Research on the Influence of Core Sensing Components on the Performance of Galvanic Dissolved Oxygen Sensors
Abstract
:1. Introduction
- Through single-factor experiments, the critical influence of the oxygen-permeable membrane on the performance of the DO sensor is deeply analyzed.
- With the help of crossover experiments, the synergistic effects of electrodes and electrolyte on DO sensors are comprehensively explored.
- From the perspective of core sensing components, it provides detailed data support and practical suggestions for optimizing sensor design.
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Method
2.2.1. Preparation of Electrolyte Solution
2.2.2. Assembly of DO Sensor
2.2.3. Experiment on the Effect of Oxygen Permeability on the Output Signal of DO Sensors
2.2.4. Experiment on the Stability of DO Sensor Output Signal Affected by Sensing Components
2.2.5. Experiment on the Response Time of DO Sensors Affected by Sensing Components
2.2.6. Evaluation Indicators and Data Processing
3. Results and Discussion
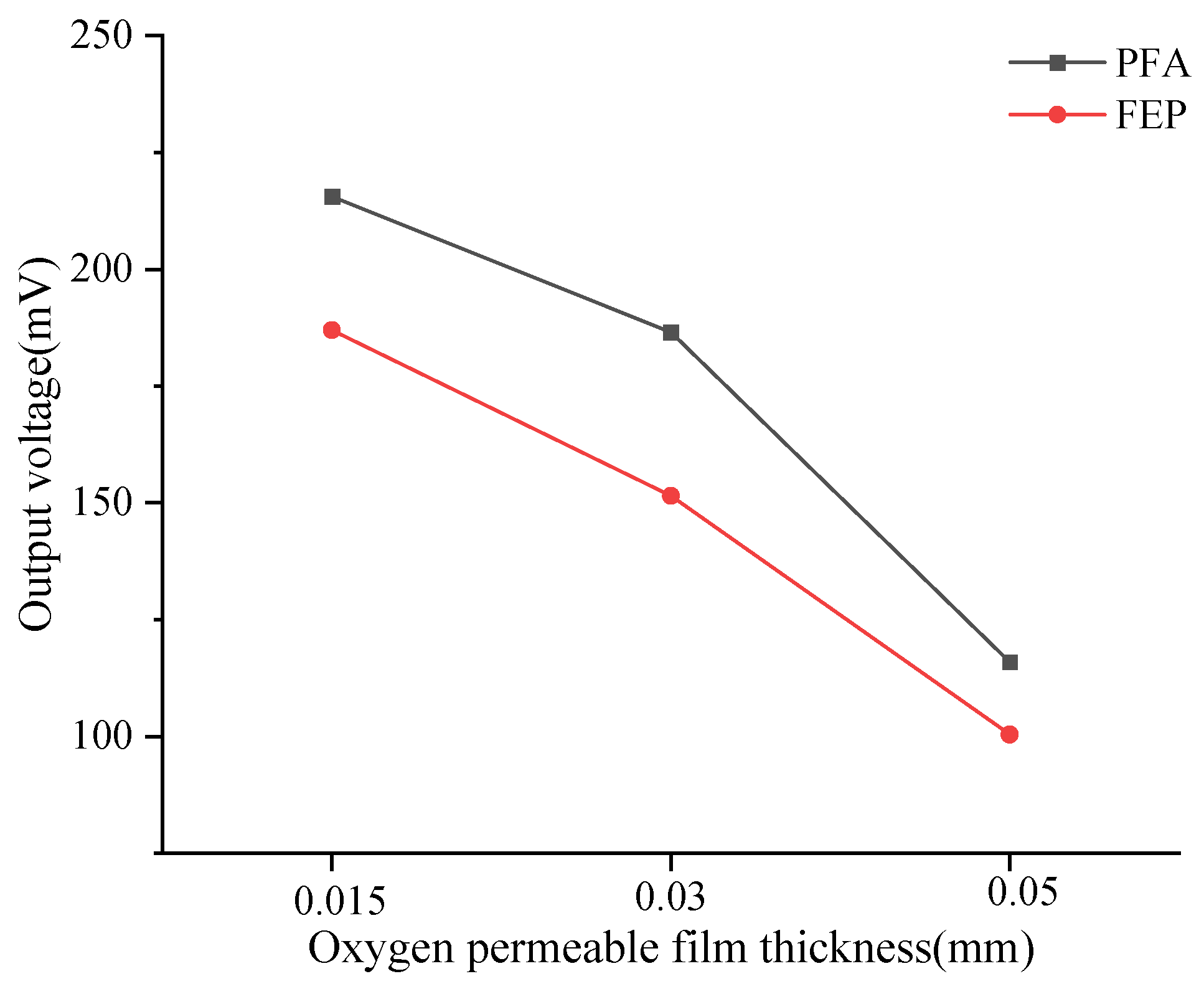
3.1. The Influence of Oxygen Permeability Efficiency on the Output Signal of DO Sensors
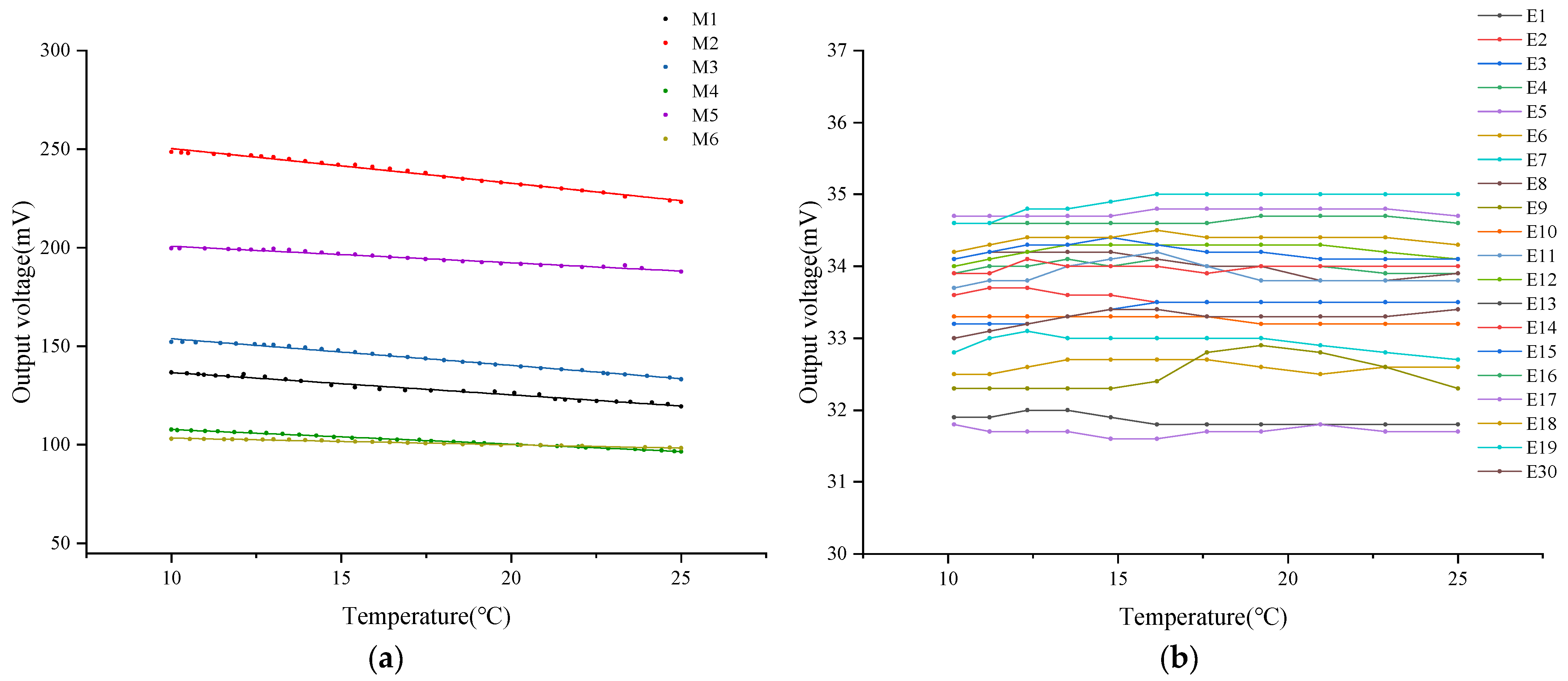
3.2. The Influence of Sensing Components on the Stability of DO Sensor Output Signals
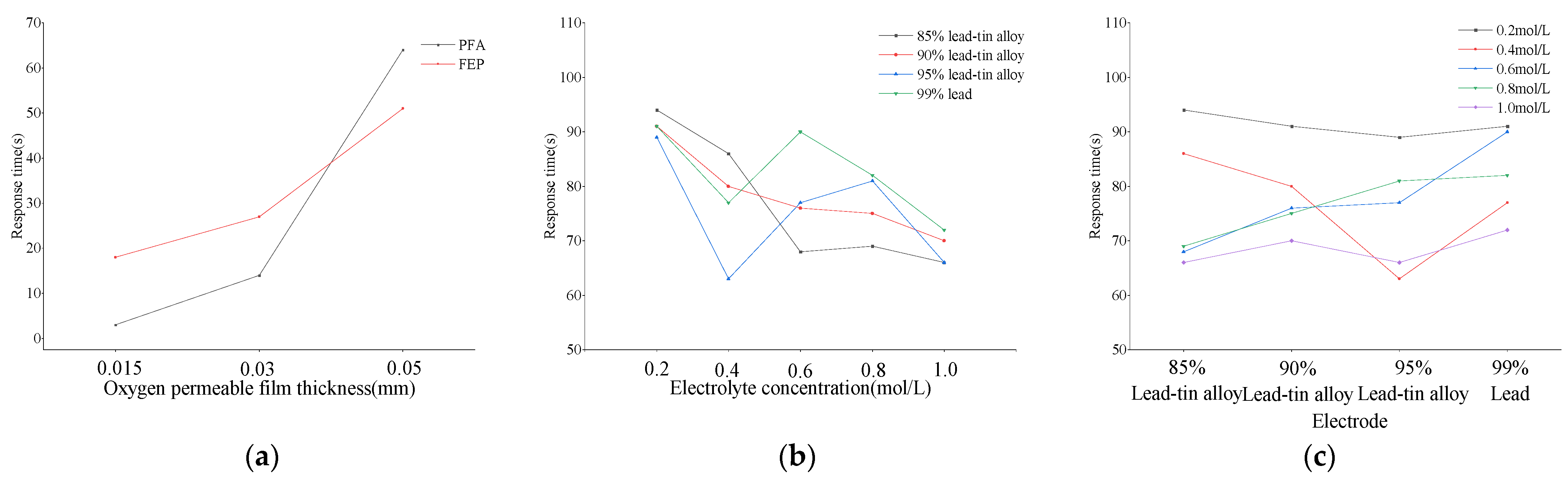
3.3. The Influence of Sensing Components on the Response Time of DO Sensors
3.4. Performance Comparison
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Bai, J.; Zhai, Y.; Zhang, K.; Wang, Y.; Tang, R.; Jorquera, M.A. Pollution Levels and Potential Ecological Risks of Trace Elements in Relation to Bacterial Community in Surface Water of Shallow Lakes in Northern China before and after Ecological Water Replenishment. J. Contam. Hydrol. 2024, 262, 104318. [Google Scholar] [CrossRef] [PubMed]
- Achtak, H.; Kouali, H.; Chaouti, A.; Dahbi, A. Use of Water Quality Parameters to Assess the Ecological Health of Atlantic Coastal Areas in the Northwestern Morocco. Reg. Stud. Mar. Sci. 2024, 71, 103427. [Google Scholar] [CrossRef]
- Liu, W.; Lin, S.; Li, X.; Li, W.; Deng, H.; Fang, H.; Li, W. Analysis of Dissolved Oxygen Influencing Factors and Concentration Prediction Using Input Variable Selection Technique: A Hybrid Machine Learning Approach. J. Environ. Manag. 2024, 357, 120777. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Khan, S.; Shaika, N.A.; Hena, S.M.; Mahmud, Y.; Haque, M.M. Ecology of Freshwater Harmful Euglenophytes: A Review. Heliyon 2024, 10, E29625. [Google Scholar] [CrossRef] [PubMed]
- Folifac, L.; Ameh, A.E.; Broadhurst, J.; Petrik, L.F.; Ojumu, T.V. Iron Nanoparticles Prepared from South African Acid Mine Drainage for the Treatment of Methylene Blue in Wastewater. Environ. Sci. Pollut. Res. 2024, 31, 38310–38322. [Google Scholar] [CrossRef] [PubMed]
- Shaghaghi, N.; Nguyen, T.; Patel, J.; Soriano, A.; Mayer, J. Doxy: Dissolved Oxygen Monitoring. In Proceedings of the 2020 IEEE Global Humanitarian Technology Conference (GHTC), Seattle, WA, USA, 29 October–1 November 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–4. [Google Scholar]
- She, D.; Allen, M.G. A Self-Powered, Biodegradable Dissolved Oxygen Microsensor. J. Microelectromech. Syst. 2020, 29, 1074–1078. [Google Scholar] [CrossRef]
- Langenfeld, N.J.; Bugbee, B. Evaluation of Three Electrochemical Dissolved Oxygen Meters. HortTechnology 2021, 31, 428–431. [Google Scholar] [CrossRef]
- Buehler, H.W.; Bucher, R. Applications of Electrochemical Sensors. In Sensors in Bioprocess Control; CRC Press: Boca Raton, FL, USA, 2020; pp. 127–172. [Google Scholar]
- Simoska, O.; Gaffney, E.M.; Minteer, S.D.; Franzetti, A.; Cristiani, P.; Grattieri, M.; Santoro, C. Recent Trends and Advances in Microbial Electrochemical Sensing Technologies: An Overview. Curr. Opin. Electrochem. 2021, 30, 100762. [Google Scholar] [CrossRef]
- Stine, J.M.; Beardslee, L.A.; Sathyam, R.M.; Bentley, W.E.; Ghodssi, R. Electrochemical Dissolved Oxygen Sensor-Integrated Platform for Wireless In Situ Bioprocess Monitoring. Sens. Actuators B Chem. 2020, 320, 128381. [Google Scholar] [CrossRef]
- Han, J.H.; Kim, S.; Choi, J.; Kang, S.; Pak, Y.K.; Pak, J.J. Development of Multi-Well-Based Electrochemical Dissolved Oxygen Sensor Array. Sens. Actuators B Chem. 2020, 306, 127465. [Google Scholar] [CrossRef]
- She, D.; Allen, M.G. A Micromachined Freestanding Electrochemical Sensor for Measuring Dissolved Oxygen. J. Microelectromech. Syst. 2019, 28, 521–531. [Google Scholar] [CrossRef]
- Leonardi, S.G.; Bonyani, M.; Ghosh, K.; Dhara, A.K.; Lombardo, L.; Donato, N.; Neri, G. Development of a Novel Cu (II) Complex Modified Electrode and a Portable Electrochemical Analyzer for the Determination of Dissolved Oxygen (DO) in Water. Chemosensors 2016, 4, 7. [Google Scholar] [CrossRef]
- Wei, Y.; Jiao, Y.; An, D.; Li, D.; Li, W.; Wei, Q. Review of Dissolved Oxygen Detection Technology: From Laboratory Analysis to Online Intelligent Detection. Sensors 2019, 19, 3995. [Google Scholar] [CrossRef]
- Zhang, D.; Fang, Y.; Miao, Z.; Ma, M.; Chen, Q. Electrochemical Determination of Dissolved Oxygen Based on Three Dimensional Electrosynthesis of Silver Nanodendrites Electrode. J. Appl. Electrochem. 2014, 44, 419–425. [Google Scholar] [CrossRef]
- Lu, D.; Bai, H.; Kong, F.; Liss, S.N.; Liao, B. Recent Advances in Membrane Aerated Biofilm Reactors. Crit. Rev. Environ. Sci. Technol. 2021, 51, 649–703. [Google Scholar] [CrossRef]
- Guo, Q.; Huang, Y.; Xu, M.; Huang, Q.; Cheng, J.; Yu, S.; Zhang, Y.; Xiao, C. PTFE Porous Membrane Technology: A Comprehensive Review. J. Membr. Sci. 2022, 664, 121115. [Google Scholar] [CrossRef]
- Yang, T.F.; Hsueh, C.Y.; Chen, B.L.; Li, W.K.; Yan, W.M. Effects of Fluorinated Ethylene Propylene Contents in a Novel Gas Diffusion Layer on Cell Performance of a Proton Exchange Membrane Fuel Cell. Int. J. Energy Res. 2022, 46, 1553–1564. [Google Scholar] [CrossRef]
- Lyubimov, A.V.; Borisov, A.G.; Korchagin, E.P.; Kozlitin, A.I.; Osipenkova, N.G. Investigation of the Process of the Electrodeposition of a Tin–Lead Alloy with a High Lead Content. Russ. Microelectron. 2021, 50, 481–485. [Google Scholar] [CrossRef]
- Casebolt, R.; Kimura, K.W.; Levine, K.; Cimada DaSilva, J.A.; Kim, J.; Dunbar, T.A.; Suntivich, J.; Hanrath, T. Effect of Electrolyte Composition and Concentration on Pulsed Potential Electrochemical CO2 Reduction. ChemElectroChem 2021, 8, 681–688. [Google Scholar] [CrossRef]
- Santos, L.S.; Landers, R.; Gushikem, Y. Application of Manganese (II) Phthalocyanine Synthesized In Situ in the SiO2/SnO2 Mixed Oxide Matrix for Determination of Dissolved Oxygen by Electrochemical Techniques. Talanta 2011, 85, 1213–1216. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, P.; Baker, G.A.; Stetter, J.; Zeng, X. Ionic Liquids as Electrolytes for the Development of a Robust Amperometric Oxygen Sensor. Anal. Chem. 2011, 83, 7066–7073. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, H.M.; Park, J.H.; Lee, S.K. Fabrication and Characterization of Micro Dissolved Oxygen Sensor Activated on Demand Using Electrolysis. Sens. Actuators B Chem. 2017, 241, 923–930. [Google Scholar] [CrossRef]
- Hossain, M.F.; McCracken, S.; Slaughter, G. Electrochemical Laser Induced Graphene-Based Oxygen Sensor. J. Electroanal. Chem. 2021, 899, 115690. [Google Scholar] [CrossRef]
- Sosna, M.; Denuault, G.; Pascal, R.W.; Prien, R.D.; Mowlem, M. Development of a Reliable Microelectrode Dissolved Oxygen Sensor. Sens. Actuators B Chem. 2007, 123, 344–351. [Google Scholar] [CrossRef]
- Luo, J.; Eitel, R. An Integrated Low Temperature Co-Fired Ceramic-Based Clark-Type Oxygen Sensor. IEEE Sens. J. 2016, 17, 1590–1595. [Google Scholar] [CrossRef]
- Liao, Q.; Wang, Y.; Chen, Y.; Wei, Y.; Wang, H. Novel Bifunctional Tantalum and Bismuth Co-Doped Perovskite BaBi0.05Co0.8Ta0.15O3−δ with High Oxygen Permeation. J. Membr. Sci. 2014, 468, 184–191. [Google Scholar] [CrossRef]
- Watanabe, K.; Takauchi, D.; Yuasa, M.; Kida, T.; Shimanoe, K.; Teraoka, Y.; Yamazoe, N. Oxygen Permeation Properties of Co-Free Perovskite-Type Oxide Membranes Based on BaFe1-yZryO3-δ. J. Electrochem. Soc. 2009, 156, E81. [Google Scholar] [CrossRef]
- Chen, J.; Asano, M.; Maekawa, Y.; Yoshida, M. Suitability of Some Fluoropolymers Used as Base Films for Preparation of Polymer Electrolyte Fuel Cell Membranes. J. Membr. Sci. 2006, 277, 249–257. [Google Scholar] [CrossRef]
- Améduri, B. The Promising Future of Fluoropolymers. Macromol. Chem. Phys. 2020, 221, 1900573. [Google Scholar] [CrossRef]
- Schafer, L. Oxygenation during Endoscopy Controlled Trial Using the Oxyguard. Gastroenterology 2000, 4, A1095. [Google Scholar] [CrossRef]
- Evans, N.T.S.; Quinton, T.H. Permeability and Diffusion Coefficient of Oxygen in Membranes for Oxygen Electrodes. Respir. Physiol. 1978, 35, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cole, H.L.; Carlson, D.J.; Miller, E.R.; Beierle, K.; Paukkunen, A.; Laine, T.K. Corrections of Humidity Measurement Errors from the Vaisala RS80 Radiosonde—Application to TOGA COARE Data. J. Atmos. Ocean. Technol. 2002, 19, 981–1002. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, P.; Zhu, M.; Deng, C.; Chen, P. The Design of Dissolved Oxygen Detection System Based on C8051F020. In Proceedings of the 2011 Second International Conference on Digital Manufacturing & Automation, Zhangjiajie, China, 5–7 August 2011; pp. 1253–1255. [Google Scholar]
- Celina, M.C.; Quintana, A. Oxygen Diffusivity and Permeation Through Polymers at Elevated Temperature. Polymer 2018, 150, 326–342. [Google Scholar] [CrossRef]
- Huang, Y.; Paul, D.R. Effect of Film Thickness on the Gas-Permeation Characteristics of Glassy Polymer Membranes. Ind. Eng. Chem. Res. 2007, 46, 2342–2347. [Google Scholar] [CrossRef]
- Gavrilović-Wohlmuther, A.; Laskos, A.; Zelger, C.; Gollas, B.; Whitehead, A.H. Effects of Electrolyte Concentration, Temperature, Flow Velocity and Current Density on Zn Deposit Morphology. J. Energy Power Eng. 2015, 9, 1019–1028. [Google Scholar] [CrossRef]
- Jordan, M. Electrodeposition of Tin-Lead Alloys. In Modern Electroplating; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 265–284. [Google Scholar]
- Dilmi, T.; Guelil, A.; Dakhouch, A. Study of Effect of Tin on the Electrochemical Properties of Cycled PbSn Alloys of Lead-Acid Battery. Alger. J. Chem. Eng. AJCE 2021, 1, 1–7. [Google Scholar]
- Gęca, I.; Korolczuk, M. A Novel Eco-Friendly and Highly Sensitive Solid Lead–Tin Microelectrode for Trace U (VI) Determination in Natural Water Samples. Sensors 2023, 23, 2552. [Google Scholar] [CrossRef]
- Gillanders, R.N.; Tedford, M.C.; Crilly, P.J.; Bailey, R.T. A composite thin film optical sensor for dissolved oxygen in contaminated aqueous environments. Anal. Chim. Acta 2005, 545, 189–194. [Google Scholar] [CrossRef]



| Equipment | Model | Production Company |
|---|---|---|
| NTC sensor | B3950 | Shenzhen Weixin Technology Co., Ltd., located in Shenzhen, China. |
| DO sensor | OxyGuard Pond Master | Denmark OxyGuard Co., Ltd., located in Copenhagen, Denmark. |
| Magnetic stirrer | C-MAG HS7 | IKA Works GmbH & Co. KG, located in Staufen, Germany. |
| Scale | YP10002B | Shanghai Lichen Instrument Technology Co., Ltd., located in Shanghai, China. |
| Insulating foam box | 300 × 205 × 255 | - |
| Water tank | 755 × 530 × 500 | Tengyi Plastics Co., Ltd., located in Dongguan, China. |
| Cylindrical bucket | 30 L | Liangbao Plastics Co., Ltd., located in Wuxi, China. |
| Heating rod | 2200 W | Xinshaoguang Electric Appliance Co., Ltd., located in Changsha, China. |
| Multimeter | VC890D | Victor Instrument Co., Ltd., located in Xi’an, China. |
| Sensor Number | Core Sensing Components | ||
|---|---|---|---|
| Oxygen-Permeable Membrane | Electrode | Electrolyte | |
| M1 | 0.015 mm PFA | OxyGuard Electrode | OxyGuard Electrolyte |
| M2 | 0.03 mm PFA | ||
| M3 | 0.05 mm PFA | ||
| M4 | 0.015 mm FEP | ||
| M5 | 0.03 mm FEP | ||
| M6 | 0.05 mm FEP | ||
| Sensor Number | Core Sensing Components | Sensor Number | Core Sensing Components | ||||
|---|---|---|---|---|---|---|---|
| Oxygen-Permeable Membrane | Electrode | Electrolyte | Oxygen-Permeable Membrane | Electrode | Electrolyte | ||
| E1 | OxyGuard Oxygen-Permeable Membrane | 85% lead–tin alloy | KCl (0.2 mol/L) | E11 | OxyGuard Oxygen-Permeable Membrane | 95% lead–tin alloy | KCl (0.2 mol/L) |
| E2 | KCl (0.4 mol/L) | E12 | KCl (0.4 mol/L) | ||||
| E3 | KCl (0.6 mol/L) | E13 | KCl (0.6 mol/L) | ||||
| E4 | KCl (0.8 mol/L) | E14 | KCl (0.8 mol/L) | ||||
| E5 | KCl (1.0 mol/L) | E15 | KCl (1.0 mol/L) | ||||
| E6 | 90% lead–tin alloy | KCl (0.2 mol/L) | E16 | 99% pure lead | KCl (0.2 mol/L) | ||
| E7 | KCl (0.4 mol/L) | E17 | KCl (0.4 mol/L) | ||||
| E8 | KCl (0.6 mol/L) | E18 | KCl (0.6 mol/L) | ||||
| E9 | KCl (0.8 mol/L) | E19 | KCl (0.8 mol/L) | ||||
| E10 | KCl (1.0 mol/L) | E20 | KCl (1.0 mol/L) | ||||
| Sensor Number | Output Voltage Parameters | ||||
|---|---|---|---|---|---|
| Mean Value (mV) | Initial Output Voltage (mV) | Final Output Voltage (mV) | Increase Amplitude | R2 | |
| M1 | 215.55 | 213.8 | 220.4 | 3.09% | 0.85 |
| M2 | 186.49 | 178.1 | 189.2 | 6.23% | 0.96 |
| M3 | 115.92 | 111.4 | 118.4 | 6.28% | 0.95 |
| M4 | 187.00 | 174.0 | 200.1 | 15.00% | 0.97 |
| M5 | 151.50 | 135.7 | 162.1 | 19.46% | 0.99 |
| M6 | 100.40 | 84.0 | 114.4 | 36.19% | 0.99 |
| Sensor Number | Output Voltage Parameters | |||
|---|---|---|---|---|
| Mean Value (mV) | Slope | Intercept | R2 | |
| M1 | 130.84 | −1.15 | 148.23 | 0.98 |
| M2 | 237.67 | −1.90 | 270.70 | 0.99 |
| M3 | 142.93 | −1.44 | 169.13 | 0.99 |
| M4 | 101.21 | −0.74 | 115.21 | 0.99 |
| M5 | 194.73 | −0.89 | 210.26 | 0.98 |
| M6 | 101.13 | −0.36 | 107.27 | 0.99 |
| Sensor Number | Output Voltage Parameters | Sensor Number | Output Voltage Parameters | ||||
|---|---|---|---|---|---|---|---|
| Mean Value (mV) | Standard Deviation | Coefficient of Variation (%) | Mean Value (mV) | Standard Deviation | Coefficient of Variation (%) | ||
| E1 | 31.86 | 0.07 | 0.24 | E11 | 33.89 | 0.15 | 0.44 |
| E2 | 33.56 | 0.07 | 0.22 | E12 | 34.21 | 0.10 | 0.30 |
| E3 | 33.39 | 0.13 | 0.39 | E13 | 34.37 | 0.07 | 0.21 |
| E4 | 33.99 | 0.06 | 0.19 | E14 | 33.98 | 0.05 | 0.10 |
| E5 | 34.74 | 0.04 | 0.14 | E15 | 34.20 | 0.09 | 0.29 |
| E6 | 32.60 | 0.07 | 0.24 | E16 | 34.62 | 0.04 | 0.12 |
| E7 | 32.93 | 0.11 | 0.34 | E17 | 31.70 | 0.06 | 0.19 |
| E8 | 34.04 | 0.14 | 0.44 | E18 | 34.37 | 0.07 | 0.21 |
| E9 | 32.48 | 0.23 | 0.71 | E19 | 34.88 | 0.15 | 0.43 |
| E10 | 33.26 | 0.04 | 0.14 | E20 | 33.27 | 0.12 | 0.36 |
| Status | Time (s) | Output Voltage (mV) | |
|---|---|---|---|
| OxyGuard DO Sensor | New DO Sensor | ||
| Saturation oxygen | - | 32.2 | 174.6 |
| Decrease to anaerobic | 19 | 29.2 | 153.1 |
| 40 | 12.5 | 106.0 | |
| 57 | 5.0 | 72.9 | |
| 85 | 1.4 | 39.4 | |
| 105 | 0.8 | 16.7 | |
| Anaerobic | - | 0.6 | 7.5 |
| Increase to saturation oxygen | 1 | 0.9 | 8.3 |
| 19 | 17.0 | 109.3 | |
| 39 | 26.9 | 151.5 | |
| 49 | 29.1 | 159.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhang, L.; Wu, Y.; Ding, W.; Liu, Y.; Zhao, S.; Gu, J. Research on the Influence of Core Sensing Components on the Performance of Galvanic Dissolved Oxygen Sensors. Sensors 2024, 24, 4155. https://doi.org/10.3390/s24134155
Liu H, Zhang L, Wu Y, Ding W, Liu Y, Zhao S, Gu J. Research on the Influence of Core Sensing Components on the Performance of Galvanic Dissolved Oxygen Sensors. Sensors. 2024; 24(13):4155. https://doi.org/10.3390/s24134155
Chicago/Turabian StyleLiu, Helai, Lingfeng Zhang, Ye Wu, Weimin Ding, Yutao Liu, Sanqin Zhao, and Jiabing Gu. 2024. "Research on the Influence of Core Sensing Components on the Performance of Galvanic Dissolved Oxygen Sensors" Sensors 24, no. 13: 4155. https://doi.org/10.3390/s24134155





