y.; Lee, C. UV-activated gas sensing properties of ZnS nanorods functionalized with Pd. Curr. Appl. Phys. 2014, 14 (Suppl. 1), S57–S62. [Google Scholar] [CrossRef]
- Ma, X.; Guo, S.; Shen, J.; Chen, Y.; Chen, C.; Sun, L.; Zhang, X.; Ruan, S. Synthesis and enhanced gas sensing properties of Au-nanoparticle decorated CdS nanowires. RSC Adv. 2016, 6, 70907. [Google Scholar] [CrossRef]
- Dun, M.; Tan, J.; Tan, W.; Tang, M.; Huang, X. CdS quantum dots supported by ultrathin porous nanosheets assembled into hollowed-out Co3O4 microspheres: A room-temperature H2S gas sensor with ultra-fast response and recovery. Sens. Actuators B Chem. 2019, 298, 126839. [Google Scholar] [CrossRef]
- Chizhov, A.S.; Rumyantseva, M.N.; Vasiliev, R.B.; Filatova, D.G.; Drozdov, K.A.; Krylov, I.V.; Marchevsky, A.; Karakulina, O.; Abakumov, A.; Gaskov, A. Visible light activation of room temperature NO2 gas sensors based on ZnO, SnO2 and In2O3 sensitized with CdSe quantum dots. Thin Solid Films 2016, 618, 253–262. [Google Scholar] [CrossRef]
- Korotcenkov, G. Current trends in nanomaterials for metal oxide-based conductometric gas sensors: Advantages and limitations. Part 1: 1D and 2D nanostructures. Nanomaterials 2020, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Liao, F.; Zhu, L.; Lu, S.; Sheng, M.; Gao, S.; Shao, M. Visible light enhanced gas sensing of CdSe nanoribbons of ethanol. CrystEngComm 2014, 16, 4231–4235. [Google Scholar] [CrossRef]
- Zhu, L.; Feng, C.; Li, F.; Zhang, D.; Li, C.; Wang, Y.; Lin, Y.; Ruan, S.; Chen, Z. Excellent gas sensing and optical properties of single-crystalline cadmium sulfide nanowires. RSC Adv. 2014, 4, 61691–61697. [Google Scholar] [CrossRef]
- Ghimpu, L.; Lupan, O.; Postica, V.; Strobel, J.; Kienle, L.; Terasa, M.-I.; Mintken, M.; Tiginyanu, I.; Marx, J.; Fiedler, B.; et al. Individual CdS-covered aerographite microtubes for room temperature VOC sensing with high selectivity. Mater. Sci. Semicond. Process. 2019, 100, 275–282. [Google Scholar] [CrossRef]
- Li, H.-Y.; Yoon, J.-W.; Lee, C.-S.; Lim, K.; Yoon, J.-W.; Lee, J.-H. Visible light assisted NO2 sensing at room temperature by CdS nanoflake array. Sens. Actuators B Chem. 2018, 255, 2963–2970. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, G.; Fan, S.; Hu, Y.; Liu, X.; Pinna, N.; Zhang, J. Mixed-dimensional heterojunction by 3D CdS nanowire arrays bridged with 2D WSe2 for ultrafast photoelectric gas sensor. ChemRxiv Mater. Chem. 2024. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Tolstoy, V.P. Current trends in nanomaterials for metal oxide-based conductometric gas sensors: Advantages and limitations—Part 2: Porous 2D nanostructures. Nanomaterials 2023, 13, 237. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Xue, Y.; Liu, X.; Liu, B.; Miao, B.; Kang, W.; Liu, S. Mesoporous ZnS hierarchical nanostructures: Facile synthesis, growth mechanism and application in gas sensing. CrystEngComm 2012, 14, 8044–8048. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, S.; Zhang, R.; Zhu, K.; Tie, Y.; Pei, S. Optimization NH3 sensing performance manifested by gas sensor based on Pr-SnS2/ZnS hierarchical nanofowers. J. Alloys Compd. 2019, 807, 151650. [Google Scholar] [CrossRef]
- Xiao, J.; Song, C.; Song, M.; Dong, W.; Li, C.; Yin, Y. Preparation and gas sensing properties of hollow ZnS microspheres. Nanosci. Nanotechnol. 2016, 16, 3026–3029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, R.; Zhu, Z.; Wang, S. Au nanoparticles decorated ZnS hollow spheres for highly improved gas sensor performances. Sens Actuators B Chem. 2017, 245, 112–121. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, S.Y.; Xu, X.L.; Jiao, H.Y.; Zhang, G.H.; Liu, L.W.; Wang, P.Y.; Gengzang, D.J.; Yao, H.H. Optimization ethanol detection performance manifested by gas sensor based on In2O3/ZnS rough microspheres. Sens Actuators B Chem. 2018, 264, 263–278. [Google Scholar] [CrossRef]
- Li, Y.; Shan, L.-X.; Lian, X.-X.; Zhou, Q.-J.; An, D.-M. Enhanced NO2 sensing performance of ZnO@ZnS core-shell structure fabricated using a solution chemical method. Ceram. Int. 2021, 47, 27411–27419. [Google Scholar] [CrossRef]
- Liu, W.; Gu, D.; Li, X. Detection of ppb-level NO2 using mesoporous ZnSe/SnO2 core-shell microspheres based chemical sensors. Sens Actuators B Chem. 2020, 320, 128365. [Google Scholar] [CrossRef]
- Tan, X.; Wang, L.; Chen, X.; Zhang, H.; Guo, J.; Dong, Z.; Qian, L.; Chen, Z.; He, C. Ultrasensitive triethylamine gas sensors with ZnSe nanospheres/nest-like Cr-doped MoO3. J. Hazard. Mater. 2024, 474, 134709. [Google Scholar] [CrossRef]
- He, P.; Fu, H.; Gong, Y.; Chen, J.; Yang, X.; Han, D.; Xiong, S.; Li, S.; An, X. SnO2@ZnS core-shell hollow spheres with enhanced room-temperature gas-sensing performance. Sens. Actuators B Chem. 2023, 388, 133809. [Google Scholar] [CrossRef]
- Singh, A.; Singh, S.; Yadav, B.C. Gigantic enhancement in response of heterostructured CeO2/CdS nanospheres based self-powered CO2 gas sensor: A comparative study. Sens. Actuators B Chem. 2023, 377, 33085. [Google Scholar] [CrossRef]
- Shan, L.-X.; Li, Y.; Wang, R.-C.; Lian, X.-X. Sensing properties of ZnS@ZnO core-shell based triethylamine sensor fabricated using solvothermal and sintering methods. J. Alloys Compd. 2023, 944, 169223. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Wang, S.; Wu, Z.; Zhang, Z. Moisture-resistant and highly selective NH3 sensor based on CdS/WS2 composite heterojunction. Langmuir 2023, 39, 10352–10366. [Google Scholar] [CrossRef] [PubMed]
- Chizhov, A.; Vasiliev, R.; Rumyantseva, M.; Krylov, I.; Drozdov, K.; Batuk, M.; Hadermann, J.; Abakumov, A.; Gaskov, A. Light-activated sub-ppm NO2 detection by hybrid ZnO/QD nanomaterials vs. charge localization in core-shell QD. Front. Mater. 2019, 6, 231. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Ko, H.; Lee, C. Light-enhanced gas sensing of ZnS-core/ZnO-shell nanowires at room temperature. J. Electroceram. 2014, 33, 75–81. [Google Scholar] [CrossRef]
- Sun, J.; Sun, L.; Han, N.; Chu, H.; Bai, H.; Shu, X.; Luo, R.; Chen, A. rGO decorated CdS/CdO composite for detection of low concentration NO2. Sens. Actuators B Chem. 2019, 299, 126832. [Google Scholar] [CrossRef]
- Rao, V.N.; Sairam, P.K.; Kim, M.-D.; Rezakazemi, M.; Aminabhavi, T.M.; Ahn, C.W.; Yang, J.-M. CdS/TiO2 nano hybrid heterostructured materials for superior hydrogen production and gas sensor applications. J. Environ. Manag. 2023, 340, 117895. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S. Highly sensitive and selective chemiresistor gas/vapor sensors based on polyaniline nanocomposite: A comprehensive review. J. Sci. Adv. Mater. Devices 2016, 1, 431–453. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, A.; Han, Y.; Li, T. Sensors based on conductive polymers and their composites: A review. Polym. Int. 2020, 69, 7–17. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, G.; Xu, J.L.; Zhang, M.; Kuo, C.C.; Wang, S.D. Conducting polymer-inorganic nanocomposite-based gas sensors: A review. Sci. Technol. Adv. Mater. 2020, 21, 768–786. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Lokhande, C.D.; Han, S.H. A room temperature liquefied petroleum gas sensor based on all-electrodeposited n-CdSe/p-polyaniline junction. Sens. Actuators B Chem. 2007, 123, 240–245. [Google Scholar] [CrossRef]
- Tatavarty, R.; Hwang, E.T.; Park, J.W.; Kwak, J.H.; Lee, J.O.; Gu, M.B. Conductive quantum dot-encapsulated electrospun nanofibers from polystyrene and polystyrene-co-maleic anhydride copolymer blend as gas sensors. React. Funct. Polym. 2011, 71, 104–108. [Google Scholar] [CrossRef]
- Mondal, S.P.; Bera, S.; Narender, G.; Ray, S.K. CdSe quantum dots-poly(3-hexylthiophene) nanocomposite sensors for selective chloroform vapor detection at room temperature. Appl. Phys. Lett. 2012, 101, 173108. [Google Scholar] [CrossRef]
- Sonawane, N.B.; Ahire, R.R.; Gurav, K.V.; Kim, J.H.; Sankapal, B.R. PEDOT:PSS shell on CdS nanowires: Room temperature LPG sensor. J. Alloys Compd. 2014, 592, 1–5. [Google Scholar] [CrossRef]
- Yeole, A.; Sen, T.; Hansora, D.P.; Mishra, S. Effect of electrical properties on gas sensitivity of polypyrrole/CdS nanocomposites. J. Appl. Polym. Sci. 2015, 132, 42379. [Google Scholar] [CrossRef]
- Talib, H.M.; Yousif, A.A.; Omran Alkhayatt, A.H. Structural, surface morphology and novel gas sensing properties of chemically deposited CdS-PVA nanocomposites; Impact of thiourea content. Chem. Phys. Impact 2024, 8, 100529. [Google Scholar] [CrossRef]
- De San Luis, A.; Aguirreurreta, Z.; Pardo, L.M.; Perez-Marquez, A.; Maudes, J.; Murillo, N.; Paulis, M.; Leiza, J.R. PS/PMMA-CdSe/ZnS quantum dots hybrid nanofibers for VOCs sensors. Isr. J. Chem. 2018, 58, 1347–1355. [Google Scholar] [CrossRef]
- Korotcenkov, G. Chemical Sensors: Comprehensive Sensor Technologies. Vol. 6: Sensors Applications; Momentum Press: New York, NY, USA, 2011. [Google Scholar]
- Korotcenkov, G.; Ivanov, M.; Brinzari, V. II–VI semiconductor-based humidity sensors. In Handbook of II–VI Semiconductor-Based Sensors and Radiation Detectors. Vol. 3: Sensors, Biosensors and Radiation Detectors; Korotcenkov, G., Ed.; Springer: Cham, Switzerland, 2023; pp. 281–303. [Google Scholar]
- Hsueh, H.-T.; Hsiao, Y.-J.; Lin, Y.-D.; Wu, C.-L. Bifacial structures of ZnS humidity sensor and C d-free CIGS photovoltaic cell as a self-powered device. IEEE Electron Dev. Lett. 2014, 35, 1272–1274. [Google Scholar] [CrossRef]
- Okur, S.; Uzar, N.; Tekguzel, N.; Erol, A.; Arıkan, M.C. Synthesis and humidity sensing analysis of ZnS nanowires. Phys. E Low-Dimens. Syst. Nanostructures 2012, 44, 1103–1107. [Google Scholar] [CrossRef]
- Fang, X.; Zhai, T.; Gautam, U.K.; Li, L.; Wua, L.; Bando, Y.; Golberg, D. ZnS nanostructures: From synthesis to applications. Prog. Mater. Sci. 2011, 56, 175–287. [Google Scholar] [CrossRef]
- Choudhari, U.; Jagtap, S. Hydrothermally synthesized ZnSe nanoparticles for relative humidity sensing application. J. Electron. Mater. 2020, 49, 5903–5916. [Google Scholar] [CrossRef]
- Dimitrov, R.I.; Moldovanska, N.; Bonev, I.K. Cadmium sulphide oxidation. Thermochim. Acta 2002, 385, 41–49. [Google Scholar] [CrossRef]
- Dimitrov, R.I.; Boyanov, B.S. Oxidation of metal sulphides and determination of characteristic temperatures by DTA and TG. J. Therm. Anal. Calorim. 2000, 61, 181–189. [Google Scholar] [CrossRef]
- Korotcenkov, G. II–VI wide-bandgap semiconductor device technology: Stability and oxidation. In Handbook of II–VI Semiconductor-Based Sensors and Radiation Detectors. Vol. 1: Materials and Technologies; Korotcenkov, G., Ed.; Springer: Cham, Switzerland, 2023; pp. 517–550. [Google Scholar] [CrossRef]
- Singh, B.P.; Singh, V.; Tyagi, R.C.; Sharma, T.P. Effect of ambient hydrogen sulfide on the physical properties of vacuum evaporated thin films of zinc sulfide. Appl. Surf. Sci. 2008, 254, 2233–2237. [Google Scholar] [CrossRef]
- Dengo, N.; De Fazio, A.F.; Weiss, M.; Marschall, R.; Dolcet, P.; Fanetti, M.; Gross, S. Thermal evolution of ZnS nanostructures: Effect of oxidation phenomena on structural features and photocatalytical performances. Inorg. Chem. 2018, 57, 13104–13114. [Google Scholar] [CrossRef] [PubMed]
- Maticiuc, N.; Kukk, M.; Spalatu, N.; Potlog, T.; Krunks, M.; Valdna, V.; Hiie, J. Comparative study of CdS films annealed in neutral, oxidizing and reducing atmospheres. Energy Procedia 2014, 44, 77–84. [Google Scholar] [CrossRef]
- Trenczek-Zajaca, A. Thermally oxidized CdS as a photoactive material. New J. Chem. 2019, 43, 8892–8902. [Google Scholar] [CrossRef]
- Ebina, A.; Takahashi, T. Studies of clean and adatom treated surfaces of II–VI compounds. J. Cryst. Growth 1982, 59, 51–64. [Google Scholar] [CrossRef]
- Khlopochkina, E.L.; Gaivoronskii, P.E.; Gavrishchuk, E.M.; Elliev, Y.E.; Yashina, E.V. Oxidation of polycrystalline zinc selenide with atmospheric oxygen. Russ. J. Appl. Chem. 2001, 74, 1079–1081. [Google Scholar] [CrossRef]
- Shanmugam, N.; Cholan, S.; Kannadasan, N.; Sathishkumar, K.; Viruthagiri, G. Effect of annealing on the ZnS nanocrystals prepared by chemical precipitation method. J. Nanomater. 2013, 2013, 351798. [Google Scholar] [CrossRef]
- Schultze, A.; Steinike, U.; Kussin, J.; Kretzschma, U. Thermal oxidation of ZnS modifications sphalerite and wurtzite. Cryst. Res. Technol. 1995, 30, 553–558. [Google Scholar] [CrossRef]
- Sasaoka, E.; Hatori, M.; Sada, N.; Uddin, M.A. Role of H2O in oxidative regeneration of ZnS formed from high-temperature desulfurization ZnO sorbent. Ind. Eng. Chem. Res. 2000, 39, 3844–3848. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, L.; Zu, B.; Guo, Y.; Xu, T.; Dou, X. CdS/ZnO core/shell nanowire-built films for enhanced photodetecting and optoelectronic gas-sensing applications. Adv. Opt. Mater. 2014, 2, 738–745. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, D. Oxidation of CdTe thin film in air coated with and without a CdCl2 layer. Phys. Status Solidi A 2012, 209, 1982–1987. [Google Scholar] [CrossRef]
- Ebina, A.; Asano, K.; Suda, Y.; Takahaski, T. Oxidation properties of II–VI compound surfaces studied by low-energy electron-loss spectroscopy and 21 eV photoemission spectroscopy. J. Vac. Sci. Technol. 1980, 17, 1074–1079. [Google Scholar] [CrossRef]
- Ebina, A.; Asano, K.; Takahaski, T. Surface properties of clean, and with adsorbed oxygen, surfaces of CdTe (110), (111), and (100) and of CdSe (0001) studied by electron-energy-loss spectroscopy and Auger-electron spectroscopy. Phys. Rev. B 1980, 22, 1980–1991. [Google Scholar] [CrossRef]
- Eom, N.S.A.; Kim, T.-S.; Choa, Y.-H.; Kim, W.-B.; Kim, B.S. Surface oxidation behaviors of Cd-rich CdSe quantum dot phosphors at high temperature. J. Nanosci. Nanotechnol. 2014, 14, 8024–8027. [Google Scholar] [CrossRef] [PubMed]
- Tikhonova, E.L.; Gaivoronskii, P.E.; Elliev, Y.E.; Gavrischuk, E.M.; Mazavin, S.M.; Yashina, E.V. Influence of conditions of zinc selenide oxidation with atmospheric oxygen on the composition of volatile products. Russ. J. Appl. Chem. 2003, 76, 1724–1727. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Zou, J.; Liu, G.; Lu, H.F.; Feng, L.F.; Lu, G.-Q.; Cheng, H.M. Silicon-induced oriented ZnS nanobelts for hydrogen sensitivity. Nanotechnology 2008, 19, 055710. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-L.; Ji, H.-M.; Wang, H.-L.; Sun, J.; Du, X.-W. Synthesis and sensing properties of ZnO/ZnS nanocages. Nanoscale Res. Lett. 2010, 5, 644. [Google Scholar] [CrossRef]
- Heiba, Z.K. X-ray quantitative analysis of the phases developed upon air annealing of ZnSe, CdSe, and CdS semiconductors. Powder Diffr. 2002, 17, 191–195. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Schwank, J.; Cerneavschi, A. Possibilities of aerosol technology for deposition of SnO2-based films with improved gas sensing characteristics. Mater. Sci. Eng. C 2002, 19, 73–77. [Google Scholar] [CrossRef]
- Kulwick, B.M. Humidity sensors. J. Am. Ceram. Soc. 1991, 74, 697–708. [Google Scholar] [CrossRef]
- Joshi, S.S.; Gujar, T.P.; Shinde, V.R.; Lokhande, C.D. Fabrication of n-CdTe/p-polyaniline heterojunction-based room temperature LPG sensor. Sens. Actuators B Chem. 2008, 132, 349–355. [Google Scholar] [CrossRef]
- BMiremadi, K.; Colbow, K.; Harima, Y. CdS photoconductivity gas sensor as an analytical tool for detection and analysis of hazardous gases in the environment. Rev. Sci. Instrum. 1997, 68, 3898–3903. [Google Scholar] [CrossRef]
- Saroch, M.; Srivastava, S.; Fink, D.; Chandra, A. Room temperature ammonia gas sensing using mixed conductor based TEMPOS structures. Sensors 2008, 8, 6355–6370. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.G.; Feng, P.; Xue, X.Y.; Shi, S.L.; Fu, X.Q.; Wang, C.; Wang, Y.G.; Wang, T.H. Room-temperature oxygen sensitivity of ZnS nanobelts. Appl. Phys. Lett. 2007, 90, 042109. [Google Scholar] [CrossRef]
- Chakraborty, S.; Pal, M. Improved ethanol sensing behaviour of cadmium sulphide nanoflakes: Beneficial effect of morphology. Sens. Actuators B Chem. 2017, 242, 1155–1164. [Google Scholar] [CrossRef]
- Saxena, N.; Kumar, P.; Gupta, V. CdS nanodroplets over silica microballs for efficient room-temperature LPG detection. Nanoscale Adv. 2019, 1, 2382–2391. [Google Scholar] [CrossRef] [PubMed]
- Nemade, K.R.; Waghuley, S.A. Ultra-violet C absorption and LPG sensing study of zinc sulphide nanoparticles deposited by a flame-assisted spray pyrolysis method. J. Taibah Univ. Sci. 2016, 10, 437–441. [Google Scholar] [CrossRef]
- Hu, L.; Wu, H. Influence of size and surface state emission on photoluminescence of CdSe quantum dots under UV irradiation. J. Lumin. 2016, 177, 307–313. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Nazzal, A.; Xiao, M. Photo-oxidation-enhanced coupling in densely packed CdSe quantum-dot films. Appl. Phys. Lett. 2003, 83, 162–164. [Google Scholar] [CrossRef]
- Kumaki, D.; Umeda, T.; Tokito, S. Influence of H2O and O2 on threshold voltage shift in organic thin-film transistors: Deprotonation of SiOH on SiO2 gate-insulator surface. Appl. Phys. Lett. 2008, 92, 093309. [Google Scholar] [CrossRef]
- Yi, X.; Liu, J.J. Surface oxidation of polycrystalline cadmium telluride thin films for Schottky barriers junction solar cells. Solid-State Electron. 1995, 38, 1151–1154. [Google Scholar] [CrossRef]
- Zázvorka, J.; Franc, J.; Statelov, M.; Pekarek, J.; Veis, M.; Moravec, P.; Masek, K. Optical and electrical study of CdZnTe surfaces passivated by KOH and NH4F solutions. Appl. Surf. Sci. 2016, 389, 1214–1219. [Google Scholar] [CrossRef]
- Alfadhili, F.K.; Phillips, A.B.; Subedi, K.K.; Perkins, C.L.; Halaoui, A.I.; Jamarkattel, M.K.; Anwar, B.M.; Liyanage, G.K.; Li, D.-B.; Grice, C.R.; et al. Back-surface passivation of CdTe solar cells using solution-processed oxidized aluminum. ACS Appl. Mater. Interfaces 2020, 12, 51337–51343. [Google Scholar] [CrossRef] [PubMed]
- Budhraja, V. Effect of SiO2 and SiNX passivation on CdTe based solar cells. Intern. J. Eng. Res. Technol. 2015, 4, 10–12. [Google Scholar] [CrossRef]
- Kephart, J.M.; Kindvall, A.; Williams, D.; Kuciauskas, D.; Dippo, P.; Munshi, A.; Sampath, W.S. Sputter-deposited oxides for interface passivation of CdTe photovoltaics. IEEE J. Photovolt. 2018, 8, 587–593. [Google Scholar] [CrossRef]
- Wu, L.; Li, Z.; Wang, D.; Lei, X.; Cai, Y.; Zeng, H.; Zhu, P.; Wang, D. CdTe surface passivation by electric field induced at the metal-oxide/CdTe interface. Sol. Energy 2021, 225, 83–90. [Google Scholar] [CrossRef]
- Korotcenkov, G. II–VI wide-bandgap semiconductor device technology: Deposition, doping, and etching. In Handbook of II–VI Semiconductor-Based Sensors and Radiation Detectors. Vol. 1: Materials and Technologies; Springer: Cham, Switzerland, 2023; pp. 465–490. [Google Scholar] [CrossRef]
- Tamura, A.; Mita, K.; Tanaka, A.; Ito, M. Mechanism of hydroxylation of metal oxide surfaces. J. Colloid Interface Sci. 2001, 243, 202–207. [Google Scholar] [CrossRef]
- Hellgren, N.; Steves, M.A.; Shallenberger, J.; O’Boyle, S.K.; Mellott, E.; Noble, A.R. Effect of etching on the oxidation of zinc selenide surfaces characterized by X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2020, 528, 146604. [Google Scholar] [CrossRef]
- Fukatsu, N. Phase equilibria in the system Cd-S-O and the stability region of a new phase 2CdS·2CdO·CdSO4. J. Electrochem. Soc. 1988, 135, 997–1003. [Google Scholar] [CrossRef]
- Sanap, V.B.; Pawar, B.H. Optical study of effect of cadmium sources on nanocrystalline CdS thin films. Chalcogenide Lett. 2010, 7, 227–231. [Google Scholar]
- Tomashyk, V.; Feychuk, P.; Shcherbak, L. Ternary Alloys Based on II–VI Semiconductor Compounds; Taylor and Francis Group: Boca Raton, FL, USA, 2013. [Google Scholar] [CrossRef]
- Soleimanian, V.; Saeedi, M.; Mokhtari, A. The influence of heat treatment on the crystallite size, dislocation density, stacking faults probability and optical band gap of nanostructured cadmium sulfide films. Mater. Sci. Semicond. Process. 2015, 30, 118–127. [Google Scholar] [CrossRef]
- Banerji, K.K. Kinetics and mechanisms of oxidation of primary alcohols by bromine. Indian J. Chem. 1973, 11, 244–245. [Google Scholar]
- Jasieniak, J.; Mulvaney, P. From Cd-Rich to Se-Rich: The manipulation of CdSe nanocrystal surface stoichiometry. J. Am. Chem. Soc. 2007, 129, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Dharmadasa, I.M.; Blomfield, C.J.; Gregory, G.E.; Young, J. Influence of chemical etching on metal contacts to II–VI compounds: CdTe and ZnSe. Intern. J. Electron. 1994, 76, 961–967. [Google Scholar] [CrossRef]
- Reese, M.O.; Perkins, C.L.; Burst, J.M.; Farrell, S.; Barnes, T.M.; Johnston, S.W.; Kuciauskas, D.; Gessert, T.A.; Metzger, W.K. Intrinsic surface passivation of CdTe. J. Appl. Phys. 2015, 118, 155305. [Google Scholar] [CrossRef]
- Dharmadasa, I.M.; Thorton, J.H.; Williams, R.H. Effects of surface treatments on Schottky barrier formation at metal n-type CdTe contacts. Appl. Phys. Lett. 1989, 54, 137–139. [Google Scholar] [CrossRef]
- Dharmadasa, I.M.; McLean, A.B.; Patterson, M.H.; Williams, R.H. Schottky barriers and interfaces on chemically etched n-CdTe single crystals. Semicond. Sci. Technol. 1987, 2, 404–412. [Google Scholar] [CrossRef]





| Semiconductor | Eg (eV) | Tmelting (°C) | Tsublimation (°C) | Toxidation (°C) |
|---|---|---|---|---|
| ZnS | 3.54–3.91 | 1827–1850 | 1178 | <500 |
| ZnSe | 2.7–2.721 | 1525 | 800–900 | <300 |
| ZnTe | 2.25–2.27 | 1238–1290 | ~600 | <300 |
| CdS | 2.42–2.46 | 1475–1750 | 980 | <450–500 |
| CdSe | 1.74–1.751 | 1268 | 600–700 | <300 |
| CdTe | 1.49–1.51 | 1092 | ~500 | <300 |
| Parameter | Material (Zn1−xCdxS) | |||||
|---|---|---|---|---|---|---|
| X = 0 | X = 0.2 | X = 0.4 | X = 0.6 | X = 0.8 | X = 1.0 | |
| Response (Ra/Rg) | 2.1 | 7.5 | 12.8 | 11.3 | 9.8 | 6.6 |
| Rise time (s) | 6 | 1 | 2 | 2 | <1 | <1 |
| Recovery time (s) | 8 | 1 | 1 | 1 | 1 | <1 |
| Sample Treatment | Cd/Te |
|---|---|
| As-received | 0.59 |
| Br2:MeOH | 0.73 |
| 3 kV Ar+ | 1.08 |
| Atomic H | 1.10 |
| Ar+, 250 °C UHV | 1.09 |
| Cd ampoule anneal | 1.29 |
| Te ampoule anneal | 1.05 |
| UV/O3 | 1.81 |
| NaOH:Na2S2O4 | 1.35 |
| KOH | 1.12 |
| Hydrazine | 0.86 |
| CH3COOH:HNO3:H2SO4 | 0.004 |
| FeCl3 | 0.03 |
| HNO3:K2Cr2O7:Cu(NO3)2 | 0.08 |
| HNO3:K2Cr2O7 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korotcenkov, G. II–VI Semiconductor-Based Conductometric Gas Sensors: Is There a Future for These Sensors? Sensors 2024, 24, 3861. https://doi.org/10.3390/s24123861
Korotcenkov G. II–VI Semiconductor-Based Conductometric Gas Sensors: Is There a Future for These Sensors? Sensors. 2024; 24(12):3861. https://doi.org/10.3390/s24123861
Chicago/Turabian StyleKorotcenkov, Ghenadii. 2024. "II–VI Semiconductor-Based Conductometric Gas Sensors: Is There a Future for These Sensors?" Sensors 24, no. 12: 3861. https://doi.org/10.3390/s24123861
APA StyleKorotcenkov, G. (2024). II–VI Semiconductor-Based Conductometric Gas Sensors: Is There a Future for These Sensors? Sensors, 24(12), 3861. https://doi.org/10.3390/s24123861






