Postural Sway Velocity of Deaf Children with and without Vestibular Dysfunction
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Sample
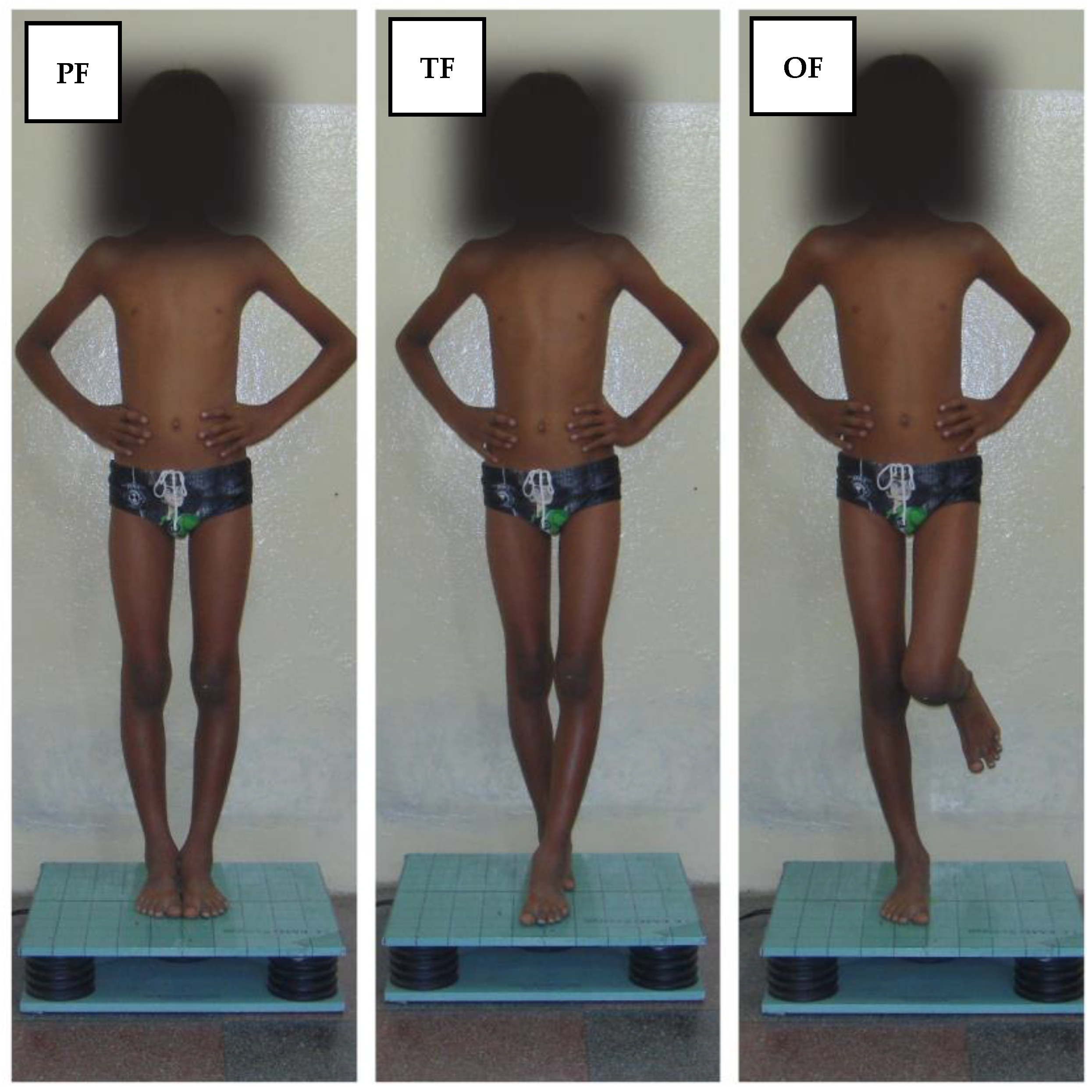
2.2. Assessment of the Postural Sway Velocity
2.3. Procedures
2.4. Hearing Assessment
2.5. Vestibular End-Organ Assessment
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plata, A.E. Understanding the roles of vision in the control of human locomotion. Gait Posture 1997, 5, 54–69. [Google Scholar] [CrossRef]
- Adkin, A.L.; Frank, J.S.; Carpenter, M.G.; Peysar, G.W. Postural control is scaled to level of postural threat. Gait Posture 2000, 12, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Nashner, L.M.; Black, F.O.; Wall, C. Adaptation to altered support and visual conditions during stance: Patients with vestibular déficits. J. Neurosci. 1982, 2, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Laufer, Y.; Ashkenazi, T.; Josman, N. The effects of a concurrent cognitive task on the postural control of young children with and without developmental coordination disorder. Gait Posture 2008, 27, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, B.; Culham, E.G.; Liston, R.A.; Grant, L. Normal variability of postural measures: Implications for the reliability of relative balance performance outcomes. Scand. J. Rehabil. Med. 1998, 30, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Pollock, A.S.; Durward, B.R.; Rowe, P.J.; Paul, J.P. What is balance? Clin. Rehabil. 2000, 14, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, R.A.; Pourmoghaddam, A.; Paloski, W.H. Sensorimotor posture control in the blind: Superior ankle proprioceptive acuity does not compensate for vision loss. Gait Posture 2013, 38, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Parreira, R.B.; Grecco, L.A.C.; Oliveira, C.S. Postural control in blind individuals: A systematic review. Gait Posture 2017, 57, 161–167. [Google Scholar] [CrossRef]
- Melo, R.S.; Lemos, A.; Raposo, M.C.F.; Monteiro, M.G.; Lambertz, D.; Ferraz, K.M. Repercussions of the hearing loss degrees and vestibular dysfunction on the static balance of children with sensorineural hearing loss. Phys. Ther. 2021, 101, pzab177. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, R.K.; Kumar, P. Vestibular evoked myogenic potentials in children with sensorineural hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 1308–1311. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; Cresswell, A.G.; Daggfeldt, K.; Thorstensson, A. Three dimensional preparatory trunk motion precedes asymmetrical upper limb movement. Gait Posture 2000, 11, 92–101. [Google Scholar] [CrossRef] [PubMed]
- De Kegel, A.; Dhooge, I.; Cambier, D.; Baetens, T.; Palmans, T.; Van Waelvelde, H. Test-retest reliability of the assessment of postural stability in typically developing children and in hearing impaired children. Gait Posture 2011, 33, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Rine, R.M.; Cornwall, G.; Gan, K.; LoCascio, C.; O’Hare, T.; Robinson, E.; Rice, M. Evidence of progressive delay of motor development in children with sensorineural hearing loss and concurrent vestibular dysfunction. Percept. Mot. Skills. 2000, 90, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Rine, R.M.; Braswell, J.; Fisher, D.; Joyce, K.; Kalar, K.; Shaffer, M. Improvement of motor development and postural control following intervention in children with sensorineural hearing loss and vestibular impairment. Int. J. Pediatr. Otorhinolaryngol. 2004, 68, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Huygen, P.L.; van Rijn, P.M.; Cremers, C.W.; Theunissen, E.J. The vestíbulo-ocular reflex in pupils at a Dutch school for the hearing impaired; findings relating to acquired causes. Int. J. Pediatr. Otorhinolaryngol. 1993, 25, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Jacot, E.; Van Den Abbeele, T.; Debre, H.R.; Wiener-Vacher, S.R. Vestibular impairments pre-and post-cochlear implant in children. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Schwab, B.; Kontorinis, G. Influencing factors on the vestibular function of deaf children and adolescents—Evaluation by means of dynamic posturography. Open Otorhinolaryngol. J. 2011, 5, 1–9. [Google Scholar] [CrossRef]
- Maes, L.; De Kegel, A.; Van Waelvelde, H.; Dhooge, I. Rotatory and collic vestibular evoked myogenic potential testing in normal-hearing and hearing-impaired children. Ear Hear. 2014, 35, 21–32. [Google Scholar] [CrossRef]
- Said, E.A.F. Vestibular assessment in children with sensorineural hearing loss using both electronystagmography and vestibular-evoked myogenic potential. Egypt. J. Otolaryngol. 2014, 30, 43–52. [Google Scholar] [CrossRef]
- Kotait, M.A.; Moaty, A.S.; Gabr, T.A. Vestibular testing in children with severe-to-profound hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2019, 125, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Kaga, K. Vestibular compensation in infants and children with congenital and acquired vestibular loss in both ears. Int. J. Pediatr. Otorhinolaryngol. 1999, 49, 215–224. [Google Scholar] [CrossRef]
- Kastanioudakis, J.; Skevas, A.; Assimakopoulos, D.; Anastasopoulos, D. Hearing loss and vestibular dysfunction in childhood from the use of streptomycin in Albania. Int. J. Pediatr. Otorhinolaryngol. 1993, 26, 109–115. [Google Scholar] [CrossRef]
- Gedik-Soyuyuce, O.; Gence-Gumus, Z.; Ozdilek, A.; Ada, M.; Korkut, N. Vestibular disorders in children: A retrospective analysis of vestibular function test findings. Int. J. Pediatr. Otorhinolaryngol. 2021, 146, 110751. [Google Scholar] [CrossRef] [PubMed]
- Elander, J.; Ullmark, T.; Ehrencrona, H.; Jonson, T.; Piccinelli, P.; Samuelsson, S.; Värendh, M. Extended genetic diagnostics for children with profound sensorineural hearing loss by implementing massive parallel sequencing. Diagnostic outcome, family experience and clinical implementation. Int. J. Pediatr. Otorhinolaryngol. 2022, 159, 111218. [Google Scholar] [CrossRef] [PubMed]
- Gadsboll, E.; Erbs, A.W.; Hougaard, D.D. Prevalence of abnormal vestibular responses in children with sensorineural hearing loss. Eur. Arch. Otorhinolaryngol. 2022, 279, 4695–4707. [Google Scholar] [CrossRef]
- An, M.H.; Yi, C.H.; Jeon, H.S.; Park, S.Y. Age-related changes of single-limb standing balance in children with and without deafness. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1539–1544. [Google Scholar] [CrossRef]
- Soylemez, E.; Ertugrul, S.; Dogan, E. Assessment of balance skills and falling risk in children with congenital bilateral profound sensorineural hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2019, 116, 75–78. [Google Scholar] [CrossRef]
- Karakoc, K.; Mujdeci, B. Evaluation of balance in children with sensorineural hearing loss according to age. Am. J. Otolaryngol. 2021, 42, 102830. [Google Scholar] [CrossRef]
- Ebrahimi, A.A.; Movallali, G.; Jamshidi, A.A.; Rahgozar, M. Postural control in deaf children. Acta Med. Iran. 2017, 55, 115–122. [Google Scholar]
- Suarez, H.; Angeli, S.; Suarez, A.; Rosales, B.; Carrera, X.; Alonso, R. Balance sensory organization in children with profound hearing loss and cochlear implants. Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.M.M.; Barros, J.F.; Sousa-Neto, B.M. Postural control in children with typical development and children with profound hearing loss. Int. J. Gen. Med. 2012, 5, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, L.S.; Mu, K. Computerized platform posturography for children: Test-retest reliability of the sensory test of the VSR system. Phys. Occup. Ther. Pediatr. 2022, 22, 101–117. [Google Scholar] [CrossRef]
- Duarte, M.; Freitas, S.M.S.F. Revision of posturography based on force plate for balance evaluation. Braz. J. Phys. Ther. 2010, 14, 183–192. [Google Scholar] [CrossRef]
- Bartlett, H.L.; Ting, L.H.; Bingham, J.T. Accuracy of force and center of pressure measures of the Wii Balance Board. Gait Posture 2014, 39, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Mentiplay, B.F.; Pua, Y.H.; Bower, K.J. Reliability and validity of the Wii Balance Board for assessment of standing balance: A systematic review. Gait Posture 2018, 61, 40–54. [Google Scholar] [CrossRef]
- De Kegel, A.; Dhooge, I.; Peersman, W.; Rijckaert, J.; Baetens, T.; Cambier, D.; Van Waelvelde, H. Construct validity of the assessment of balance in children who are developing typically and children with hearing impairments. Phys. Ther. 2010, 90, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.S.; Lemos, A.; Raposo, M.C.F.; Belian, R.B.; Ferraz, K.M. Balance performance of children and adolescents with sensorineural hearing loss: Repercussions of hearing loss degrees and etiological factors. Int. J. Pediatr. Otorhinolaryngol. 2018, 110, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Banerjee, S.; Biswas, R. A study on the dynamic balance of schoolchildren in India with varying degrees of hearing impairments. J. Phys. Educ. Sport. 2022, 22, 1177–1189. [Google Scholar] [CrossRef]
- Chilosi, A.M.; Comparini, A.; Scusa, M.F.; Berrettini, S.; Forli, F.; Battini, R.; Cioni, G. Neurodevelopmental disorders in children with severe to profound sensorineural hearing loss: A clinical study. Dev. Med. Child. Neurol. 2010, 52, 856–862. [Google Scholar] [CrossRef]
- Lisboa, T.R.; Jurkiewicz, A.L.; Zeigelboim, B.S.; Martins-Bassetto, J.; Klagenberg, K.F. Vestibular findings in children with hearing loss. Int. Arch. Otorhinolaryngol. 2005, 9, 271–279. [Google Scholar]
- Zhou, G.; Kenna, M.A.; Stevens, K.; Licameli, G. Assessment of saccular function in children with sensorineural hearing loss. Arch. Otolaryngol. Head. Neck Surg. 2009, 135, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; Dhooge, I.; Dhondt, C.; Vanaudenaerde, S.; Sucaet, M.; Rombaut, L.; Maes, L. Vestibular infant screening (VIS)—Flanders: Results after 1.5 years of vestibular screening in hearing-impaired children. Sci. Rep. 2020, 10, 21011. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.A.E.; Metwally, S.A.A.; Tantawy, M.A.; Hussieny, N.L.; Ibrahim, I.T.H.; Hegazy, M.T.A.; Abdelaziz, N.N. Cervical vestibular evoked myogenic potential (Cvemp) in children with sensorineural hearing loss. Egypt. J. Hosp. Med. 2022, 89, 7125–7131. [Google Scholar] [CrossRef]
- Martens, S.; Dhooge, I.; Dhondt, C.; Vanaudenaerde, S.; Sucaet, M.; Van Hoecke, H.; Maes, L. Three years of vestibular infant screening in infants with sensorineural hearing loss. Pediatrics 2022, 150, e2021055340. [Google Scholar] [CrossRef] [PubMed]
- Cushing, S.L.; Papsin, B.C.; Rutka, J.A.; James, L.A.; Gordon, K.A. Evidence of vestibular and balance dysfunction in children with profound sensorineural hearing loss. Laryngoscope 2008, 118, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Jafari, Z.; Asad-Malayeri, S. The effect of saccular function on static balance ability of profound hearing-impaired children. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 919–924. [Google Scholar] [CrossRef]
- Wolter, N.E.; Gordon, K.A.; Papsin, B.C.; Cushing, S.L. Vestibular and balance impairment contributes to cochlear implant failure in children. Otol. Neurotol. 2015, 36, 1029–1034. [Google Scholar] [CrossRef]
- Apeksha, K.; Singh, S.; Rathnamala, M.; Varalakshmi, S.; Preethu, D.J.; Kavya, V.; Thejasvi, M.A. Balance assessment of children with sensorineural hearing loss. Indian. J. Otolaryngology Head. Neck Surg. 2021, 73, 12–17. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Woollacott, M.H. The growth of stability: Postural control from a development perspective. J. Mot. Behav. 1985, 17, 131–147. [Google Scholar] [CrossRef]
- Melo, R.S.; Freire, M.E.A.; Santos, S.E.; Damasceno, H.A.M.; Raposo, M.C.F. Static balance in students with normal hearing and with sensorineural hearing loss. Rev. Neurocienc. 2015, 23, 241–247. [Google Scholar] [CrossRef]
- Open Source Epidemiologic Statistics for Public Health. Available online: www.openepi.com (accessed on 29 August 2023).
- Verbecque, E.; Vereeck, L.; Hallemans, A. Postural sway in children: A literature review. Gait Posture 2016, 49, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Verbecque, E.; Costa, P.H.L.; Meyns, P.; Desloovere, K.; Vereeck, L.; Hallemans, A. Age-related changes in postural sway in preschoolers. Gait Posture 2016, 44, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Sember, V.; Groselj, J.; Pajek, M. Balance tests in pre-adolescent children: Retest reliability, construct validity, and relative ability. Int. J. Environ. Res. Public. Health 2020, 17, 5474. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, A.W.; Armitano-Lago, C.N.; Cone, B.L.; Bonnette, S.; Rhea, C.K.; Cummins-Sebree, S.; Riley, M.A. Postural control development from late childhood through young adulthood. Gait Posture 2021, 86, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Sonobe, M.; Inoue, Y. Center of mass estimation using a force platform and inertial sensors for balance evaluation in quiet standing. Sensors 2023, 23, 4933. [Google Scholar] [CrossRef] [PubMed]
- British Society of Audiology Recommendation. Descriptors for puretone audiograms. Br. J. Audiol. 1988, 22, 123. [Google Scholar] [CrossRef]
- Maes, L.; Dhooge, I.; De Vel, E.; D’haenens, W.; Bockstael, A.; Kepples, H.; Vinck, B.M. Normative data and test-retest reliability of the sinusoidal harmonic acceleration test, pseudorandom rotation test and velocity step test. J. Vestib. Res. 2008, 18, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Albertino, S.; Bittar, R.S.M.; Bottino, M.A.; Ganança, M.M.; Gonçalves, D.U.; Greters, M.E.; Ganança, F.F. Air caloric test reference values. Braz. J. Otorhinolayngol. 2012, 78, 2. [Google Scholar] [CrossRef]
- Verrechia, L.; Barret, K.G.; Karltorp, E. The feasibility, validity and reliability of a child friendly vestibular assessment in infants and children candidates to cochlear implant. Int. J. Pediatr. Otorhinolaryngol. 2020, 135, 110093. [Google Scholar] [CrossRef]
- Singh, A.; Heet, H.; Guggenheim, D.S.; Lim, M.; Garg, B.; Bao, M.; Riska, K.M. A systematic review on the association between vestibular dysfunction and balance performance in children with hearing loss. Ear Hear. 2022, 43, 712–721. [Google Scholar] [CrossRef]
- Shen, M.; Xue, S.; Wei, X.; Chen, B.; Kong, Y.; Li, Y. Characteristics of vestibular-evoked myogenic potentials in children with vestibular malformation and severe sensorineural hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2024, 176, 111781. [Google Scholar] [CrossRef] [PubMed]
- Derlich, M.; Krecisz, K.; Kuczynski, M. Attention demand and postural control in children with hearing deficit. Res. Dev. Disabil. 2011, 32, 1808–1813. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.S.; Lemos, A.; Macky, C.F.S.T.; Raposo, M.C.F.; Ferraz, K.M. Postural control assessment in students with normal hearing and sensorineural hearing loss. Braz. J. Otorhinolaryngol. 2015, 81, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Mehrem, E.; Khaireldin, A.; Essa, M.; Allah, M.G.; Lobbos, B.; Kamel, R. Sensorineural hearing loss imprint on postural control: A pediatric and adolescent innovative study. NeuroRehabilitation 2023, 53, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Wolter, N.E.; Cushing, S.L.; Vilchez-Madrigal, L.D.; James, A.L.; Campos, J.; Papsin, B.C.; Gordon, K.A. Unilateral hearing loss is associated with impaired balance in children: A pilot study. Otol. Neurotol. 2016, 37, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Zarei, H.; Norasteh, A.A.; Lieberman, L.J.; Ertel, M.W.; Brian, A. Balance control in individuals with hearing impairment: A systematic review and meta-analysis. Audiol. Neurootol. 2024, 29, 30–48. [Google Scholar] [CrossRef] [PubMed]
- Easton, R.D.; Greene, A.J.; DiZio, P.; Lackner, J.R. Auditory cues for orientation and postural control in sighted and congenitally blind people. Exp. Brain Res. 1998, 118, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Yost, W.A. Relationship between postural stability and spatial hearing. J. Am. Acad. Audiol. 2013, 24, 782–788. [Google Scholar] [CrossRef]
- Gandemer, L.; Parseihian, G.; Kronland-Martinet, R.; Bourdin, C. Spatial cues provided by sound improve postural stabilization: Evidence of a spatial auditory map? Front. Neurosci. 2017, 11, 357. [Google Scholar] [CrossRef]
- Karim, A.M.; Rumalla, K.; King, L.A.; Hullar, T.E. The effect of spatial auditory landmarks on ambulation. Gait Posture 2018, 60, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Cushing, S.L.; Chia, R.; James, A.L.; Papsin, B.C.; Gordon, K.A. A test of static and dynamic balance function in children with cochlear implants: The vestibular Olympics. Arch. Otolaryngol. Head. Neck Surg. 2008, 134, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Cushing, S.L.; Pothier, D.; Hughes, C.; Hubbard, B.J.; Gordon, K.A.; Papsin, B.C. Providing auditory cues to improve stability in children who are deaf. Laryngoscope 2012, 122, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Mazaheryazdi, M.; Moossavi, A.; Sarrafzadah, J.; Talebian, S.; Jalaie, S. Study of the effects of hearing on static and dynamic postural function in children using cochlear implants. Int. J. Pediatr. Otorhinolaryngol. 2017, 100, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Wolter, N.E.; Gordon, K.A.; Campos, J.; Madrigal, L.D.V.; Papsin, B.C.; Cushing, S.L. Impact of the sensory environment on balance in children with bilateral cochleovestibular loss. Hear. Res. 2021, 400, 108134. [Google Scholar] [CrossRef] [PubMed]
- Hamzehpour, F.; Absalan, A.; Pirasteh, E.; Sharafi, Z.; Arbabsarjoo, H. Investigating the effect of hearing aid use on the balance status of children with severe to profound congenital hearing loss using the pediatric clinical test of sensory interaction for balance. J. Am. Acad. Audiol. 2021, 32, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Zarei, H.; Norasteh, A.A.; King, L. The effect of auditory cues on static postural control: A systematic review and meta-analysis. Audiol. Neurotol. 2022, 27, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Suarez, H.; Alonso, R.; Arocena, S.; Ferreira, E.; Roman, C.S.; Suarez, A.; Lapilover, V. Sensorimotor interaction in deaf children. Relationship between gait performance and hearing input during childhood assessed in pre-lingual cochlear implant users. Acta Otolaryngol. 2017, 137, 346–351. [Google Scholar] [CrossRef]
- Horowitz, G.; Ungar, O.J.; Levit, Y.; Himmelfarb, M.; Handzel, O. The impact of condutive hearing loss on balance. Clin. Otolaryngol. 2020, 45, 106–110. [Google Scholar] [CrossRef]
- Siedlecka, B.; Sobrera, M.; Sikora, A.; Drzewowska, I. The influence of sounds on posture control. Acta Bioeng. Biomech. 2015, 17, 96–102. [Google Scholar]
- Mohammadi, M.; Enayati, Z.; Shaabani, M.; Vahedi, M. Stationary auditory White noise improves postural control in healthy adults: A novel study on head-shaking. J. Vestib. Res. 2022, 32, 99–112. [Google Scholar] [CrossRef]
- Morris, B.; Cosetti, M.; Kelly, J.; Yang, J.; Harel, D.; Medlin, A.; Lubetzky, A.V. Differing postural control patterns in individuals with bilateral and unilateral hearing loss. Am. J. Otolaryngol. 2023, 44, 103866. [Google Scholar] [CrossRef] [PubMed]
- Suarez, H.; Ferreira, E.; Arocena, S.; Pintos, B.G.; Quinteros, M.; Suarez, S.; Gonzalez, M.P. Motor and cognitive performances in pre-lingual cochlear implant adolescents, related with vestibular function and auditory input. Acta Otolaryngol. 2019, 139, 367–372. [Google Scholar] [CrossRef]
- Suarez, A.; Ferreira, E.; Pintos, B.G.; Arocena, S.; Suarez, H. Postural control characterization according to age auditory in cochlear implants users. Cochlear Implant Int. 2021, 22, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Lubetzky, A.V. Balance, falls, and hearing loss: Is it time for a paradigm shift? JAMA Otolaryngol. Head Neck Surg. 2020, 146, 535–536. [Google Scholar] [CrossRef]
- De Kegel, A.; Maes, L.; Baetens, T.; Dhooge, I.; Van Waelvelde, H. The influence of a vestibular dysfunction on the motor development of hearing-impaired children. Laryngoscope 2012, 122, 2837–2843. [Google Scholar] [CrossRef]
- Maes, L.; De Kegel, A.; Van Waelvelde, H.; Dhooge, I. Association between vestibular function and motor performance in hearing-impaired children. Otol. Neurotol. 2014, 35, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, M.; Gordon, K.A.; Polonenko, M.; Blaser, S.I.; Papsin, B.C.; Cushing, S.L. Vestibular and balance function is often impaired in children with profound unilateral sensorineural hearing loss. Hear. Res. 2019, 372, 52–61. [Google Scholar] [CrossRef]
- Ionescu, E.; Reynard, P.; Goulème, N.; Becaud, C.; Spruyt, K.; Ortega-Solis, J.; Thai-Van, H. How sacculo-collic function assessed by cervical vestibular evoked myogenic potentials correlates with the quality of postural control in hearing impaired children? Int. J. Pediatr. Otorhinolaryngol. 2020, 130, 109840. [Google Scholar] [CrossRef]
- Chisari, D.; Vitkovic, J.; Clark, R.; Rance, G. Vestibular function and balance performance in children with sensorineural hearing loss. Int. J. Audiol. 2023, 1–9. [Google Scholar] [CrossRef]
- Livingstone, N.; McPhillips, M. Motor skill deficits in children with partial hearing. Dev. Med. Child. Neurol. 2011, 53, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Jelsma, J.; Rogers, C. Motor proficiency and dynamic visual acuity in children with bilateral sensorineural hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Fellinger, M.J.; Holzinger, D.; Aigner, M.; Beitel, C.; Fellinger, J. Motor performance and correlates of mental health in children who are deaf or hard of hearing. Dev. Med. Child. Neurol. 2015, 57, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Peñeñory, V.M.; Manresa-Yee, C.; Riquelme, I.; Collazos, C.A.; Fardoun, H.M. Scoping review of systems to train psychomotor skills in hearing impaired children. Sensors 2018, 18, 2546. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.S.; Silva, P.W.A.; Tassitano, R.M.; Macky, C.F.S.T.; Silva, L.V.C. Balance and gait evaluation: Comparative study between deaf and hearing students. Rev. Paul. Pediatr. 2012, 30, 385–391. [Google Scholar] [CrossRef]
- Majlesi, M.; Azadian, E.; Farahpour, N.; Jafarnezhad, A.A.; Rashedi, H. Lower limb muscle activity during gait in individuals with hearing loss. Australas. Phys. Eng. Sci. Med. 2017, 40, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Jafarnezhadgero, A.A.; Majlesi, M.; Azadian, E. Gait ground reaction force characteristics in deaf and hearing children. Gait Posture 2017, 53, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.S. Gait performance of children and adolescents with sensorineural hearing loss. Gait Posture 2017, 57, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Hartman, E.; Houwen, S.; Visscher, C. Motor skill performance and sports participation in deaf elementary school children. Adapt. Phys. Act. Q. 2011, 28, 132–145. [Google Scholar] [CrossRef]
- Engel-Yeger, B.; Hamed-Daher, S. Comparing participation in out of school activities between children with visual impairments, children with hearing impairments and typical peers. Res. Dev. Disabil. 2013, 34, 3124–3132. [Google Scholar] [CrossRef]
- Melo, R.S.; Lemos, A.; Paiva, G.S.; Ithamar, L.; Lima, M.C.; Eickmann, S.H.; Belian, R.B. Vestibular rehabilitation exercises programs to improve the postural control, balance and gait of children with sensorineural hearing loss: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2019, 127, 109650. [Google Scholar] [CrossRef] [PubMed]
- Yigider, A.P.; Yilmaz, S.; Ulusoy, H.; Kara, T.; Kufeciler, L.; Kaya, K.H. Emotional and behavioral problems in children and adolescents with hearing loss and their effects on quality of life. Int. J. Pediatr. Otorhinolaryngol. 2020, 137, 110245. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, S.C.P.M.; Rieffe, C.; Kouwenberg, M.; Soede, W.; Briaire, J.J.; Frijns, J.H.M. Depression in hearing-impaired children. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Nakamura, M.; Shinjo, Y.; Kaga, L. Vestibular-evoked myogenic potentials in cochlear implant children. Acta Otolaryngol. 2006, 126, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Cushing, S.L.; Gordon, K.A.; Rutka, J.A.; James, A.L.; Papsin, B.C. Vestibular end-organ dysfunction in children with sensorineural hearing loss and cochlear implants: An expanded cohort and etiologic assessment. Otol. Neutotol. 2013, 34, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Thierry, B.; Blanchard, M.; Leboulanger, N.; Parodi, M.; Wiener-Vacher, S.R.; Garabedian, E.N.; Loundon, N. Cochlear implantation and vestibular function in children. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.D.; Zhang, X.T.; Zhang, Q.; Hu, J.; Chen, Y.F.; Xu, M. Ocular and cervical vestibular-evoked myogenic potential in children with cochlear implant. Clin. Neurophysiol. 2015, 126, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Kolkaila, E.A.; Helal, D.S.E.; El-Mahallawi, T.H.; El-Garib, A.M. Combined cervical and ocular vestibular evoked myogenic potentials in cochlear implanted children. Egypt. J. Ear Nose Throat Allied Sci. 2023, 24, 1–7. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Q.; Xiao, Q.; Zhang, Y.; Chen, Z.; Liu, S.; Zhang, Q. Vestibular dysfunction in pediatric patients with cochlear implantation: A systematic review and meta-analysis. Front. Neurol. 2022, 13, 996580. [Google Scholar] [CrossRef]
- Huang, M.W.; Hsu, C.J.; Kuan, C.C.; Chang, W.H. Static balance function in children with cochlear implants. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 700–703. [Google Scholar] [CrossRef]
- Oyewumi, M.; Wolter, N.E.; Heon, E.; Gordon, K.A.; Papsin, B.C.; Cushing, S.L. Using balance function to screen for vestibular impairment in children with sensorineural hearing loss and cochlear implants. Otol. Neurotol. 2016, 37, 926–932. [Google Scholar] [CrossRef]
- Kelly, A.; Liu, Z.; Leonard, S.; Toner, F.; Adams, M.; Toner, J. Balance in children following cochlear implantation. Cochlear Implant. Int. 2018, 19, 22–25. [Google Scholar] [CrossRef]
- Bayat, A.; Farhadi, M.; Emamdjomeh, H.; Nadimi, Z.; Mirmomeni, G.; Saki, N. Influence of cochlear implantation on balance function in pediatrics. Int. Tinnitus J. 2020, 24, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Mujdeci, B.; Onder, S.; Allusoglu, S.; Boynuergi, S.; Kum, O.; Atan, D. The effects of age at cochlear implantation on balance in children. a pilot study. Int. J. Artif. Organs. 2021, 44, 440–445. [Google Scholar] [CrossRef]
- Forli, F.; Giuntini, G.; Ciabotti, A.; Bruschini, L.; Lofkvist, U.; Berrettini, S. How does a bilingual environment affect the results in children with cochlear implants compared to monolingual-matched children? An Italian follow-up study. Int. J. Pediatr. Otorhinolaryngol. 2018, 105, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann-Muller, D.; Kuhn, H.; Matthies, C.; Hagen, R.; Shehata-Dieler, W. Outcomes after cochlear implant provision in children with cochlear nerve hypoplasia or aplasia. Int. J. Pediatr. Otorhinolaryngol. 2018, 112, 132–140. [Google Scholar] [CrossRef]
- Zeitler, D.M.; Sladen, D.P.; DeJong, M.D.; Torres, J.H.; Dorman, M.F.; Carlson, M.L. Cochlear implantation for single-sided deafness in children and adolescents. Int. J. Pediatr. Otorhinolaryngol. 2019, 118, 128–133. [Google Scholar] [CrossRef]
- Lee, Y.; Sim, H. Bilateral cochlear implantation versus unilateral cochlear implantation in deaf children: Effects of sentence context and listening conditions on recognition of spoken words in sentences. Int. J. Pediatr. Otorhinolaryngol. 2020, 137, 110237. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.D.; Cushing, S.L.; Papsin, B.C.; Gordon, K.A. Hearing and speech benefits of cochlear implantation in children: A review of the literature. Int. J. Pediatr. Otorhinolaryngol. 2020, 133, 109984. [Google Scholar] [CrossRef]
- Méndez, M.D.C.L.; Gacía, L.F.; González, M.T.D. Effectiveness of rhythmic training on linguistics skill development in deaf children and adolescents with cochlear implants: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2023, 169, 111561. [Google Scholar] [CrossRef]
- Rine, R.M. Vestibular rehabilitation for children. Semin. Hear. 2018, 39, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.C.; Silva, A.L.S. Pediatric vestibular rehabilitation: A case study. Pediatr. Phys. Ther. 2019, 31, e14–e19. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, Y.; Rezazadeh, N.; Moossavi, A.; Haghgoo, H.A.; Moghadam, S.F.; Pishyareh, E.; Khodabandelou, Y. Introduction of pediatric balance therapy in children with vestibular dysfunction: Review of indications, mechanisms, and key exercises. Iran. Rehabil. J. 2016, 14, 5–14. [Google Scholar] [CrossRef]
- Zhou, Y.; Qi, J. Effectiveness of interventions on improving balance in children and adolescentes with hearing impairment: A systematic review. Front. Physiol. 2022, 13, 876974. [Google Scholar] [CrossRef] [PubMed]
- Zarei, H.; Norasteh, A.A.; Lieberman, L.J.; Ertel, M.W.; Brian, A. The impacts of exercise training programs in balance in children with hearing loss: A systematic review and meta-analysis. J. Bodyw. Mov. Ther. 2024, 37, 296–307. [Google Scholar] [CrossRef]
- Melo, R.S.; Tavares-Netto, A.R.; Delgado, A.; Wiesiolek, C.C.; Ferraz, K.M.; Belian, R.B. Does the practice of sports or recreational activities improve the balance and gait of children and adolescents with sensorineural hearing loss? a systematic review. Gait Posture 2020, 77, 144–155. [Google Scholar] [CrossRef]
- Melo, R.S.; Lemos, A.; Delgado, A.; Raposo, M.C.F.; Ferraz, K.M.; Belian, R.B. Use of virtual reality-based games to improve balance and gait of children and adolescents with sensorineural hearing loss: A systematic review and meta-analysis. Sensors 2023, 23, 6601. [Google Scholar] [CrossRef]
| Children with Normal Hearing | Children with Hearing Loss | p-Value | |||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Volunteers | 65 | (100) | 65 | (100) | |
| Sexes: | |||||
| - Female | 35 | (53.8) | 35 | (53.8) | |
| - Male | 30 | (46.2) | 30 | (46.2) | |
| Age, Years (Mean) | 9.0 ± 1.45 | 9.0 ± 1.45 | 1.000 a | ||
| Height | 1.34 ± 0.08 | 1.35 ± 0.09 | 0.573 a | ||
| Weight | 31.9 ± 8.71 | 31.7 ± 9.04 | 0.601 a | ||
| Body Mass Index (BMI) | 17.2 ± 3.27 | 16.8 ± 3.26 | 0.903 a | ||
| Z-Score (BMI) | 0.85 ± 1.24 | 0.53 ± 2.46 | 0.354 a | ||
| Handedness: | |||||
| - Right-Handed | 62 | (95.4) | 60 | (92.3) | 0.359 b |
| - Left-Handed | 3 | (4.6) | 5 | (7.7) | |
| Degrees of Hearing Loss: | |||||
| - Mild and Moderate | -- | -- | 17 | (26.2) | |
| - Severe and Profound | -- | -- | 48 | (73.8) | |
| Vestibular Dysfunction | -- | -- | 27 | (41.5) | |
| Hearing AIDS Users | -- | -- | 2 | (3.1) | |
| Cochlear Implant Users | -- | -- | 5 | (7.7) | |
| Etiology of Hearing Loss: | |||||
| - Unknown | -- | -- | 23 | (35.4) | |
| - Rubella | -- | -- | 18 | (27.7) | |
| - Prematurity | -- | -- | 7 | (10.8) | |
| - Consanguinity of Parents | -- | -- | 6 | (9.2) | |
| - Use of Ototoxic Drugs | -- | -- | 5 | (7.7) | |
| - Hypoxia Peri or Postnatal | -- | -- | 5 | (7.7) | |
| - Meningitis Postnatal | -- | -- | 1 | (1.5) | |
| Antero-Posterior | Medio-Lateral | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal Hearing (n = 65) | Hearing Loss (n = 60) | Normal Hearing (n = 65) | Hearing Loss (n = 60) | ||||||
| Mean ± SD | Mean ± SD | DM (CI) | p-Value | Mean ± SD | Mean ± SD | DM (CI) | p-Value | ||
| Parallel feet | 1.56 ± 0.55 | 4.95 ± 2.42 | −3.39 (−4.00 to −2.77) | 0.001 a | 1.45 ± 0.52 | 1.97 ± 1.09 | −0.52 (−0.81 to −0.22) | 0.001 a | |
| Eyes Open | Tandem feet | 2.84 ± 1.14 | 4.94 ± 3.12 | −2.10 (−2.91 to −1.28) | 0.000 a | 2.09 ± 0.62 | 3.30 ± 1.84 | −1.21 (−1.68 to −0.73) | 0.000 a |
| One foot | 3.87 ± 1.43 | 5.29 ± 2.97 | −1.42 (−2.23 to −0.60) | 0.003 a | 3.49 ± 1.01 | 4.83 ± 2.52 | −1.34 (−2.01 to −0.66) | 0.001 a | |
| Parallel feet | 2.00 ± 0.75 | 2.91 ± 3.28 | −0.91 (−1.73 to 0.08) | 0.050 a | 1.83 ± 0.63 | 2.43 ± 1.65 | −0.60 (−1.03 to −0.16) | 0.002 a | |
| Eyes Closed | Tandem feet | 4.61 ± 2.21 | 5.84 ± 2.83 | −1.23 (−2.12 to −0.33) | 0.005 a | 3.71 ± 1.51 | 4.90 ± 2.28 | −1.19 (−1.86 to −0.51) | 0.000 a |
| One foot | 8.56 ± 4.21 | 8.58 ± 3.91 | −0.02 (−1.46 to 1.42) | 0.994 a | 6.93 ± 2.23 | 8.18 ± 3.69 | −1.25 (−2.32 to −0.18) | 0.246 a | |
| Normal Hearing (n = 65) | SNHL (MM) (n = 12) | SNHL (SP) (n = 26) | NH versus SNHL-MM | NH versus SNHL-SP | SNHL: MM versus SP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | DM | CI 95% | p-Value | DM | CI 95% | p-Value | DM | CI 95% | p-Value | ||
| Parallel feet | 1.56 ± 0.55 | 1.85 ± 0.74 | 2.73 ± 2.24 | −0.29 | −0.65 to 0.07 | 0.308 a | −1.17 | −1.75 to −0.58 | 0.001 a | −0.88 | −2.23 to 0.47 | 0.218 a | |
| Eyes Open | Tandem feet | 2.84 ± 1.14 | 3.52 ± 1.72 | 4.75 ± 2.91 | −0.68 | −1.45 to 0.09 | 0.241 a | −1.91 | −2.74 to −1.07 | 0.002 a | −1.23 | −3.07 to 0.61 | 0.218 a |
| One foot | 3.87 ± 1.43 | 4.23 ± 1.87 | 5.74 ± 3.35 | −0.36 | −1.30 to 0.58 | 0.668 a | −1.87 | −2.86 to −0.87 | 0.003 a | −1.50 | −3.61 to 0.59 | 0.146 a | |
| Parallel feet | 2.00 ± 0.75 | 2.23 ± 0.70 | 2.25 ± 0.98 | −0.23 | −0.69 to 0.23 | 0.211 a | −0.25 | −0.62 to 0.12 | 0.416 a | −0.02 | −0.65 to 0.61 | 0.588 a | |
| Eyes Closed | Tandem feet | 4.61 ± 2.21 | 5.63 ± 3.78 | 5.59 ± 1.90 | −1.02 | −2.58 to 0.54 | 0.365 a | −0.98 | −1.96 to 0.09 | 0.011 a | 0.04 | −1.81 to 1.89 | 0.429 a |
| One foot | 8.56 ± 4.21 | 8.26 ± 4.12 | 8.01 ± 3.47 | 0.30 | −2.32 to 2.92 | 0.705 a | 0.55 | −1.30 to 2.40 | 0.513 a | 0.25 | −2.35 to 2.85 | 0.745 a | |
| Normal Hearing (n = 65) | SNHL (MM) (n = 12) | SNHL (SP) (n = 26) | NH versus SNHL-MM | NH versus SNHL-SP | SNHL: MM versus SP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | DM | CI 95% | p-Value | DM | CI 95% | p-Value | DM | CI 95% | p-Value | ||
| Parallel feet | 1.45 ± 0.52 | 1.62 ± 0.63 | 2.04 ± 0.77 | −0.17 | −0.50 to 0.16 | 0.474 a | −0.59 | −0.86 to −0.31 | 0.000 a | −0.42 | −0.93 to 0.09 | 0.129 a | |
| Eyes Open | Tandem feet | 2.09 ± 0.62 | 2.81 ± 1.22 | 3.00 ± 1.26 | −0.72 | −1.18 to −0.25 | 0.074 a | −0.91 | −1.30 to −0.51 | 0.001 a | −0.19 | −1.07 to 0.69 | 0.631 a |
| One foot | 3.49 ± 1.01 | 3.97 ± 1.39 | 4.94 ± 2.12 | −0.48 | −1.15 to 0.19 | 0.273 a | −1.45 | −2.10 to −0.79 | 0.001 a | −0.97 | −2.33 to 0.39 | 0.174 a | |
| Parallel feet | 1.83 ± 0.63 | 2.07 ± 0.70 | 2.12 ± 0.78 | −0.24 | −0.64 to 0.16 | 0.088 a | −0.29 | −0.60 to 0.02 | 0.043 a | −0.05 | −0.58 to 0.48 | 0.816 a | |
| Eyes Closed | Tandem feet | 3.71 ± 1.51 | 4.51 ± 1.85 | 4.71 ± 1.28 | −0.80 | −1.77 to 0.17 | 0.070 a | −1.00 | −1.66 to −0.33 | 0.001 a | −0.20 | −1.24 to 0.84 | 0.297 a |
| One foot | 6.93 ± 2.23 | 7.55 ± 2.41 | 7.90 ± 3.57 | −0.62 | −2.03 to 0.79 | 0.565 a | −0.97 | −2.20 to 0.26 | 0.809 a | −0.35 | −2.65 to 1.95 | 0.914 a | |
| Normal Hearing (n = 65) | Hearing Loss (NVF) (n = 38) | Hearing Loss (VD) (n = 22) | NH versus SNHL-NVF | NH versus SNHL-VD | SNHL: NVF versus VD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | DM | CI 95% | p-Value | DM | CI 95% | p-Value | DM | CI 95% | p-Value | ||
| Parallel feet | 1.56 ± 0.55 | 2.45 ± 1.93 | 9.26 ± 6.58 | −0.89 | −1.39 to −0.38 | 0.001 a | −7.70 | −9.32 to −6.07 | 0.069 a | −6.81 | −9.08 to −4.53 | 0.824 a | |
| Eyes Open | Tandem feet | 2.84 ± 1.14 | 4.36 ± 2.63 | 5.94 ± 3.67 | −1.52 | −2.26 to −0.77 | 0.003 a | −3.10 | −4.11 to −2.08 | 0.001 a | −1.58 | −3.21 to 0.05 | 0.109 a |
| One foot | 3.87 ± 1.43 | 5.26 ± 3.02 | 5.33 ± 2.97 | −1.39 | −2.26 to −0.51 | 0.011 a | −1.46 | −2.40 to −0.51 | 0.029 a | −0.07 | −1.67 to 1.53 | 0.860 a | |
| Parallel feet | 2.00 ± 0.75 | 2.23 ± 0.89 | 4.10 ± 5.15 | −0.23 | −0.55 to 0.09 | 0.215 a | −2.10 | −3.39 to −0.80 | 0.036 a | −1.87 | −3.57 to −0.16 | 0.311 a | |
| Eyes Closed | Tandem feet | 4.61 ± 2.21 | 5.60 ± 2.58 | 6.26 ± 3.23 | −0.99 | −1.94 to −0.03 | 0.016 a | −1.65 | −2.87 to −0.42 | 0.033 a | −0.66 | −2.17 to 0.85 | 0.570 a |
| One foot | 8.56 ± 4.21 | 8.09 ± 3.63 | 9.38 ± 4.31 | 0.47 | 1.15 to 2.09 | 0.488 a | −0.82 | −2.89 to 1.25 | 0.329 a | −1.29 | −3.37 to 0.79 | 0.073 a | |
| Normal Hearing (n = 65) | Hearing Loss (NVF) (n = 38) | Hearing Loss (VD) (n = 22) | NH versus SNHL-NVF | NH versus SNHL-VD | SNHL: NVF versus VD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | DM | CI 95% | p-Value | DM | CI 95% | p-Value | DM | CI 95% | p-Value | ||
| Parallel feet | 1.45 ± 0.52 | 1.91 ± 0.75 | 2.08 ± 1.52 | −0.46 | −0.70 to −0.21 | 0.001 a | −0.63 | −1.06 to −0.19 | 0.068 a | −0.17 | −0.75 to 0.41 | 0.485 a | |
| Eyes Open | Tandem feet | 2.09 ± 0.62 | 2.94 ± 1.24 | 3.93 ± 2.49 | −0.85 | −1.21 to −0.48 | 0.001 a | −1.84 | −2.50 to −1.17 | 0.000 a | −0.99 | −1.95 to −0.02 | 0.187 a |
| One foot | 3.49 ± 1.01 | 4.63 ± 1.96 | 5.17 ± 3.29 | −1.14 | −1.72 to −0.55 | 0.001 a | −1.68 | −2.58 to −0.77 | 0.056 a | −0.54 | −1.89 to 0.81 | 0.957 a | |
| Parallel feet | 1.83 ± 0.63 | 2.11 ± 0.75 | 3.00 ± 2.48 | −0.28 | −0.55 to −0.06 | 0.016 a | −1.17 | −1.83 to −0.50 | 0.008 a | −0.89 | −1.75 to −0.02 | 0.165 a | |
| Eyes Closed | Tandem feet | 3.71 ± 1.51 | 4.65 ± 1.46 | 5.34 ± 3.25 | −0.94 | −1.54 to −0.33 | 0.000 a | −1.63 | −2.65 to −0.60 | 0.009 a | −0.69 | −1.91 to 0.53 | 0.602 a |
| One foot | 6.93 ± 2.23 | 7.79 ± 3.22 | 8.86 ± 4.39 | −0.86 | −1.92 to 0.20 | 0.640 a | −1.93 | −3.36 to −0.49 | 0.105 a | −1.07 | −3.04 to 0.90 | 0.315 a | |
| Antero-Posterior (n = 5) | Medio-Lateral (n = 5) | ||
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Parallel feet | 4.60 ± 2.18 | 2.56 ± 0.44 | |
| Eyes Open | Tandem feet | 5.96 ± 1.31 | 4.13 ± 1.11 |
| One foot | 5.77 ± 0.98 | 6.50 ± 2.09 | |
| Parallel feet | 2.27 ± 0.29 | 2.03 ± 0.16 | |
| Eyes Closed | Tandem feet | 6.57 ± 1.17 | 4.66 ± 0.34 |
| One foot | 7.41 ± 1.29 | 8.51 ± 2.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, R.S.; Lemos, A.; Wiesiolek, C.C.; Soares, L.G.M.; Raposo, M.C.F.; Lambertz, D.; Belian, R.B.; Ferraz, K.M. Postural Sway Velocity of Deaf Children with and without Vestibular Dysfunction. Sensors 2024, 24, 3888. https://doi.org/10.3390/s24123888
Melo RS, Lemos A, Wiesiolek CC, Soares LGM, Raposo MCF, Lambertz D, Belian RB, Ferraz KM. Postural Sway Velocity of Deaf Children with and without Vestibular Dysfunction. Sensors. 2024; 24(12):3888. https://doi.org/10.3390/s24123888
Chicago/Turabian StyleMelo, Renato S., Andrea Lemos, Carine Carolina Wiesiolek, Lucas Gallindo Martins Soares, Maria Cristina Falcão Raposo, Daniel Lambertz, Rosalie Barreto Belian, and Karla Mônica Ferraz. 2024. "Postural Sway Velocity of Deaf Children with and without Vestibular Dysfunction" Sensors 24, no. 12: 3888. https://doi.org/10.3390/s24123888









