Preparation and Mechanism Analysis of High-Performance Humidity Sensor Based on Eu-Doped TiO2
Abstract
1. Introduction
2. Experimental Details
2.1. Materials and Characterizations
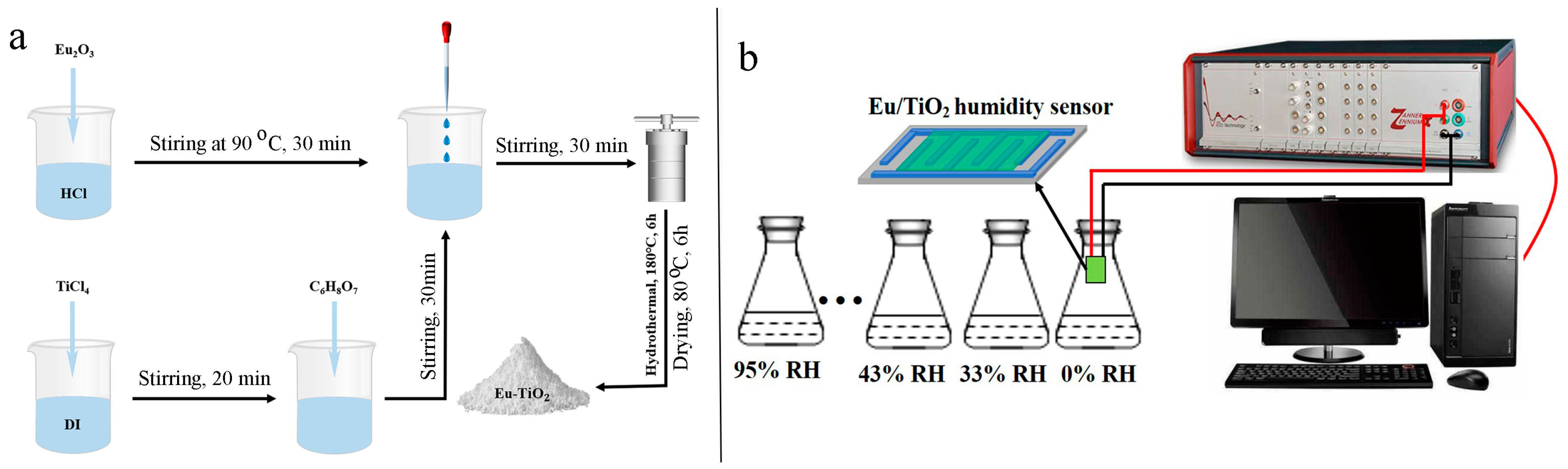
2.2. Preparation of Eu-TiO2
2.3. Humidity Sensor Tests
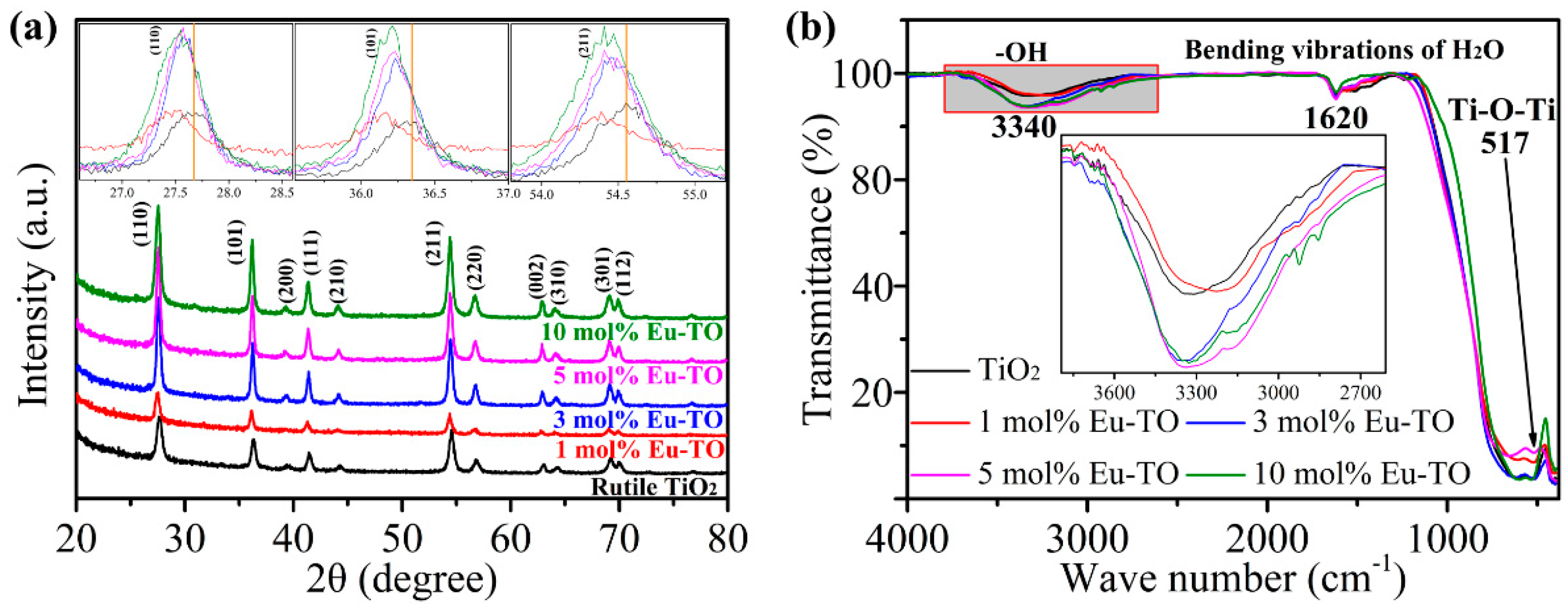
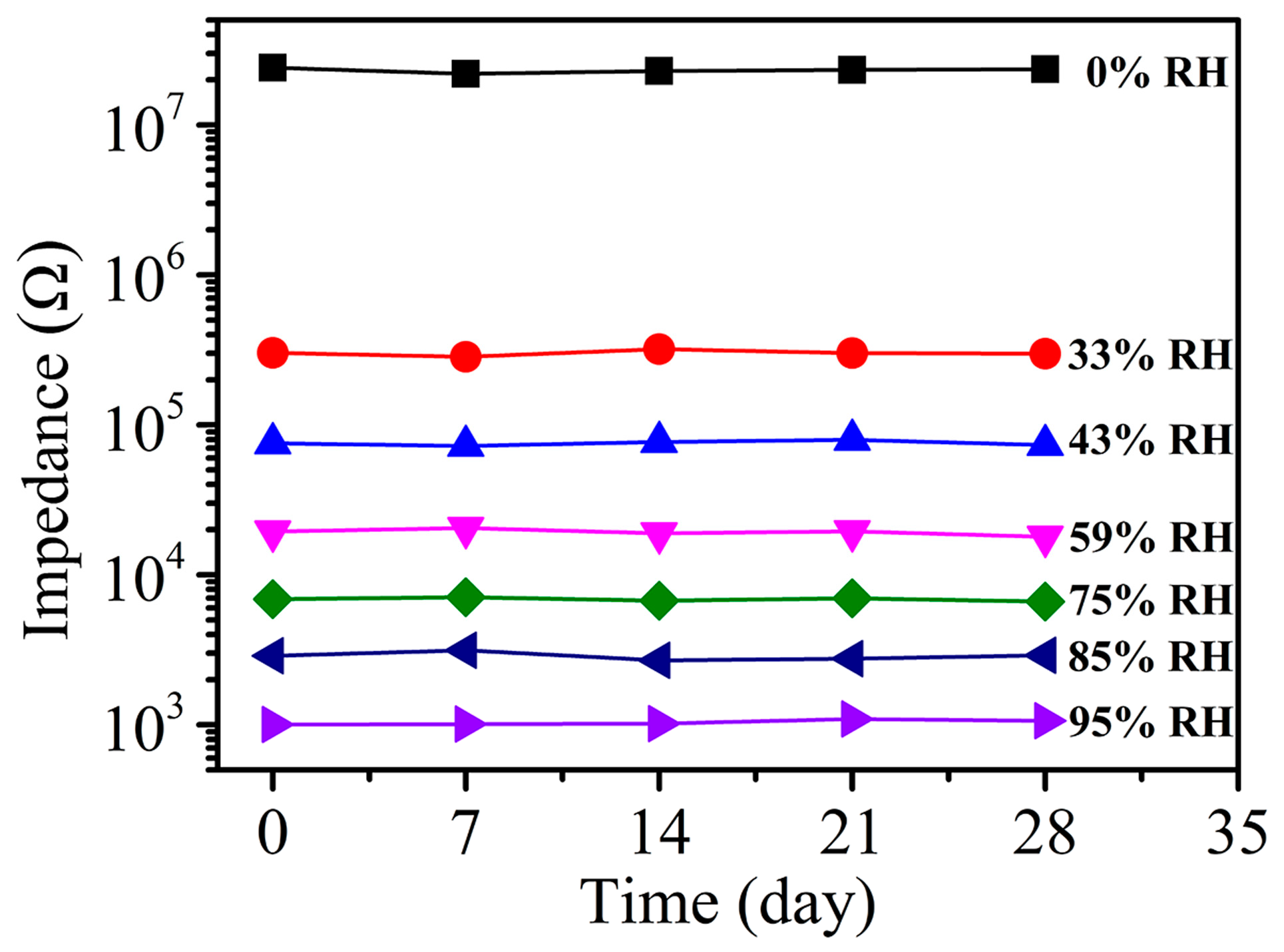
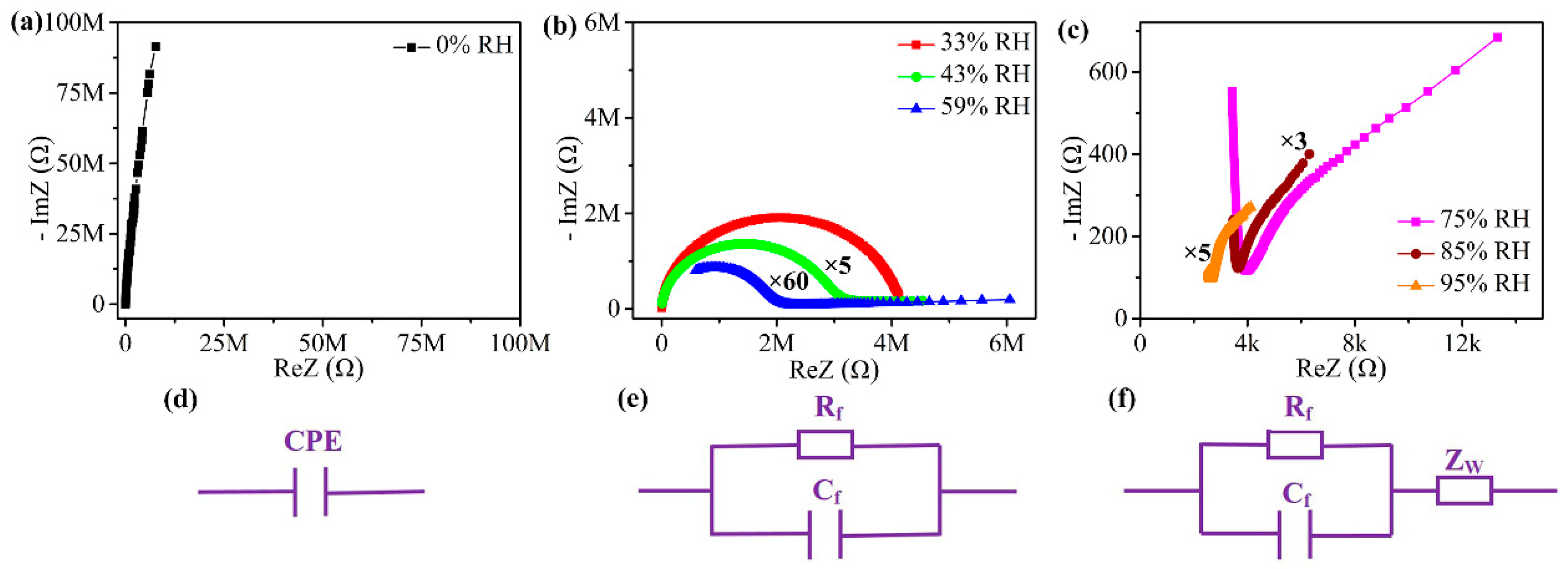
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Squillaci, M.A.; Ferlauto, L.; Zagranyarski, Y.; Milita, S.; Müllen, K.; Samorì, P. Self-Assembly of an Amphiphilic π-Conjugated Dyad into Fibers: Ultrafast and Ultrasensitive Humidity Sensor. Adv. Mater. 2015, 27, 3170–3174. [Google Scholar] [CrossRef]
- Kano, S.; Kim, K.; Fujii, M. Fast-Response and Flexible Nanocrystal-Based Humidity Sensor for Monitoring Human Respiration and Water Evaporation on Skin. ACS Sens. 2017, 2, 828–833. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, H.; Chen, C.; Lin, C. Investigation of humidity sensor based on Au modified ZnO nanosheets via hydrothermal method and first principle. Sens. Actuators B Chem. 2019, 287, 526–534. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Z.; Umar, A.; Wang, Y.; Tian, T.; Shang, Y.; Fan, Y.; Qi, Q.; Xu, D. Supramolecularly Modified Graphene for Ultrafast Responsive and Highly Stable Humidity Sensor. J. Phys. Chem. C 2015, 119, 28640–28647. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Man, J.; Chen, C. Preparation of high performance Fe-doped SnO2 humidity sensor and its application in respiration detection. Sens. Actuators A Phys. 2023, 362, 114644. [Google Scholar] [CrossRef]
- Guo, Y.; Xi, H.; Gu, Z.; Li, M.; Li, X.; Gao, D. A self-powered PVA-based flexible humidity sensor with humidity-related voltage output for multifunctional applications. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130700. [Google Scholar] [CrossRef]
- Muhammad, Y.; Shah, M.; Safi, M.A.; Khattak, S.G.; Aziz, A.; Hassan, H. Highly selective and sensitive humidity sensor using reduced graphene oxide based iron oxide nanocomposites. Mater. Sci. Eng. B 2024, 303, 117324. [Google Scholar] [CrossRef]
- Li, N.; Jiang, Y.; Zhou, C.; Xiao, Y.; Meng, B.; Wang, Z.; Huang, D.; Xing, C.; Peng, Z. High-Performance Humidity Sensor Based on Urchin-Like Composite of Ti3C2 MXene-Derived TiO2 Nanowires. ACS Appl. Mater. Interfaces 2019, 11, 38116–38125. [Google Scholar] [CrossRef]
- Dou, Y.; Li, C.; Luo, W.; Qian, L.; Wang, L.; Li, D.; Li, H.; Li, M. Surface acoustic wave relative humidity sensor based on GO/TiO2 sensitive film. Sens. Actuators A Phys. 2024, 365, 114906. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, H.; Gu, W.; Chen, C. Preparation and mechanism investigation of highly sensitive humidity sensor based on Ag/TiO2. Curr. Appl. Phys. 2022, 43, 57–65. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.; Guo, Y.; Lv, X.; Dou, X. 2D TiO2 nanosheets for ultrasensitive humidity sensing application benefited by abundant surface oxygen vacancy defects. Sens. Actuators B Chem. 2018, 262, 350–358. [Google Scholar] [CrossRef]
- Chen, X.; Hu, H.; Yu, F.; Peng, D.; Ke, Y. MoS2 compounded bidirectionally with TiO2 for hydrogen evolution reaction with enhanced humidity sensing performance. Mater. Sci. Semicond. Process. 2018, 82, 75–81. [Google Scholar] [CrossRef]
- Jeong, H.; Noh, Y.; Lee, D. Highly Stable and Sensitive Resistive Flexible Humidity Sensors by Means of Roll-to-Roll Printed Electrodes and Flower-like TiO2 Nanostructures. Ceram. Int. 2018, 45, 985–992. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of temperature and humidity on the sensing performance of TiO2 nanowirebased ethanol vapor sensors. Nanotechnology 2021, 32, 325501. [Google Scholar] [CrossRef]
- Joshi, K.; Rawat, M.; Gautam, S.K.; Singh, R.G.; Ramola, R.C.; Singh, F. Band gap widening and narrowing in Cu-doped ZnO thin films. J. Alloys Compd. 2016, 680, 252–258. [Google Scholar] [CrossRef]
- Quan, Z.; Liu, X.; Qi, Y.; Song, Z.; Qi, S.; Zhou, G.; Xu, X. Robust room temperature ferromagnetism and band gap tuning in nonmagnetic Mg doped ZnO films. Appl. Surf. Sci. 2016, 399, 751–757. [Google Scholar] [CrossRef]
- Liao, Z.; Yuan, Z.; Gan, H.; Meng, F. Doped rare earth elements enhance gas sensing properties of hollow spiral-like Mn(OH)F semiconductor sensors for acetate esters detection. Sens. Actuators B Chem. 2024, 412, 135825. [Google Scholar] [CrossRef]
- Kalaiezhily, R.K.; Saravanan, G.V.; Asvini, N.; Vijayan, K. Tuning violet to green emission in luminomagnetic Dy,Er co-doped ZnO nanoparticles. Ceram. Int. 2018, 44, 19560–19569. [Google Scholar] [CrossRef]
- Pavón, F.; Urbieta, A.; Fernández, P. Luminescence and light guiding properties of Er and Li codoped ZnO nanostructures. J. Lumin. 2018, 195, 396–401. [Google Scholar] [CrossRef]
- Mondal, D.; Bhattachary, D.; Mondal, T.; Kundu, M.; Sarkar, S.; Mandal, T.K.; Paul, B.K.; Das, S. Rare earth ion-infused α-MnO2 nano-rods for excellent EMI shielding efficiency: Experimental and theoretical insights. Sustain. Mater. Technol. 2023, 38, 00772. [Google Scholar] [CrossRef]
- Aslan, F. Increasing the photoelectric conversion efficiency of dye-sensitized solar cells by doping SrAl2O4:Eu+2, Dy+3 to TiO2-based photoanodes. Physica B 2023, 668, 415267. [Google Scholar] [CrossRef]
- Zhumanova, K.; Serik, L.; Molkenova, A.; Atabaev, T.S. UV light blocking and conversion by porous europium-doped titanium dioxide (TiO2-Eu) thin films for potential protection of photovoltaic devices. Mater. Today Chem. 2022, 26, 101171. [Google Scholar] [CrossRef]
- Hernandez, J.V.; Coste, S.; Murillo, A.G.; Romo, F.C.; Kassiba, A. Effects of metal doping (Cu, Ag, Eu) on the electronic and optical behavior of nanostructured TiO2. J. Alloys Compd. 2017, 710, 355–363. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, H.; Zhang, J. Synthesis of high response gold/titanium dioxide humidity sensor and its application in human respiration. Ceram. Int. 2021, 47, 30880–30887. [Google Scholar] [CrossRef]
- Trandafilovi, L.V.; Jovanovi, D.J.; Zhang, X.S.; Ptasinska, S.; Dramicanin, M.D. Enhanced photocatalytic degradation of methylene blue and methyl orange by ZnO:Eu nanoparticles. Appl. Catal. B Environ. 2016, 203, 740–752. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Y.; Hao, H.; Xu, Y.; Huang, J.; Sun, D.; Li, Q. Rape Pollen-Templated Synthesis of C,N Self-Doped Hierarchical TiO2 for Selective Hydrogenation of 1,3-Butadiene. ACS Sustain. Chem. Eng. 2017, 6, 882–888. [Google Scholar] [CrossRef]
- Abdullah, S.A.; Sahdan, M.Z.; Nayan, N.; Embong, Z.; Adriyanto, F. Neutron beam interaction with rutile TiO2 single crystal (1 1 1): Raman and XPS study on Ti3+-oxygen vacancy formation. Mater. Lett. 2020, 263, 127143. [Google Scholar] [CrossRef]
- Chi, M.; Sun, X.; Achintya, S.; Dabis, Z.; Tatarchuk, B. A quantitative XPS examination of UV induced surface modification of TiO2 sorbents for the increased saturation capacity of sulfur heterocycles. Fuel 2019, 238, 454–461. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.; Zhan, Z.; Wang, L.; Feng, S.; Chai, X.; Lu, W.; Shen, J.; Weng, Z.; Sun, J. Catalyst-Free, Selective Growth of ZnO Nanowires on SiO2 by Chemical Vapor Deposition for Transfer-Free Fabrication of UV Photodetectors. ACS Appl. Mater. Interfaces 2015, 7, 20264–20271. [Google Scholar] [CrossRef]
- Pawbake, A.S.; Waykar, R.; Late, D.J.; Jadkar, S.R. Highly Transparent Wafer-Scale Synthesis of Crystalline WS2 Nanoparticle Thin Film for Photodetector and Humidity-Sensing Applications. ACS Appl. Mater. Interfaces 2016, 8, 3359–3365. [Google Scholar] [CrossRef]
- Duan, Z.; Li, J.; Yuan, Z.; Jiang, Y.; Tai, H. Capacitive humidity sensor based on zirconium phosphate nanoplates film with wide sensing range and high response. Sens. Actuators B. Chem. 2023, 394, 134445. [Google Scholar] [CrossRef]
- Chethan, B.; Prasad, V.; Mathew, S.; Jan, H. Quick responding humidity sensor based on polyaniline/reduced graphene oxide composite. Synth. Met. 2024, 307, 117644. [Google Scholar] [CrossRef]
- Wang, S.; Yan, H.; Zheng, H.; He, Y.; Guo, X.; Li, S.; Yang, C. Fast response humidity sensor based on chitosan/graphene oxide/tin dioxide composite. Sens. Actuators B. Chem. 2023, 392, 34070. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, H. Preparation and performance study of humidity sensor based on defect-controlled TiO2/CdS heterostructure. Sens. Actuators B Chem. 2024, 404, 135321. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Chen, C.; Zhang, L.; Ma, X.; Li, X.; Wang, J. Preparation of silver nanoparticles modified SnO2 humidity sensor for tobacco storage environment detection. Sens. Actuators B Chem. 2024, 409, 135612. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.; Ji, J.; Zhang, H.; Xu, Z.; Feng, Z.; Wu, W.; Wang, Y. Non-contact humidity monitoring: Boosting the performance of all-printed humidity sensor using PDDA-modified Ti3C2Tx nanoribbons. Chem. Eng. J. 2024, 485, 149633. [Google Scholar] [CrossRef]
- Yang, F.; Li, P. Preparation and humidity sensing performance study of SnO2 in situ loaded rGO. Mater. Sci. Eng. B 2023, 290, 116329. [Google Scholar] [CrossRef]
- Na, B.; Guo, C.; Wang, T. Multifunctional and highly sensitive humidity sensor based on KCl/Sm2O3 nanoflowers with ultrafast response. Sens. Actuators B Chem. 2023, 385, 133701. [Google Scholar] [CrossRef]
- Yu, X.; Chen, X.; Ding, X.; Chen, X.; Yu, X.; Zhao, X. High-sensitivity and low-hysteresis humidity sensor based on hydrothermally reduced graphene oxide/nanodiamond. Sens. Actuators B Chem. 2018, 283, 761–768. [Google Scholar] [CrossRef]
- Zhang, D.; Zong, X.; Wu, Z.; Zhang, Y. Ultrahigh-performance impedance humidity sensor based on layer-by-layer self-assembled tin disulfide/titanium dioxide nanohybrid film. Sens. Actuators B Chem. 2018, 266, 52–62. [Google Scholar] [CrossRef]
- Ying, Z.; Yu, C.; Zhang, Y.; Cheng, X.; Feng, C.; Chen, L.; Zhou, J.; Ruan, S. A novel humidity sensor based on NaTaO3 nanocrystalline. Sens. Actuators B Chem. 2012, 174, 485–489. [Google Scholar]
- Peng, X.; Chu, J.; Aldalbahi, A.; Rivera, M.; Wang, L.; Duan, S.; Feng, P. A flexible humidity sensor based on KC–MWCNTs composites. Appl. Surf. Sci. 2016, 387, 149–154. [Google Scholar] [CrossRef]





| Materials | Response | Res./Rec. Time (s) | Sensing Range (% RH) | Refs. |
|---|---|---|---|---|
| rGO/Fe2O3 | 2715.541% | 5 s/10 s | 40–80% RH | [7] |
| ZrP | 91.5% | 57 s/9 s | 10.9–91.5% RH | [31] |
| PANI/RGO | 10.52% | 3 s/4 s | 7–97% RH | [32] |
| CS/GO/SnO2 | 72,683% | 8 s/8 s | 15–95% RH | [33] |
| TiO2/CdS | 63,459 | 5 s/7 s | 11–95% RH | [34] |
| Ag/SnO2 | 24,292.3 | 5/8 s | 11–95% RH | [35] |
| PDDA/Ti3C2Tx | 48,813% | 8.794 s/2.656 s | 11–97% RH | [36] |
| Fe/SnO2 | 145,290 | 10 s/8 s | 11–95% RH | [5] |
| SnO2/rGO | 3.84 × 105% | 45 s/8 s | 11–95% RH | [37] |
| KCl/Sm2O3 | 127,121 | 0.1 s/21 s | 11–95% RH | [38] |
| Eu/TiO2 | 23,997 | 3 s/13.1 s | 11–95% RH | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Chen, C.; Zhang, H. Preparation and Mechanism Analysis of High-Performance Humidity Sensor Based on Eu-Doped TiO2. Sensors 2024, 24, 4142. https://doi.org/10.3390/s24134142
Zhang L, Chen C, Zhang H. Preparation and Mechanism Analysis of High-Performance Humidity Sensor Based on Eu-Doped TiO2. Sensors. 2024; 24(13):4142. https://doi.org/10.3390/s24134142
Chicago/Turabian StyleZhang, Ling, Chu Chen, and Hongyan Zhang. 2024. "Preparation and Mechanism Analysis of High-Performance Humidity Sensor Based on Eu-Doped TiO2" Sensors 24, no. 13: 4142. https://doi.org/10.3390/s24134142
APA StyleZhang, L., Chen, C., & Zhang, H. (2024). Preparation and Mechanism Analysis of High-Performance Humidity Sensor Based on Eu-Doped TiO2. Sensors, 24(13), 4142. https://doi.org/10.3390/s24134142







