Carbazolyl-Modified Neutral Ir(III) Complexes for Efficient Detection of Picric Acid in Aqueous Media
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Instruments
2.2. Synthesis and Characterizations of 1 and 2
2.3. Sample Preparation and Sensing of PA
3. Results and Discussion
3.1. Photophysical and AIPE Properties
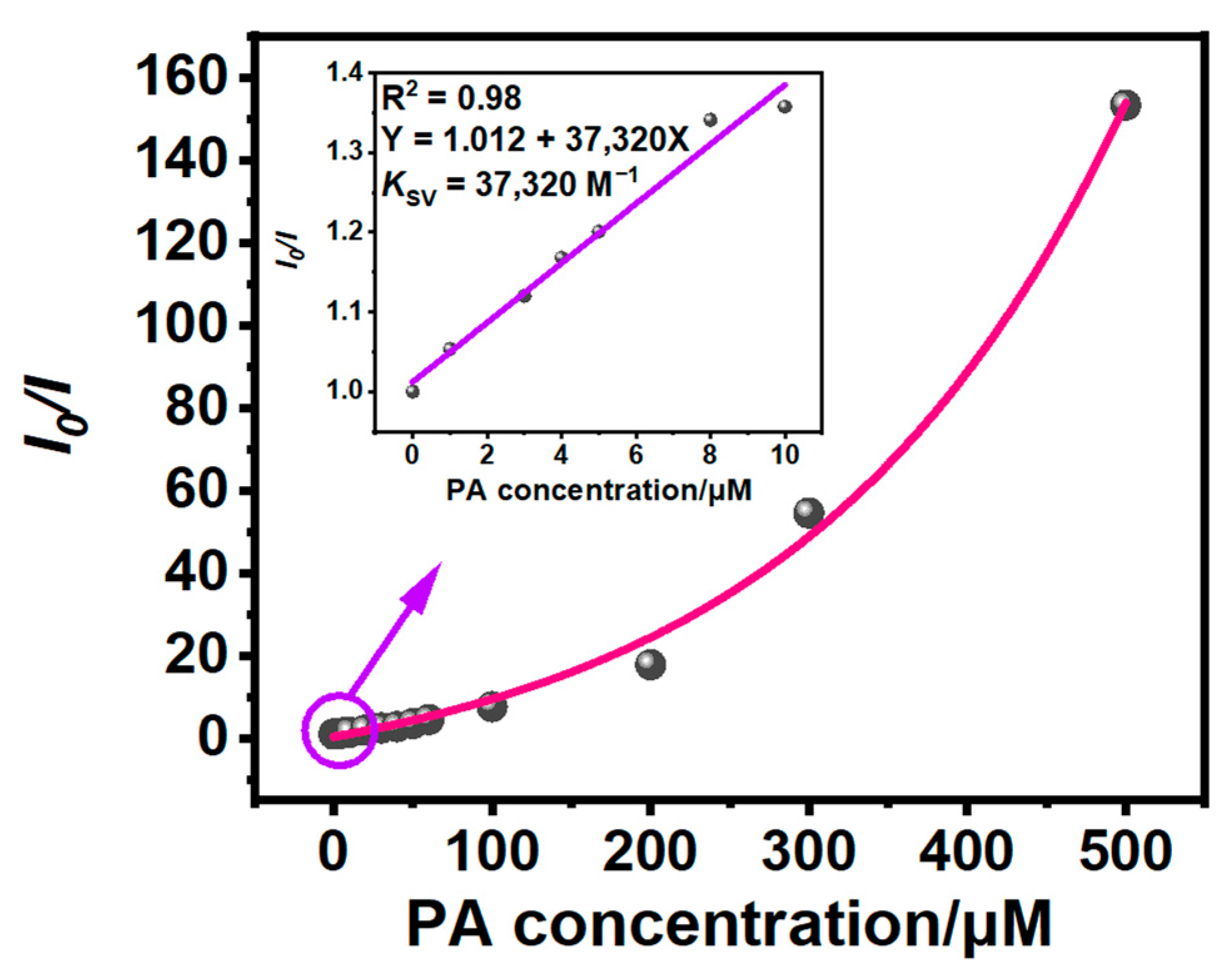
3.2. Detection of PA
3.3. Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, J.; Xie, Z.; Lam, J.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Cui, H.; Wang, Y.; Wang, C.; Li, Y.; Wang, C. Biocompatible AIE material from natural resources: Chitosan and its multifunctional applications. Carbohydr. Polym. 2020, 227, 115338. [Google Scholar] [CrossRef] [PubMed]
- Wagalgave, S.; Birajdar, S.; Malegaonkar, J.; Bhosale, S. Chapter eight-patented AIE materials for biomedical applications. Prog. Mol. Biol. Transl. 2021, 185, 199–223. [Google Scholar]
- Guan, W.; Chen, J.; Liu, J.; Shi, B.; Yao, H.; Zhang, Y.; Wei, T.; Lin, Q. Macrocycles-assembled AIE supramolecular polymer networks. Coord. Chem. Rev. 2024, 507, 215717. [Google Scholar] [CrossRef]
- Hao, Y.; Ji, F.; Li, T.; Tian, M.; Han, X.; Chai, F. Portable smartphone platform utilizing AIE-featured carbon dots for multivariate visual detection for Cu2+, Hg2+ and BSA in real samples. Food Chem. 2024, 446, 138843. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, Z.; Lam, J.; Law, C.; Tang, B. Silole-containing polyacetylenes. Synthesis, thermal stability, tight emission, nanodimensional aggregation, and restricted intramolecular rotation. Macromolecules 2003, 36, 1108–1117. [Google Scholar] [CrossRef]
- Baldo, M.; O’Brien, D.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.; Forrest, S. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151–154. [Google Scholar] [CrossRef]
- Caminade, A.; Hameau, A.; Turrin, C.; Laurent, R.; Majoral, J. Dendritic metal complexes for bioimaging. Coord. Chem. Rev. 2021, 430, 213739. [Google Scholar] [CrossRef]
- Huynh, M.; Vinck, R.; Gibert, B.; Gasser, G. Strategies for the nuclear delivery of metal complexes to cancer cells. Adv. Mater. 2024, 36, 2311437. [Google Scholar] [CrossRef]
- Wen, L.; Hou, X.; Shan, G.; Song, W.; Zhang, S.; Sun, H.; Su, Z. Rational molecular design of aggregation-induced emission cationic Ir(III) phosphors achieving supersensitive and selective detection of nitroaromatic explosives. J. Mater. Chem. C 2017, 5, 10847–10854. [Google Scholar] [CrossRef]
- Sainaba, A.; Saha, R.; Venkateswarulu, M.; Zangrando, E.; Mukherjee, P. Pt(II) tetrafacial barrel with aggregation-induced emission for sensing. Inorg. Chem. 2024, 63, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Brunner, K.; Dijken, A.; Börner, H.; Bantiaansen, J.; Kiggen, N.; Langeveld, B. Carbazole compounds as host materials for triplet emitters in organic light-emitting diodes: Tuning the HOMO level without influencing the triplet energy in small molecules. J. Am. Chem. Soc. 2004, 126, 6035–6042. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Shan, G.; Fu, Q.; Su, Z. Tuning emission of AIE-Active organometallic Ir(III) complexes by simple modulation of strength of donor/acceptor on ancillary ligands. Organometallics 2016, 35, 3996–4001. [Google Scholar] [CrossRef]
- Mao, H.; Yang, Y.; Zhao, K.; Duan, Y.; Song, W.; Shan, G.; Su, Z. Fine-tuning emission color of aggregation-induced emission-active Ir(III) phosphors through simple ligand modification. Dyes Pigment. 2021, 192, 109439. [Google Scholar] [CrossRef]
- Ma, Q.; Dong, W.; Ma, Z.; Lv, X.; Li, Y.; Duan, Q. Synthesis of phosphorescent iridium(III) complex containing carbazole and its sensing property towards nitro-aromatic compounds. Mater. Lett. 2019, 249, 120–123. [Google Scholar] [CrossRef]
- Dong, W.; Ma, Q.; Ma, Z.; Duan, Q.; Lü, X.; Qiu, N.; Fei, T.; Su, Z. Phosphorescent iridium(III) complex based photoluminescence sensor for sensitive and selective detection of picric acid. Dyes Pigment. 2020, 172, 107799. [Google Scholar] [CrossRef]
- Di, L.; Xing, Y.; Yang, Z.; Qiao, C.; Xia, Z. Photostable aggregation-induced emission of iridium(III) complex realizing robust and high-resolution imaging of latent fingerprints. Sens. Actuat. B Chem. 2023, 375, 132898. [Google Scholar] [CrossRef]
- Verbitskiy, E.; Gorbunov, E.; Baranova, A.; Lugovik, K.; Khokhlov, K.; Cheprakova, E.; Kim, G.; Rusinov, G.; Chupakhin, O.; Charushin, V. New 2H-[1,2,3]triazolo[4,5-e][1,2,4]triazolo[1,5-a]pyrimidine derivatives as luminescent fluorophores for detection of nitroaromatic explosives. Tetrahedron 2016, 72, 4954–4961. [Google Scholar] [CrossRef]
- Verbitskiy, E.; Baranova, A.; Lugovik, K.; Khokhlov, K.; Cheprakova, E.; Shafikov, M.; Rusinov, G.; Chupakhin, O.; Charushin, V. New 4,5-di(hetero)arylpyrimidines as sensing elements for detection of nitroaromatic explosives in vapor phase. Dyes Pigment. 2017, 137, 360–371. [Google Scholar] [CrossRef]
- Wang, H.; Chen, C.; Liu, Y.; Wu, Y.; Yuan, Y.; Zhou, Q. A highly sensitive and selective chemosensor for 2,4,6-trinitrophenol based on L-cysteine-coated cadmium sulfide quantum dots. Talanta 2019, 198, 242–248. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.; Chen, G.; Chen, Y.; Liu, D.; Liao, J. A red-emitting COF ionic exchanged with green-emitting Tb(III) complex anion: Synthesis, characterization, ratiometric emission sensing, and removal of picric acid. Front. Chem. 2022, 10, 865304. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, Y.; Shu, Y.; Wang, J.; Qiu, H. Fabrication and application of 2,4,6-trinitrophenol sensors based on fluorescent functional materials. J. Hazard. Mater. 2022, 425, 127987. [Google Scholar] [CrossRef]
- James, T.; Sandanayake, K.; Shinkai, S. Chiral discrimination of monosaccharides using a fluorescent molecular sensor. Nature 1995, 374, 345–347. [Google Scholar] [CrossRef]
- Lachance, B.; Robidoux, P.; Hawari, J.; Ampleman, G.; Thiboutot, S.; Sunahara, G. Cytotoxic and genotoxic effects of energetic compounds on bacterial and mammalian cells in vitro. Mutat. Res. Fund. Mol. Mech. Mutagen. 1999, 444, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Ferrand, Y.; Crump, M.; Davis, A. A synthetic lectin analog for biomimetic disaccharide recognition. Science 2007, 318, 619–622. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.; Guo, L.; Cao, D. Fluorescent polymer nanotubes as bifunctional materials for selective sensing and fast removal of picric acid. Sens. Actuators B Chem. 2018, 274, 102–109. [Google Scholar] [CrossRef]
- Liu, C.; Rao, X.; Lv, X.; Qiu, J.; Jin, Z. Substituent effects on the photophysical and electrochemical properties of iridium(III) complexes containing an arylcarbazolyl moiety. Dye. Pigment. 2014, 109, 13–20. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.; Gao, Z.; Wang, L.; Tang, Y.; Liu, J.; Liu, C. Supramolecular copolymers under kinetic, thermodynamic, or pathway-switching control. Angew. Chem. Int. Ed. 2023, 62, e202302581. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Y.; Cai, R.; Zhang, H.; Liu, C. Controllable 1D, 2D and 3D supramolecular assemblies of Ir(III) complexes. Mater. Chem. Front. 2023, 7, 5915–5923. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, Q.; Shi, Y.; Tang, B.; Che, C.; Liu, C. Three-component multiblock 1D supramolecular copolymers of Ir(III) complexes with controllable sequences. Angew. Chem. Int. Ed. 2023, 62, e202312844. [Google Scholar] [CrossRef]
- Yan, Y.; Jia, W.; Cai, R.; Liu, C. An AIPE-active fluorinated cationic platinum(II) complex for efficient detection of picric acid in aqueous media. Chin. Chem. Lett. 2024, 35, 108819. [Google Scholar] [CrossRef]
- He, P.; Chen, Y.; Li, X.; Yan, Y.; Liu, C. Aggregation-induced emission-active Ir(III) complexes for sensing picric acid in water. Chemosensors 2023, 11, 177. [Google Scholar] [CrossRef]
- He, P.; Chen, Y.; Li, X.; Yan, Y.; Liu, C. AIPE-active cationic Ir(III) complexes for efficient detection of 2,4,6-trinitrophenol and oxygen. Dalton Trans. 2023, 52, 128–135. [Google Scholar] [CrossRef]
- Tamayo, A.; Alleyne, B.; Djurovich, P.; Lamansky, S.; Tsyba, I.; Ho, N.; Bau, R.; Thompson, M. Synthesis and characterization of facial and meridional tris-cyclometalated iridium(III) complexes. J. Am. Chem. Soc. 2003, 125, 7377–7387. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, S.; Wang, S.; Li, Y. Emissive properties and aggregation-induced emission enhancement of excited-state intramolecular proton-transfer compounds. Comptes Rendus Chim. 2011, 14, 789–798. [Google Scholar] [CrossRef]
- Chu, Z.; Fan, Z.; Zhang, X.; Tan, X.; Li, D.; Chen, G.; Zhao, Q. A comparison of ACQ, AIE and AEE-based polymers loaded on polyurethane foams as sensors for explosives detection. Sensors 2018, 18, 1565. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, Z.; Wu, Z.; Huang, X. A soluble porous coordination polymer for fluorescence sensing of explosives and toxic anions under homogeneous environment. Sensors 2023, 23, 9719. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Tian, Y.; Wang, X.; Gong, D.; Guo, Y.; Iqbal, K.; Wang, Z.; Liu, W.; Qin, W. Carbon dots prepared by solid state method via citric acid and 1,10-phenanthroline for selective and sensing detection of Fe2+ and Fe3+. Sens. Actuators B Chem. 2016, 237, 408–415. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]







| Complex 2 | Intensity |
|---|---|
| X1 | 101.59 |
| X2 | 101.52 |
| X3 | 101.33 |
| X4 | 101.37 |
| X5 | 101.33 |
| X6 | 101.22 |
| X7 | 101.20 |
| X8 | 101.39 |
| X9 | 101.21 |
| X10 | 101.49 |
| X11 | 101.28 |
| X | 101.36 |
| σ | 0.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhang, L.; Shi, Y.; Liu, C. Carbazolyl-Modified Neutral Ir(III) Complexes for Efficient Detection of Picric Acid in Aqueous Media. Sensors 2024, 24, 4074. https://doi.org/10.3390/s24134074
Xu J, Zhang L, Shi Y, Liu C. Carbazolyl-Modified Neutral Ir(III) Complexes for Efficient Detection of Picric Acid in Aqueous Media. Sensors. 2024; 24(13):4074. https://doi.org/10.3390/s24134074
Chicago/Turabian StyleXu, Jiangchao, Liyan Zhang, Yusheng Shi, and Chun Liu. 2024. "Carbazolyl-Modified Neutral Ir(III) Complexes for Efficient Detection of Picric Acid in Aqueous Media" Sensors 24, no. 13: 4074. https://doi.org/10.3390/s24134074







