Advances in Nanomaterials and Colorimetric Detection of Arsenic in Water: Review and Future Perspectives
Abstract
:1. Introduction
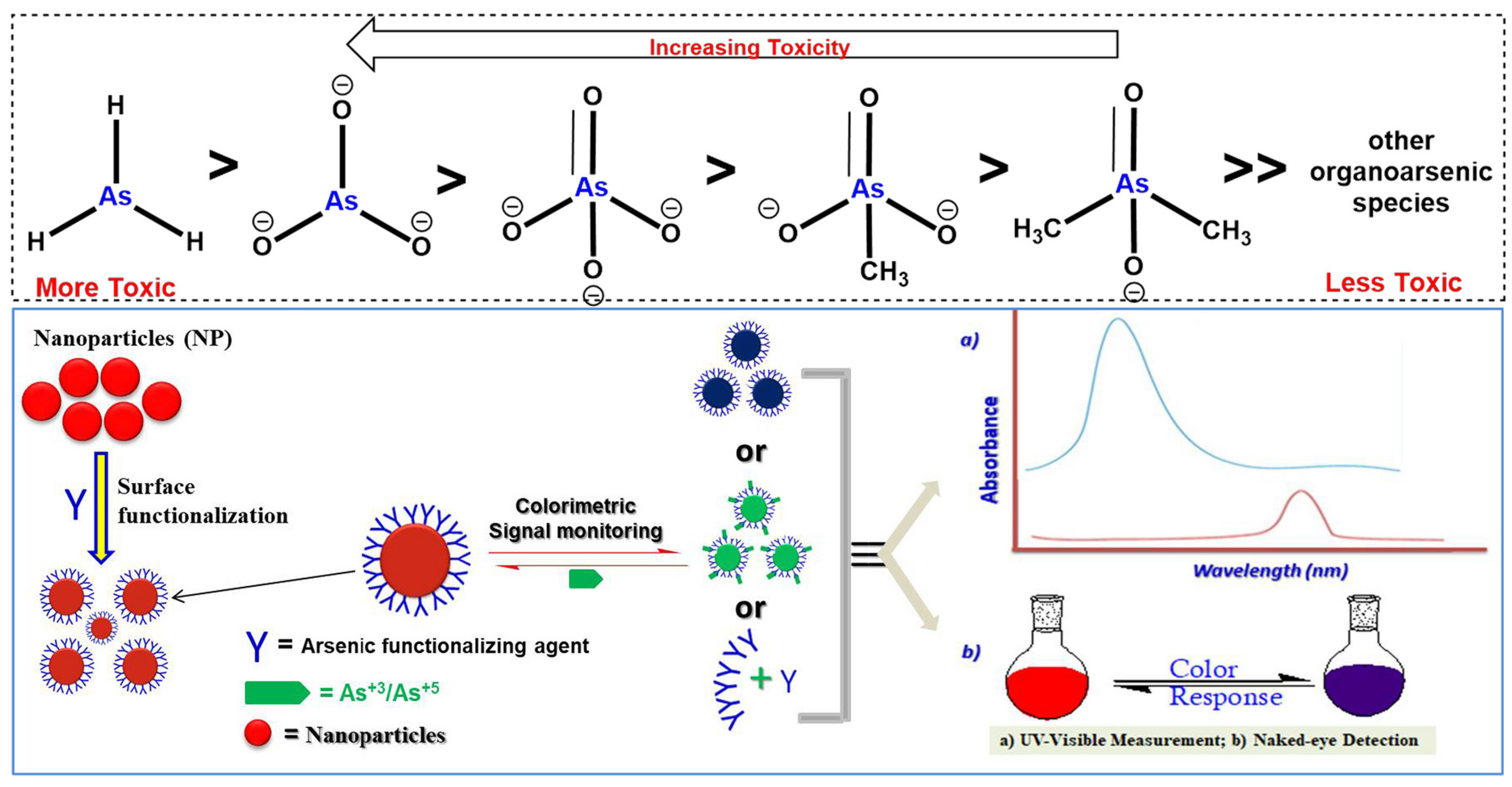
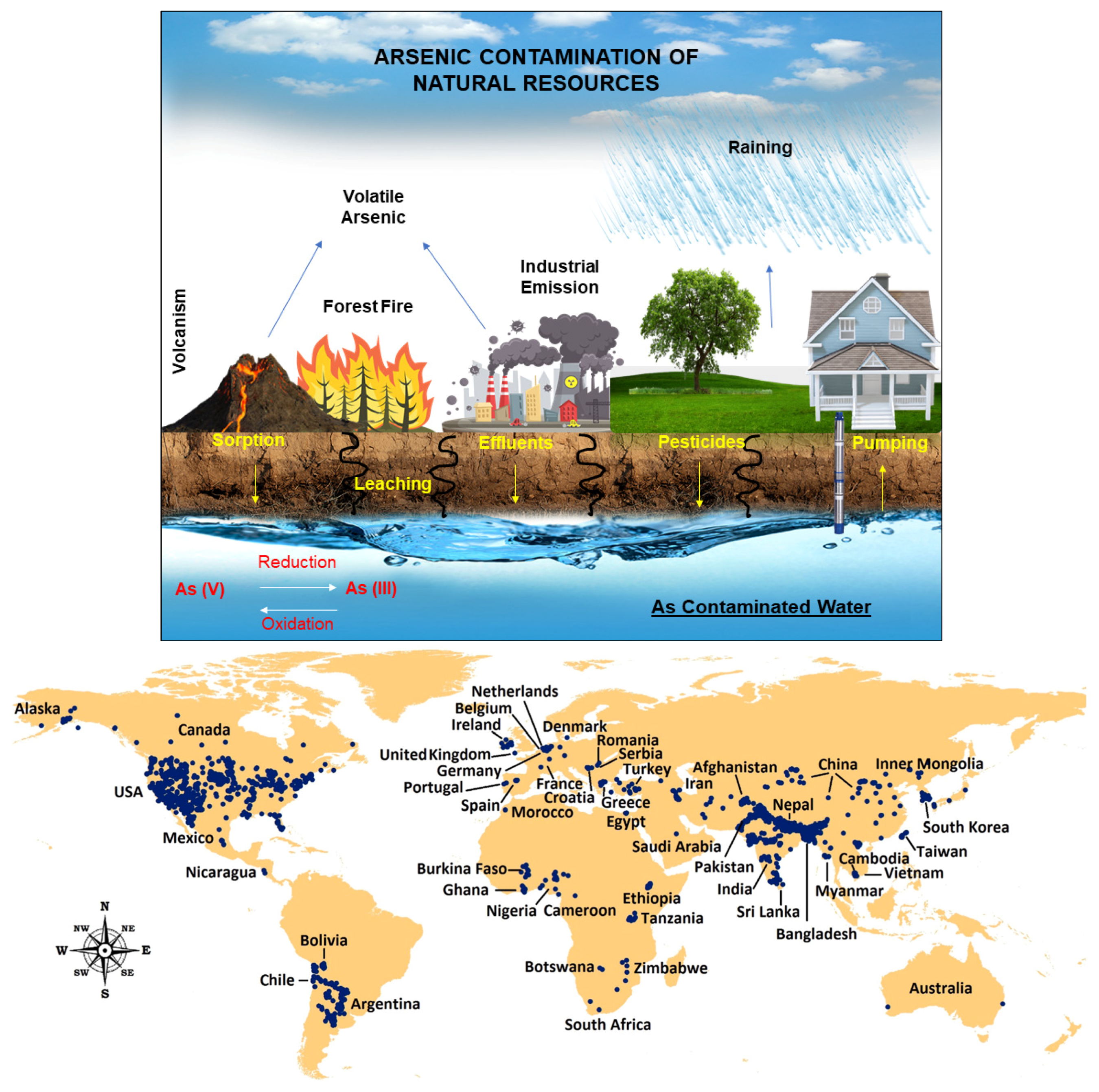
Arsenic Contamination and Its Impact on Public Health
2. Colorimetric Sensing
2.1. Marsh Reaction and Gutzeit Method
2.2. Molybdenum Blue-Based Methods
2.3. Methylene Blue-Based Methods
3. Nanomaterials and Colorimetric Advancements
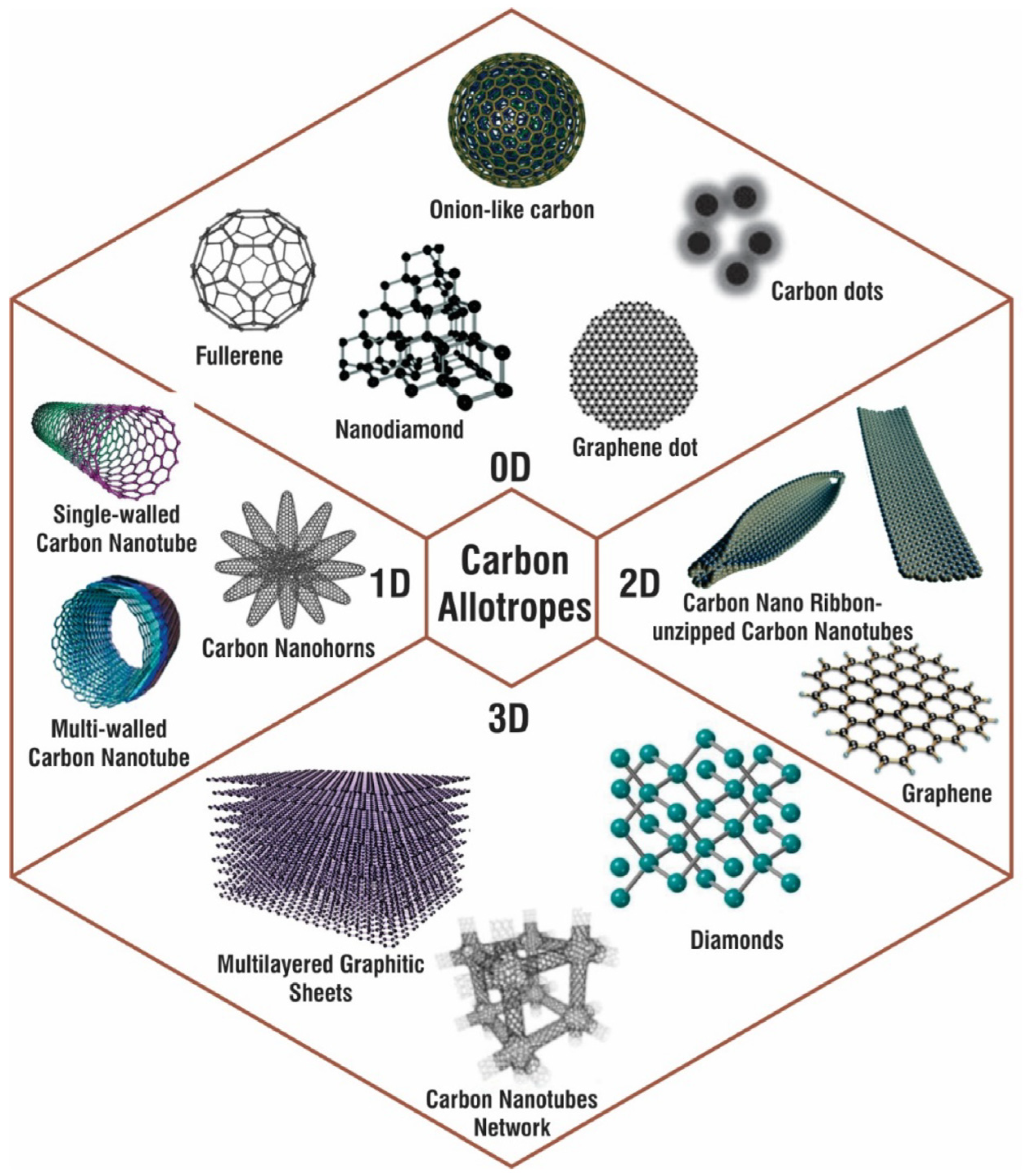
3.1. Carbon Nanomaterials
3.2. Metallic Nanoparticles (MNPs)
3.2.1. Gold-Based Nanomaterials (AuNPs)
3.2.2. Silver-Based Nanomaterials
3.3. Metal Oxide-Based Nanocomposites
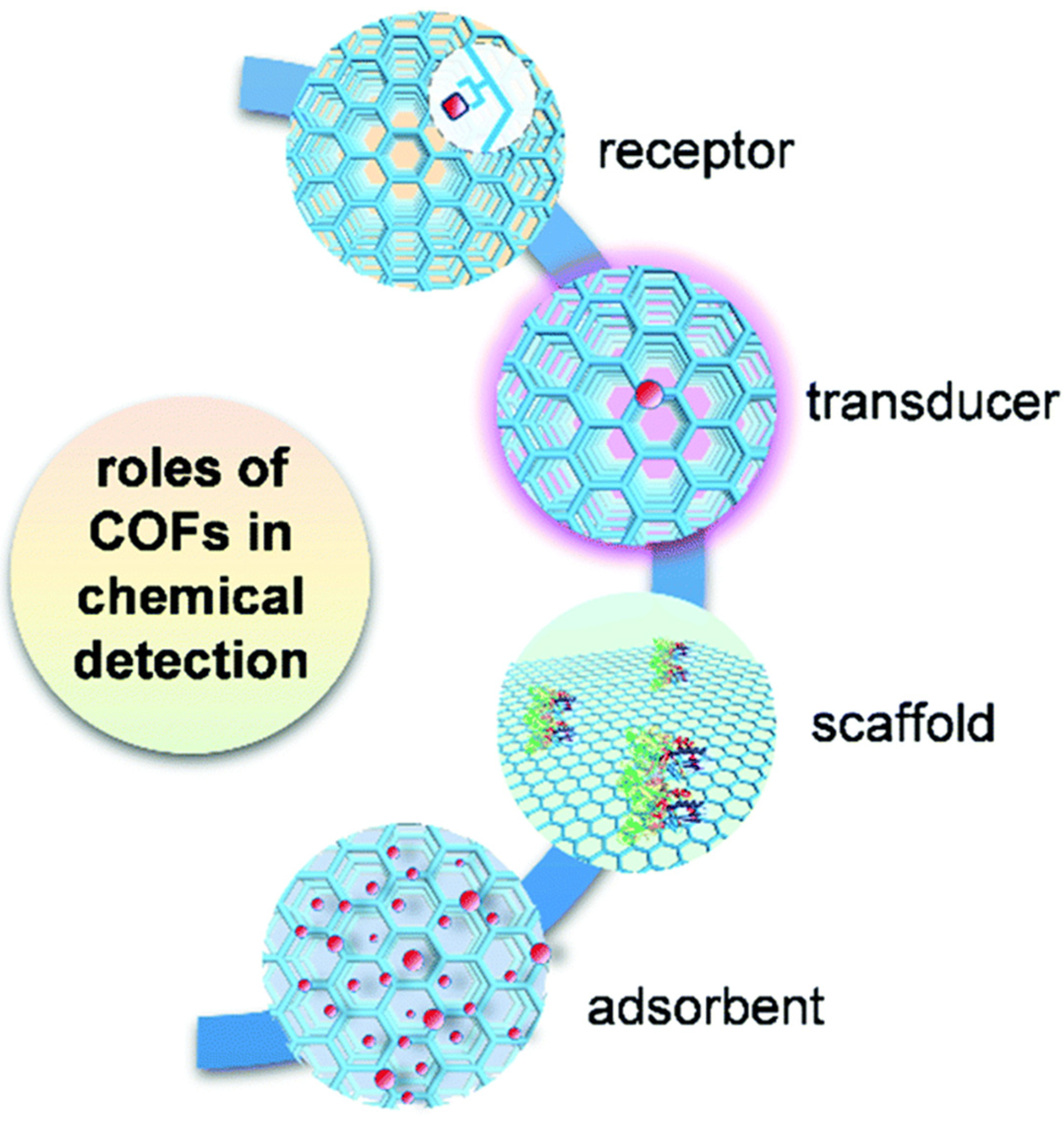
3.4. Metal–Organic Frameworks (MOFs) and Covalent Organic Frameworks (COFs)
3.5. Other Materials and Combinations
4. Challenges and Future Prospects
4.1. Challenges
- Interferences and Matrix Effects: Despite the sensitivity exhibited by certain nanomaterials-based systems, their susceptibility to interference from other metal ions commonly present in groundwater poses a significant challenge. Ensuring the specificity of nanomaterial-based detection systems towards arsenic ions is crucial to avoid false positives or interference from other ions in water. This could be very crucial due to the complex environmental sample matrices in real-world analysis and can significantly influence the performance. Functionalization of nanomaterials can impart selectivity towards arsenic and minimize interference from other contaminants in water samples. Also, addressing matrix effects through sample pre-treatment techniques or matrix-matched calibration standards is crucial for reliable arsenic detection in diverse environmental analysis.
- Stability and Reproducibility: Maintaining the stability of nanomaterials and ensuring reproducibility of results are critical challenges that need to be addressed and validated to make these detection systems reliable for long-term use and practical application. Results variation is very common among nanomaterial-based methods/systems and needs to be further explored and validated with statistically significant datasets including the consistency of nanomaterials, integration with other sensor/system components, and the capability to adapt to broader sample types.
- Cost and Integration with Existing Infrastructure: While nanomaterial-based detection systems hold promise for arsenic detection, ensuring their cost-effectiveness and portability remains imperative. The affordability of nano-systems is crucial for widespread adoption, especially in resource-limited settings. Additionally, enhancing portability can enable on-site testing, facilitating timely interventions to ensure water safety. Scaling up the production of nanomaterials for large-scale deployment in arsenic detection systems while maintaining quality control and safety are challenges which need to be addressed. Integration of nanomaterial-based detection systems with existing water testing and treatment infrastructure and regulatory frameworks may pose logistical challenges and require collaborative efforts between researchers, policymakers, and stakeholders.
- Environmental Impact: The environmental impact of nanomaterials used in detection systems needs to be thoroughly evaluated to prevent any adverse effects on ecosystems and human health.
4.2. Prospects
- Nanomaterials have exhibited immense potential in the field of colorimetric detection of arsenic in water due to their unique properties such as high surface area, tuneable optical properties, and high sensitivities. They are rapid, cost-effective and can offer high precision towards arsenic detection due to their large surface area to volume ratio, enabling the detection of low As concentrations. Nanomaterial-based detection systems can be designed to be portable and user-friendly, enabling their deployment in remote or resource-limited areas where arsenic contamination is prevalent.
- Future research should emphasize the creation of cost-effective, user-friendly, greener, and environmentally sustainable nanoparticles/nanomaterials. Prioritizing selectivity, sensitivity, low detection limits, anti-interference capabilities, and simplicity in design will ensure widespread accessibility and application of arsenic detection technologies. Focus on reliable and consistent synthetic methods yielding nanoparticles of regular size is crucial, as nanoparticle size plays a key role in As(III) detection to improve their practical application.
- Advanced materials such as carbon dots (CDs), MXene, graphene, and MOFs/COFs offer distinct features that hold promising potential for advancing arsenic colorimetric detection. CDs are capable of exhibiting high sensitivity due to their tuneable properties and easy surface functionalization for selective arsenic binding [116]. MXene materials boast high surface areas and conductivity, which could facilitate efficient arsenic adsorption and detection. Graphene’s large surface-to-volume ratio, conductivity, and chemical tunability are of interest, while MOFs/COFs are capable of providing tailorable pore structures for precise arsenic sorption and integration with signal transduction mechanisms.
- Paper-based sensors could offer a promising solution for arsenic detection in water due to their simplicity, low cost, portability, and potential integration with existing infrastructure. These sensors involve immobilizing selective receptors or indicators onto paper substrates, undergoing a colorimetric change in the presence of arsenic ions. Further improvements could enhance specificity and sensitivity, and integration with smartphone technologies could enable real-time monitoring and remote sensing. Paper-based sensors hold great promise especially in resource-limited or remote areas where conventional methods are impractical.
- Microfluidic technology could offer innovative solutions for arsenic detection in water, leveraging miniaturized fluid-handling systems. Advantages include miniaturization, automation, precise fluid flow control, and integration of multiple functions on a single device. Microfluidic platforms efficiently handle small sample volumes, reducing reagent consumption and waste. It can help in multiple samples analysis simultaneously to enhance monitoring capabilities.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hong, Y.-S.; Song, K.-H.; Chung, J.-Y. Health effects of chronic arsenic exposure. J. Prev. Med. Public Health 2014, 47, 245–252. [Google Scholar] [CrossRef]
- Kanel, S.R.; Das, T.K.; Varma, R.S.; Kurwadkar, S.; Chakraborty, S.; Joshi, T.P.; Bezbaruah, A.N.; Nadagouda, M.N. Arsenic Contamination in Groundwater: Geochemical Basis of Treatment Technologies. ACS Environ. Au 2023, 3, 135–152. [Google Scholar] [CrossRef]
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Aposhian, H.V.; Graziano, J.H.; Thompson, C.; Suk, W.A. The Broad Scope of Health Effects from Chronic Arsenic Exposure: Update on a Worldwide Public Health Problem. Environ. Health Perspect. 2013, 121, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.S. 1—Arsenic: Chemistry, Occurrence, and Exposure. In Handbook of Arsenic Toxicology; Flora, S.J.S., Ed.; Academic Press: Oxford, UK, 2015; pp. 1–49. [Google Scholar]
- ATSDR. 2022 ATSDR Substance Priority List. 2022. Available online: https://www.atsdr.cdc.gov/spl/index.html#2022spl (accessed on 5 October 2023).
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Authority, E.F.S.; Arcella, D.; Cascio, C.; Gómez Ruiz, J.Á. Chronic dietary exposure to inorganic arsenic. EFSA J. 2021, 19, e06380. [Google Scholar] [CrossRef]
- WHO. Preventing Disease through Healthy Environments: Exposure to Arsenic: A Major Public Health Concern. 2019. Available online: https://iris.who.int/handle/10665/329482 (accessed on 22 March 2024).
- EPA. Drinking Water Parameters: Microbiological, Chemical and Indicator Parameters in the 2014 Drinking Water Regulations 2014. Available online: https://www.epa.ie/publications/compliance--enforcement/drinking-water/drinking-water-parameters.php (accessed on 10 November 2023).
- WHO. Chemical Fact Sheets: Arsenic. 2022. Available online: https://www.who.int/publications/m/item/chemical-fact-sheets--arsenic (accessed on 8 September 2023).
- Behari, J.R.; Prakash, R. Determination of total arsenic content in water by atomic absorption spectroscopy (AAS) using vapour generation assembly (VGA). Chemosphere 2006, 63, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodas, D.; Corns, W.T.; Chen, B.; Stockwell, P.B. Atomic Fluorescence Spectrometry: A suitable detection technique in speciation studies for arsenic, selenium, antimony and mercury. J. Anal. At. Spectrom. 2010, 25, 933–946. [Google Scholar] [CrossRef]
- Chen, B.; Krachler, M.; Gonzalez, Z.I.; Shotyk, W. Improved determination of arsenic in environmental and geological specimens using HG-AFS. J. Anal. At. Spectrom. 2005, 20, 95–102. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Hirata, S. Simultaneous determination of arsenic(III) and arsenic(V) by flow injection–inductively coupled plasma–atomic emission spectrometry (ICP-AES) with ultrasonic nebulization. Anal. Bioanal. Chem. 2003, 375, 139–144. [Google Scholar] [CrossRef]
- Nookabkaew, S.; Rangkadilok, N.; Mahidol, C.; Promsuk, G.; Satayavivad, J. Determination of Arsenic Species in Rice from Thailand and Other Asian Countries Using Simple Extraction and HPLC-ICP-MS Analysis. J. Agric. Food Chem. 2013, 61, 6991–6998. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, J.S.; Lee, J.Y.; Jeon, J.S.; Kim, J.W.; Russo, R.E.; Gonzalez, J.; Yoo, J.H.; Kim, K.S.; Yang, J.S.; et al. Analysis of arsenic in rice grains using ICP-MS and fs LA-ICP-MS. J. Anal. At. Spectrom. 2014, 29, 1233–1237. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.-R.; Wen, S.-H.; Liang, R.-P.; Qiu, J.-D. Optical sensors for inorganic arsenic detection. TrAC Trends Anal. Chem. 2019, 118, 869–879. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, H.; Wang, Z.; Cao, H.; Zhang, K.; Li, X.; Yang, Z. Nanomaterial-based aptamer sensors for arsenic detection. Biosens. Bioelectron. 2020, 148, 111785. [Google Scholar] [CrossRef]
- Nearing, M.M.; Koch, I.; Reimer, K.J. Complementary arsenic speciation methods: A review. Spectrochim. Acta Part B At. Spectrosc. 2014, 99, 150–162. [Google Scholar] [CrossRef]
- Guo, J.; Cao, W.; Lang, G.; Sun, Q.; Nan, T.; Li, X.; Ren, Y.; Li, Z. Worldwide Distribution, Health Risk, Treatment Technology, and Development Tendency of Geogenic High-Arsenic Groundwater. Water 2024, 16, 478. [Google Scholar] [CrossRef]
- Boyden, H.; Gillan, M.; Molina, J.; Gadgil, A.; Tseng, W. Community Perceptions of Arsenic Contaminated Drinking Water and Preferences for Risk Communication in California’s San Joaquin Valley. Int. J. Environ. Res. Public Health 2023, 20, 813. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhin, K.H.; Kahsay, M.H.; Wegahita, N.K.; Teklu, T.; Berhe, B.A.; Gebru, A.G.; Tesfay, A.H.; Asgedom, A.G. Nanomaterial-based optical colorimetric sensors for rapid monitoring of inorganic arsenic species: A review. Discov. Nano 2024, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Raju, N.J. Arsenic in the geo-environment: A review of sources, geochemical processes, toxicity and removal technologies. Environ. Res. 2022, 203, 111782. [Google Scholar] [CrossRef]
- Singh, A.P.; Goel, R.K.; Kaur, T. Mechanisms pertaining to arsenic toxicity. Toxicol. Int. 2011, 18, 87–93. [Google Scholar] [CrossRef]
- Huang, L.; Wu, H.; van der Kuijp, T.J. The health effects of exposure to arsenic-contaminated drinking water: A review by global geographical distribution. Int. J. Environ. Health Res. 2015, 25, 432–452. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Hara, T.O.; Tian, F.; Singh, B. Review of analytical techniques for arsenic detection and determination in drinking water. Environ. Sci. Adv. 2023, 2, 171–195. [Google Scholar] [CrossRef]
- Yadav, M.K.; Saidulu, D.; Gupta, A.K.; Ghosal, P.S.; Mukherjee, A. Status and management of arsenic pollution in groundwater: A comprehensive appraisal of recent global scenario, human health impacts, sustainable field-scale treatment technologies. J. Environ. Chem. Eng. 2021, 9, 105203. [Google Scholar] [CrossRef]
- WHO. 10 Chemicals of Public Health Concern. 2020. Available online: https://www.who.int/news-room/photo-story/photo-story-detail/10-chemicals-of-public-health-concern (accessed on 18 October 2023).
- Sinha, D.; Prasad, P. Health effects inflicted by chronic low-level arsenic contamination in groundwater: A global public health challenge. J. Appl. Toxicol. 2020, 40, 87–131. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Arsenic: A Review on a Great Health Issue Worldwide. Appl. Sci. 2022, 12, 6184. [Google Scholar] [CrossRef]
- Bundschuh, J.; Niazi, N.K.; Alam, M.A.; Berg, M.; Herath, I.; Tomaszewska, B.; Maity, J.P.; Ok, Y.S. Global arsenic dilemma and sustainability. J. Hazard. Mater. 2022, 436, 129197. [Google Scholar] [CrossRef]
- Mohammed Abdul, K.S.; Jayasinghe, S.S.; Chandana, E.P.S.; Jayasumana, C.; De Silva, P.M.C.S. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef]
- Kolya, H.; Hashitsume, K.; Kang, C.-W. Recent Advances in Colorimetric Detection of Arsenic Using Metal-Based Nanoparticles. Toxics 2021, 9, 143. [Google Scholar] [CrossRef]
- Mazumder, D.N.G. 6—Health effects of chronic arsenic toxicity. In Handbook of Arsenic Toxicology, 2nd ed.; Flora, S.J.S., Ed.; Academic Press: Oxford, UK, 2023; pp. 151–192. [Google Scholar]
- Rajiv, S.V.; George, M.; Nandakumar, G. Dermatological manifestations of arsenic exposure. J. Ski. Sex. Transm. Dis. 2023, 5, 14–21. [Google Scholar] [CrossRef]
- Mitra, A.; Chatterjee, S.; Gupta, D.K. Environmental Arsenic Exposure and Human Health Risk. In Arsenic Water Resources Contamination: Challenges and Solutions; Fares, A., Singh, S.K., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 103–129. [Google Scholar]
- Hughes, M.F.; Beck, B.D.; Chen, Y.; Lewis, A.S.; Thomas, D.J. Arsenic Exposure and Toxicology: A Historical Perspective. Toxicol. Sci. 2011, 123, 305–332. [Google Scholar] [CrossRef]
- Nawarathne, M.; Weerasinghe, R.; Dharmarathne, C. Colorimetric and Fluorometric detection of arsenic: Arsenate and arsenite. Anal. Methods Environ. Chem. J. 2023, 6, 29–57. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mukherjee, D.; Sengupta, M.K.; Chowdhury, U.K.; Lodh, D.; Chanda, C.R.; Roy, S.; Selim, M.; Quamruzzaman, Q.; Milton, A.H.; et al. Effectiveness and Reliability of Arsenic Field Testing Kits: Are the Million Dollar Screening Projects Effective or Not? Environ. Sci. Technol. 2002, 36, 5385–5394. [Google Scholar] [CrossRef] [PubMed]
- Pande, S.P.; Deshpande, L.S.; Kaul, S. Laboratory and field assessment of arsenic testing field kits in Bangladesh and West Bengal, India. Environ. Monit. Assess. 2001, 68, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kinniburgh, D.G.; Kosmus, W. Arsenic contamination in groundwater: Some analytical considerations. Talanta 2002, 58, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Steinmaus, C.M.; George, C.M.; Kalman, D.A.; Smith, A.H. Evaluation of two new arsenic field test kits capable of detecting arsenic water concentrations close to 10 μg/L. Environ. Sci. Technol. 2006, 40, 3362–3366. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh-Amiri, A.; Fowlie, P.; Kazi, A.; Siraj, S.; Ahmed, S.; Akbor, A. Validation of analysis of arsenic in water samples using Wagtech Digital Arsenator. Sci. Total Environ. 2011, 409, 2662–2667. [Google Scholar] [CrossRef] [PubMed]
- Ibnul, N.K.; Tripp, C.P. A simple solution to the problem of selective detection of phosphate and arsenate by the molybdenum blue method. Talanta 2022, 238, 123043. [Google Scholar] [CrossRef] [PubMed]
- Yogarajah, N.; Tsai, S.S.H. Detection of trace arsenic in drinking water: Challenges and opportunities for microfluidics. Environ. Sci. Water Res. Technol. 2015, 1, 426–447. [Google Scholar] [CrossRef]
- Okazaki, T.; Kuramitz, H.; Hata, N.; Taguchi, S.; Murai, K.; Okauchi, K. Visual colorimetry for determination of trace arsenic in groundwater based on improved molybdenum blue spectrophotometry. Anal. Methods 2015, 7, 2794–2799. [Google Scholar] [CrossRef]
- He, Y.; Liu, J.; Duan, Y.; Yuan, X.; Ma, L.; Dhar, R.; Zheng, Y. A critical review of on-site inorganic arsenic screening methods. J. Environ. Sci. 2022, 125, 453–469. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Kundu, S.; Mandal, M.; Pal, T. Silver and gold nanocluster catalyzed reduction of methylene blue by arsine in a micellar medium. Langmuir 2002, 18, 8756–8760. [Google Scholar] [CrossRef]
- Reddy, R.R.; Rodriguez, G.D.; Webster, T.M.; Abedin, M.J.; Karim, M.R.; Raskin, L.; Hayes, K.F. Evaluation of arsenic field test kits for drinking water: Recommendations for improvement and implications for arsenic affected regions such as Bangladesh. Water Res. 2020, 170, 115325. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Niu, X.; Li, X.; Li, Z.; Du, D.; Lin, Y. Nanomaterial-based sensors and biosensors for enhanced inorganic arsenic detection: A functional perspective. Sens. Actuators B Chem. 2020, 315, 128100. [Google Scholar] [CrossRef]
- Thakkar, S.; Dumée, L.F.; Gupta, M.; Singh, B.R.; Yang, W. Nano–enabled sensors for detection of arsenic in water. Water Res. 2021, 188, 116538. [Google Scholar] [CrossRef]
- Salek Maghsoudi, A.; Hassani, S.; Mirnia, K.; Abdollahi, M. Recent Advances in Nanotechnology-Based Biosensors Development for Detection of Arsenic, Lead, Mercury, and Cadmium. Int. J. Nanomed. 2021, 16, 803–832. [Google Scholar] [CrossRef]
- Devi, P.; Thakur, A.; Lai, R.Y.; Saini, S.; Jain, R.; Kumar, P. Progress in the materials for optical detection of arsenic in water. TrAC Trends Anal. Chem. 2019, 110, 97–115. [Google Scholar] [CrossRef]
- Saeed, A.A.; Singh, B.; Nooredeen Abbas, M.; Dempsey, E. Evaluation of Bismuth Modified Carbon Thread Electrode for Simultaneous and Highly Sensitive Cd (II) and Pb (II) Determination. Electroanalysis 2016, 28, 2205–2213. [Google Scholar] [CrossRef]
- Singh, B.; Dempsey, E.; Dickinson, C.; Laffir, F. Inside/outside Pt nanoparticles decoration of functionalised carbon nanofibers (Pt19.2/f-CNF80.8) for sensitive non-enzymatic electrochemical glucose detection. Analyst 2012, 137, 1639–1648. [Google Scholar] [CrossRef]
- Singh, B.; Dempsey, E.; Laffir, F. Carbon nanochips and nanotubes decorated PtAuPd-based nanocomposites for glucose sensing: Role of support material and efficient Pt utilisation. Sens. Actuators B Chem. 2014, 205, 401–410. [Google Scholar] [CrossRef]
- Singh, B.; Laffir, F.; Dickinson, C.; McCormac, T.; Dempsey, E. Carbon Supported Cobalt and Nickel Based Nanomaterials for Direct Uric Acid Determination. Electroanalysis 2011, 23, 79–89. [Google Scholar] [CrossRef]
- Singh, B.; Laffir, F.; McCormac, T.; Dempsey, E. PtAu/C based bimetallic nanocomposites for non-enzymatic electrochemical glucose detection. Sens. Actuators B Chem. 2010, 150, 80–92. [Google Scholar] [CrossRef]
- Singh, B.; Seddon, B.; Dempsey, E.; Redington, W.; Dickinson, C. Porous Core-Shell Platinum-Silver Nanocatalyst for the Electrooxidation of Methanol. Electroanalysis 2015, 27, 135–143. [Google Scholar] [CrossRef]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.Ó.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef] [PubMed]
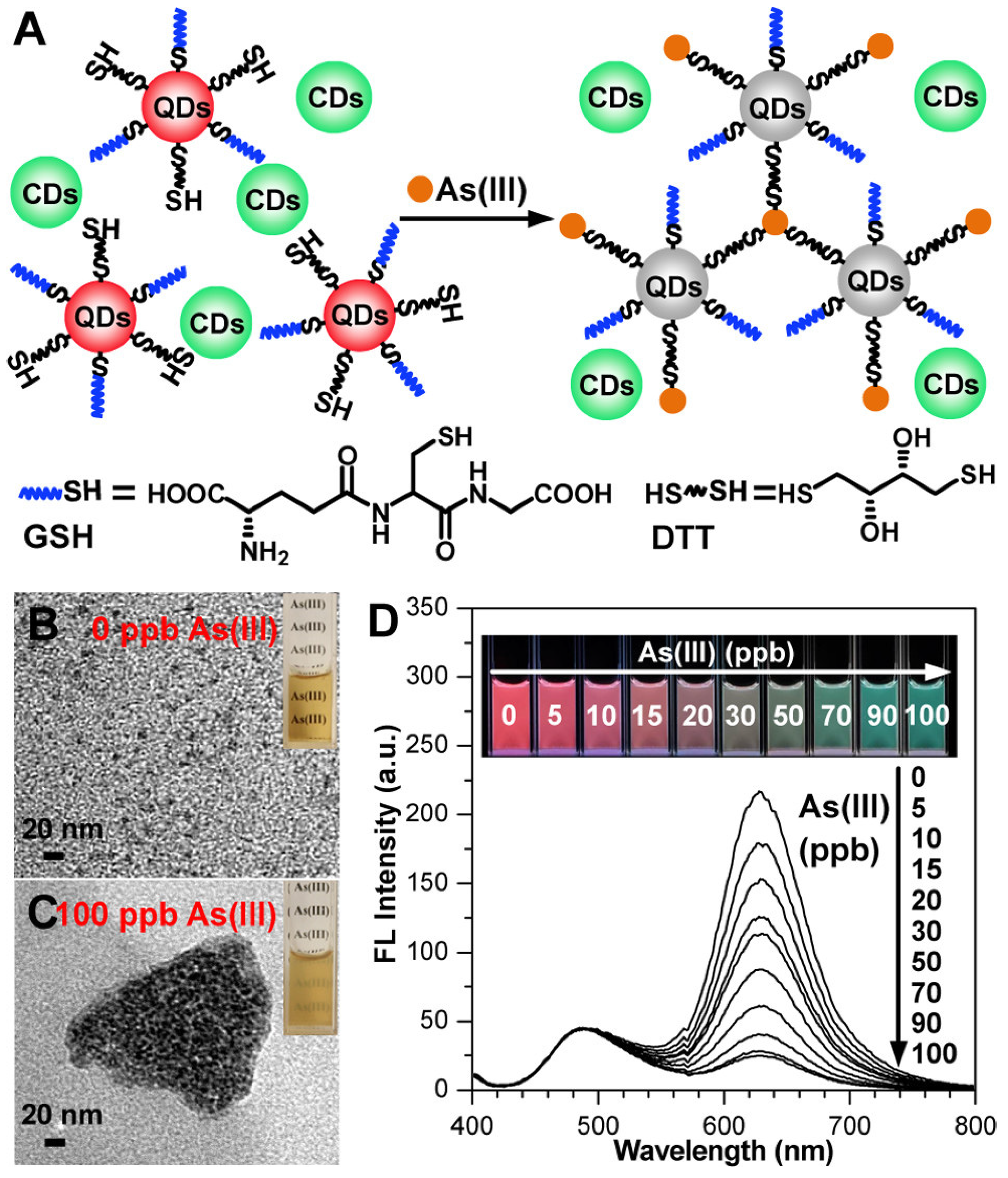
- He, X.; Li, Y.; Yang, C.; Lu, L.; Nie, Y.; Tian, X. Carbon dots–MnO2 nanocomposites for As(iii) detection in groundwater with high sensitivity and selectivity. Anal. Methods 2020, 12, 5572–5580. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, X.; Liu, C.; Zhang, R.; Gu, X.; Guan, G.; Jiang, C.; Zhang, L.; Du, S.; Liu, B.; et al. Color-Multiplexing-Based Fluorescent Test Paper: Dosage-Sensitive Visualization of Arsenic(III) with Discernable Scale as Low as 5 ppb. Anal. Chem. 2016, 88, 6105–6109. [Google Scholar] [CrossRef]
- Al-Rekabi, S.H.; Mustapha Kamil, Y.; Abu Bakar, M.H.; Fen, Y.W.; Lim, H.N.; Kanagesan, S.; Mahdi, M.A. Hydrous ferric oxide-magnetite-reduced graphene oxide nanocomposite for optical detection of arsenic using surface plasmon resonance. Opt. Laser Technol. 2019, 111, 417–423. [Google Scholar] [CrossRef]
- Pathan, S.; Jalal, M.; Prasad, S.; Bose, S. Aggregation-induced enhanced photoluminescence in magnetic graphene oxide quantum dots as a fluorescence probe for As(iii) sensing. J. Mater. Chem. A 2019, 7, 8510–8520. [Google Scholar] [CrossRef]
- Naseh, M.F.; Singh, N.; Ansari, J.R.; Kumar, A.; Sarkar, T.; Datta, A. L-cysteine functionalized graphene quantum dots for sub-ppb detection of As (III). Nanotechnology 2022, 33, 065504. [Google Scholar] [CrossRef]
- Kusuma, S.A.F.; Harmonis, J.A.; Pratiwi, R.; Hasanah, A.N. Gold Nanoparticle-Based Colorimetric Sensors: Properties and Application in Detection of Heavy Metals and Biological Molecules. Sensors 2023, 23, 8172. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chen, C.-P.; Wu, T.-H.; Yang, C.-H.; Lin, C.-W.; Chen, C.-Y. Gold Nanoparticle-Based Colorimetric Strategies for Chemical and Biological Sensing Applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef]
- Sun, J.; Lu, Y.; He, L.; Pang, J.; Yang, F.; Liu, Y. Colorimetric sensor array based on gold nanoparticles: Design principles and recent advances. TrAC Trends Anal. Chem. 2020, 122, 115754. [Google Scholar] [CrossRef]
- Zheng, B.; Li, J.; Zheng, Z.; Zhang, C.; Huang, C.; Hong, J.; Li, Y.; Wang, J. Rapid colorimetric detection of arsenic (III) by glutathione functionalized gold nanoparticles based on RGB extracting system. Opt. Laser Technol. 2021, 133, 106522. [Google Scholar] [CrossRef]
- Domínguez-González, R.; González Varela, L.; Bermejo-Barrera, P. Functionalized gold nanoparticles for the detection of arsenic in water. Talanta 2014, 118, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, J.R.; Arbneshi, T.; Afrin Khan, S.; Neely, A.; Candice, P.; Varisli, B.; Washington, M.; McAfee, S.; Robinson, B.; Banerjee, S.; et al. Use of Gold Nanoparticles in a Simple Colorimetric and Ultrasensitive Dynamic Light Scattering Assay: Selective Detection of Arsenic in Groundwater. Angew. Chem. Int. Ed. 2009, 48, 9668–9671. [Google Scholar] [CrossRef] [PubMed]
- Deepa, A.; Padmini, V. Highly Efficient Colorimetric Sensor for Selective and Sensitive Detection of Arsenite Ion (III) in Aqueous Medium. J. Fluoresc. 2019, 29, 813–818. [Google Scholar] [CrossRef]
- Nath, P.; Priyadarshni, N.; Chanda, N. Europium-Coordinated Gold Nanoparticles on Paper for the Colorimetric Detection of Arsenic(III, V) in Aqueous Solution. ACS Appl. Nano Mater. 2018, 1, 73–81. [Google Scholar] [CrossRef]
- Tan, Z.-Q.; Liu, J.-F.; Yin, Y.-G.; Shi, Q.-T.; Jing, C.-Y.; Jiang, G.-B. Colorimetric Au Nanoparticle Probe for Speciation Test of Arsenite and Arsenate Inspired by Selective Interaction between Phosphonium Ionic Liquid and Arsenite. ACS Appl. Mater. Interfaces 2014, 6, 19833–19839. [Google Scholar] [CrossRef]
- Paul, D.; Dutta, S.; Biswas, R. LSPR enhanced gasoline sensing with a U-bent optical fiber. J. Phys. D Appl. Phys. 2016, 49, 305104. [Google Scholar] [CrossRef]
- Boruah, B.S.; Daimari, N.K.; Biswas, R. Functionalized silver nanoparticles as an effective medium towards trace determination of arsenic (III) in aqueous solution. Results Phys. 2019, 12, 2061–2065. [Google Scholar] [CrossRef]
- Das Chakraborty, S.; Mondal, S.; Satpati, B.; Pal, U.; De, S.K.; Bhattacharya, M.; Ray, S.; Senapati, D. Wide Range Morphological Transition of Silver Nanoprisms by Selective Interaction with As (III): Tuning–Detuning of Surface Plasmon Offers to Decode the Mechanism. J. Phys. Chem. C 2019, 123, 11044–11054. [Google Scholar] [CrossRef]
- Saadati, A.; Farshchi, F.; Hasanzadeh, M.; Liu, Y.; Seidi, F. Colorimetric and naked-eye detection of arsenic(iii) using a paper-based microfluidic device decorated with silver nanoparticles. RSC Adv. 2022, 12, 21836–21850. [Google Scholar] [CrossRef] [PubMed]
- Babu Christus, A.A.; Panneerselvam, P.; Ravikumar, A. Novel, sensitive and selective colorimetric detection of arsenate in aqueous solution by a Fenton-like reaction of Fe3O4 nanoparticles. Anal. Methods 2018, 10, 4378–4386. [Google Scholar] [CrossRef]
- Li, B.; Chrzanowski, M.; Zhang, Y.; Ma, S. Applications of metal-organic frameworks featuring multi-functional sites. Coord. Chem. Rev. 2016, 307, 106–129. [Google Scholar] [CrossRef]
- Mohan, B.; Kumari, R.; Virender; Singh, G.; Singh, K.; Pombeiro, A.J.L.; Yang, X.; Ren, P. Covalent organic frameworks (COFs) and metal–organic frameworks (MOFs) as electrochemical sensors for the efficient detection of pharmaceutical residues. Environ. Int. 2023, 175, 107928. [Google Scholar] [CrossRef] [PubMed]
- Audu, C.O.; Nguyen, H.G.T.; Chang, C.-Y.; Katz, M.J.; Mao, L.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. The dual capture of AsV and AsIII by UiO-66 and analogues. Chem. Sci. 2016, 7, 6492–6498. [Google Scholar] [CrossRef]
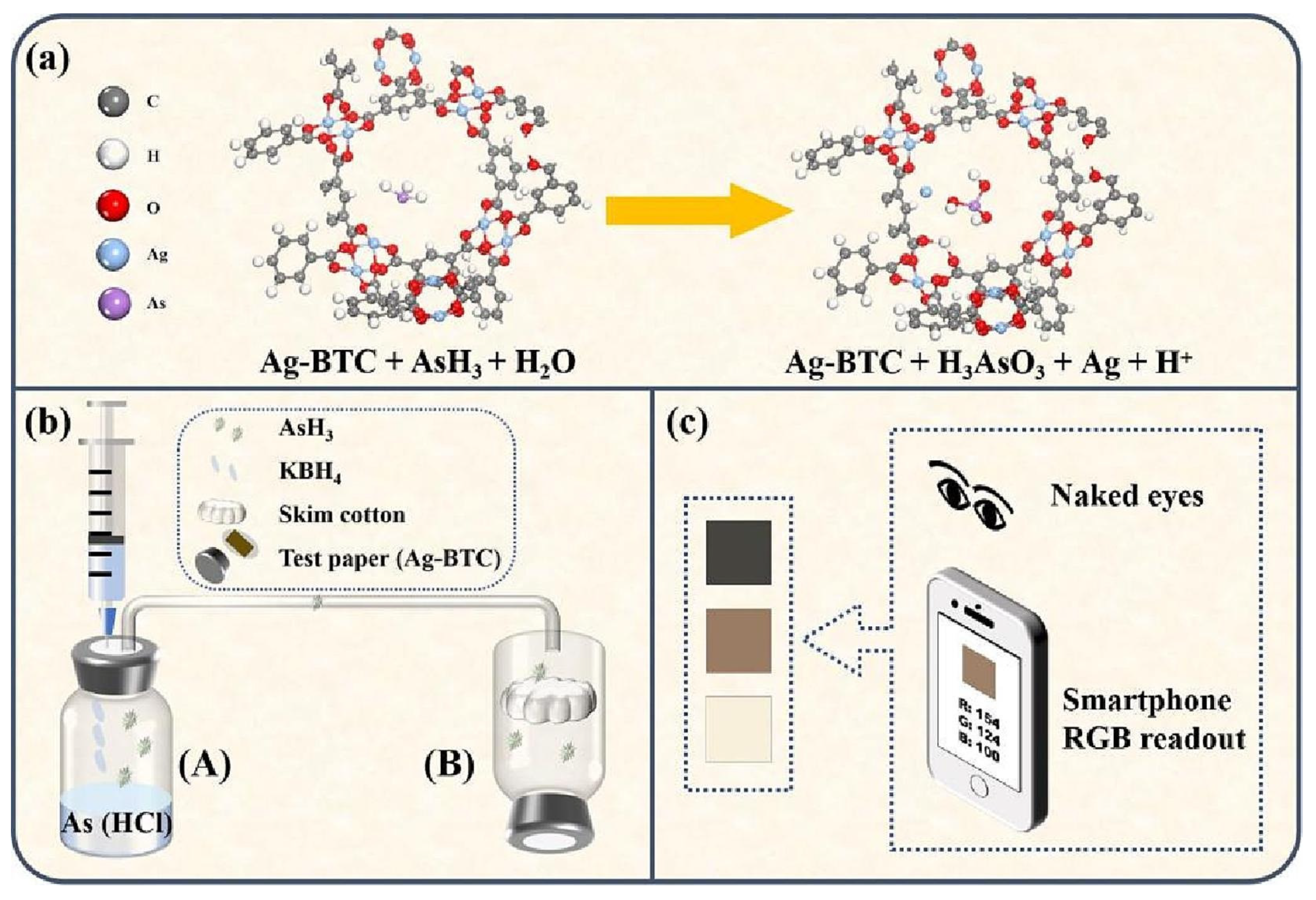
- Zou, Z.; Ye, S.; Xiao, J.; Jiang, C.; Zhang, S.; Tan, C.; Xiong, X.; Huang, K. Ag-containing metal organic framework reacted with AsH3: Mechanism and application for inorganic arsenic detection by hydride generation-smartphone RGB readout colorimetric system. Food Chem. 2023, 428, 136806. [Google Scholar] [CrossRef]
- Meng, Z.; Mirica, K.A. Covalent organic frameworks as multifunctional materials for chemical detection. Chem. Soc. Rev. 2021, 50, 13498–13558. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, G. A covalent organic framework containing bipyridine groups as a fluorescent chemical probe for the ultrasensitive detection of arsenic (III). J. Photochem. Photobiol. A Chem. 2021, 421, 113528. [Google Scholar] [CrossRef]
- Ge, K.; Liu, J.; Fang, G.; Wang, P.; Zhang, D.; Wang, S. A Colorimetric Probe Based on Functionalized Gold Nanorods for Sensitive and Selective Detection of As(III) Ions. Sensors 2018, 18, 2372. [Google Scholar] [CrossRef]
- Matsunaga, K.; Okuyama, Y.; Hirano, R.; Okabe, S.; Takahashi, M.; Satoh, H. Development of a simple analytical method to determine arsenite using a DNA aptamer and gold nanoparticles. Chemosphere 2019, 224, 538–543. [Google Scholar] [CrossRef]
- Boruah, B.S.; Biswas, R.; Deb, P. A green colorimetric approach towards detection of arsenic (III): A pervasive environmental pollutant. Opt. Laser Technol. 2019, 111, 825–829. [Google Scholar] [CrossRef]
- Boruah, B.S.; Daimari, N.K.; Biswas, R. Mangifera Indica Leaf Extract Mediated Gold Nanoparticles: A Novel Platform for Sensing of As(III). IEEE Sens. Lett. 2019, 3, 1–3. [Google Scholar] [CrossRef]
- Boruah, B.S.; Biswas, R. Selective detection of arsenic (III) based on colorimetric approach in aqueous medium using functionalized gold nanoparticles unit. Mater. Res. Express 2018, 5, 015059. [Google Scholar] [CrossRef]
- Gong, L.; Du, B.; Pan, L.; Liu, Q.; Yang, K.; Wang, W.; Zhao, H.; Wu, L.; He, Y. Colorimetric aggregation assay for arsenic(III) using gold nanoparticles. Microchim. Acta 2017, 184, 1185–1190. [Google Scholar] [CrossRef]
- Shrivas, K.; Patel, S.; Sinha, D.; Thakur, S.S.; Patle, T.K.; Kant, T.; Dewangan, K.; Satnami, M.L.; Nirmalkar, J.; Kumar, S. Colorimetric and smartphone-integrated paper device for on-site determination of arsenic (III) using sucrose modified gold nanoparticles as a nanoprobe. Microchim. Acta 2020, 187, 173. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.; Liu, J.; Dumée, L.F.; Singh, B.R.; Shukla, S.; Yang, W. Arsenic ion assisted core–satellites nano-assembly of gold nanoparticles for its colorimetric determination in water. J. Water Process Eng. 2022, 48, 102833. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Ding, J.; Hayat, K.; Zhan, X.; Zhou, P.; Zhang, D. Label-free colorimetric assay for arsenic(III) determination based on a truncated short ssDNA and gold nanoparticles. Microchim. Acta 2021, 188, 38. [Google Scholar] [CrossRef] [PubMed]
- Harisha, K.S.; Narayana, B.; Sangappa, Y. Highly selective and sensitive colorimetric detection of arsenic(III) in aqueous solution using green synthesized unmodified gold nanoparticles. J. Dispers. Sci. Technol. 2023, 44, 132–143. [Google Scholar] [CrossRef]
- Thao Nguyen, N.L.; Park, C.Y.; Park, J.P.; Kailasa, S.K.; Park, T.J. Synergistic molecular assembly of an aptamer and surfactant on gold nanoparticles for the colorimetric detection of trace levels of As3+ ions in real samples. New J. Chem. 2018, 42, 11530–11538. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, L.; Zhan, S.; Wang, F.; Zhou, P. Ultrasensitive aptamer biosensor for arsenic(iii) detection in aqueous solution based on surfactant-induced aggregation of gold nanoparticles. Analyst 2012, 137, 4171–4178. [Google Scholar] [CrossRef]
- Siangproh, W.; Chailapakul, O.; Songsrirote, K. Simple and fast colorimetric detection of inorganic arsenic selectively adsorbed onto ferrihydrite-coated silica gel using silver nanoplates. Talanta 2016, 153, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Shi, Y.; Zhang, R.; Zhao, F.; Liu, F.; Liu, L. Simple, rapid and label-free colorimetric assay for arsenic based on unmodified gold nanoparticles and a phytochelatin-like peptide. Anal. Methods 2012, 4, 3937–3941. [Google Scholar] [CrossRef]
- Ghodake, G.; Vassiliadis, V.S.; Choi, J.-H.; Jang, J.; Lee, D.S. Facile Synthesis of Gold Nanoparticles by Amino Acid Asparagine: Selective Sensing of Arsenic. J. Nanosci. Nanotechnol. 2015, 15, 7235–7239. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, X.; Niu, X.; Pan, J. Colorimetric detection and membrane removal of arsenate by a multifunctional L-arginine modified FeOOH. Sep. Purif. Technol. 2021, 258, 118021. [Google Scholar] [CrossRef]
- Wang, J.; Tao, H.; Lu, T.; Wu, Y. Adsorption enhanced the oxidase-mimicking catalytic activity of octahedral-shape Mn3O4 nanoparticles as a novel colorimetric chemosensor for ultrasensitive and selective detection of arsenic. J. Colloid Interface Sci. 2021, 584, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.-H.; Zhong, X.-L.; Wu, Y.-D.; Liang, R.-P.; Zhang, L.; Qiu, J.-D. Colorimetric Assay Conversion to Highly Sensitive Electrochemical Assay for Bimodal Detection of Arsenate Based on Cobalt Oxyhydroxide Nanozyme via Arsenate Absorption. Anal. Chem. 2019, 91, 6487–6497. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Ramrakhiani, L.; Mukherjee, D.; Mishra, U.; Halder, A.; Mandal, A.K.; Ghosh, S. Green synthesis of iron oxide nanoparticles for arsenic remediation in water and sludge utilization. Clean Technol. Environ. Policy 2019, 21, 795–813. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. DNA adsorption by magnetic iron oxide nanoparticles and its application for arsenate detection. Chem. Commun. 2014, 50, 8568–8570. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, G.; Xia, T.; Su, X. Ultrasensitive fluorescent nanosensor for arsenate assay and removal using oligonucleotide-functionalized CuInS2 quantum dot@magnetic Fe3O4 nanoparticles composite. Sens. Actuators B Chem. 2015, 220, 1205–1211. [Google Scholar] [CrossRef]
- Zhong, X.-L.; Wen, S.-H.; Wang, Y.; Luo, Y.-X.; Li, Z.-M.; Liang, R.-P.; Zhang, L.; Qiu, J.-D. Colorimetric and electrochemical arsenate assays by exploiting the peroxidase-like activity of FeOOH nanorods. Microchim. Acta 2019, 186, 732. [Google Scholar] [CrossRef]
- Vaid, K.; Dhiman, J.; Kumar, S.; Kim, K.-H.; Kumar, V. Mixed metal (cobalt/molybdenum) based metal-organic frameworks for highly sensitive and specific sensing of arsenic (V): Spectroscopic versus paper-based approaches. Chem. Eng. J. 2021, 426, 131243. [Google Scholar] [CrossRef]
- Banerjee, S.; Kumar, N.P.; Srinivas, A.; Roy, S. Core-shell Fe3O4@Au nanocomposite as dual-functional optical probe and potential removal system for arsenic (III) from Water. J. Hazard. Mater. 2019, 375, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Yu, M.; Lv, J.; Wang, L.; Zhou, P. Colorimetric Detection of Trace Arsenic(III) in Aqueous Solution Using Arsenic Aptamer and Gold Nanoparticles. Aust. J. Chem. 2014, 67, 813–818. [Google Scholar] [CrossRef]
- Xu, X.; Wang, L.; Zou, X.; Wu, S.; Pan, J.; Li, X.; Niu, X. Highly sensitive colorimetric detection of arsenite based on reassembly-induced oxidase-mimicking activity inhibition of dithiothreitol-capped Pd nanozyme. Sens. Actuators B Chem. 2019, 298, 126876. [Google Scholar] [CrossRef]
- Bhat, A.; Ravi, K.; Tian, F.; Singh, B. Arsenic Contamination Needs Serious Attention: An Opinion and Global Scenario. Pollutants 2024, 4, 196–211. [Google Scholar] [CrossRef]
- Huang, Y.; Miu, Q.; Kwong, R.W.M.; Zhang, D.; Fan, Y.; Zhou, M.; Yan, X.; Jia, J.; Yan, B.; Li, C. Leveraging the One Health concept for arsenic sustainability. Eco-Environ. Health 2024. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Costa, M. Arsenic: A Global Environmental Challenge. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 47–63. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]

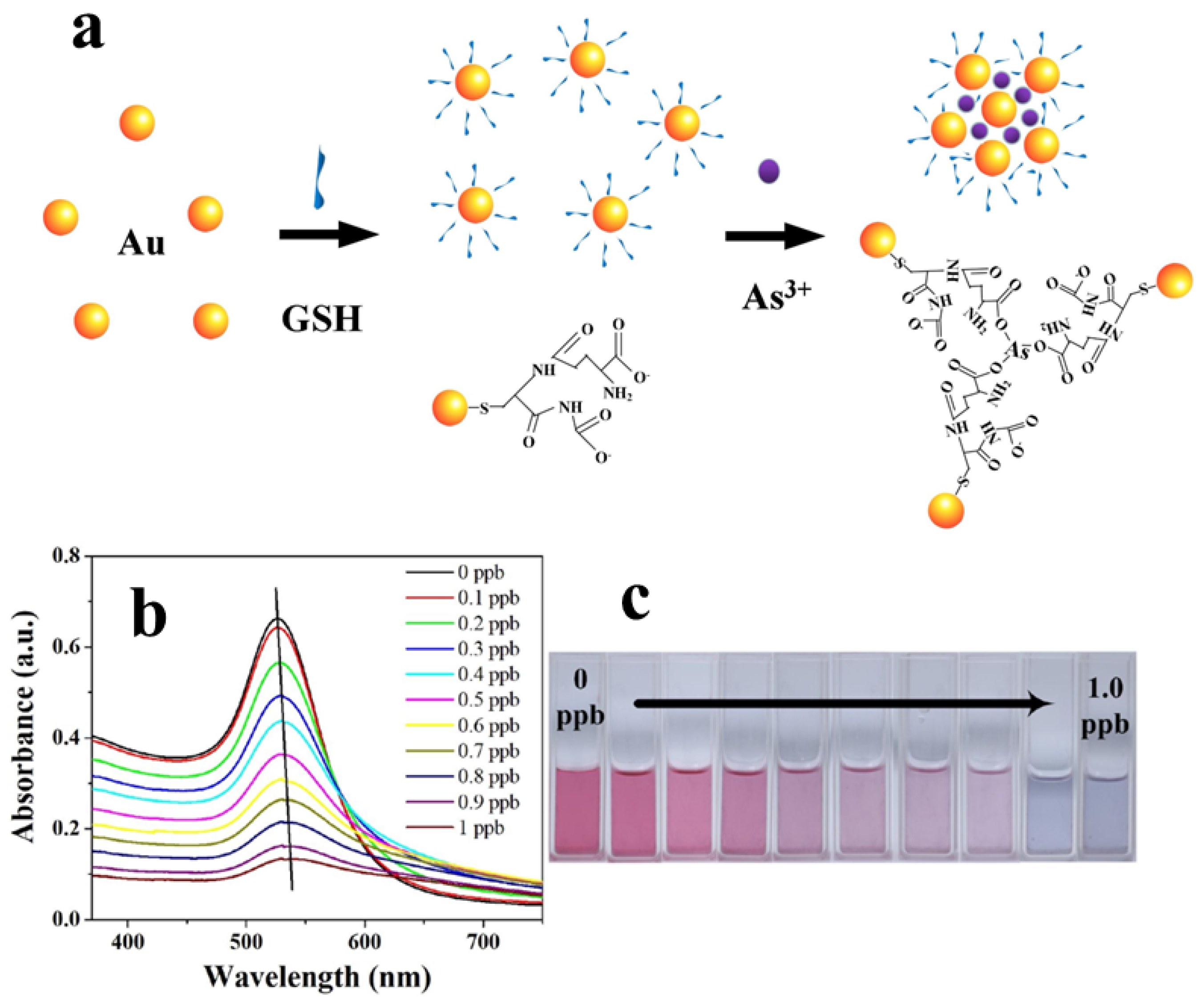
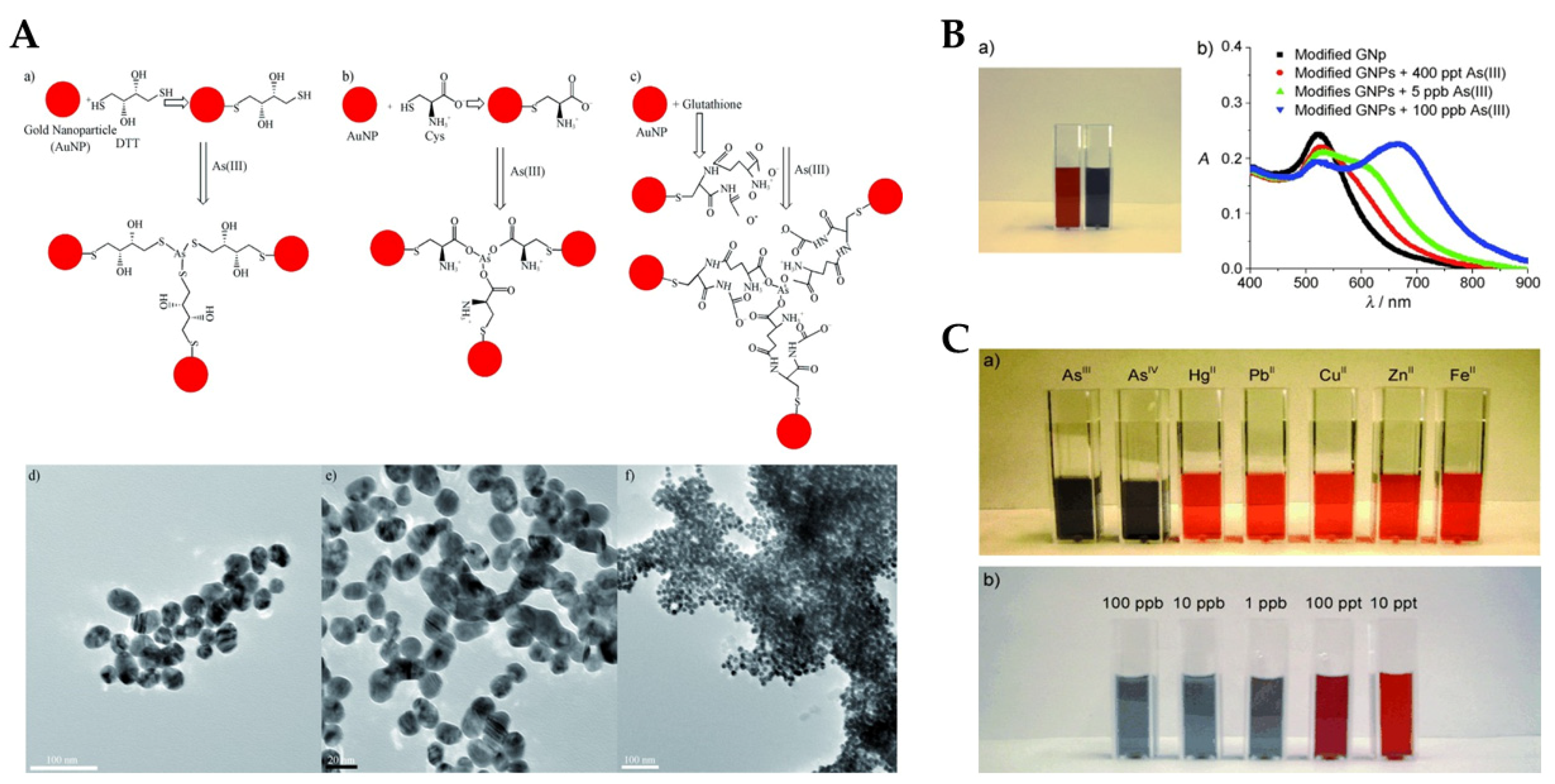
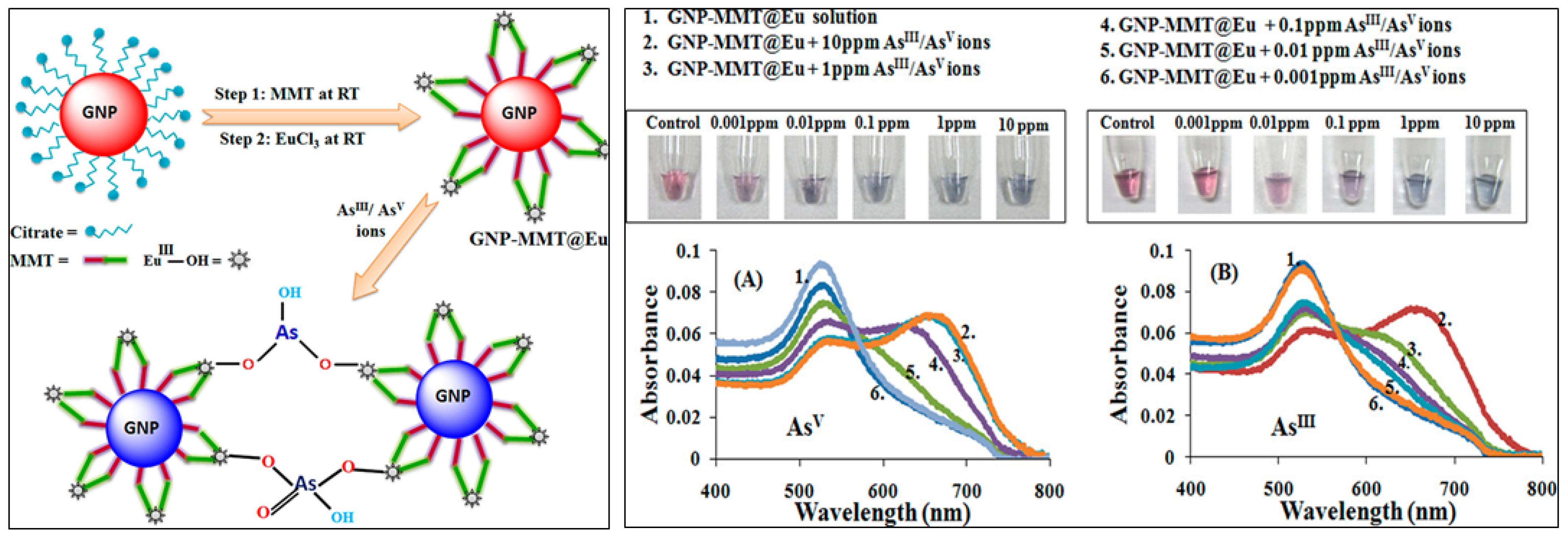


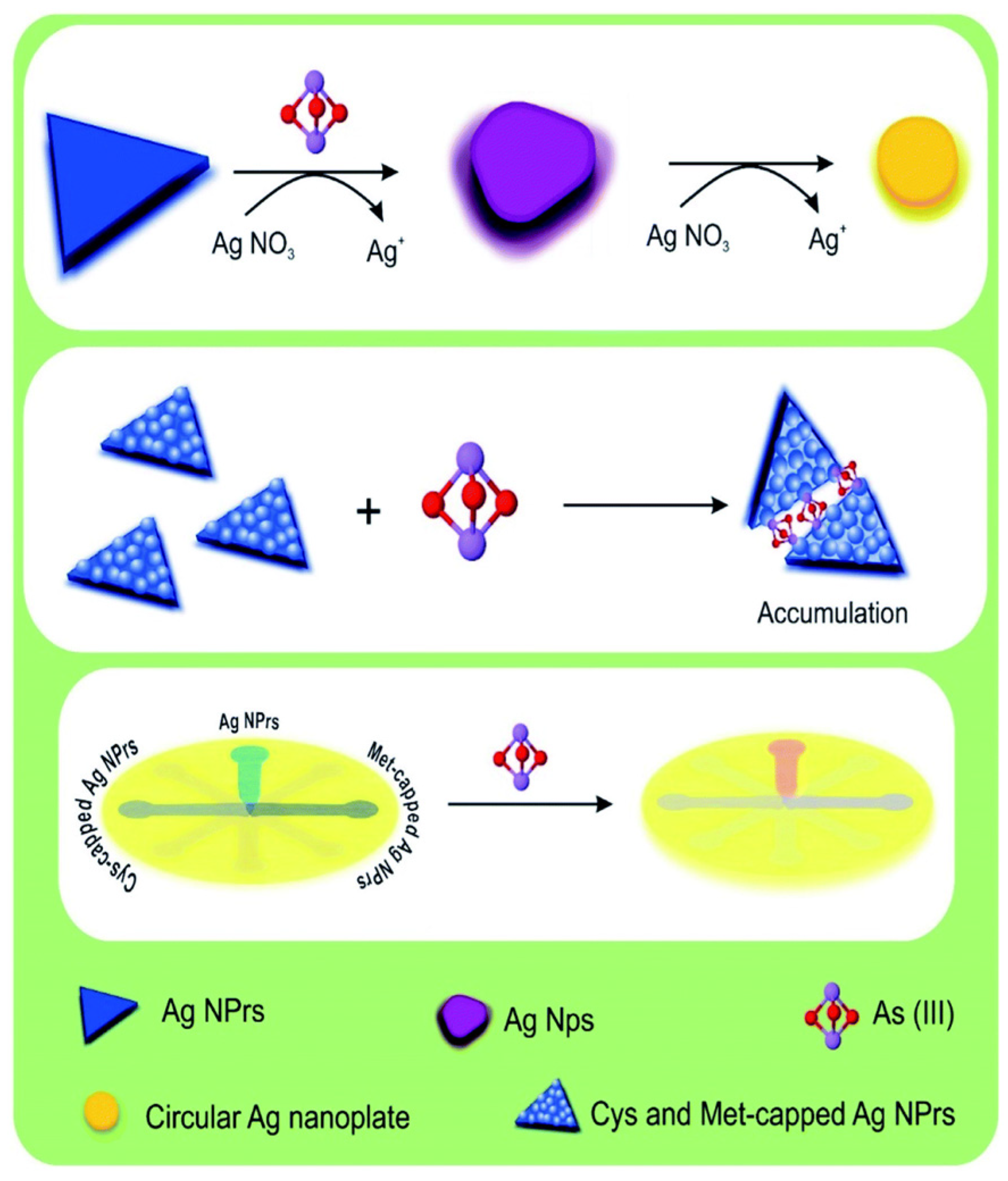

| Commercial Colorimetric Kit | Theoretical LOD (ppb) | Practical LOD (ppb) | Reliability (ppb) | Cost per Sample (USD) | Time per Sample (min) | Features | Ref. |
|---|---|---|---|---|---|---|---|
| NIPSOM | 10 | >20 | Unreliable < 70 | 0.4 | 5 | Colour sensitivity to yellow; working quickly. | [40,41] |
| Merck | 10 | >50 | Unreliable < 70 | 0.5–1.00 | 30 | Colour sensitivity to yellow; working quickly. | [40,41,42] |
| GPL | 10 | NA | Unreliable < 70 | 0.4 | 20 | Colour sensitivity to yellow; working quickly. | [40] |
| AAIH and PH | 50 | >50 | Unreliable < 70 | 0.4 | NA | Colour sensitivity to yellow; working quickly. | [40,41] |
| AAN | 10 | >20 | Unreliable < 70 | 0.4 | 30 | Colour sensitivity to yellow; working quickly. | [40,41,42] |
| Quick As | 5 | NA | Can identify samples > 15 | 1.00–2.00 | NA | Colour sensitivity to yellow; working quickly. | [43] |
| Hach Ez | 10 | NA | Can identify samples > 15 | <1–2.00 | 20–40 | Colour sensitivity to yellow; working quickly. | [42,43] |
| Arsenator | 0.5–2 | NA | Found to be correct 85% of the time, more reliable at lower concentrations | 1.00 | 20 | Ability to make accurate dilutions | [42,44] |
| No | Metal Nanoparticles | Analyte | Detection Method | Limit of Detection (ppb) | Range of Detection (ppb) | Reference |
|---|---|---|---|---|---|---|
| 1 | Dithiothreitol coated Au-Nanorods | As(III) | UV-Vis spectrometry | 10 | 10–100.1 | [87] |
| 2 | AuNPs DNA aptamer | As(III) | UV-Vis spectrometry | 161 | 76.6–766 | [88] |
| 3 | GSH-functionalized AuNPs | As(III) | RGB extracting software | 0.12 | - | [70] |
| 4 | Glucose–AuNPs | As(III) | Naked eye | 0.53 | 1–14 | [89] |
| 5 | Mangifera indica leaf extract–AuNPs | As(III) | UV-Vis spectrometry | 1.2 | - | [90] |
| 6 | AuNPs-PEG | As(III) | UV-Vis spectrometry | 5.0 | - | [91] |
| 7 | Citrate-capped AuNPs | As(III) | UV-Vis spectrometry | 1.8 | 4–100 | [92] |
| 8 | Sucrose–AuNPs | As(III) | UV-Vis spectrometry | 20 | 50–3000 | [93] |
| 9 | GNP-MMT@Eu | As(III), As(V) | Naked eye | ≤10.0 | - | [74] |
| 10 | PEG–AgNPs | As(III) | UV–Vis spectrometry | 1.0 | 5–13 | [77] |
| 11 | Amino acid smart nanoprobe AuNPs | As(III) | UV–Vis spectrometry | 0.22 | 1–200 | [94] |
| 12 | ssDNA (Apt-21) AuNPs | As(III) | UV–Vis spectrometry | 0.18 | 1–100 | [95] |
| 13 | ss-AuNPs | As(III) | UV–Vis spectrometry | 2.94 | 1–10 | [96] |
| 14 | Ars-3 aptamer-CTAB-AuNPs | As(III) | UV-Vis spectrometry | 16.9 | 1–100 | [97] |
| 15 | RS-based Aptasensor with Aggregated AuNPs | As(III) | Naked eye | 40 | 1–1500 | [98] |
| 16 | GSH/DTT/Asn–AgNPs | As(III), As(V) | UV-Vis spectrometry | 0.36 | 0.4–20 | [71] |
| 17 | Ag-containing MOF | As(III) | Naked eye | 50 | n.a. | [84] |
| 18 | AgNPls-SiO2-Fh | As(III), As(V) | Naked eye | 500 | 500–3000 | [99] |
| 19 | Peptide–AuNPs | As(III), As(V) | Naked eye | 1.54 | n.a. | [100] |
| 20 | Asparagine–AuNPs | As(III) | UV-Vis spectrometry | 100 | 100–2000 | [101] |
| 21 | L-arginine-modified FeOOH | As(V) | UV-Vis spectrometry | 0.42 | 0.67–3333.33 | [102] |
| 22 | Oxidase-mimicking activity of Mn3O4 NPs | As(III), As(V) | Naked eye | 1.32 | 5–1000 | [103] |
| 23 | Cobalt oxyhydroxide (CoOOH) nanoflakes | As(V) | UV-Vis spectrometry | 3.72 | 4–500 | [104] |
| 24 | α-Fe2O3 | As(V) | UV-Vis spectrometry | - | 100–2000 | [105] |
| 25 | DNA-functionalized Fe3O4 nanoparticles | As(V) | Fluorescence quenching | 0.95 | - | [106] |
| 26 | CuInS2 quantum dots@magnetic Fe3O4 | As(V) | Fluorescence quenching | 10 | 0.015–15,384.6 | [107] |
| 27 | Iron oxyhydroxide (FeOOH) nanorods | As(V) | UV-Vis spectrometry | 0.012 | 0.04–200 | [108] |
| 28 | Co/Mo-MOF | As(V) | Naked eye | 0.02 | 0.05–106 | [109] |
| 29 | Fe3O4 (core)-gold (shell)-thiol ligands | As(III) | UV-Vis spectrometry | 0.86 | - | [110] |
| 30 | Aptamer–AsIII complex | As(III) | - | 1.26 | 1.26–200 | [111] |
| 31 | Dithiothreitol-capped Pd nanoparticles | As(III) | Naked eye | 0.035 | 0.033–333.3 | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, A.; Tian, F.; Singh, B. Advances in Nanomaterials and Colorimetric Detection of Arsenic in Water: Review and Future Perspectives. Sensors 2024, 24, 3889. https://doi.org/10.3390/s24123889
Bhat A, Tian F, Singh B. Advances in Nanomaterials and Colorimetric Detection of Arsenic in Water: Review and Future Perspectives. Sensors. 2024; 24(12):3889. https://doi.org/10.3390/s24123889
Chicago/Turabian StyleBhat, Abhijnan, Furong Tian, and Baljit Singh. 2024. "Advances in Nanomaterials and Colorimetric Detection of Arsenic in Water: Review and Future Perspectives" Sensors 24, no. 12: 3889. https://doi.org/10.3390/s24123889








