An Overall Automated Architecture Based on the Tapping Test Measurement Protocol: Hand Dexterity Assessment through an Innovative Objective Method
Abstract
1. Introduction
2. Motivation and Contribution of the Work
- Proposal of an automated measurement protocol to assess coordinative abilities through tapping test-based exercises;
- Computation of a large number of features to give a quantitative, objective, and exhaustive movement assessment;
- Comparative analysis of the proposed tasks to obtain general outcomes about the analyzed population samples.
3. The Proposed Measurement Architecture
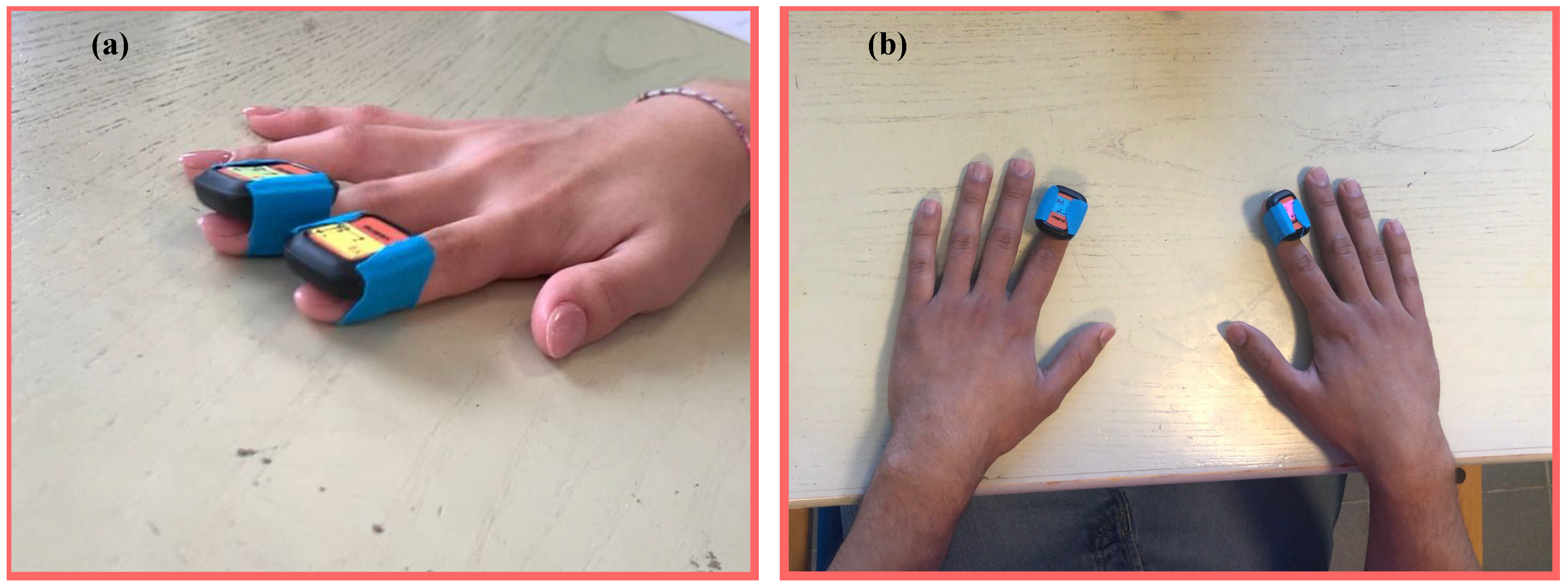
3.1. The Measurement Protocol
- Alternate uni-manual (UniALT): tapping is performed by alternating the index and middle fingers of the dominant hand.
- Simultaneous uni-manual (UniSIM): tapping is performed by simultaneously moving the index and middle fingers of the dominant hand.
- Alternate bi-manual (BimALT): tapping is performed by alternately moving the index fingers of the right and left hands.
- Simultaneous bi-manual (BimSIM): tapping is performed by simultaneously moving the index fingers of both hands.
3.1.1. Uni-Manual
3.1.2. Bi-Manual
3.1.3. Participants
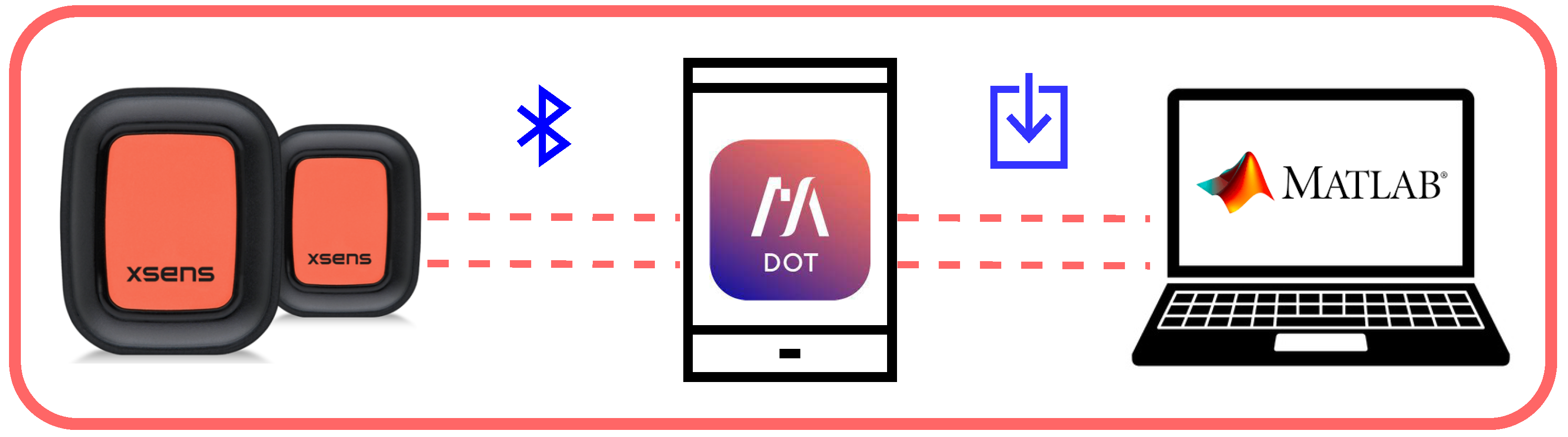
3.2. The Adopted Measurement Platform
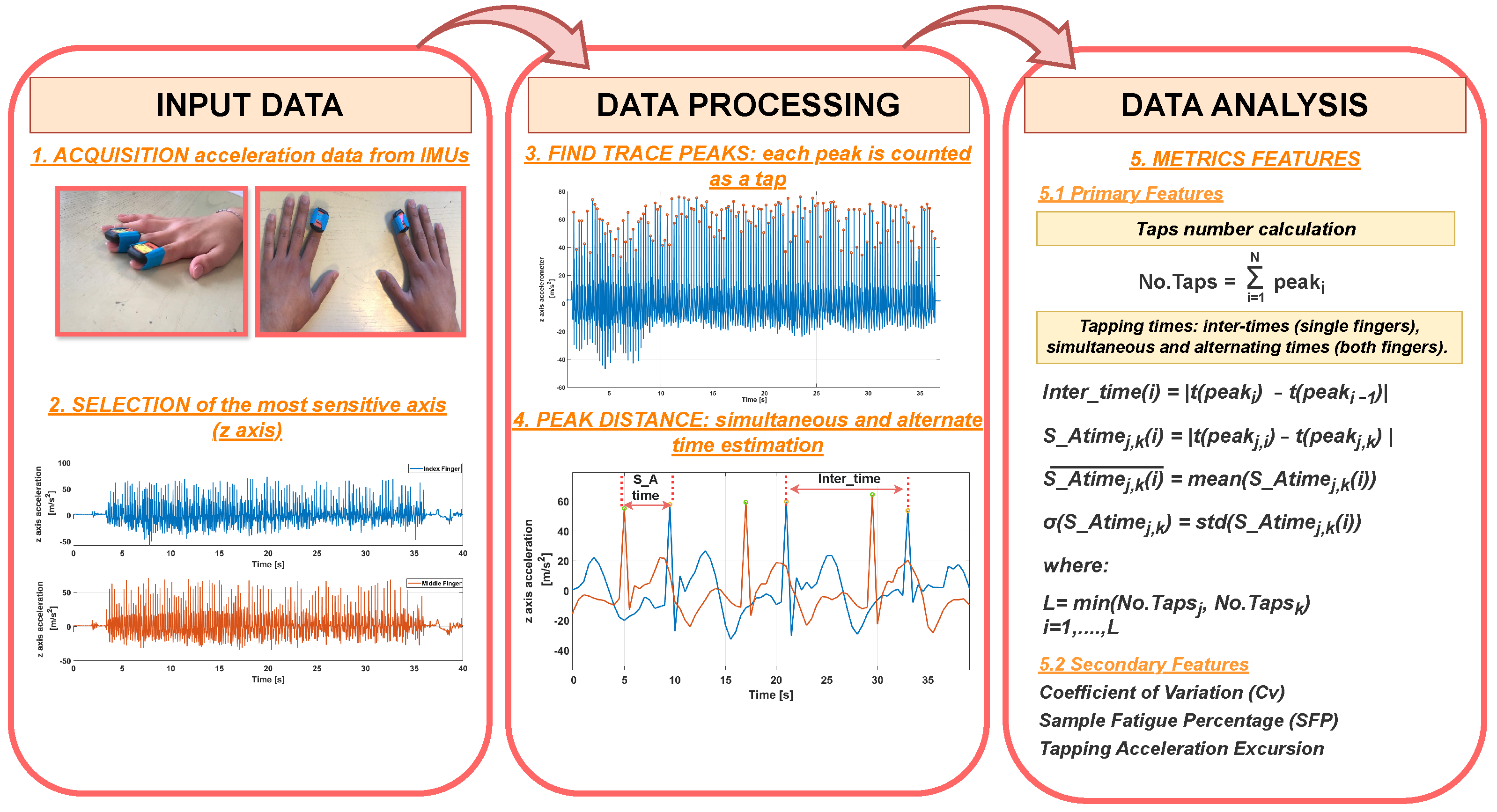
3.3. The Data Analysis
| Algorithm 1 Data analysis: Computation of metrics features. |
|
4. Results
4.1. Experimental Characterization
- Position A: a fixed position from the metacarpal joint of the index finger (2 cm) is chosen to place the IMU.
- Position B: IMU placed on the distal phalanx of an index finger, without considering joint distance.
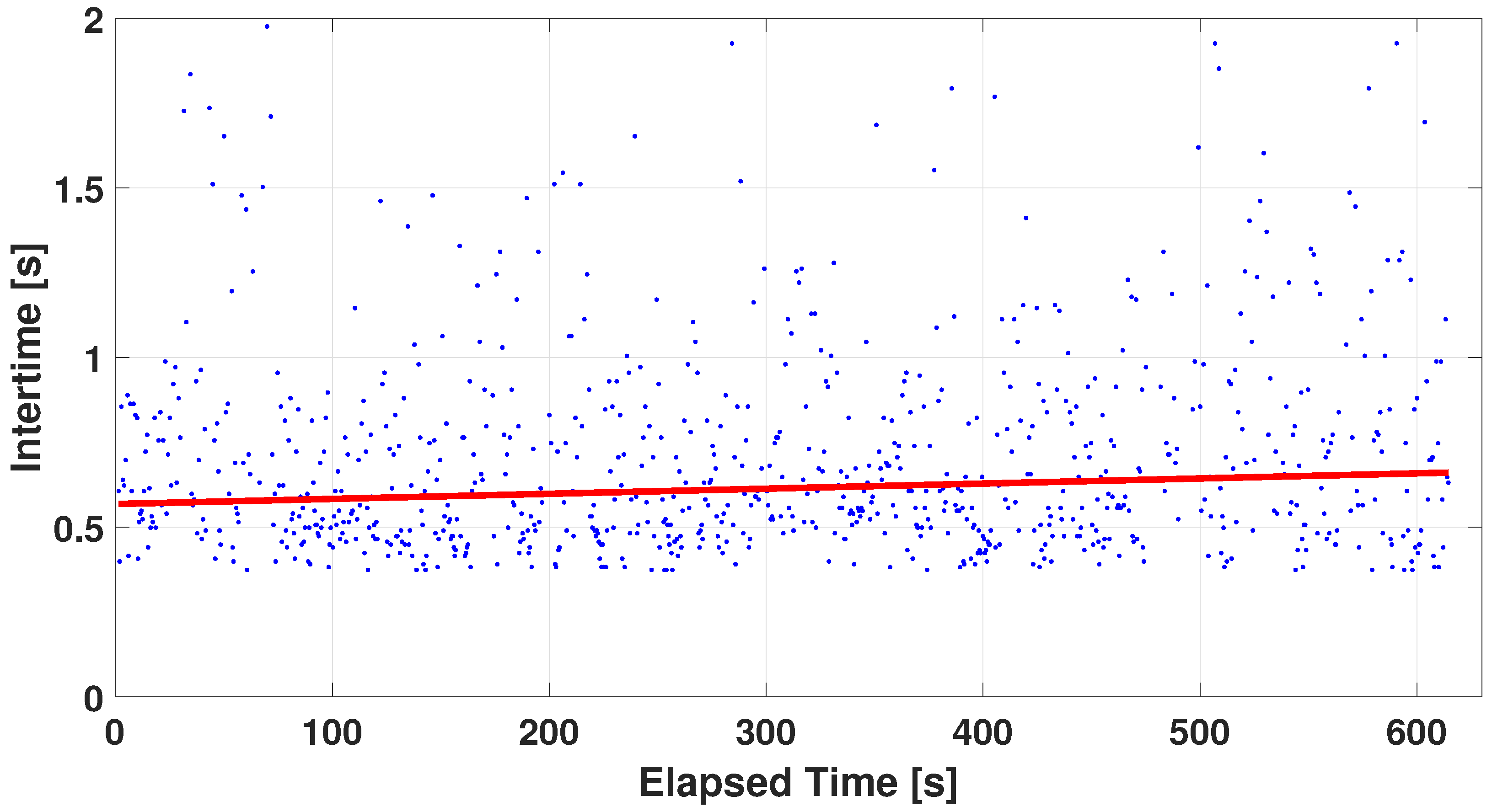
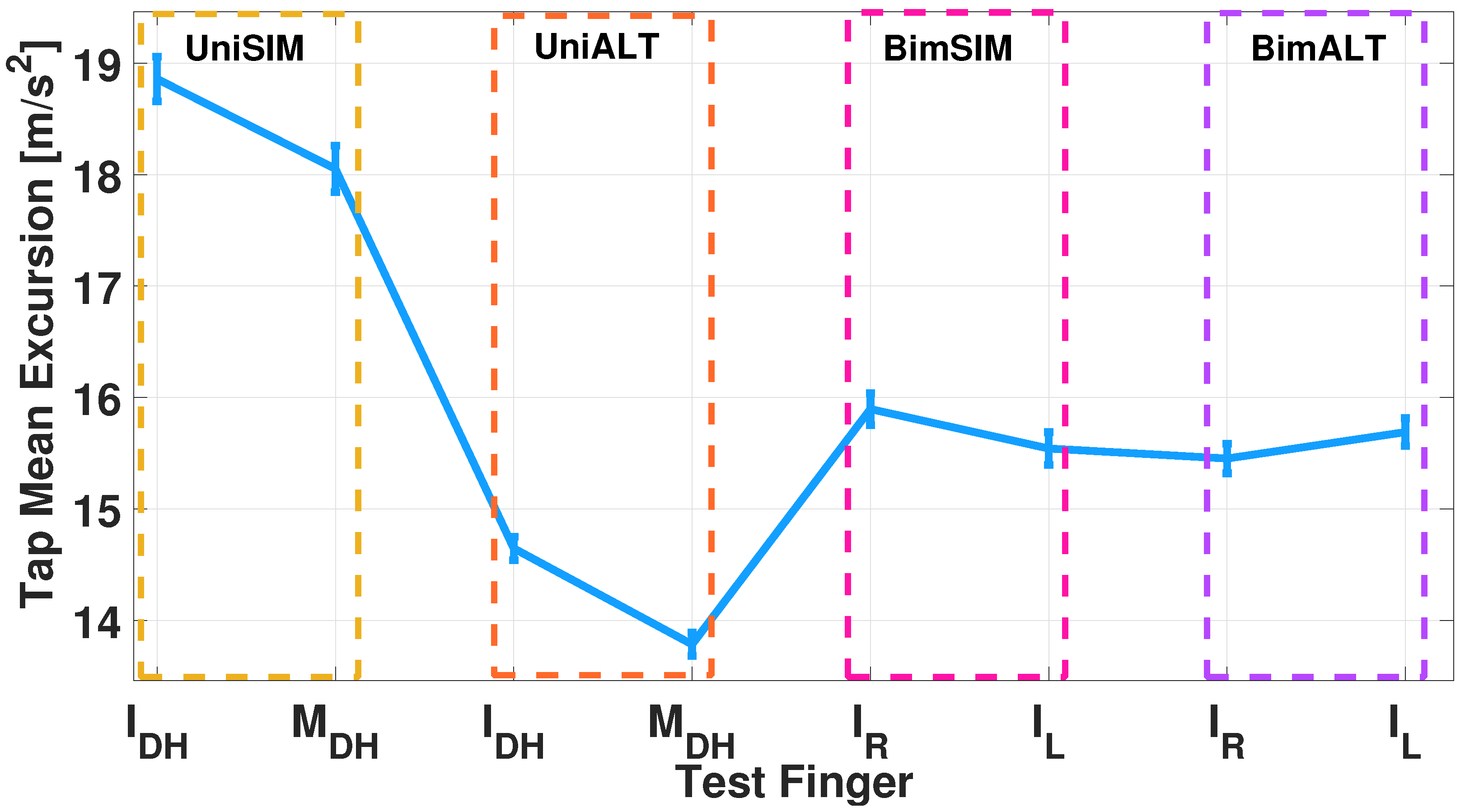
4.2. Measurement Protocol Results
4.2.1. Single-Finger Analysis
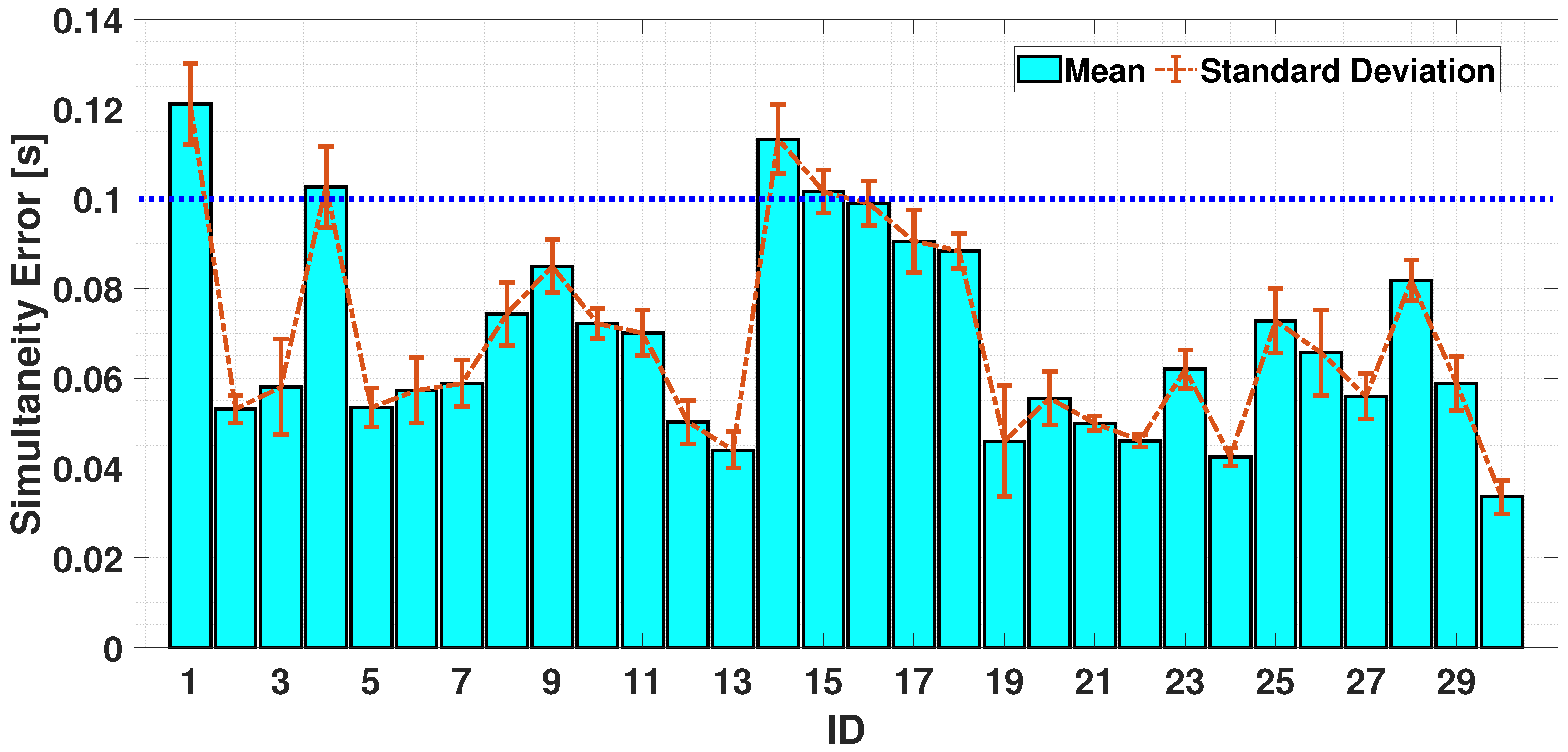
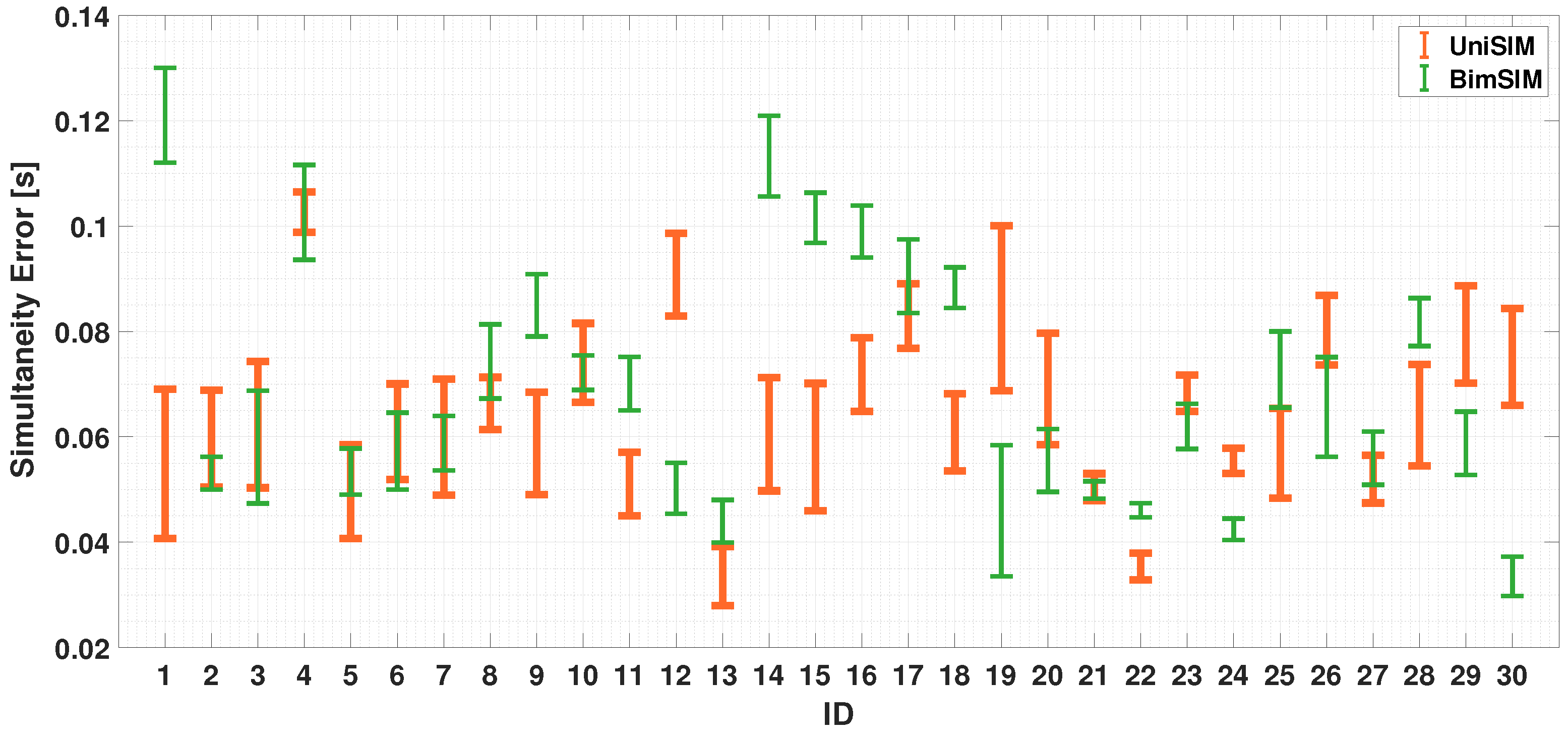
4.2.2. Dual-Finger Analysis—A Simultaneous Case
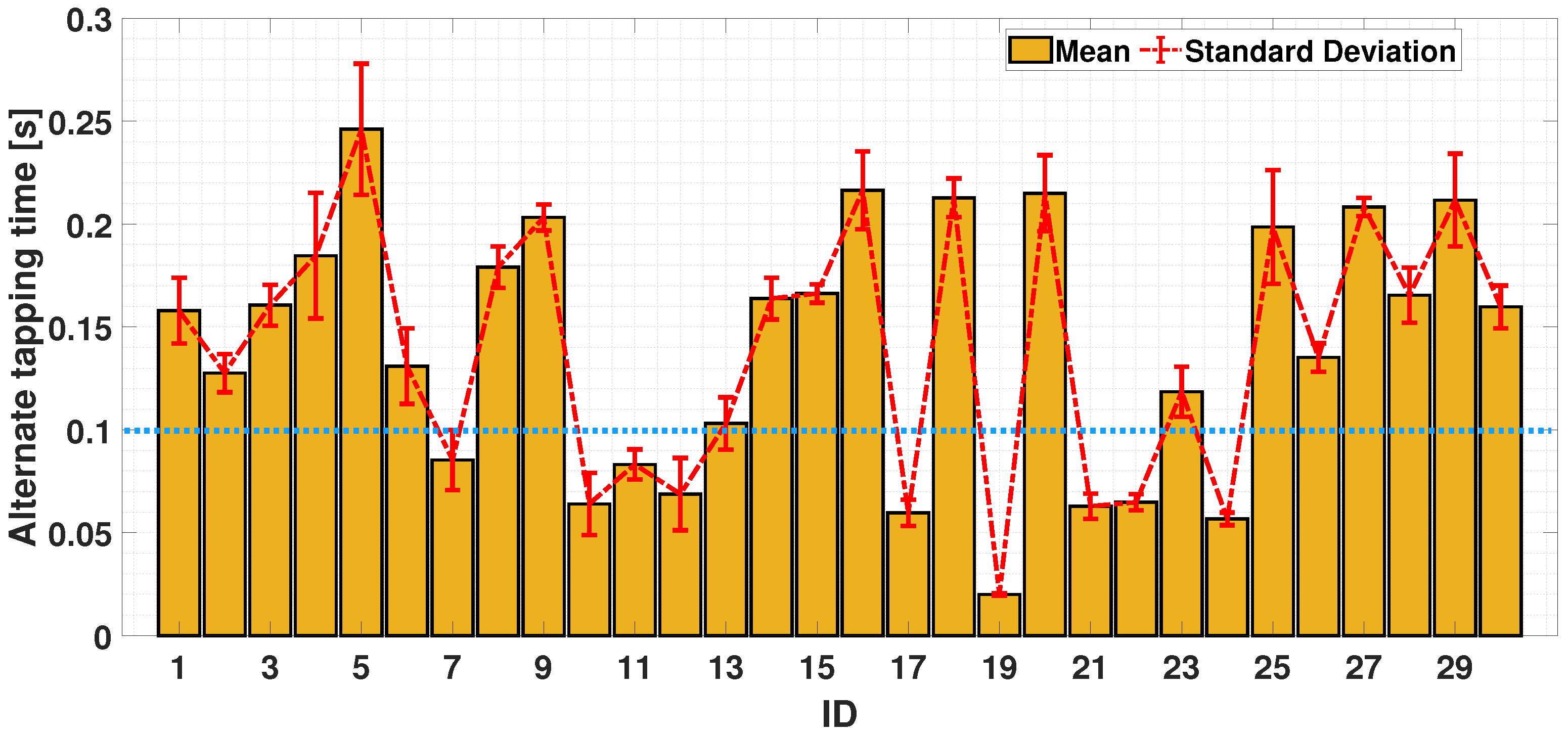
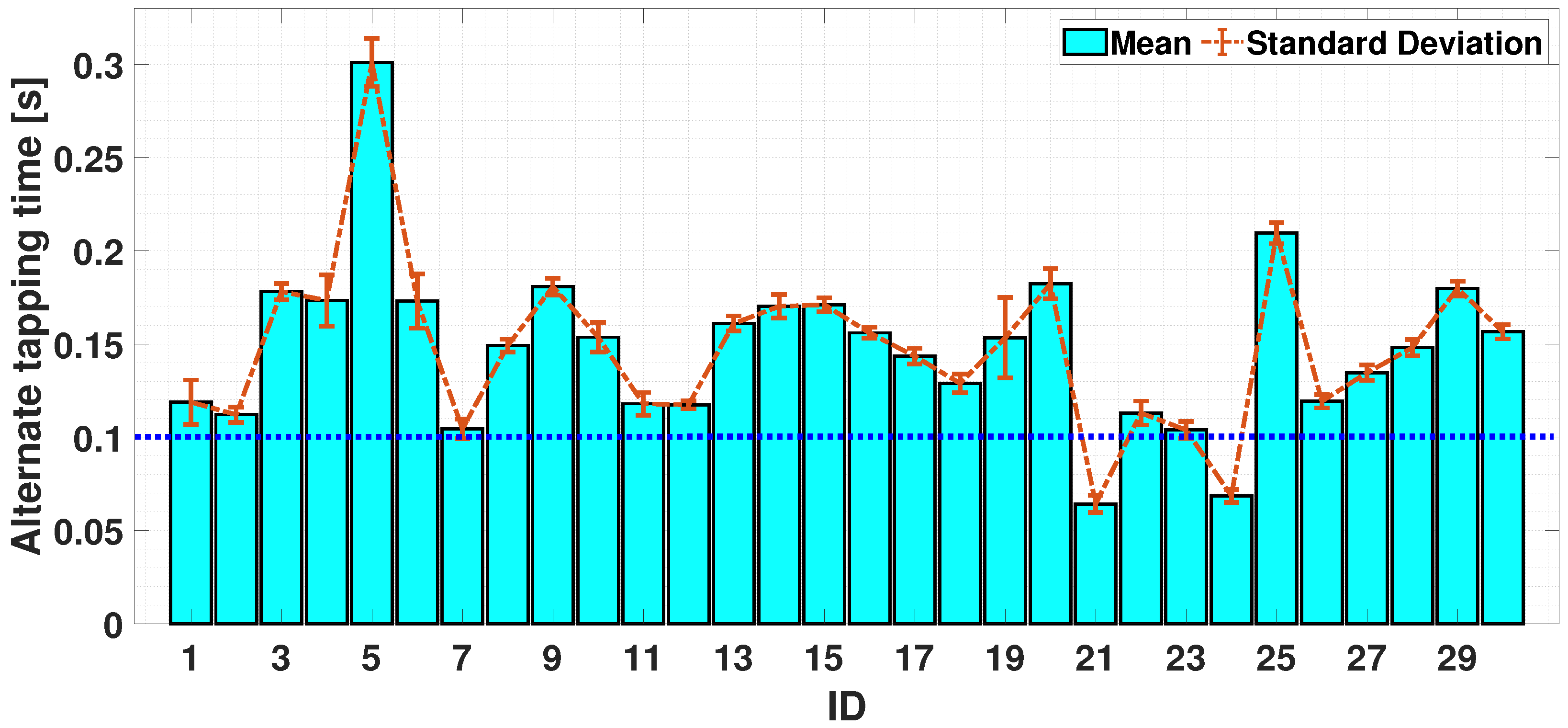
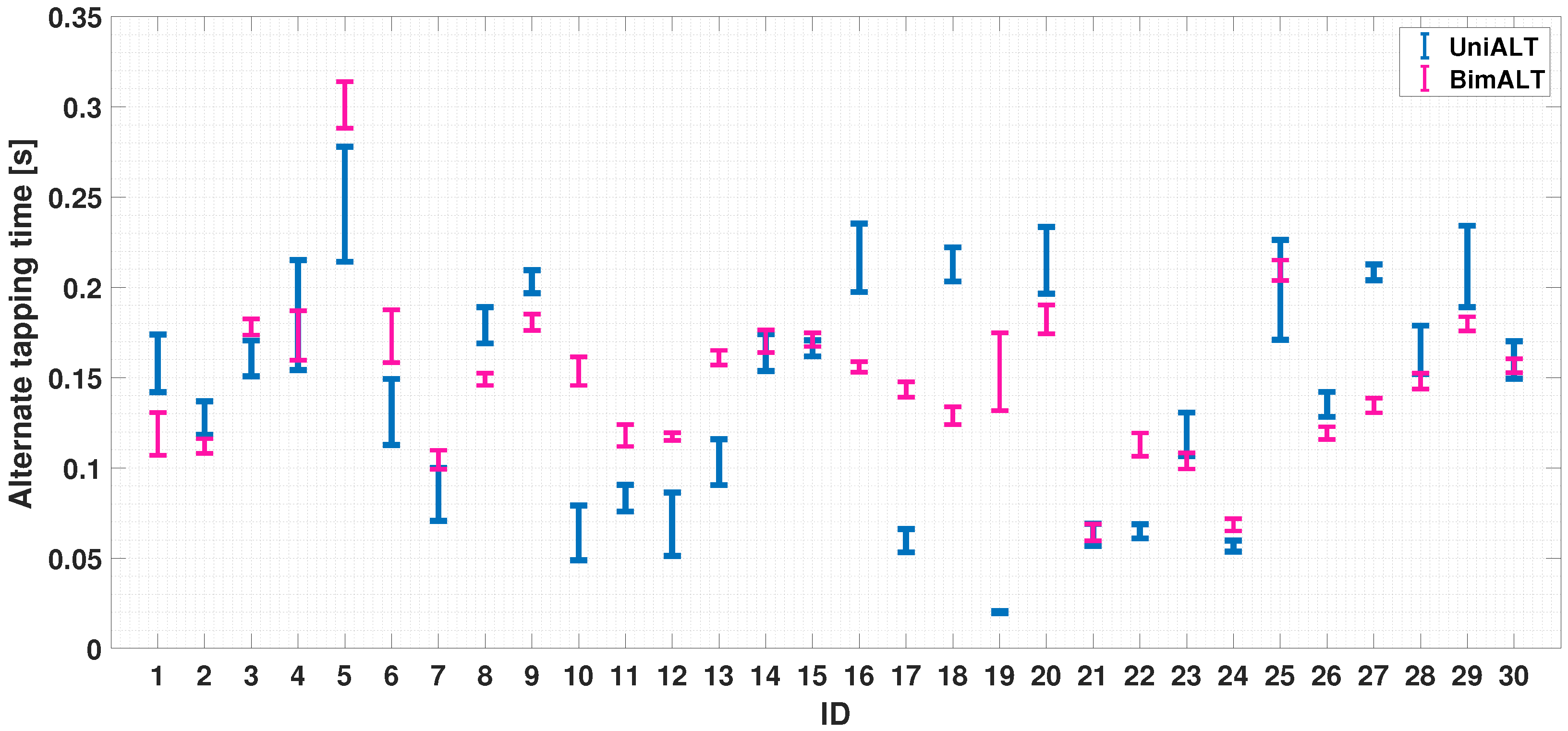
4.2.3. Dual-Finger Analysis—An Alternate Case
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stănciulescu, R. Dexterity, Mobility and Flexibility, Essential Components in the Development of the Military Students’ Motor Skills. Int. Conf. Knowl.-Based Organ. 2023, 29, 242–246. [Google Scholar] [CrossRef]
- Stergiou, N.; Decker, L.M. Human movement variability, nonlinear dynamics, and pathology: Is there a connection? Hum. Mov. Sci. 2011, 30, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xiong, C.; Huang, X.; Sun, R.; Xiong, Y. Kinematic analysis and dexterity evaluation of upper extremity in activities of daily living. Gait Posture 2010, 32, 475–481. [Google Scholar] [PubMed]
- Alamri, A.; Eid, M.; Iglesias, R.; Shirmohammadi, S.; El Saddik, A. Haptic virtual rehabilitation exercises for poststroke diagnosis. IEEE Trans. Instrum. Meas. 2008, 57, 1876–1884. [Google Scholar] [CrossRef]
- Morris, M.E.; Bilney, B.; Miller, K.J.; Nijkrake, M.J.; Munneke, M.; McGinley, J.L. Hand dexterity assessment in Parkinson’s disease: Construct validity of the 9-Hole peg test for the more affected hand. Disabil. Rehabil. 2021, 43, 3834–3838. [Google Scholar]
- Voola, S.I.; Hema, S. Effectiveness of Tele Rehabilitation on Manual Dexterity and its Impact on Quality of Life in Patients with Parkinson’s Disease: A Pilot Study. Indian J. Physiother. Occup. Ther. 2020, 14, 186–193. [Google Scholar] [CrossRef]
- Reed, J.C.; Reed, H.B. The halstead—Reitan neuropsychological battery. In Contemporary Approaches to Neuropsychological Assessment; Springer: Boston, MA, USA, 1997; pp. 93–129. [Google Scholar]
- Axelrod, B.N.; Meyers, J.E.; Davis, J.J. Finger tapping test performance as a measure of performance validity. Clin. Neuropsychol. 2014, 28, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Pathirana, P.N.; Horne, M.; Power, L.; Szmulewicz, D.J. Entropy-based analysis of rhythmic tapping for the quantitative assessment of cerebellar ataxia. Biomed. Signal Process. Control 2020, 59, 101916. [Google Scholar] [CrossRef]
- Suzumura, S.; Kanada, Y.; Osawa, A.; Sugioka, J.; Maeda, N.; Nagahama, T.; Shiramoto, K.; Kuno, K.; Kizuka, S.; Sano, Y.; et al. Assessment of finger motor function that reflects the severity of cognitive function. Fujita Med. J. 2021, 7, 122–129. [Google Scholar]
- Suzumura, S.; Osawa, A.; Maeda, N.; Sano, Y.; Kandori, A.; Mizuguchi, T.; Yin, Y.; Kondo, I. Differences among patients with Alzheimer’s disease, older adults with mild cognitive impairment and healthy older adults in finger dexterity. Geriatr. Gerontol. Int. 2018, 18, 907–914. [Google Scholar] [CrossRef]
- Waqar, A.; Ahmad, I.; Habibi, D.; Hart, N.; Phung, Q.V. Enhancing Athlete Tracking Using Data Fusion in Wearable Technologies. IEEE Trans. Instrum. Meas. 2021, 70, 4004013. [Google Scholar] [CrossRef]
- Talitckii, A.; Kovalenko, E.; Shcherbak, A.; Anikina, A.; Bril, E.; Zimniakova, O.; Semenov, M.; Dylov, D.V.; Somov, A. Comparative Study of Wearable Sensors, Video, and Handwriting to Detect Parkinson’s Disease. IEEE Trans. Instrum. Meas. 2022, 71, 2509910. [Google Scholar] [CrossRef]
- Carissimo, C.; Cerro, G.; Di Libero, T.; Ferrigno, L.; Marino, A.; Rodio, A. Objective evaluation of coordinative abilities and training effectiveness in sports scenarios: An automated measurement protocol. IEEE Access 2023, 11, 76996–77008. [Google Scholar] [CrossRef]
- Di Libero, T.; Carissimo, C.; Cerro, G.; Abbatecola, A.M.; Marino, A.; Miele, G.; Ferrigno, L.; Rodio, A. Motor abilities analysis using a standardized tapping test enhanced by a detailed processing stage: Gender and age comparison. In Proceedings of the 2023 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Jeju, Republic of Korea, 14–16 June 2023; pp. 1–6. [Google Scholar] [CrossRef]
- Di Libero, T.; Carissimo, C.; Zagaglia, A.; Cerro, G.; Ferrigno, L.; Rodio, A. Assessment of coordinative abilities through upper extremity wearable device technology. In Proceedings of the 2022 IEEE International Workshop on Sport, Technology and Research, STAR 2022-Proceedings, Trento, Italy, 6–8 July 2022; pp. 175–179. [Google Scholar] [CrossRef]
- Schmitt, L. Finger-tapping test. In Encyclopedia of Autism Spectrum Disorders; Springer: Cham, Switzerland, 2021; p. 2030. [Google Scholar]
- Mitrushina, M.; Boone, K.B.; Razani, J.; D’Elia, L.F. Handbook of Normative Data for Neuropsychological Assessment; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Akram, N.; Li, H.; Ben-Joseph, A.; Budu, C.; Gallagher, D.A.; Bestwick, J.P.; Schrag, A.; Noyce, A.J.; Simonet, C. Developing and assessing a new web-based tapping test for measuring distal movement in Parkinson’s disease: A Distal Finger Tapping test. Sci. Rep. 2022, 12, 386. [Google Scholar] [PubMed]
- Coleman, A.R.; Moberg, P.J. Comparison of the Halstead-Reitan and infrared light beam finger tappers. Assessment 1997, 4, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zeng, W.; Yu, T.; Xu, Y.; Xiao, Y.; Cao, X.; Cao, Z. Vision-based finger tapping test in patients with Parkinson’s disease via spatial-temporal 3D hand pose estimation. IEEE J. Biomed. Health Inform. 2022, 26, 3848–3859. [Google Scholar] [CrossRef] [PubMed]
- Chettouf, S.; Rueda-Delgado, L.M.; de Vries, R.; Ritter, P.; Daffertshofer, A. Are unimanual movements bilateral? Neurosci. Biobehav. Rev. 2020, 113, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Koeneke, S.; Lutz, K.; Wüstenberg, T.; Jäncke, L. Bimanual versus unimanual coordination: What makes the difference? Neuroimage 2004, 22, 1336–1350. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, A.P.; Carpenter, A.F. Coding of movements in the motor cortex. Curr. Opin. Neurobiol. 2015, 33, 34–39. [Google Scholar] [CrossRef]
- Di Libero, T.; Langiano, E.; Carissimo, C.; Ferrara, M.; Diotaiuti, P.; Rodio, A. Technological support for people with Parkinson’s disease: A narrative review. J. Gerontol. Geriatr. 2023, 71, 87–101. [Google Scholar] [CrossRef]
- Serrien, D.J. Coordination constraints during bimanual versus unimanual performance conditions. Neuropsychologia 2008, 46, 419–425. [Google Scholar] [CrossRef]
- Lang, C.E.; Schieber, M.H. Differential impairment of individuated finger movements in humans after damage to the motor cortex or the corticospinal tract. J. Neurophysiol. 2003, 90, 1160–1170. [Google Scholar] [CrossRef]
- Sainburg, R.L.; Schaefer, S.Y. Interlimb differences in control of movement extent. J. Neurophysiol. 2004, 92, 1374–1383. [Google Scholar] [CrossRef]
- Shim, J.K.; Lay, B.S.; Zatsiorsky, V.M.; Latash, M.L. Age-related changes in finger coordination in static prehension tasks. J. Appl. Physiol. 2004, 97, 213–224. [Google Scholar] [CrossRef]
- Zhao, M.; Marino, M.; Samogin, J.; Swinnen, S.P.; Mantini, D. Hand, foot and lip representations in primary sensorimotor cortex: A high-density electroencephalography study. Sci. Rep. 2019, 9, 19464. [Google Scholar] [CrossRef]
- Yokoi, A.; Arbuckle, S.A.; Diedrichsen, J. The role of human primary motor cortex in the production of skilled finger sequences. J. Neurosci. 2018, 38, 1430–1442. [Google Scholar] [CrossRef]
- Schieber, M.H.; Santello, M. Hand function: Peripheral and central constraints on performance. J. Appl. Physiol. 2004, 96, 2293–2300. [Google Scholar] [CrossRef]
- Wiegel, P.; Kurz, A.; Leukel, C. Evidence that distinct human primary motor cortex circuits control discrete and rhythmic movements. J. Physiol. 2020, 598, 1235–1251. [Google Scholar] [CrossRef]
- François-Brosseau, F.E.; Martinu, K.; Strafella, A.P.; Petrides, M.; Simard, F.; Monchi, O. Basal ganglia and frontal involvement in self-generated and externally-triggered finger movements in the dominant and non-dominant hand. Eur. J. Neurosci. 2009, 29, 1277–1286. [Google Scholar] [CrossRef]
- Kornhuber, H.H.; Deecke, L. Brain potential changes in voluntary and passive movements in humans: Readiness potential and reafferent potentials. Pflügers Arch.-Eur. J. Physiol. 2016, 468, 1115–1124. [Google Scholar] [CrossRef]
- Van Vugt, F.T.; Altenmüller, E. On the one hand or on the other: Trade-off in timing precision in bimanual musical scale playing. Adv. Cogn. Psychol. 2019, 15, 216. [Google Scholar] [CrossRef]
- Saito, Y.; Maezawa, T.; Kawahara, J.I. Beat patterns determine inter-hand differences in synchronization error in a bimanual coordination tapping task. i-Perception 2021, 12, 20416695211053882. [Google Scholar] [CrossRef]
- Sisti, H.M.; Beebe, A.; Bishop, M.; Gabrielsson, E. A brief review of motor imagery and bimanual coordination. Front. Hum. Neurosci. 2022, 16, 1037410. [Google Scholar] [CrossRef]
- Movella. Xsens Products. Available online: https://www.movella.com/products/xsens (accessed on 24 August 2023).
- MATLABTM. Find Abrupt Changes. Available online: https://it.mathworks.com/help/signal/ref/findchangepts.html (accessed on 1 March 2024).
- Mawase, F.; Uehara, S.; Bastian, A.J.; Celnik, P. Motor learning enhances use-dependent plasticity. J. Neurosci. 2017, 37, 2673–2685. [Google Scholar] [CrossRef]
- Mollà-Casanova, S.; Llorens, R.; Borrego, A.; Salinas-Martínez, B.; Serra-Añó, P. Validity, reliability, and sensitivity to motor impairment severity of a multi-touch app designed to assess hand mobility, coordination, and function after stroke. J. Neuroeng. Rehabil. 2021, 18, 70. [Google Scholar] [CrossRef]
- Roalf, D.R.; Rupert, P.; Mechanic-Hamilton, D.; Brennan, L.; Duda, J.E.; Weintraub, D.; Trojanowski, J.Q.; Wolk, D.; Moberg, P.J. Quantitative assessment of finger tapping characteristics in mild cognitive impairment, Alzheimer’s disease, and Parkinson’s disease. J. Neurol. 2018, 265, 1365–1375. [Google Scholar] [CrossRef]
- Lövdén, M.; Garzón, B.; Lindenberger, U. Human skill learning: Expansion, exploration, selection, and refinement. Curr. Opin. Behav. Sci. 2020, 36, 163–168. [Google Scholar] [CrossRef]
- Crichton, P. Penfield’s homunculus. J. Neurol. Neurosurg. Psychiatry 1994, 57, 525, Erratum in: J. Neurol. Neurosurg. Psychiatry 1994, 57, 772. [Google Scholar] [CrossRef]
- Makai-Bölöni, S.; Thijssen, E.; van Brummelen, E.M.; Groeneveld, G.J.; Doll, R.J. Touchscreen-based finger tapping: Repeatability and configuration effects on tapping performance. PLoS ONE 2021, 16, e0260783. [Google Scholar] [CrossRef]
- Naito, E.; Morita, T.; Asada, M. Importance of the primary motor cortex in development of human hand/finger dexterity. Cereb. Cortex Commun. 2020, 1, tgaa085. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, Y.; Wang, X.; Wei, H.; Huang, Q.; Li, L. The correlations between kinematic profiles and cerebral hemodynamics suggest changes of motor coordination in single and bilateral finger movement. Front. Hum. Neurosci. 2022, 16, 957364. [Google Scholar] [CrossRef]
- Afreen, S.; Mazhar, K.; Malik, R.; Asif, I. Role of Neuroplasticity in Neurorehabilitation. Asia Pac. J. Allied Health Sci. Vol. 2021, 4, 46–51. [Google Scholar]
- Machan, T.; Krupps, K. The Neuroplastic Adaptation Trident Model: A suggested novel framework for ACL rehabilitation. Int. J. Sport. Phys. Ther. 2021, 16, 896. [Google Scholar] [CrossRef]
- Pauwels, L.; Gooijers, J. The Role of the Corpus Callosum (Micro) Structure in Bimanual Coordination: A Literature Review Update. J. Mot. Behav. 2023, 55, 525–537. [Google Scholar] [CrossRef]
- Tomita, Y.; Tanaka, S.; Takahashi, S.; Takeuchi, N. Detecting cognitive decline in community-dwelling older adults using simple cognitive and motor performance tests. Geriatr. Gerontol. Int. 2020, 20, 212–217. [Google Scholar] [CrossRef]
- Carey, J.R.; Bhatt, E.; Nagpal, A. Neuroplasticity promoted by task complexity. Exerc. Sport Sci. Rev. 2005, 33, 24–31. [Google Scholar]
- Ivanenko, Y.P.; Cappellini, G.; Solopova, I.; Grishin, A.; MacLellan, M.J.; Poppele, R.; Lacquaniti, F. Plasticity and modular control of locomotor patterns in neurological disorders with motor deficits. Front. Comput. Neurosci. 2013, 7, 123. [Google Scholar] [CrossRef]
- Yue, Z.; Zhang, X.; Wang, J. Hand rehabilitation robotics on poststroke motor recovery. Behav. Neurol. 2017, 2017, 3908135. [Google Scholar] [CrossRef]
- Kotseva, K.; De Backer, G.; De Bacquer, D.; Rydén, L.; Hoes, A.; Grobbee, D.; Maggioni, A.; Marques-Vidal, P.; Jennings, C.; Abreu, A.; et al. Primary prevention efforts are poorly developed in people at high cardiovascular risk: A report from the European Society of Cardiology EURObservational Research Programme EUROASPIRE V survey in 16 European countries. Eur. J. Prev. Cardiol. 2021, 28, 370–379. [Google Scholar]



| Mean ± St.Dev | |
|---|---|
| Age | 25.67 ± 0.33 |
| Index Finger Length [cm] | 10.03 ± 0.12 |
| Middle Finger Length [cm] | 11.07 ± 0.12 |
| [%] Configuration A | [%] Configuration B | |
|---|---|---|
| ID 1 | 10.60 | 7.40 |
| ID 2 | 3.31 | 4.54 |
| ID 3 | 6.16 | 5.05 |
| ID 4 | 8.58 | 5.03 |
| ID 5 | 3.27 | 1.71 |
| ID 6 | 7.40 | 5.53 |
| ID 7 | 4.54 | 4.91 |
| ID 8 | 5.05 | 4.61 |
| ID 9 | 5.03 | 2.76 |
| ID 10 | 1.71 | 0.78 |
| Mean value | 5.57 | 4.23 |
| Test UniSIM | Mean | Dev.Std |
|---|---|---|
| Index | −0.006 | 0.010 |
| Middle | −0.005 | 0.010 |
| Test UniALT | Mean | Dev.Std |
| Index | 0.006 | 0.060 |
| Middle | 0.000 | 0.033 |
| Test BimSIM | Mean | Dev.Std |
|---|---|---|
| Index R | −0.0022 | 0.0075 |
| Index L | −0.0033 | 0.0062 |
| Test BimALT | Mean | Dev.Std |
| Index R | 0.000 | 0.013 |
| Index L | −0.001 | 0.017 |
| Test UniSIM | Test UniALT | Test BimSIM | Test BimALT | ||
|---|---|---|---|---|---|
| Index | 0.29 | 0.54 | Index R | 0.38 | 0.48 |
| Middle | 0.31 | 0.50 | Index L | 0.30 | 0.47 |
| UniSIM vs. UniALT | UniSIM vs. BimSIM | UniSIM vs. BimALT | |
|---|---|---|---|
| Right Index | 22.33% | 15.70% | 18.06% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Libero, T.; Carissimo, C.; Cerro , G.; Abbatecola , A.M.; Marino, A.; Miele , G.; Ferrigno , L.; Rodio, A. An Overall Automated Architecture Based on the Tapping Test Measurement Protocol: Hand Dexterity Assessment through an Innovative Objective Method. Sensors 2024, 24, 4133. https://doi.org/10.3390/s24134133
Di Libero T, Carissimo C, Cerro G, Abbatecola AM, Marino A, Miele G, Ferrigno L, Rodio A. An Overall Automated Architecture Based on the Tapping Test Measurement Protocol: Hand Dexterity Assessment through an Innovative Objective Method. Sensors. 2024; 24(13):4133. https://doi.org/10.3390/s24134133
Chicago/Turabian StyleDi Libero, Tommaso, Chiara Carissimo, Gianni Cerro , Angela Marie Abbatecola , Alessandro Marino, Gianfranco Miele , Luigi Ferrigno , and Angelo Rodio. 2024. "An Overall Automated Architecture Based on the Tapping Test Measurement Protocol: Hand Dexterity Assessment through an Innovative Objective Method" Sensors 24, no. 13: 4133. https://doi.org/10.3390/s24134133
APA StyleDi Libero, T., Carissimo, C., Cerro , G., Abbatecola , A. M., Marino, A., Miele , G., Ferrigno , L., & Rodio, A. (2024). An Overall Automated Architecture Based on the Tapping Test Measurement Protocol: Hand Dexterity Assessment through an Innovative Objective Method. Sensors, 24(13), 4133. https://doi.org/10.3390/s24134133











