Associations of Physical Activity and Heart Rate Variability from a Two-Week ECG Monitor with Cognitive Function and Dementia: The ARIC Neurocognitive Study
Abstract
:1. Introduction
2. Methods
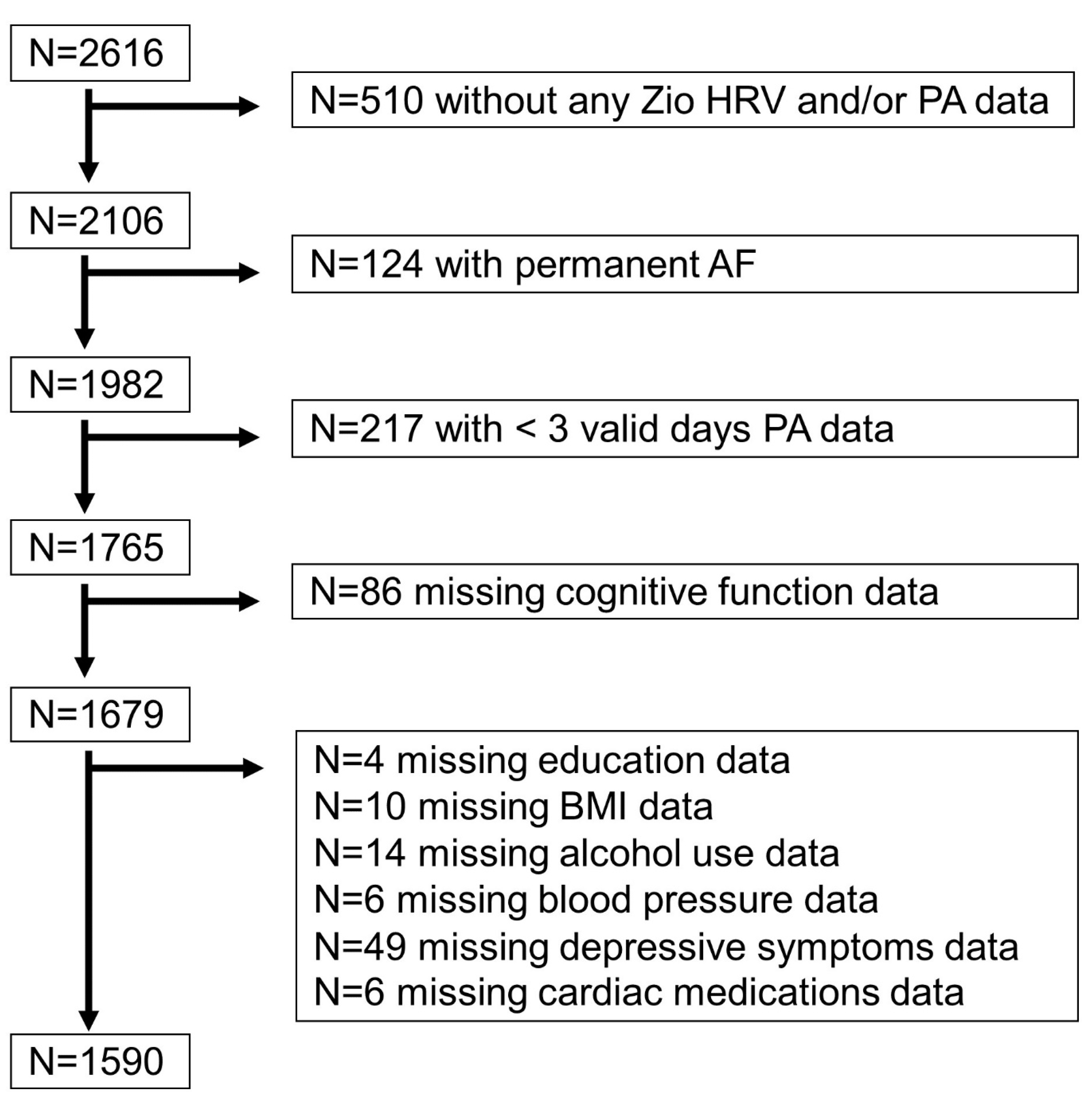
2.1. Study Population
2.2. Heart Rate Variability
2.3. Physical Activity
2.4. Cognitive Test Factor Scores
2.5. Mild Cognitive Impairment or Dementia Status
2.6. Other Covariates
2.7. Statistical Methods
3. Results
3.1. Sample Characteristics
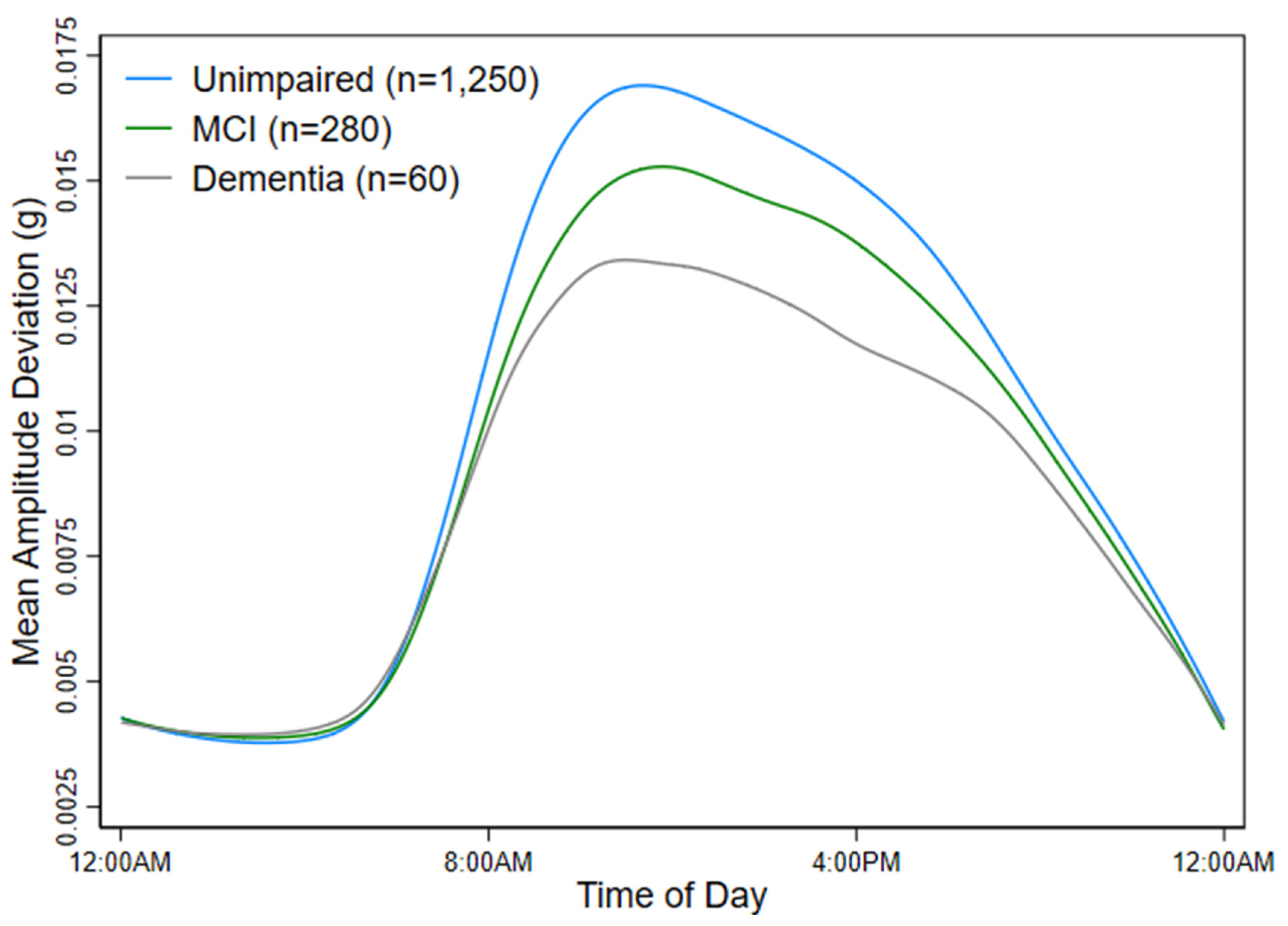
3.2. Physical Activity and Cognitive Function
3.3. Heart Rate Variability and Cognitive Function
3.4. Independent Effects of Physical Activity and Heart Rate Variability Measures
3.5. Exclusion of Stroke, Intermittent Atrial Fibrillation, Beta Blockers, and Calcium Channel Blockers
3.6. Sensitivity Analyses Restricted by Cognitive Status
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Li, Q.; Cong, W.; Mu, S.; Zhan, R.; Zhong, S.; Zhao, M.; Zhao, C.; Kang, K.; Zhou, Z. Effect of physical activity on risk of Alzheimer’s disease: A systematic review and meta-analysis of twenty-nine prospective cohort studies. Ageing Res. Rev. 2023, 92, 102127. [Google Scholar] [CrossRef] [PubMed]
- Vítor, J.; Melita, C.; Rodrigues, M.; de Sousa, D.A.; Costa, J.; Ferro, J.M.; Verdelho, A. Physical activity in vascular cognitive impairment: Systematic review with meta-analysis. J. Stroke Cerebrovasc. Dis. 2023, 32, 107133. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Liu, F.; Wanigatunga, A.A.; Schrack, J.A. Assessment of Physical Activity in Adults Using Wrist Accelerometers. Epidemiol. Rev. 2022, 43, 65–93. [Google Scholar] [CrossRef]
- Maher, C.; Szeto, K.; Arnold, J. The use of accelerometer-based wearable activity monitors in clinical settings: Current practice, barriers, enablers, and future opportunities. BMC Health Serv. Res. 2021, 21, 1064. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, A.; Urbanek, J.K.; Etzkorn, L.; Parikh, R.; Soliman, E.Z.; Wanigatunga, A.A.; Gabriel, K.P.; Coresh, J.; Schrack, J.A.; Chen, L.Y. Validation of a Zio XT Patch Accelerometer for the Objective Assessment of Physical Activity in the Atherosclerosis Risk in Communities (ARIC) Study. Sensors 2024, 24, 761. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 290215. [Google Scholar] [CrossRef]
- Nicolini, P.; Ciulla, M.M.; Malfatto, G.; Abbate, C.; Mari, D.; Rossi, P.D.; Pettenuzzo, E.; Magrini, F.; Consonni, D.; Lombardi, F. Autonomic Dysfunction in Mild Cognitive Impairment: Evidence from Power Spectral Analysis of Heart Rate Variability in a Cross-Sectional Case-Control Study. PLoS ONE 2014, 9, e96656. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Favieri, F.; Casagrande, M. Heart Rate Variability and Cognitive Function: A Systematic Review. Front. Neurosci. 2019, 13, 436204. [Google Scholar] [CrossRef]
- Imbimbo, C.; Spallazzi, M.; Ferrari-Pellegrini, F.; Villa, A.; Zilioli, A.; Mutti, C.; Parrino, L.; Lazzeroni, D. Heart rate variability and cognitive performance in adults with cardiovascular risk. Cereb. Circ. Cogn. Behav. 2022, 3, 100136. [Google Scholar] [CrossRef]
- Meessen, J.; Sütterlin, S.; Gauggel, S.; Forkmann, T. Learning by heart—The relationship between resting vagal tone and metacognitive judgments: A pilot study. Cogn. Process. 2018, 19, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Magnon, V.; Vallet, G.T.; Benson, A.; Mermillod, M.; Chausse, P.; Lacroix, A.; Bouillon-Minois, J.-B.; Dutheil, F. Does heart rate variability predict better executive functioning? A systematic review and meta-analysis. Cortex 2022, 155, 218–236. [Google Scholar] [CrossRef] [PubMed]
- de Vilhena Toledo, M.A.; Junqueira, L.F. Cardiac autonomic modulation and cognitive status in Alzheimer’s disease. Clin. Auton. Res. 2010, 20, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.Y.; Elliott, T.; Knowles, M.; Howard, R. Heart rate variability in relation to cognition and behavior in neurodegenerative diseases: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 73, 101539. [Google Scholar] [CrossRef] [PubMed]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T. Heart rate variability: Measurement and clinical utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Folsom, A.R.; Coresh, J.; Sharrett, A.R.; Couper, D.; Wagenknecht, L.E.; Mosley, T.H.; Ballantyne, C.M.; Boerwinkle, E.A.; Rosamond, W.D.; et al. The ARIC (Atherosclerosis Risk in Communities) Study: JACC Focus Seminar 3/8. J. Am. Coll. Cardiol. 2021, 77, 2939–2959. [Google Scholar] [CrossRef] [PubMed]
- Vähä-Ypyä, H.; Vasankari, T.; Husu, P.; Suni, J.; Sievänen, H. A universal, accurate intensity-based classification of different physical activities using raw data of accelerometer. Clin. Physiol. Funct. Imaging 2015, 35, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Karas, M.; Muschelli, J.; Leroux, A.; Urbanek, J.K.; Wanigatunga, A.A.; Bai, J.; Crainiceanu, C.M.; Schrack, J.A. Comparison of Accelerometry-Based Measures of Physical Activity: Retrospective Observational Data Analysis Study. JMIR mHealth uHealth 2022, 10, e38077. [Google Scholar] [CrossRef] [PubMed]
- Karas, M.; Schrack, J.; Urbanek, J. arctools: Processing and Physical Activity Summaries of Minute Level Activity Data 2021. Available online: https://cran.r-project.org/package=arctools/arctools.pdf (accessed on 1 June 2023).
- Gross, A.L.; Power, M.C.; Albert, M.S.; Deal, J.A.; Gottesman, R.F.; Griswold, M.; Wruck, L.M.; Mosley, T.H., Jr.; Coresh, J.; Sharrett, A.R. Application of latent variable methods to the study of cognitive decline when tests change over time. Epidemiology 2015, 26, 878–887. [Google Scholar] [CrossRef]
- Reitan, R.M. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Percept. Mot. Skills 1958, 8, 271. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Adult Intelligence Scale-Revised; Psychological Corporation: New York, NY, USA, 1981; Volume 1. [Google Scholar]
- Wechsler, D. Wechsler Memory Scale-Revised; Psychological Corporation: San Antonio, TX, USA, 1987. [Google Scholar]
- Ryan, J.; Lopez, S. Understanding psychological assessment. Perspectives on individual differences. In Wechsler Adult Intelligence Scale-III; Dorfman, W., Hersen, M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2001; p. 23. [Google Scholar]
- Benton, A.; Hamsher, K. Multilingual Aphasia Examination; University of Iowa: Iowa Cita, IA, USA, 1976. [Google Scholar]
- Williams, B.W.; Mack, W.; Henderson, V.W. Boston Naming Test in Alzheimer’s disease. Neuropsychologia 1989, 27, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current Version and Scoring Rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef]
- Pfeffer, R.I.; Kurosaki, T.T.; Harrah, C.H.; Chance, J.M.; Filos, S. Measurement of Functional Activities in Older Adults in the Community. J. Gerontol. 1982, 37, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Gottesman, R.F.; Sharrett, A.R.; Wruck, L.M.; Windham, B.G.; Coker, L.; Schneider, A.L.C.; Hengrui, S.; Alonso, A.; Coresh, J.; et al. Mild cognitive impairment and dementia prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study. Alzheimer’s Dement. 2016, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Radloff, L.S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Phillips, C. Lifestyle Modulators of Neuroplasticity: How Physical Activity, Mental Engagement, and Diet Promote Cognitive Health during Aging. Neural Plast. 2017, 2017, 3589271. [Google Scholar] [CrossRef]
- Varma, V.R.; Watts, A. Daily Physical Activity Patterns During the Early Stage of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 55, 659–667. [Google Scholar] [CrossRef]
- De la Rosa, A.; Olaso-Gonzalez, G.; Arc-Chagnaud, C.; Millan, F.; Salvador-Pascual, A.; García-Lucerga, C.; Blasco-Lafarga, C.; Garcia-Dominguez, E.; Carretero, A.; Correas, A.G.; et al. Physical exercise in the prevention and treatment of Alzheimer’s disease. J. Sport Health Sci. 2020, 9, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Rennie, K.L. Effects of Moderate and Vigorous Physical Activity on Heart Rate Variability in a British Study of Civil Servants. Am. J. Epidemiol. 2003, 158, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Allan, J.L.; McMinn, D.; Daly, M. A bidirectional relationship between executive function and health behavior: Evidence, implications, and future directions. Front. Neurosci. 2016, 10, 210626. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Ren, P.; Wang, X.; Anthony, M.; Tadin, D.; Heffner, K.L. Cortical thickness is associated with altered autonomic function in cognitively impaired and non-impaired older adults. J. Physiol. 2017, 595, 6969–6978. [Google Scholar] [CrossRef] [PubMed]
- Hautala, A.J.; Karjalainen, J.; Kiviniemi, A.M.; Kinnunen, H.; Makikallio, T.H.; Huikuri, H.V.; Tulppo, M.P. Physical activity and heart rate variability measured simultaneously during waking hours. Am. J. Physiol.-Heart Circ. Physiol. 2010, 298, H874–H880. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D. Neural mechanisms of autonomic, affective, and cognitive integration. J. Comp. Neurol. 2005, 493, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Aftyka, J.; Staszewski, J.; Dębiec, A.; Pogoda-Wesołowska, A.; Żebrowski, J. Heart rate variability as a predictor of stroke course, functional outcome, and medical complications: A systematic review. Front. Physiol. 2023, 14, 1115164. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Junejo, R.T.; Thomas, G.N.; Fisher, J.P.; Lip, G.Y.H. Heart rate variability in patients with atrial fibrillation and hypertension. Eur. J. Clin. Investig. 2021, 51, e13361. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Khandoker, A.H.; Alkhodari, M.; Hadjileontiadis, L.J.; Jelinek, H.F. Investigating the effects of beta-blockers on circadian heart rhythm using heart rate variability in ischemic heart disease with preserved ejection fraction. Sci. Rep. 2023, 13, 5828. [Google Scholar] [CrossRef]
- Lo, J.W.; Crawford, J.D.; Desmond, D.W.; Bae, H.-J.; Lim, J.-S.; Godefroy, O.; Roussel, M.; Kang, Y.; Jahng, S.; Köhler, S.; et al. Long-Term Cognitive Decline After Stroke: An Individual Participant Data Meta-Analysis. Stroke 2022, 53, 1318–1327. [Google Scholar] [CrossRef]
- Eguchi, K.; Schwartz, J.E.; Pickering, T.G.; Hoshide, S.; Ishikawa, J.; Shimada, K.; Kario, K. Increased heart rate variability during sleep is a predictor for future cardiovascular events in patients with type 2 diabetes. Hypertens. Res. 2010, 33, 737–742. [Google Scholar] [CrossRef]
- Malik, J.; Soliman, E.Z.; Wu, H.T. An adaptive QRS detection algorithm for ultra-long-term ECG recordings. J. Electrocardiol. 2020, 60, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.E.W.; Behar, J.; Andreotti, F.; Clifford, G.D.; Oster, J. Multimodal heart beat detection using signal quality indices. Physiol. Meas. 2015, 36, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Wu, H.T. Extract Fetal ECG from Single-Lead Abdominal ECG by De-Shape Short Time Fourier Transform and Nonlocal Median. Front. Appl. Math. Stat. 2017, 3. [Google Scholar] [CrossRef]
- Xu, X.; Schuckers, S.; CHIME Study Group. Collaborative Home Infant Monitoring Evaluation. Automatic detection of artifacts in heart period data. J. Electrocardiol. 2001, 34, 205–210. [Google Scholar] [CrossRef]
- Petrėnas, A.; Marozas, V.; Sörnmo, L. Low-complexity detection of atrial fibrillation in continuous long-term monitoring. Comput. Biol. Med. 2015, 65, 184–191. [Google Scholar] [CrossRef]
- The Task Force of the European Society of Cardiology, The North American Society of Pacing. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
| Overall (N = 1590) | Low LTMAD (N = 530) | Medium LTMAD (N = 530) | High LTMAD (N = 530) | |
|---|---|---|---|---|
| Age (years), mean (SD) | 78.8 (4.5) | 80.5 (5.0) | 78.8 (4.3) | 77.1 (3.4) |
| Female, % | 58 | 65 | 59 | 48 |
| Black, % | 32 | 42 | 29 | 26 |
| Education, % | ||||
| Grade school | 3 | 5 | 2 | 3 |
| Some high school | 7 | 9 | 6 | 7 |
| High school graduate | 28 | 30 | 27 | 26 |
| Vocational school | 10 | 9 | 11 | 10 |
| At least some college | 34 | 30 | 38 | 33 |
| Grad/prof school | 18 | 17 | 16 | 22 |
| BMI (kg/m2), mean (SD) | 28.4 (5.4) | 29.9 (6.2) | 28.5 (5.2) | 26.8 (4.0) |
| Alcohol use, % | ||||
| Current | 52 | 42 | 53 | 60 |
| Former | 28 | 31 | 29 | 23 |
| Ever | 20 | 27 | 18 | 16 |
| Smoking, % | ||||
| Current | 8 | 9 | 8 | 6 |
| Former | 50 | 45 | 52 | 52 |
| Never | 43 | 46 | 41 | 42 |
| BP (mm Hg), mean (SD) | ||||
| Systolic | 134.6 (18.7) | 135.5 (19.6) | 135.4 (18.6) | 133.0 (17.9) |
| Diastolic | 67.7 (10.4) | 67.2 (10.3) | 67.7 (10.7) | 68.3 (10.3) |
| Diabetes, % | 30 | 38 | 30 | 23 |
| Heart Failure, % | 6 | 12 | 5 | 2 |
| Cardiac medications, % | 50 | 59 | 50 | 42 |
| CES-D, mean (SD) | 2.5 (2.8) | 3.2 (3.1) | 2.5 (2.8) | 2.0 (2.2) |
| TMAD, mean (SD) | 16.6 (4.7) | 12.0 (1.7) | 16.0 (1.2) | 21.9 (3.6) |
| SDNN (ms), mean (SD) | 125.3 (39.9) | 119.0 (42.3) | 123.3 (37.2) | 133.7 (38.5) |
| rMSSD (ms), mean (SD) | 47.2 (46.9) | 52.1 (52.4) | 43.8 (38.7) | 45.6 (48.2) |
| Cognitive status, % | ||||
| Unimpaired | 79 | 72 | 80 | 84 |
| MCI | 17 | 22 | 17 | 14 |
| Dementia | 4 | 6 | 3 | 2 |
| Overall (N = 1590) | Low SDNN (N = 530) | Medium SDNN (N = 530) | High SDNN (N = 530) | |
|---|---|---|---|---|
| Age (years), mean (SD) | 78.8 (4.5) | 78.8 (4.5) | 78.7 (4.4) | 78.8 (4.4) |
| Female, % | 58 | 69 | 60 | 44 |
| Black, % | 32 | 36 | 34 | 26 |
| Education, % | ||||
| Grade school | 3 | 3 | 4 | 3 |
| Some high school | 7 | 8 | 8 | 6 |
| High school graduate | 28 | 29 | 27 | 26 |
| Vocational school | 10 | 11 | 10 | 9 |
| At least some college | 34 | 32 | 34 | 35 |
| Grad/prof school | 18 | 16 | 17 | 21 |
| BMI (kg/m2), mean (SD) | 28.4 (5.4) | 29.2 (6.0) | 28.2 (5.1) | 27.8 (4.8) |
| Alcohol use, % | ||||
| Current | 52 | 49 | 50 | 56 |
| Former | 28 | 28 | 31 | 25 |
| Ever | 20 | 23 | 19 | 19 |
| Smoking, % | ||||
| Current | 8 | 10 | 6 | 7 |
| Former | 50 | 48 | 51 | 49 |
| Never | 43 | 43 | 43 | 43 |
| BP (mm Hg), mean (SD) | ||||
| Systolic | 134.6 (18.7) | 135.1 (19.4) | 135.2 (18.5) | 133.7 (18.3) |
| Diastolic | 67.7 (10.4) | 68.8 (10.9) | 67.3 (9.9) | 67.0 (10.4) |
| Diabetes, % | 30 | 37 | 30 | 24 |
| Heart Failure, % | 6 | 9 | 5 | 4 |
| Cardiac medications, % | 50 | 59 | 49 | 44 |
| CES-D, mean (SD) | 2.5 (2.8) | 2.8 (3.0) | 2.4 (2.7) | 2.4 (2.6) |
| TMAD, mean (SD) | 16.6 (4.7) | 15.1 (3.9) | 16.9 (4.5) | 17.9 (5.3) |
| SDNN (ms), mean (SD) | 125.3 (39.9) | 89.0 (15.9) | 119.8 (9.9) | 167.1 (36.3) |
| rMSSD (ms), mean (SD) | 47.2 (46.9) | 32.2 (21.3) | 41.6 (28.0) | 67.8 (68.5) |
| Cognitive status, % | ||||
| Unimpaired | 79 | 79 | 79 | 79 |
| MCI | 17 | 18 | 17 | 18 |
| Dementia | 4 | 4 | 4 | 4 |
| Global Cognition β (95% CI) | Executive Function β (95% CI) | Memory β (95% CI) | Language β (95% CI) | |
|---|---|---|---|---|
| LTMAD | 0.30 (0.16, 0.44) | 0.38 (0.22, 0.53) | 0.14 (−0.04, 0.32) | 0.15 (−0.02, 0.32) |
| Log SDNN | 0.03 (−0.24, 0.30) | 0.19 (−0.10, 0.48) | −0.21 (−0.56, 0.13) | −0.15 (−0.47, 0.17) |
| Log rMSSD | −0.12 (−0.2, 0.010) | −0.08 (−0.2, 0.06) | −0.16 (−0.3, 0.004) | −0.11 (−0.26, 0.05) |
| Unimpaired (N = 1250) | Mild Cognitive Impairment (N = 280) OR (95% CI) | Dementia (N = 60) OR (95% CI) | |
|---|---|---|---|
| LTMAD | REF | 0.38 (0.22, 0.67) a | 0.25 (0.08, 0.74) |
| Log SDNN | REF | 0.84 (0.30, 2.36) | 3.39 (0.40, 28.37) |
| Log rMSSD | REF | 1.40 (0.87, 2.27) | 1.24 (0.46, 3.35) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, F.R.; Wu, H.-T.; Etzkorn, L.; Rooney, M.R.; Soliman, E.Z.; Deal, J.A.; Crainiceanu, C.; Spira, A.P.; Wanigatunga, A.A.; Schrack, J.A.; et al. Associations of Physical Activity and Heart Rate Variability from a Two-Week ECG Monitor with Cognitive Function and Dementia: The ARIC Neurocognitive Study. Sensors 2024, 24, 4060. https://doi.org/10.3390/s24134060
Marino FR, Wu H-T, Etzkorn L, Rooney MR, Soliman EZ, Deal JA, Crainiceanu C, Spira AP, Wanigatunga AA, Schrack JA, et al. Associations of Physical Activity and Heart Rate Variability from a Two-Week ECG Monitor with Cognitive Function and Dementia: The ARIC Neurocognitive Study. Sensors. 2024; 24(13):4060. https://doi.org/10.3390/s24134060
Chicago/Turabian StyleMarino, Francesca R., Hau-Tieng Wu, Lacey Etzkorn, Mary R. Rooney, Elsayed Z. Soliman, Jennifer A. Deal, Ciprian Crainiceanu, Adam P. Spira, Amal A. Wanigatunga, Jennifer A. Schrack, and et al. 2024. "Associations of Physical Activity and Heart Rate Variability from a Two-Week ECG Monitor with Cognitive Function and Dementia: The ARIC Neurocognitive Study" Sensors 24, no. 13: 4060. https://doi.org/10.3390/s24134060






