Intervention-Induced Changes in Balance and Task-Dependent Neural Activity in Adults with Acquired Brain Injury: A Pilot Randomized Control Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Group Allocation
2.4. Outcome Measures
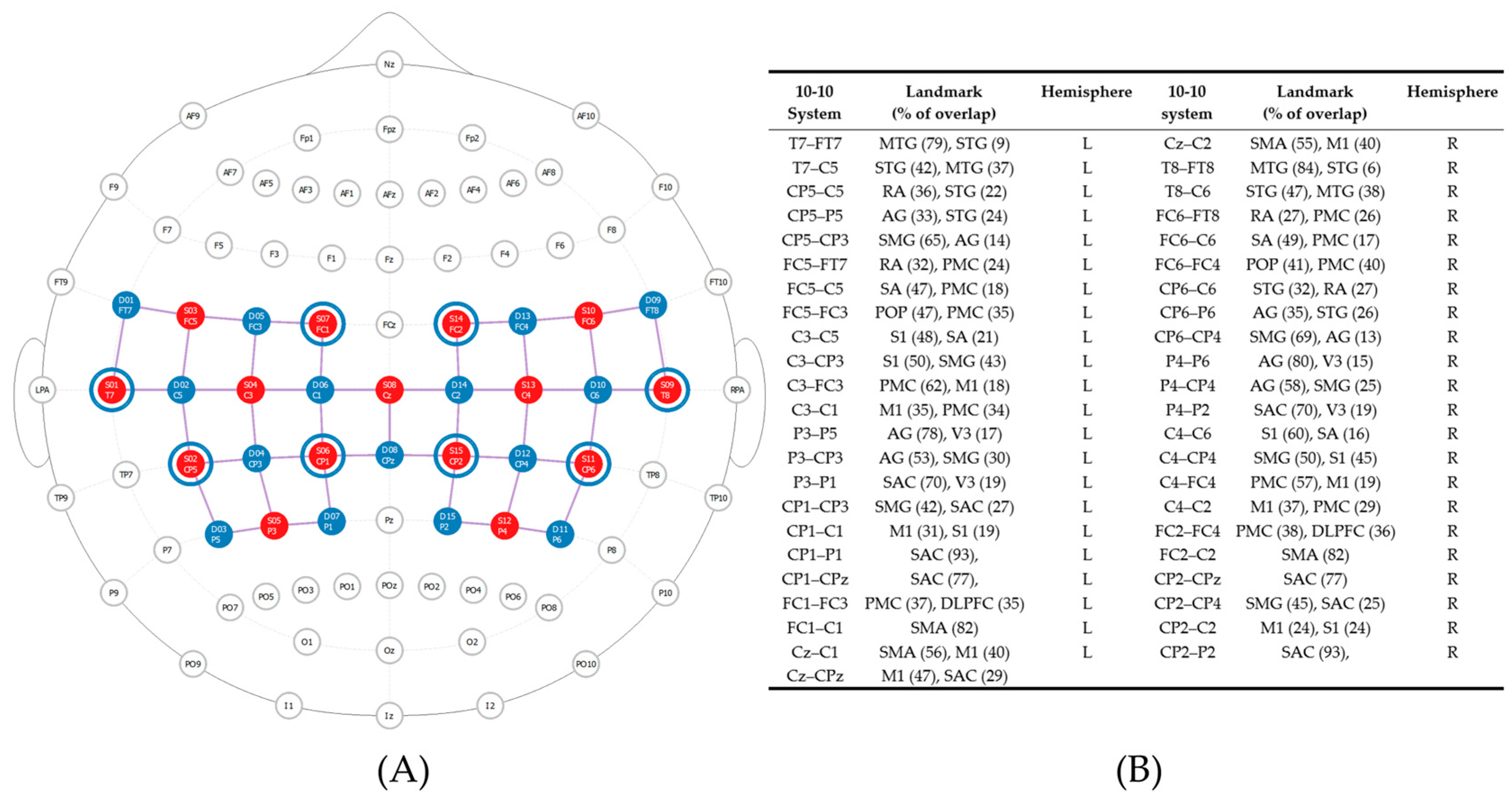
2.4.1. Anatomical Brain Scans
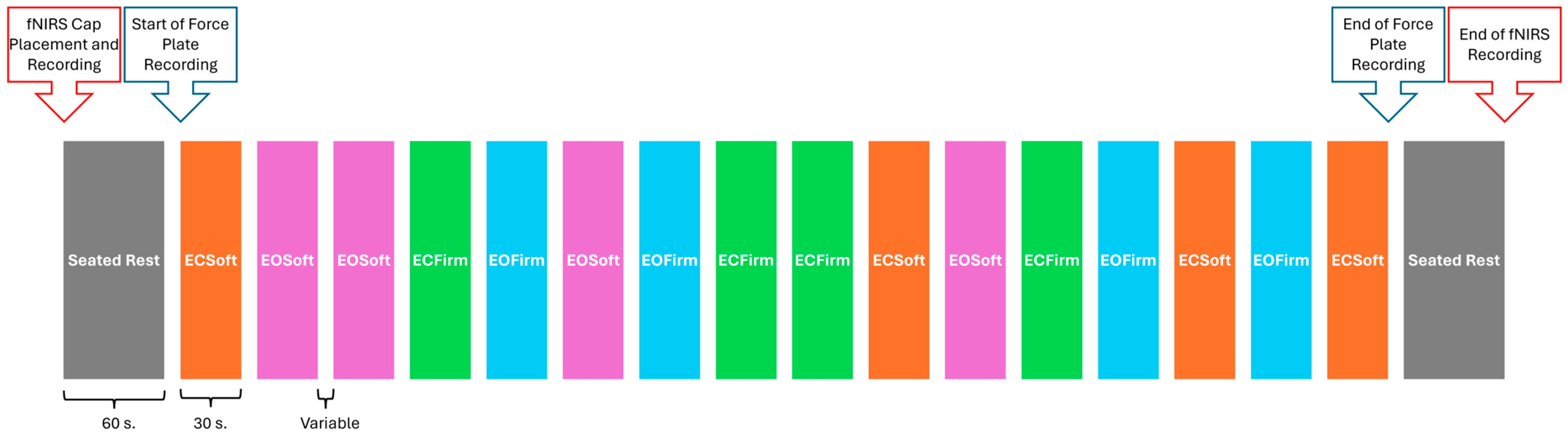
2.4.2. Balance Performance and Task-Dependent Neural Activity
Measures
Procedure
2.4.3. Force Plate Balance Data Processing
2.4.4. fNIRS Task-Dependent Neural Activity Data Processing
fNIRS Processing Steps
fNIRS Data Quality
2.5. Study Intervention
2.6. Statistical Analysis
3. Results
3.1. Baseline Differences between Groups
3.2. Balance—Center of Pressure
3.2.1. Balance—Main Effects of Time
3.2.2. Balance—Interaction Effects and Linear Contrasts
3.2.3. Balance—Simple (Within-Group) Effects
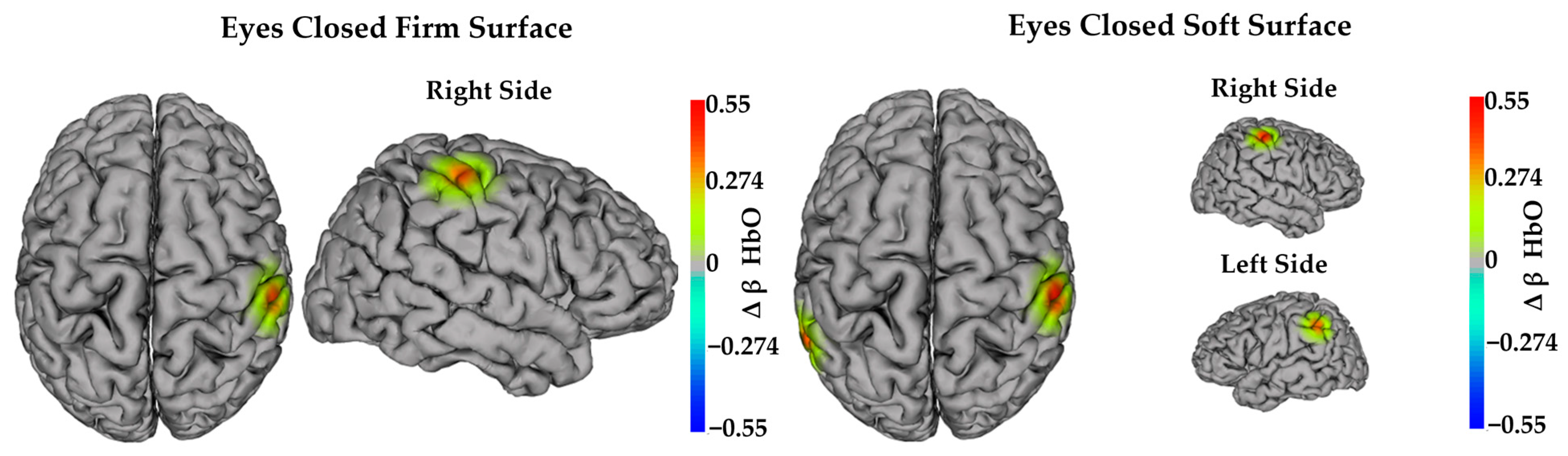
3.3. Task-Dependent Neural Activity
3.3.1. Task-Dependent Neural Activity—Main Effects of Time
3.3.2. Task-Dependent Neural Activity—Interaction Effects and Linear Contrasts
3.3.3. Task-Dependent Activity—Simple (Within-Group) Effects
4. Discussion
Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brain Injury Association of America. What Is the Difference between an Acquired Brain Injury and a Traumatic Brain Injury? Available online: https://www.biausa.org/brain-injury/about-brain-injury/nbiic/what-is-the-difference-between-an-acquired-brain-injury-and-a-traumatic-brain-injury (accessed on 19 February 2024).
- Goldman, L.; Siddiqui, E.M.; Khan, A.; Jahan, S.; Rehman, M.U.; Mehan, S.; Sharma, R.; Budkin, S.; Kumar, S.N.; Sahu, A.; et al. Understanding Acquired Brain Injury: A Review. Biomedicines 2022, 10, 2167. [Google Scholar] [CrossRef] [PubMed]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The Epidemiology and Impact of Traumatic Brain Injury: A Brief Overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics—2023 Update: A Report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [PubMed]
- Hoofien, D.; Gilboa, A.; Vakil, E.; Donovick, P.J. Traumatic Brain Injury (TBI) 10–20 Years Later: A Comprehensive Outcome Study of Psychiatric Symptomatology, Cognitive Abilities and Psychosocial Functioning. Brain Inj. 2001, 15, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Dikmen, S.S.; Machamer, J.E.; Powell, J.M.; Temkin, N.R. Outcome 3 to 5 Years After Moderate to Severe Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2003, 84, 1449–1457. [Google Scholar] [CrossRef]
- Whiteneck, G.G.; Cuthbert, J.P.; Corrigan, J.D.; Bogner, J.A. Prevalence of Self-Reported Lifetime History of Traumatic Brain Injury and Associated Disability: A Statewide Population-Based Survey. J. Head Trauma Rehabil. 2016, 31, E55. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.; Stewart, W.; Dams-O’Connor, K.; Diaz-Arrastia, R.; Horton, L.; Menon, D.K.; Polinder, S. The Chronic and Evolving Neurological Consequences of Traumatic Brain Injury. Lancet Neurol. 2017, 16, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Ingersoll, C.D.; Armstrong, C.W. The Effects of Closed-Head Injury on Postural Sway. Med. Sci. Sports Exerc. 1992, 24, 739. [Google Scholar] [CrossRef]
- Johnson, L.; Williams, G.; Sherrington, C.; Pilli, K.; Chagpar, S.; Auchettl, A.; Beard, J.; Gill, R.; Vassallo, G.; Rushworth, N.; et al. The Effect of Physical Activity on Health Outcomes in People with Moderate-to-Severe Traumatic Brain Injury: A Rapid Systematic Review with Meta-Analysis. BMC Public Health 2023, 23, 63. [Google Scholar] [CrossRef]
- Schmid, A.A.; Van Puymbroeck, M.; Altenburger, P.A.; Schalk, N.L.; Dierks, T.A.; Miller, K.K.; Damush, T.M.; Bravata, D.M.; Williams, L.S. Poststroke Balance Improves With Yoga. Stroke 2012, 43, 2402–2407. [Google Scholar] [CrossRef]
- Stephens, J.A.; Van Puymbroeck, M.; Sample, P.L.; Schmid, A.A. Yoga Improves Balance, Mobility, and Perceived Occupational Performance in Adults with Chronic Brain Injury: A Preliminary Investigation. Complement. Ther. Clin. Pract. 2020, 40, 101172. [Google Scholar] [CrossRef]
- Schmid, A.A.; Miller, K.K.; Van Puymbroeck, M.; Schalk, N. Feasibility and Results of a Case Study of Yoga to Improve Physical Functioning in People with Chronic Traumatic Brain Injury. Disabil. Rehabil. 2016, 38, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Gothe, N.P.; Khan, I.; Hayes, J.; Erlenbach, E.; Damoiseaux, J.S. Yoga Effects on Brain Health: A Systematic Review of the Current Literature. Brain Plast. 2019, 5, 105–122. [Google Scholar] [CrossRef]
- Gothe, N.P.; Hayes, J.M.; Temali, C.; Damoiseaux, J.S. Differences in Brain Structure and Function Among Yoga Practitioners and Controls. Front. Integr. Neurosci. 2018, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Surgent, O.J.; Dadalko, O.I.; Pickett, K.A.; Travers, B.G. Balance and the Brain: A Review of Structural Brain Correlates of Postural Balance and Balance Training in Humans. Gait Posture 2019, 71, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, B.W.; Bekkers, E.M.J.; Gilat, M.; De Rond, V.; Hardwick, R.M.; Nieuwboer, A. Functional Neuroimaging of Human Postural Control: A Systematic Review with Meta-Analysis. Neurosci. Biobehav. Rev. 2020, 115, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, Y.; Okada, H.; Yoshikawa, E.; Nobezawa, S.; Futatsubashi, M. Brain Activation during Maintenance of Standing Postures in Humans. Brain 1999, 122, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, Y.; Okada, H.; Yoshikawa, E.; Futatsubashi, M.; Nobezawa, S. Absolute Changes in Regional Cerebral Blood Flow in Association with Upright Posture in Humans: An Orthostatic PET Study. J. Nucl. Med. 2021, 42, 707–712. [Google Scholar]
- Pinti, P.; Aichelburg, C.; Gilbert, S.; Hamilton, A.; Hirsch, J.; Burgess, P.; Tachtsidis, I. A Review on the Use of Wearable Functional Near-Infrared Spectroscopy in Naturalistic Environments. Jpn. Psychol. Res. 2018, 60, 347–373. [Google Scholar] [CrossRef]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The Present and Future Use of Functional Near-infrared Spectroscopy (fNIRS) for Cognitive Neuroscience. Ann. N. Y. Acad. Sci. 2020, 1464, 5–29. [Google Scholar] [CrossRef]
- Scholkmann, F.; Kleiser, S.; Metz, A.J.; Zimmermann, R.; Mata Pavia, J.; Wolf, U.; Wolf, M. A Review on Continuous Wave Functional Near-Infrared Spectroscopy and Imaging Instrumentation and Methodology. NeuroImage 2014, 85, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Stephens, J.A.; Press, D.; Atkins, J.; Duffy, J.R.; Thomas, M.L.; Weaver, J.A.; Schmid, A.A. Feasibility of Acquiring Neuroimaging Data from Adults with Acquired Brain Injuries before and after a Yoga Intervention. Brain Sci. 2023, 13, 1413. [Google Scholar] [CrossRef] [PubMed]
- Herold, F.; Wiegel, P.; Scholkmann, F.; Thiers, A.; Hamacher, D.; Schega, L. Functional Near-Infrared Spectroscopy in Movement Science: A Systematic Review on Cortical Activity in Postural and Walking Tasks. Neurophoton 2017, 4, 041403. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Miyai, I.; Hattori, N.; Hatakenaka, M.; Yagura, H.; Kawano, T.; Kubota, K. Cortical Control of Postural Balance in Patients with Hemiplegic Stroke. NeuroReport 2012, 23, 314. [Google Scholar] [CrossRef] [PubMed]
- Helmich, I.; Berger, A.; Lausberg, H. Neural Control of Posture in Individuals with Persisting Postconcussion Symptoms. Med. Sci. Sports Exerc. 2016, 48, 2362–2369. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, H.; Mihara, M.; Hattori, N.; Hatakenaka, M.; Kawano, T.; Yagura, H.; Miyai, I.; Mochizuki, H. Cortical Changes Underlying Balance Recovery in Patients with Hemiplegic Stroke. NeuroImage 2014, 85, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Leach, H.J.; Hidde, M.C.; Portz, J.D.; Van Puymbroeck, M.; Sharp, J.L.; Fox, A.L.; Schmid, A.A.; Fruhauf, C.A. Matching Exercise Volume in Active Control Groups for Yoga Interventions. Altern. Ther. Health Med. 2023, 29, 237–241. [Google Scholar]
- Stephens, J.A.; Hernandez-Sarabia, J.A.; Sharp, J.L.; Leach, H.J.; Bell, C.; Thomas, M.L.; Buryznska, A.Z.; Weaver, J.A.; Schmid, A.A. Adaptive Yoga versus Low-Impact Exercise for Adults with Chronic Acquired Brain Injury: A Pilot Randomized Control Trial Protocol. Front. Hum. Neurosci. 2023, 17, 1291094. [Google Scholar] [CrossRef] [PubMed]
- King, P.R.; Donnelly, K.T.; Donnelly, J.P.; Dunnam, M.; Warner, G.; Kittleson, C.J.; Bradshaw, C.B.; Alt, M.; Meier, S.T. Psychometric Study of the Neurobehavioral Symptom Inventory. J. Rehabil. Res. Dev. 2012, 49, 879–888. [Google Scholar] [CrossRef]
- Goble, D.J.; Khan, E.; Baweja, H.S.; O’Connor, S.M. A Point of Application Study to Determine the Accuracy, Precision and Reliability of a Low-Cost Balance Plate for Center of Pressure Measurement. J. Biomech. 2018, 71, 277–280. [Google Scholar] [CrossRef]
- O’Connor, S.M.; Baweja, H.S.; Goble, D.J. Validating the BTrackS Balance Plate as a Low Cost Alternative for the Measurement of Sway-Induced Center of Pressure. J. Biomech. 2016, 49, 4142–4145. [Google Scholar] [CrossRef] [PubMed]
- Richmond, S.B.; Dames, K.D.; Goble, D.J.; Fling, B.W. Leveling the Playing Field: Evaluation of a Portable Instrument for Quantifying Balance Performance. J. Biomech. 2018, 75, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Zimeo Morais, G.A.; Balardin, J.B.; Sato, J.R. fNIRS Optodes’ Location Decider (fOLD): A Toolbox for Probe Arrangement Guided by Brain Regions-of-Interest. Sci. Rep. 2018, 8, 3341. [Google Scholar] [CrossRef] [PubMed]
- Brigadoi, S.; Cooper, R.J. How Short Is Short? Optimum Source–Detector Distance for Short-Separation Channels in Functional near-Infrared Spectroscopy. NPh 2015, 2, 025005. [Google Scholar] [CrossRef] [PubMed]
- Tachtsidis, I.; Scholkmann, F. False Positives and False Negatives in Functional Near-Infrared Spectroscopy: Issues, Challenges, and the Way Forward. Neurophoton 2016, 3, 031405. [Google Scholar] [CrossRef] [PubMed]
- Peirce, J.; Gray, J.R.; Simpson, S.; MacAskill, M.; Höchenberger, R.; Sogo, H.; Kastman, E.; Lindeløv, J.K. PsychoPy2: Experiments in Behavior Made Easy. Behav. Res. 2019, 51, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Peirce, J.W. PsychoPy—Psychophysics Software in Python. J. Neurosci. Methods 2007, 162, 8–13. [Google Scholar] [CrossRef]
- Peirce, J.W. Generating Stimuli for Neuroscience Using PsychoPy. Front. Neuroinform. 2008, 2, 343. [Google Scholar] [CrossRef]
- Goble, D.J.; Brown, E.C.; Marks, C.R.C.; Baweja, H.S. Expanded Normative Data for the Balance Tracking System Modified Clinical Test of Sensory Integration and Balance Protocol. Med. Devices Sens. 2020, 3, e10084. [Google Scholar] [CrossRef]
- Prieto, T.E.; Myklebust, J.B.; Hoffmann, R.G.; Lovett, E.G.; Myklebust, B.M. Measures of Postural Steadiness: Differences between Healthy Young and Elderly Adults. IEEE Trans. Biomed. Eng. 1996, 43, 956–966. [Google Scholar] [CrossRef]
- Baker, W.B.; Parthasarathy, A.B.; Busch, D.R.; Mesquita, R.C.; Greenberg, J.H.; Yodh, A.G. Modified Beer-Lambert Law for Blood Flow. Biomed. Opt. Express 2014, 5, 4053. [Google Scholar] [CrossRef] [PubMed]
- Fishburn, F.A.; Ludlum, R.S.; Vaidya, C.J.; Medvedev, A.V. Temporal Derivative Distribution Repair (TDDR): A Motion Correction Method for fNIRS. NeuroImage 2019, 184, 171–179. [Google Scholar] [CrossRef]
- Plichta, M.M.; Herrmann, M.J.; Baehne, C.G.; Ehlis, A.-C.; Richter, M.M.; Pauli, P.; Fallgatter, A.J. Event-Related Functional near-Infrared Spectroscopy (fNIRS) Based on Craniocerebral Correlations: Reproducibility of Activation? Hum. Brain Mapp. 2007, 28, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Pollonini, L.; Olds, C.; Abaya, H.; Bortfeld, H.; Beauchamp, M.S.; Oghalai, J.S. Auditory Cortex Activation to Natural Speech and Simulated Cochlear Implant Speech Measured with Functional Near-Infrared Spectroscopy. Hear. Res. 2014, 309, 84–93. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R.J.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A Second Update of Codes and MET Values. Med. Sci. Sports Exerc. 2011, 43, 1575. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Ontario, CA, USA, 1998. [Google Scholar]
- Wang, Y.; Yan, J.; Wen, J.; Yu, T.; Li, X. An Intracranial Electroencephalography (iEEG) Brain Function Mapping Tool with an Application to Epilepsy Surgery Evaluation. Front. Neuroinform. 2016, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.; Preacher, K.J. On Effect Size. Psychol. Methods 2012, 17, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, J.A. Qualitative Descriptors of Strength of Association and Effect Size. J. Soc. Serv. Res. 1996, 21, 37–59. [Google Scholar] [CrossRef]
- Berg, K.; Wood-Dauphinee, S.; Williams, J. The Balance Scale: Reliability Assessment with Elderly Residents and Patients with an Acute Stroke. Scand. J. Rehabil. Med. 1995, 27, 27–36. [Google Scholar]
- Hyndman, D.; Ashburn, A.; Yardley, L.; Stack, E. Interference between Balance, Gait and Cognitive Task Performance among People with Stroke Living in the Community. Disabil. Rehabil. 2006, 28, 849–856. [Google Scholar] [CrossRef]
- Palmisano, S.; Fasotti, L.; Bertens, D. Neurobehavioral Initiation and Motivation Problems After Acquired Brain Injury. Front. Neurol. 2020, 11, 23. [Google Scholar] [CrossRef]
- Strotzer, M. One Century of Brain Mapping Using Brodmann Areas. Clin. Neuroradiol. 2009, 19, 179–186. [Google Scholar] [CrossRef]
- Takakura, H.; Nishijo, H.; Ishikawa, A.; Shojaku, H. Cerebral Hemodynamic Responses During Dynamic Posturography: Analysis with a Multichannel Near-Infrared Spectroscopy System. Front. Hum. Neurosci. 2015, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Clower, D.M.; West, R.A.; Lynch, J.C.; Strick, P.L. The Inferior Parietal Lobule Is the Target of Output from the Superior Colliculus, Hippocampus, and Cerebellum. J. Neurosci. 2001, 21, 6283–6291. [Google Scholar] [CrossRef]
- Kantak, S.S.; Stinear, J.W.; Buch, E.R.; Cohen, L.G. Rewiring the Brain: Potential Role of the Premotor Cortex in Motor Control, Learning, and Recovery of Function Following Brain Injury. Neurorehabil. Neural Repair 2012, 26, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Nachev, P.; Kennard, C.; Husain, M. Functional Role of the Supplementary and Pre-Supplementary Motor Areas. Nat. Rev. Neurosci. 2008, 9, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Karim, H.; Fuhrman, S.I.; Sparto, P.; Furman, J.; Huppert, T. Functional Brain Imaging of Multi-Sensory Vestibular Processing during Computerized Dynamic Posturography Using near-Infrared Spectroscopy. NeuroImage 2013, 74, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Jahn, K.; Deutschländer, A.; Stephan, T.; Kalla, R.; Wiesmann, M.; Strupp, M.; Brandt, T. Imaging Human Supraspinal Locomotor Centers in Brainstem and Cerebellum. NeuroImage 2008, 39, 786–792. [Google Scholar] [CrossRef]
- Tomaiuolo, F.; MacDonald, J.D.; Caramanos, Z.; Posner, G.; Chiavaras, M.; Evans, A.C.; Petrides, M. Morphology, Morphometry and Probability Mapping of the Pars Opercularis of the Inferior Frontal Gyrus: An in Vivo MRI Analysis. Eur. J. Neurosci. 1999, 11, 3033–3046. [Google Scholar] [CrossRef]
- Lobel, E.; Kleine, J.F.; Bihan, D.L.; Leroy-Willig, A.; Berthoz, A. Functional MRI of Galvanic Vestibular Stimulation. J. Neurophysiol. 1998, 80, 2699–2709. [Google Scholar] [CrossRef]



| Groups | |||
|---|---|---|---|
| Yoga (n = 13) | Exercise (n = 10) | ||
| Mean (SD) | Mean (SD) | p-Value | |
| Age (years) | 59.15 ± 15.32 | 41.00 ± 20.81 | 0.03 * |
| Sessions Attended | 13.31 ± 2.87 | 12.70 ± 2.36 | 0.58 |
| Time Since First Brain Injury (years) | 20.23 ± 20.88 | 5.58 ± 5.64 | 0.03 * |
| Sex | n (%) | n (%) | |
| Male | 3 (23) | 5 (50) | 0.13 |
| Female | 10 (77) | 5 (50) | |
| Race/Ethnicity | |||
| White | 13 (100) | 10 (100) | NA |
| Educational Level | |||
| High School | 0 (00) | 3 (30) | 0.15 |
| Some College | 3 (23) | 1 (10) | |
| College Graduate | 6 (46) | 2 (20) | |
| Some Post-Graduate | 1 (08) | 0 (00) | |
| Post-Graduate Degree | 3 (23) | 4 (40) | |
| ABI Type | |||
| Aneurysm | 0 (00) | 1 (10) | 0.94 |
| Carcinoma | 1 (08) | 0 (00) | |
| Hypoxia | 1 (08) | 1 (10) | |
| Stroke | 4 (31) | 3 (30) | |
| TBI | 7 (54) | 5 (50) | |
| Previous Rehabilitation | |||
| Yes | 10 (77) | 8 (80) | 1.00 |
| No | 3 (23) | 2 (20) | |
| Self-Reported Loss of Balance | |||
| Moderate | 10 (77) | 7 (70) | 0.81 |
| Moderate to Severe | 2 (15) | 1 (10) | |
| Severe | 1 (08) | 2 (20) | |
| Depression | |||
| Yes | 10 (77) | 7 (70) | 1.00 |
| No | 3 (23) | 3 (30) | |
| ADD/ADHD | |||
| Yes | 3 (23) | 1 (10) | 0.60 |
| No | 10 (77) | 9 (90) | |
| Sensory Processing Difficulties | |||
| Yes | 2 (15) | 3 (30) | 0.62 |
| No | 11 (85) | 7 (70) | |
| Fainting Episodes? | |||
| Yes | 7 (54) | 4 (40) | 0.68 |
| No | 6 (46) | 6 (60) | |
| Yoga | Exercise | |||||||
|---|---|---|---|---|---|---|---|---|
| Condition | Est. Mean ± SE Δ (cm) | F(df) | p | dCohen | Est. Mean ± SE Δ (cm) | F(df) | p | dCohen |
| APRange EOFirm | −0.61 ± 0.24 | 6.19(1,19.54) | 0.022 * | 0.49 | −0.58 ± 0.28 | 4.25(1,19.21) | 0.053 | 0.46 |
| APRange EOSoft | −0.73 ± 0.26 | 7.57(1,18.56) | 0.013 * | 0.36 | −0.71 ± 0.33 | 4.44(1,18.99) | 0.049 | 0.35 |
| APRange ECFirm | −0.55 ± 0.33 | 2.71(1,19.16) | 0.116 | 0.27 | −0.26 ± 0.39 | 0.44(1,18.92) | 0.517 | 0.12 |
| APRange ECSoft | −1.05 ± 0.43 | 5.91(1,15.65) | 0.028 | 0.42 | −0.92 ± 0.53 | 2.95(1,16.29) | 0.105 | 0.37 |
| COPLength EOFirm | −1.97 ± 4.86 | 0.16(1,20.18) | 0.690 | 0.07 | −8.98 ± 5.82 | 2.39(1,20.52) | 0.138 | 0.30 |
| COPLength EOSoft | −4.06 ± 5.47 | 0.50(1,19.23) | 0.488 | 0.10 | −8.42 ± 7.26 | 1.34(1,19.71) | 0.260 | 0.20 |
| COPLength ECFirm | −8.04 ± 4.71 | 2.91(1,20.07) | 0.103 | 0.18 | −2.42 ± 5.65 | 0.18(1,20.22) | 0.673 | 0.05 |
| COPLength ECSoft | −17.52 ± 8.46 | 4.29(1,16.41) | 0.054 | 0.28 | −29.24 ± 10.53 | 7.70(1,16.82) | 0.013 * | 0.47 |
| MLRange EOFirm | −0.28 ± 0.23 | 1.60(1,19.73) | 0.221 | 0.20 | −0.33 ± 0.27 | 1.54(1,20.06) | 0.229 | 0.24 |
| MLRange EOSoft | −0.52 ± 0.30 | 3.02(1,18.55) | 0.099 | 0.22 | −0.19 ± 0.38 | 0.25(1,18.96) | 0.622 | 0.08 |
| MLRange ECFirm | −0.58 ± 0.30 | 3.74(1,19.48) | 0.068 | 0.13 | 0.06 ± 0.36 | 0.03(1,19.72) | 0.869 | 0.16 |
| MLRange ECSoft | −1.35 ± 0.40 | 11.45(1,16.06) | 0.004 * | 0.50 | −1.63 ± 0.50 | 10.73(1,16.52) | 0.005 * | 0.60 |
| Interaction Effects Group Change (Post − Pre) | Linear Contrast | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Channel (10-10) | Landmark (Hemisphere) | Balance Condition | F(df) | p | Δ Exercise (Mean ± SE) | Δ Yoga (Mean ± SE) | t(df) | p | Estimated Difference ± SE (ΔE − ΔY) |
| P4–P6 | AG (R) | EOFirm | 4.62(1,21.01) | 0.043 | −0.170 ± 0.121 | 0.174 ± 0.105 | −2.15(21.01) | 0.043 | −0.334 ± 0.160 |
| P4–P2 | SAC (R) | EOFirm | 5.87(1,21.40) | 0.024 | −0.326 ± 0.151 | 0.157 ± 0.130 | −2.42(21.40) | 0.024 | −0.483 ± 0.199 |
| T7–FT7 | MTG (L) | EOSoft | 4.42(1,19.56) | 0.049 | −0.199 ± 0.149 | 0.215 ± 0.129 | −2.10(19.56) | 0.049 | −0.414 ± 0.197 |
| FC5–FC3 | POP (L) | EOSoft | 4.69(1,18.13) | 0.044 | 0.058 ± 0.141 | −0.353 ± 0.127 | 2.17(18.13) | 0.044 | 0.411 ± 0.190 |
| C4–C6 | S1 (R) | EOSoft | 5.13(1,20.56) | 0.034 | 0.111 ± 0.114 | −0.226 ± 0.096 | 2.26(20.56) | 0.034 | 0.338 ± 0.149 |
| CP2–CP4 | SMG (R) | EOSoft | 5.85(1,18.82) | 0.026 | 0.100 ± 0.102 | −0.227 ± 0.088 | 2.42(18.82) | 0.026 | 0.326 ± 0.135 |
| T7–C5 | STG (L) | ECSoft | 6.54(1,20.75) | 0.018 | −0.247 ± 0.142 | 0.229 ± 0.120 | −2.56(20.75) | 0.018 | −0.476 ± 0.186 |
| CP5–C5 | RA (L) | ECSoft | 4.76(1,20.86) | 0.041 | 0.179 ± 0.127 | −0.182 ± 0.107 | 2.18(20.86) | 0.041 | 0.362 ± 0.166 |
| CP5–CP3 | SMG (L) | ECSoft | 11.21(1,20.76) | 0.003 | 0.355 ± 0.102 | −0.092 ± 0.086 | 3.35(20.76) | 0.003 | 0.447 ± 0.134 |
| P3–CP3 | AG (L) | ECSoft | 9.19(1,21.09) | 0.006 | 0.172 ± 0.103 | −0.236 ± 0.087 | 3.03(21.09) | 0.006 | 0.408 ± 0.134 |
| CP6–CP4 | SMG (R) | ECSoft | 5.02(1,20.64) | 0.036 | 0.189 ± 0.113 | −0.141 ± 0.095 | 2.24(20.64) | 0.036 | 0.330 ± 0.147 |
| C4–C6 | S1 (R) | ECSoft | 6.14(1,20.45) | 0.022 | 0.243 ± 0.118 | −0.139 ± 0.099 | 2.48(20.45) | 0.022 | 0.382 ± 0.154 |
| C4–CP4 | SMG (R) | ECSoft | 7.40(1,21.06) | 0.013 | 0.405 ± 0.131 | −0.060 ± 0.110 | 2.72(21.06) | 0.013 | 0.465 ± 0.171 |
| C4–C2 | M1 (R) | ECSoft | 4.79(1,17.12) | 0.043 | 0.316 ± 0.170 | −0.169 ± 0.142 | 2.19(17.12) | 0.043 | 0.485 ± 0.222 |
| CP2–CP4 | SMG (R) | ECSoft | 6.72(1,20.89) | 0.017 | 0.272 ± 0.121 | −0.137 ± 0.102 | 2.59(20.89) | 0.017 | 0.409 ± 0.158 |
| Interaction Effects | Group Change (Post − Pre) | Linear Contrast | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Channel (10-10) | Landmark (Hemisphere) | Balance Condition | F(df) | p | Δ Exercise (Mean ± SE) | Δ Yoga (Mean ± SE) | t(df) | p | Estimated Difference ± SE (ΔE − ΔY) |
| CP5–P5 | AG (L) | EOFirm | 5.79(1,16.42) | 0.028 | 0.240 ± 0.134 | −0.174 ± 0.109 | 2.41(16.42) | 0.028 | 0.415 ± 0.172 |
| Cz–C2 | SMA (R) | EOFirm | 5.74(1,20.36) | 0.026 | 0.225 ± 0.211 | −0.436 ± 0.179 | 2.40(20.36) | 0.026 | 0.662 ± 0.276 |
| T8–C6 | STG (R) | EOFirm | 5.89(1,17.26) | 0.026 | 0.196 ± 0.157 | −0.291 ± 0.126 | 2.43(17.26) | 0.026 | 0.488 ± 0.201 |
| FC2–FC4 | PMC (R) | EOFirm | 4.48(1,21.02) | 0.046 | 0.094 ± 0.145 | −0.309 ± 0.123 | 2.12(21.02) | 0.046 | 0.403 ± 0.190 |
| FC2–C2 | SMA (R) | EOFirm | 6.97(1,21.01) | 0.015 | 0.332 ± 0.177 | −0.282 ± 0.151 | 2.64(21.01) | 0.015 | 0.614 ± 0.232 |
| CP5–P5 | AG (L) | EOSoft | 7.40(1,16.59) | 0.015 | 0.340 ± 0.160 | −0.223 ± 0.131 | 2.72(16.59) | 0.015 | 0.563 ± 0.207 |
| CP5–CP3 | SMG (L) | EOSoft | 5.13(1,18.11) | 0.036 | 0.418 ± 0.137 | 0.023 ± 0.108 | 2.26(18.11) | 0.036 | 0.395 ± 0.174 |
| FC5–FC3 | POP (L) | EOSoft | 6.96(1,20.70) | 0.015 | 0.355 ± 0.184 | −0.283 ± 0.156 | 2.64(20.70) | 0.015 | 0.638 ± 0.242 |
| Cz–C2 | SMA (R) | EOSoft | 9.60(1,20.85) | 0.005 | 0.287 ± 0.210 | −0.566 ± 0.178 | 3.10(20.85) | 0.005 | 0.854 ± 0.276 |
| T8–C6 | STG (R) | EOSoft | 7.80(1,20.34) | 0.011 | 0.368 ± 0.139 | −0.138 ± 0.117 | 2.79(20.34) | 0.011 | 0.507 ± 0.181 |
| FC6–FT8 | RA (R) | EOSoft | 6.28(1,20.81) | 0.021 | 0.336 ± 0.151 | −0.158 ± 0.127 | 2.51(20.81) | 0.021 | 0.494 ± 0.197 |
| CP6–CP4 | SMG (R) | EOSoft | 5.26(1,20.46) | 0.033 | 0.277 ± 0.156 | −0.194 ± 0.133 | 2.29(20.46) | 0.033 | 0.471 ± 0.206 |
| P4–CP4 | AG (R) | EOSoft | 6.84(1,20.71) | 0.016 | 0.153 ± 0.164 | −0.412 ± 0.140 | 2.62(20.71) | 0.016 | 0.565 ± 0.216 |
| C4–C6 | S1 (R) | EOSoft | 4.59(1,17.16) | 0.047 | 0.128 ± 0.126 | −0.241 ± 0.117 | 2.14(17.16) | 0.047 | 0.368 ± 0.172 |
| Cz–C2 | SMA (R) | ECFirm | 12.06(1,19.52) | 0.002 | 0.272 ± 0.161 | −0.461 ± 0.136 | 3.47(17.52) | 0.002 | 0.733 ± 0.211 |
| CP6–CP4 | SMG (R) | ECFirm | 6.53(1,20.72) | 0.019 | 0.185 ± 0.148 | −0.309 ± 0.125 | 2.56(20.72) | 0.019 | 0.494 ± 0.193 |
| CP5–P5 | AG (L) | ECSoft | 4.46(1,17.65) | 0.049 | 0.192 ± 0.138 | −0.185 ± 0.113 | 2.11(17.65) | 0.049 | 0.377 ± 0.179 |
| Cz–C2 | SMA (R) | ECSoft | 9.21(1,20.25) | 0.006 | 0.212 ± 0.167 | −0.453 ± 0.141 | 3.03(20.25) | 0.006 | 0.665 ± 0.219 |
| Yoga | Exercise | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Channel (10-10) | Balance Condition | Mean Δ ± SE (Post − Pre) | F(df) | p | dCohen | Mean Δ ± SE (Post − Pre) | F(df) | p | dCohen |
| FC5–FC3 | EOSoft | −0.353 ± 0.127 | 7.76(1,18.39) | 0.012 * | 0.81 | 0.058 ± 0.141 | 0.17(1,17.92) | 0.686 | 0.13 |
| FC1–FC3 | EOSoft | −0.352 ± 0.111 | 10.04(1,20.30) | 0.005 * | 0.95 | −0.122 ± 0.131 | 0.86(1,21.19) | 0.364 | 0.32 |
| CP2–CP4 | EOSoft | −0.227 ± 0.088 | 6.63(1,18.56) | 0.019 * | 0.72 | 0.100 ± 0.102 | 0.95(1,19.02) | 0.342 | 0.31 |
| FC1–C1 | ECFirm | 0.334 ± 0.095 | 13.02(1,20.37) | 0.002 * | 1.08 | 0.219 ± 0.112 | 3.80(1,21.29) | 0.065 | 0.68 |
| C4–CP4 | ECFirm | 0.102 ± 0.115 | 0.78(1,18.32) | 0.388 | 0.27 | 0.423 ± 0.142 | 8.81(1,19.02) | 0.008 * | 1.11 |
| CP5–CP3 | ECSoft | −0.092 ± 0.086 | 1.14(1,20.35) | 0.297 | 0.27 | 0.355 ± 0.102 | 12.08(1,21.05) | 0.002 * | 1.01 |
| P3–CP3 | ECSoft | −0.236 ± 0.087 | 7.39(1,20.55) | 0.013 * | 0.085 | 0.172 ± 0.103 | 2.79(1,21.49) | 0.109 | 0.61 |
| C4–CP4 | ECSoft | −0.060 ± 0.110 | 0.294(1,20.52) | 0.594 | 0.17 | 0.405 ± 0.131 | 9.63(1,21.45) | 0.005 * | 1.10 |
| Yoga | Exercise | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Channel (10-10) | Balance Condition | Mean Δ ± SE (Post − Pre) | F(df) | p | dCohen | Mean Δ ± SE (Post − Pre) | F(df) | p | dCohen |
| Cz–C2 | EOFirm | −0.436 ± 0.179 | 5.97(1,19.78) | 0.024 * | 0.81 | 0.225 ± 0.211 | 1.14(1,20.79) | 0.297 | 0.41 |
| FC2–FC4 | EOFirm | −0.309 ± 0.123 | 6.31(1,20.50) | 0.020 * | 0.75 | 0.094 ± 0.145 | 0.42(1,21.40) | 0.524 | 0.22 |
| P4–CP4 | EOFirm | −0.493 ± 0.141 | 12.14(1,20.09) | 0.002 * | 1.33 | −0.166 ± 0.166 | 1.00(1,21.19) | 0.329 | 0.44 |
| CP5–CP3 | EOSoft | 0.023 ± 0.108 | 0.05(1,17.08) | 0.831 | 0.05 | 0.418 ± 0.137 | 9.31(1,18.78) | 0.007 * | 0.94 |
| T8–C6 | EOSoft | −0.138 ± 0.117 | 1.40(1,19.88) | 0.251 | 0.32 | 0.368 ± 0.139 | 7.05(1,20.68) | 0.015 * | 0.85 |
| Cz–C2 | EOSoft | −0.566 ± 0.178 | 10.11(1,20.28) | 0.005 * | 1.03 | 0.287 ± 0.210 | 1.87(1,21.27) | 0.186 | 0.51 |
| P4–CP4 | EOSoft | −0.412 ± 0.140 | 8.65(1,20.08) | 0.008 * | 1.10 | 0.153 ± 0.164 | 0.86(1,21.18) | 0.363 | 0.40 |
| Cz–C2 | ECFirm | −0.461 ± 0.136 | 11.47(1,10.04) | 0.003 * | 0.95 | 0.272 + 0.161 | 2.84(1,19.87) | 0.107 | 0.55 |
| CP6–CP4 | ECFirm | −0.309 ± 0.125 | 6.11(1,20.16) | 0.022 * | 0.79 | 0.185 ± 0.148 | 1.58(1,21.14) | 0.223 | 0.47 |
| P4–CP4 | ECFirm | −0.407 ± 0.139 | 8.51(1,20.69) | 0.008 * | 1.10 | −0.028 ± 0.163 | 0.28(1,21.79) | 0.868 | 0.07 |
| C4–FC4 | ECFirm | −0.263 ± 0.099 | 7.01(1,19.00) | 0.016 * | 0.63 | −0.017 ± 0.125 | 0.19(1,19.68) | 0.892 | 0.04 |
| Cz–C2 | ECSoft | −0.453 ± 0.141 | 10.27(1,19.73) | 0.005 * | 0.95 | 0.212 ± 0.167 | 1.60(1,20.62) | 0.220 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Sarabia, J.A.; Schmid, A.A.; Sharp, J.L.; Stephens, J.A. Intervention-Induced Changes in Balance and Task-Dependent Neural Activity in Adults with Acquired Brain Injury: A Pilot Randomized Control Trial. Sensors 2024, 24, 4047. https://doi.org/10.3390/s24134047
Hernandez-Sarabia JA, Schmid AA, Sharp JL, Stephens JA. Intervention-Induced Changes in Balance and Task-Dependent Neural Activity in Adults with Acquired Brain Injury: A Pilot Randomized Control Trial. Sensors. 2024; 24(13):4047. https://doi.org/10.3390/s24134047
Chicago/Turabian StyleHernandez-Sarabia, Jesus A., Arlene A. Schmid, Julia L. Sharp, and Jaclyn A. Stephens. 2024. "Intervention-Induced Changes in Balance and Task-Dependent Neural Activity in Adults with Acquired Brain Injury: A Pilot Randomized Control Trial" Sensors 24, no. 13: 4047. https://doi.org/10.3390/s24134047
APA StyleHernandez-Sarabia, J. A., Schmid, A. A., Sharp, J. L., & Stephens, J. A. (2024). Intervention-Induced Changes in Balance and Task-Dependent Neural Activity in Adults with Acquired Brain Injury: A Pilot Randomized Control Trial. Sensors, 24(13), 4047. https://doi.org/10.3390/s24134047







