An Inertial-Based Wearable System for Monitoring Vital Signs during Sleep
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dataset
2.2. IMU-Based Wearable System
2.3. IMU-Derived Vital Signs
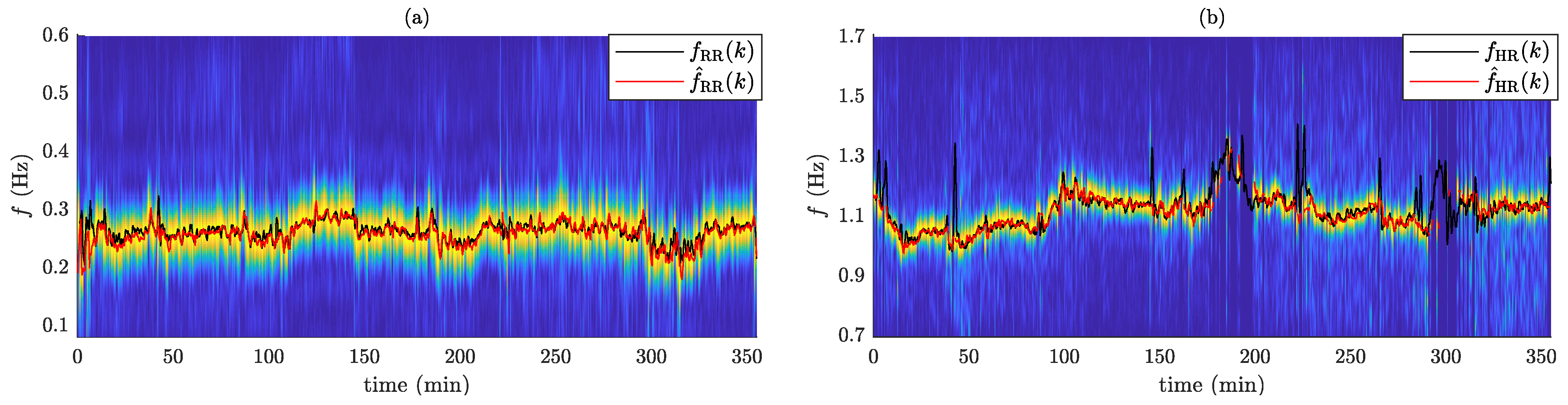
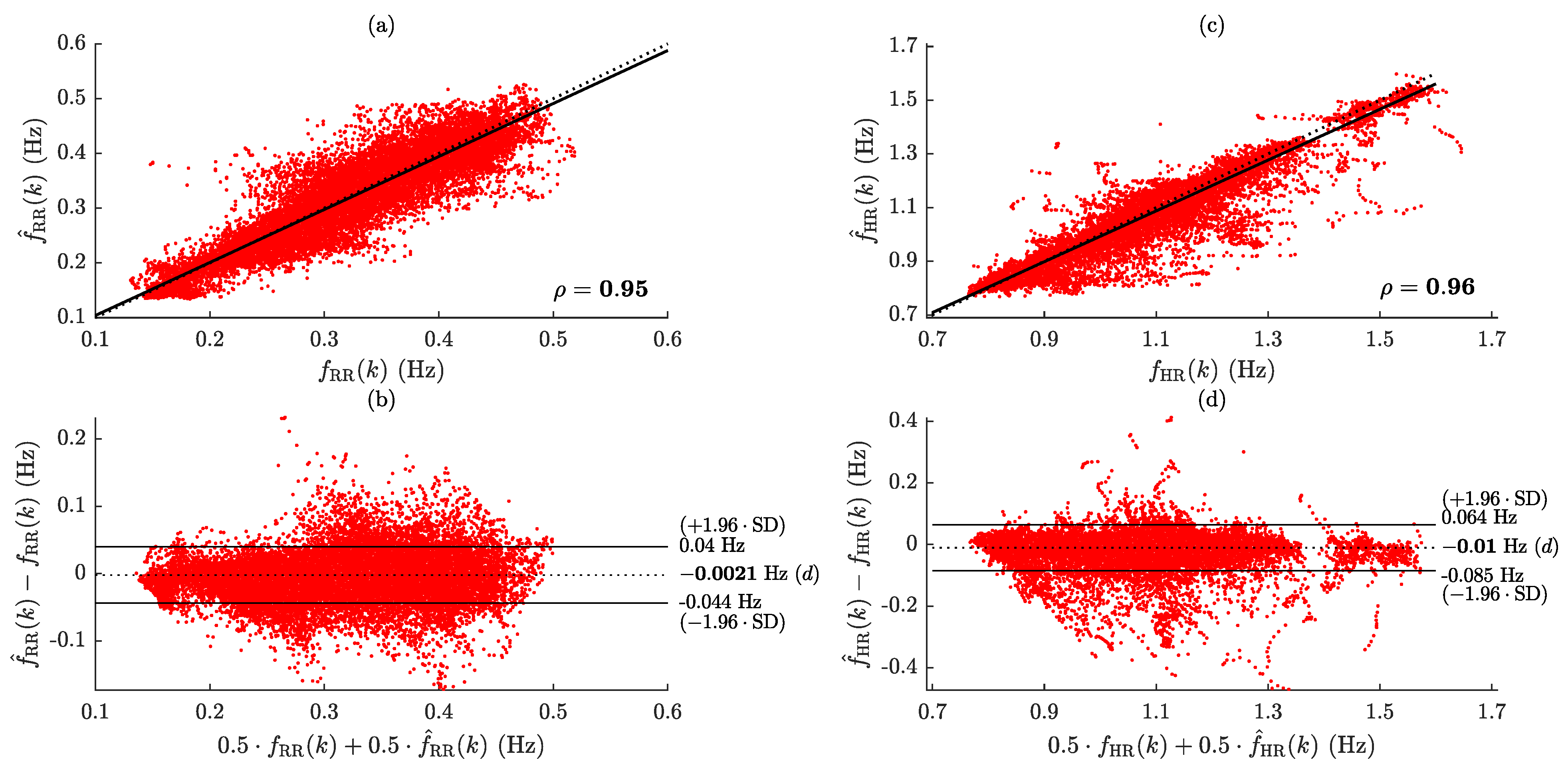
2.4. Performance Evaluation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RR | Respiratory rate |
| HR | Heart rate |
| ICU | Intensive care unit |
| ECG | Electrocardiogram |
| BCG | Ballistocardiogram |
| SCG | Seismocardiogram |
| GCG | Gyrocardiogram |
| SDB | Sleep-disordered breathing |
| SNR | Signal-to-noise ratio |
| PCA | Principal component analysis |
| IMU | Inertial measurement units |
| AHI | Apnea–hypopnea index |
| OSA | Obstructive sleep apnea |
| PSG | Polysomnography |
| PD | Parkinson’s disease |
| MAE | Mean absolute error |
| IQR | interquartile range |
References
- Andersen, L.W.; Holmberg, M.J.; Berg, K.M.; Donnino, M.W.; Granfeldt, A. In-hospital cardiac arrest: A review. JAMA 2019, 321, 1200–1210. [Google Scholar] [CrossRef]
- Cretikos, M.A.; Bellomo, R.; Hillman, K.; Chen, J.; Finfer, S.; Flabouris, A. Respiratory rate: The neglected vital sign. Med. J. Aust. 2008, 188, 657–659. [Google Scholar] [CrossRef]
- Smith, M.B.; Chiovaro, J.C.; O’Neil, M.; Kansagara, D.; Quiñones, A.R.; Freeman, M.; Motu’apuaka, M.L.; Slatore, C.G. Early warning system scores for clinical deterioration in hospitalized patients: A systematic review. Ann. Am. Thorac. Soc. 2014, 11, 1454–1465. [Google Scholar] [CrossRef]
- Barfod, C.; Lauritzen, M.M.P.; Danker, J.K.; Sölétormos, G.; Forberg, J.L.; Berlac, P.A.; Lippert, F.; Lundstrøm, L.H.; Antonsen, K.; Lange, K.H.W. Abnormal vital signs are strong predictors for intensive care unit admission and in-hospital mortality in adults triaged in the emergency department-a prospective cohort study. Scand. J. Trauma Resusc. Emerg. Med. 2012, 20, 1–10. [Google Scholar] [CrossRef]
- Brekke, I.J.; Puntervoll, L.H.; Pedersen, P.B.; Kellett, J.; Brabrand, M. The value of vital sign trends in predicting and monitoring clinical deterioration: A systematic review. PLoS ONE 2019, 14, e0210875. [Google Scholar] [CrossRef]
- Petit, C.; Bezemer, R.; Atallah, L. A review of recent advances in data analytics for post-operative patient deterioration detection. J. Clin. Monit. Comput. 2018, 32, 391–402. [Google Scholar] [CrossRef]
- Joshi, M.; Ashrafian, H.; Aufegger, L.; Khan, S.; Arora, S.; Cooke, G.; Darzi, A. Wearable sensors to improve detection of patient deterioration. Expert Rev. Med. Devices 2019, 16, 145–154. [Google Scholar] [CrossRef]
- Huhn, S.; Axt, M.; Gunga, H.C.; Maggioni, M.A.; Munga, S.; Obor, D.; Sié, A.; Boudo, V.; Bunker, A.; Sauerborn, R.; et al. The impact of wearable technologies in health research: Scoping review. JMIR Mhealth Uhealth 2022, 10, e34384. [Google Scholar] [CrossRef]
- Ramasamy, S.; Balan, A. Wearable sensors for ECG measurement: A review. Sens. Rev. 2018, 38, 412–419. [Google Scholar] [CrossRef]
- Bailón, R.; Sornmo, L.; Laguna, P. A robust method for ECG-based estimation of the respiratory frequency during stress testing. IEEE. Trans. Biomed. Eng. 2006, 53, 1273–1285. [Google Scholar] [CrossRef]
- Varon, C.; Morales, J.; Lázaro, J.; Orini, M.; Deviaene, M.; Kontaxis, S.; Testelmans, D.; Buyse, B.; Borzée, P.; Sörnmo, L.; et al. A Comparative Study of ECG-derived Respiration in Ambulatory Monitoring using the Single-lead ECG. Sci. Rep. 2020, 10, 5704. [Google Scholar] [CrossRef]
- Knight, S.; Lipoth, J.; Namvari, M.; Gu, C.; Hedayati, M.; Syed-Abdul, S.; Spiteri, R.J. The accuracy of wearable photoplethysmography sensors for telehealth monitoring: A scoping review. Telemed. J. e-Health 2023, 29, 813–828. [Google Scholar] [CrossRef]
- Lázaro, J.; Gil, E.; Bailón, R.; Mincholé, A.; Laguna, P. Deriving respiration from photoplethysmographic pulse width. Med. Biol. Eng. Comput. 2013, 51, 233–242. [Google Scholar] [CrossRef]
- Charlton, P.H.; Allen, J.; Bailón, R.; Baker, S.; Behar, J.A.; Chen, F.; Clifford, G.D.; Clifton, D.A.; Davies, H.J.; Ding, C.; et al. The 2023 wearable photoplethysmography roadmap. Physiol. Meas. 2023, 44, 111001. [Google Scholar] [CrossRef]
- Balasubramaniam, S.L.; Wang, Y.; Ryan, L.; Hossain, J.; Rahman, T.; Shaffer, T.H. Age-related ranges of respiratory inductance plethysmography (RIP) reference values for infants and children. Paediatr. Respir. Rev. 2019, 29, 60–67. [Google Scholar] [CrossRef]
- Soon, S.; Svavarsdottir, H.; Downey, C.; Jayne, D.G. Wearable devices for remote vital signs monitoring in the outpatient setting: An overview of the field. BMJ Innov. 2020, 6, 55. [Google Scholar] [CrossRef]
- Giovangrandi, L.; Inan, O.T.; Wiard, R.M.; Etemadi, M.; Kovacs, G.T. Ballistocardiography—A method worth revisiting. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 4279–4282. [Google Scholar]
- Inan, O.T.; Migeotte, P.F.; Park, K.S.; Etemadi, M.; Tavakolian, K.; Casanella, R.; Zanetti, J.; Tank, J.; Funtova, I.; Prisk, G.K.; et al. Ballistocardiography and seismocardiography: A review of recent advances. IEEE J. Biomed. Health Inform. 2014, 19, 1414–1427. [Google Scholar] [CrossRef]
- Sadek, I.; Biswas, J.; Abdulrazak, B. Ballistocardiogram signal processing: A review. Health Inf. Sci. Syst. 2019, 7, 10. [Google Scholar] [CrossRef]
- Taebi, A.; Solar, B.E.; Bomar, A.J.; Sandler, R.H.; Mansy, H.A. Recent advances in seismocardiography. Vibration 2019, 2, 64–86. [Google Scholar] [CrossRef]
- Jafari Tadi, M.; Lehtonen, E.; Saraste, A.; Tuominen, J.; Koskinen, J.; Teräs, M.; Airaksinen, J.; Pänkäälä, M.; Koivisto, T. Gyrocardiography: A new non-invasive monitoring method for the assessment of cardiac mechanics and the estimation of hemodynamic variables. Sci. Rep. 2017, 7, 6823. [Google Scholar] [CrossRef]
- Liu, H.; Allen, J.; Zheng, D.; Chen, F. Recent development of respiratory rate measurement technologies. Physiol. Meas. 2019, 40, 07TR01. [Google Scholar] [CrossRef]
- Reinvuo, T.; Hannula, M.; Sorvoja, H.; Alasaarela, E.; Myllyla, R. Measurement of respiratory rate with high-resolution accelerometer and EMFit pressure sensor. In Proceedings of the IEEE SAS 2006, Houston, TX, USA, 7–9 February 2006; pp. 192–195. [Google Scholar]
- Bieber, G.; Haescher, M.; Vahl, M. Sensor requirements for activity recognition on smart watches. In Proceedings of the PETRA 2013, Island of Rhodes, Greece, 29–31 May 2013; pp. 1–6. [Google Scholar]
- Cesareo, A.; Previtali, Y.; Biffi, E.; Aliverti, A. Assessment of breathing parameters using an inertial measurement unit (IMU)-based system. Sensors 2018, 19, 88. [Google Scholar] [CrossRef]
- D’Mello, Y.; Skoric, J.; Xu, S.; Roche, P.J.; Lortie, M.; Gagnon, S.; Plant, D.V. Real-time cardiac beat detection and heart rate monitoring from combined seismocardiography and gyrocardiography. Sensors 2019, 19, 3472. [Google Scholar] [CrossRef]
- Hughes, S.; Liu, H.; Zheng, D. Influences of sensor placement site and subject posture on measurement of respiratory frequency using triaxial accelerometers. Front. Physiol. 2020, 11, 823. [Google Scholar] [CrossRef]
- Schipper, F.; van Sloun, R.J.; Grassi, A.; Derkx, R.; Overeem, S.; Fonseca, P. Estimation of respiratory rate and effort from a chest-worn accelerometer using constrained and recursive principal component analysis. Physiol. Meas. 2021, 42, 045004. [Google Scholar] [CrossRef]
- Zhao, C.; Zeng, W.; Hu, D.; Liu, H. Robust heart rate monitoring by a single wrist-worn accelerometer based on signal decomposition. IEEE Sens. J. 2021, 21, 15962–15971. [Google Scholar] [CrossRef]
- Romano, C.; Schena, E.; Formica, D.; Massaroni, C. Comparison between chest-worn accelerometer and gyroscope performance for heart rate and respiratory rate monitoring. Biosensors 2022, 12, 834. [Google Scholar] [CrossRef]
- Liu, G.Z.; Guo, Y.W.; Zhu, Q.S.; Huang, B.Y.; Wang, L. Estimation of respiration rate from three-dimensional acceleration data based on body sensor network. Telemed. J. e-Health 2011, 17, 705–711. [Google Scholar] [CrossRef]
- Kawamoto, K.; Kuriyama, H.; Tajima, S. Actigraphic detection of REM sleep based on respiratory rate estimation. J. Med. Biol. Eng. 2013, 2, 20–25. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, G.Z. Monitoring cardio-respiratory and posture movements during sleep: What can be achieved by a single motion sensor. In Proceedings of the BSN 2015, Cambridge, MA, USA, 9–12 June 2015; pp. 1–6. [Google Scholar]
- Hernandez, J.; McDuff, D.; Picard, R.W. Biowatch: Estimation of heart and breathing rates from wrist motions. In Proceedings of the PervasiveHealth 2015, Istanbul, Turkey, 20–23 May 2015; pp. 169–176. [Google Scholar]
- Ryser, F.; Hanassab, S.; Lambercy, O.; Werth, E.; Gassert, R. Respiratory analysis during sleep using a chest-worn accelerometer: A machine learning approach. Biomed. Signal Process. Control 2022, 78, 104014. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.; Whang, M. An enhanced method to estimate heart rate from seismocardiography via ensemble averaging of body movements at six degrees of freedom. Sensors 2018, 18, 238. [Google Scholar] [CrossRef]
- Yoon, J.W.; Noh, Y.S.; Kwon, Y.S.; Kim, W.K.; Yoon, H.R. Improvement of dynamic respiration monitoring through sensor fusion of accelerometer and gyro-sensor. J. Electr. Eng. Technol. 2014, 9, 334–343. [Google Scholar] [CrossRef]
- Cesareo, A.; Biffi, E.; Cuesta-Frau, D.; D’Angelo, M.G.; Aliverti, A. A novel acquisition platform for long-term breathing frequency monitoring based on inertial measurement units. Med. Biol. Eng. Comput. 2020, 58, 785–804. [Google Scholar] [CrossRef]
- De Fazio, R.; Greco, M.R.; De Vittorio, M.; Visconti, P. A Differential Inertial Wearable Device for Breathing Parameter Detection: Hardware and Firmware Development, Experimental Characterization. Sensors 2022, 22, 9953. [Google Scholar] [CrossRef]
- Jarchi, D.; Rodgers, S.J.; Tarassenko, L.; Clifton, D.A. Accelerometry-based estimation of respiratory rate for post-intensive care patient monitoring. IEEE Sens. J. 2018, 18, 4981–4989. [Google Scholar] [CrossRef]
- Zschocke, J.; Kluge, M.; Pelikan, L.; Graf, A.; Glos, M.; Müller, A.; Mikolajczyk, R.; Bartsch, R.P.; Penzel, T.; Kantelhardt, J.W. Detection and analysis of pulse waves during sleep via wrist-worn actigraphy. PLoS ONE 2019, 14, e0226843. [Google Scholar] [CrossRef]
- Doheny, E.P.; Lowery, M.M.; Russell, A.; Ryan, S. Estimation of respiration rate and sleeping position using a wearable accelerometer. In Proceedings of the EMBC 2020, Montreal, QC, Canada, 20–24 July 2020; pp. 4668–4671. [Google Scholar]
- Massaroni, C.; Nicolò, A.; Lo Presti, D.; Sacchetti, M.; Silvestri, S.; Schena, E. Contact-based methods for measuring respiratory rate. Sensors 2019, 19, 908. [Google Scholar] [CrossRef]
- De Fazio, R.; Stabile, M.; De Vittorio, M.; Velázquez, R.; Visconti, P. An overview of wearable piezoresistive and inertial sensors for respiration rate monitoring. Electronics 2021, 10, 2178. [Google Scholar] [CrossRef]
- Rahmani, M.H.; Berkvens, R.; Weyn, M. Chest-worn inertial sensors: A survey of applications and methods. Sensors 2021, 21, 2875. [Google Scholar] [CrossRef]
- Kontaxis, S.; Lázaro, J.; Corino, V.D.; Sandberg, F.; Bailón, R.; Laguna, P.; Sörnmo, L. ECG-derived respiratory rate in atrial fibrillation. IEEE. Trans. Biomed. Eng. 2019, 67, 905–914. [Google Scholar] [CrossRef]
- Kostikis, N.; Rigas, G.; Konitsiotis, S.; Fotiadis, D.I. Configurable offline sensor placement identification for a medical device monitoring Parkinson’s disease. Sensors 2021, 21, 7801. [Google Scholar] [CrossRef]
- Antonini, A.; Reichmann, H.; Gentile, G.; Garon, M.; Tedesco, C.; Frank, A.; Falkenburger, B.; Konitsiotis, S.; Tsamis, K.; Rigas, G.; et al. Toward objective monitoring of Parkinson’s disease motor symptoms using a wearable device: Wearability and performance evaluation of PDMonitor®. Front. Neurol. 2023, 14, 1080752. [Google Scholar] [CrossRef]
- Ates, H.C.; Nguyen, P.Q.; Gonzalez-Macia, L.; Morales-Narváez, E.; Güder, F.; Collins, J.J.; Dincer, C. End-to-end design of wearable sensors. Nat. Rev. Mater. 2022, 7, 887–907. [Google Scholar] [CrossRef]
- Mokhlespour Esfahani, M.I.; Nussbaum, M.A. Preferred placement and usability of a smart textile system vs. inertial measurement units for activity monitoring. Sensors 2018, 18, 2501. [Google Scholar] [CrossRef]
- O’Day, J.; Lee, M.; Seagers, K.; Hoffman, S.; Jih-Schiff, A.; Kidziński, Ł.; Delp, S.; Bronte-Stewart, H. Assessing inertial measurement unit locations for freezing of gait detection and patient preference. J. Neuroeng. Rehabil. 2022, 19, 20. [Google Scholar] [CrossRef]
- Bricout, A.; Fontecave-Jallon, J.; Pépin, J.L.; Guméry, P.Y. Accelerometry-derived respiratory index estimating apnea-hypopnea index for sleep apnea screening. Comput. Methods Programs Biomed. 2021, 207, 106209. [Google Scholar] [CrossRef]
- Bates, A.; Ling, M.J.; Mann, J.; Arvind, D.K. Respiratory rate and flow waveform estimation from tri-axial accelerometer data. In Proceedings of the BSN 2010, Singapore, 7–9 June 2010; pp. 144–150. [Google Scholar]
- Chase, J.D.; Busa, M.A.; Staudenmayer, J.W.; Sirard, J.R. Sleep measurement using wrist-worn accelerometer data compared with polysomnography. Sensors 2022, 22, 5041. [Google Scholar] [CrossRef]
- Boe, A.J.; McGee Koch, L.L.; O’Brien, M.K.; Shawen, N.; Rogers, J.A.; Lieber, R.L.; Reid, K.J.; Zee, P.C.; Jayaraman, A. Automating sleep stage classification using wireless, wearable sensors. NPJ Digit. Med. 2019, 2, 131. [Google Scholar] [CrossRef]
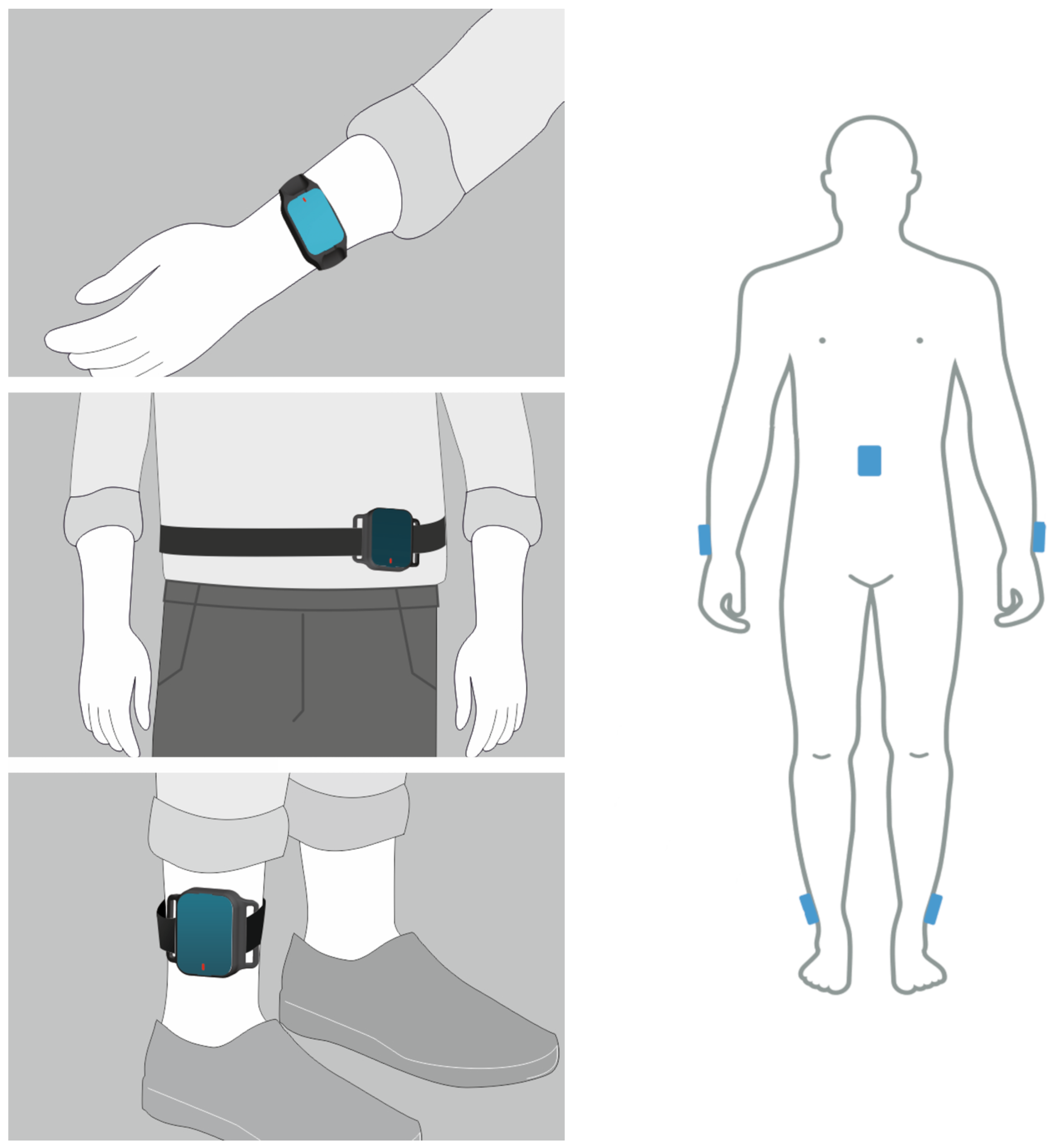


| Aggregated Demographics | Values |
|---|---|
| Number of participants | 23 |
| Sex (Male/Female) | 15/8 |
| Age (years) | 46.6 (14.9) |
| Body mass index (kg/m2) | 29.5 (8.1) |
| AHI (events/hour) | 27.5 (33.0) |
| No OSA () | 6 |
| Mild OSA () | 9 |
| Moderate OSA () | 1 |
| Severe OSA () | 7 |
| RR | HR | |||
|---|---|---|---|---|
| ID | MAE (Hz) | MAE (Hz) | ||
| 1 | ||||
| 2 | ||||
| 3 | ||||
| 4 | ||||
| 5 | ||||
| 6 | ||||
| 7 | ||||
| 8 | ||||
| 9 | ||||
| 10 | ||||
| 11 | ||||
| 12 | ||||
| 13 | ||||
| 14 | ||||
| 15 | ||||
| 16 | ||||
| 17 | 0 | |||
| 18 | 100 | |||
| 19 | ||||
| 20 | 0 | |||
| 21 | ||||
| 22 | ||||
| 23 | ||||
| Median | ||||
| IQR | ||||
| Study | Sensor | Location | Participants | Methodology | Rate | Performance Metrics |
|---|---|---|---|---|---|---|
| [31] | ACC | Chest | 12, | Axes fusion | RR | |
| [32] | ACC | Wrist | 34, | Axis selection | RR | , |
| [33] | ACC | Chest | 7, | Axes fusion | RR HR | |
| [34] | ACC & GYRO | Wrist | 3, | Axis selection and rate fusion | HR | min−1, |
| [35] | ACC | Chest | 13, | Axes fusion | RR | min−1 |
| [41] | ACC | Wrist | 182, | Axis selection | HR | , |
| [42] | ACC | Chest | 11, | Axis selection | RR | min−1 |
| Current | ACC & GYRO | Waist | 23, | Axes fusion | RR HR | min−1, , min−1, , |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontaxis, S.; Kanellos, F.; Ntanis, A.; Kostikis, N.; Konitsiotis, S.; Rigas, G. An Inertial-Based Wearable System for Monitoring Vital Signs during Sleep. Sensors 2024, 24, 4139. https://doi.org/10.3390/s24134139
Kontaxis S, Kanellos F, Ntanis A, Kostikis N, Konitsiotis S, Rigas G. An Inertial-Based Wearable System for Monitoring Vital Signs during Sleep. Sensors. 2024; 24(13):4139. https://doi.org/10.3390/s24134139
Chicago/Turabian StyleKontaxis, Spyridon, Foivos Kanellos, Adamantios Ntanis, Nicholas Kostikis, Spyridon Konitsiotis, and George Rigas. 2024. "An Inertial-Based Wearable System for Monitoring Vital Signs during Sleep" Sensors 24, no. 13: 4139. https://doi.org/10.3390/s24134139
APA StyleKontaxis, S., Kanellos, F., Ntanis, A., Kostikis, N., Konitsiotis, S., & Rigas, G. (2024). An Inertial-Based Wearable System for Monitoring Vital Signs during Sleep. Sensors, 24(13), 4139. https://doi.org/10.3390/s24134139






