A Novel AI Approach for Assessing Stress Levels in Patients with Type 2 Diabetes Mellitus Based on the Acquisition of Physiological Parameters Acquired during Daily Life
Abstract
1. Introduction
2. Related Work
2.1. Impact of Stress on Diabetes
2.2. Stress and Blood Pressure
2.3. Correlation between Diabetes and Blood Pressure
2.4. Stress Management
3. Materials and Methods
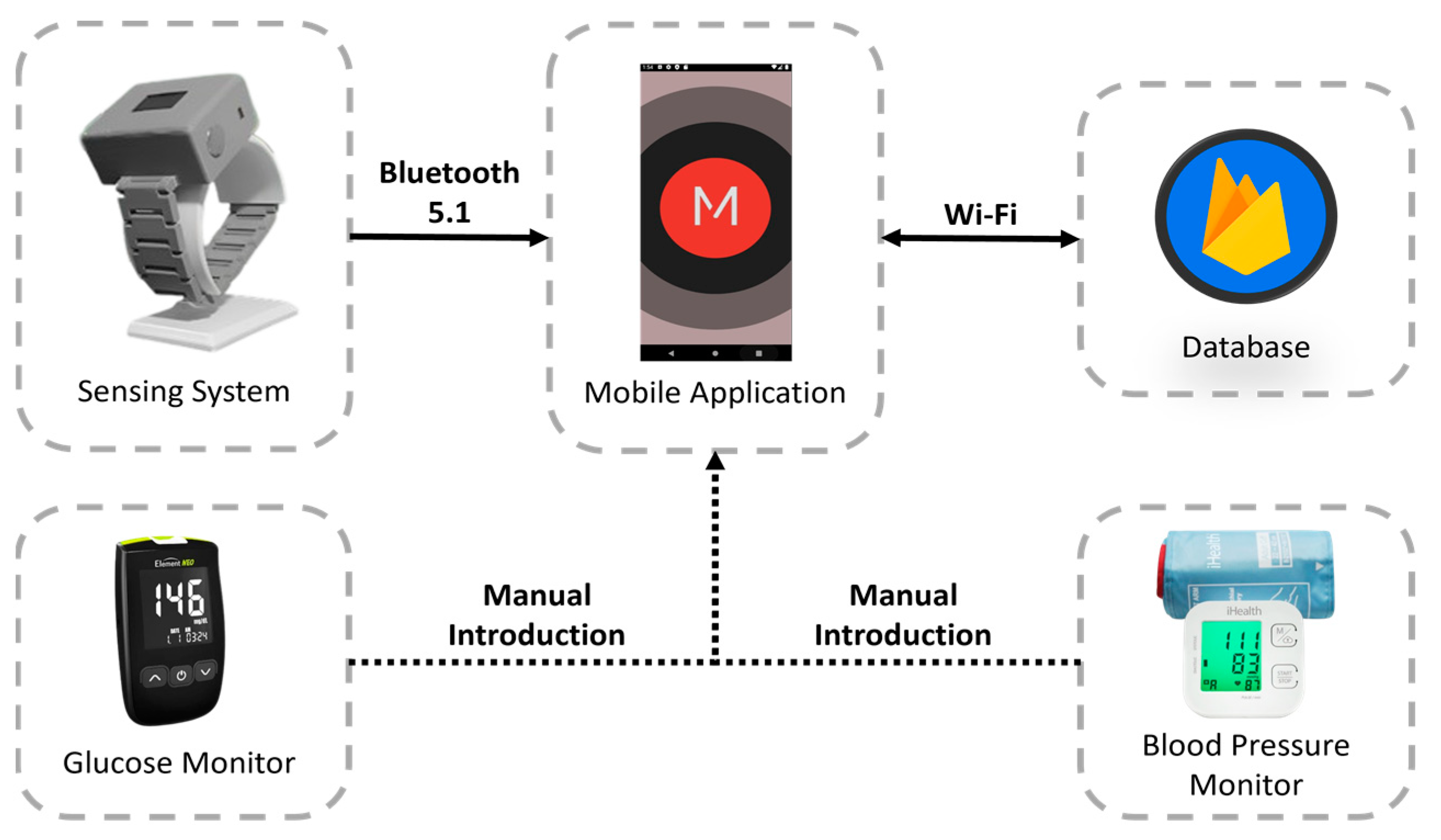
3.1. Proposed Healthcare System
- Sensing system: wearable device developed for the acquisition of physiological parameters and the establishment of a communication channel with the mobile application, allowing users to be authenticated and data to be stored locally or remotely, depending on the availability of the network.
- Mobile application: A user interface is provided to access the data stored in the database. This interface allows the entry of data such as glucose and blood pressure levels. It also includes a feature to classify stress levels, user authentication, and clinical advice based on the analysis of physiological indicators.
- Database: tasked with the remote storage of user-specific data and information. The Google NoSQL database “Firebase” was selected for the proposed system because of its benefits in developing mobile and web applications, including its interoperability with IOS, Android, Web, Unity, and C++.
- Glucose monitor: Element NEO is a straightforward glucose monitor designed to assist users in managing diabetes by incorporating functions that reduce the likelihood of complications associated with this condition. With its backlit display, ergonomic design, and illumination at the test strip insertion point, this device is suitable for all types of users. This device has been certified by Common Era (CE) [28].
- Blood pressure monitor: measures BP and pulse rate and allows users to check the results directly on the screen, which changes color depending on the level of blood pressure (red, yellow, and green). This device has been certified by the U.S. Food and Drug Administration (FDA) and CE [29].
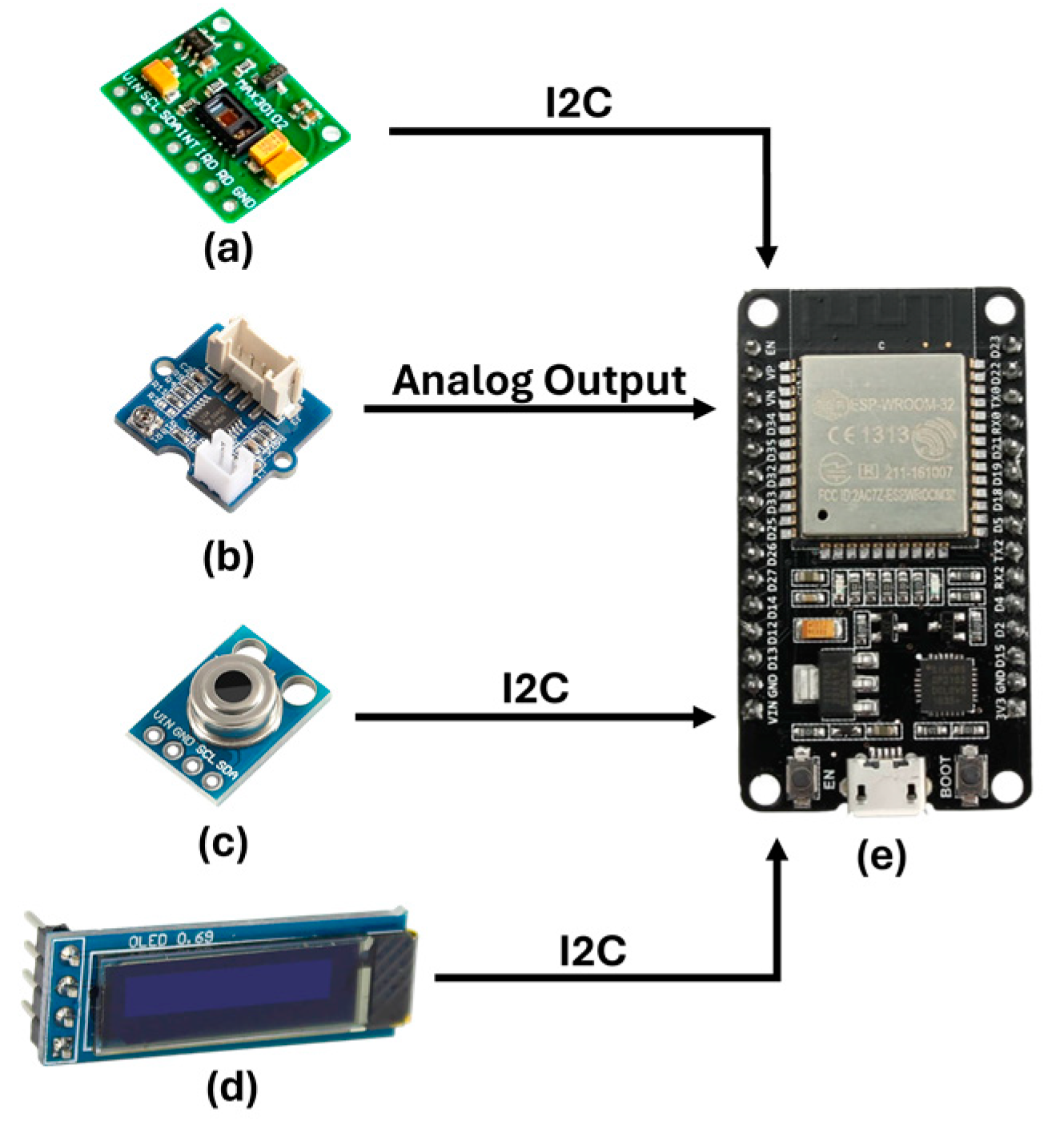
3.1.1. Sensing System
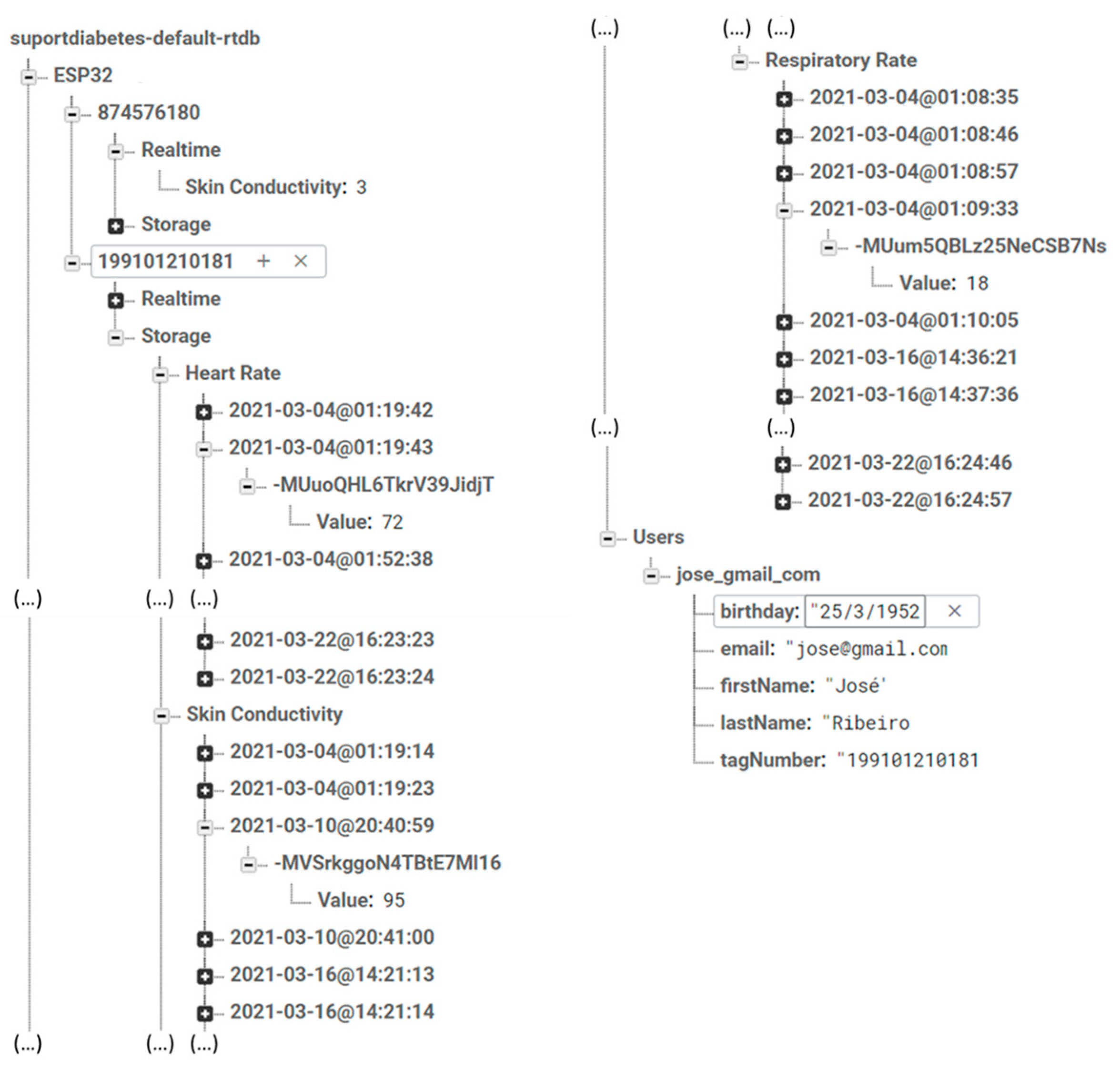
3.1.2. Database
3.1.3. Mobile Application
3.1.4. Glucose and Blood Pressure Monitors
3.2. Stress Assessment
3.3. Blood Glucose Levels
3.4. Experimental Procedure
4. Results and Discussion
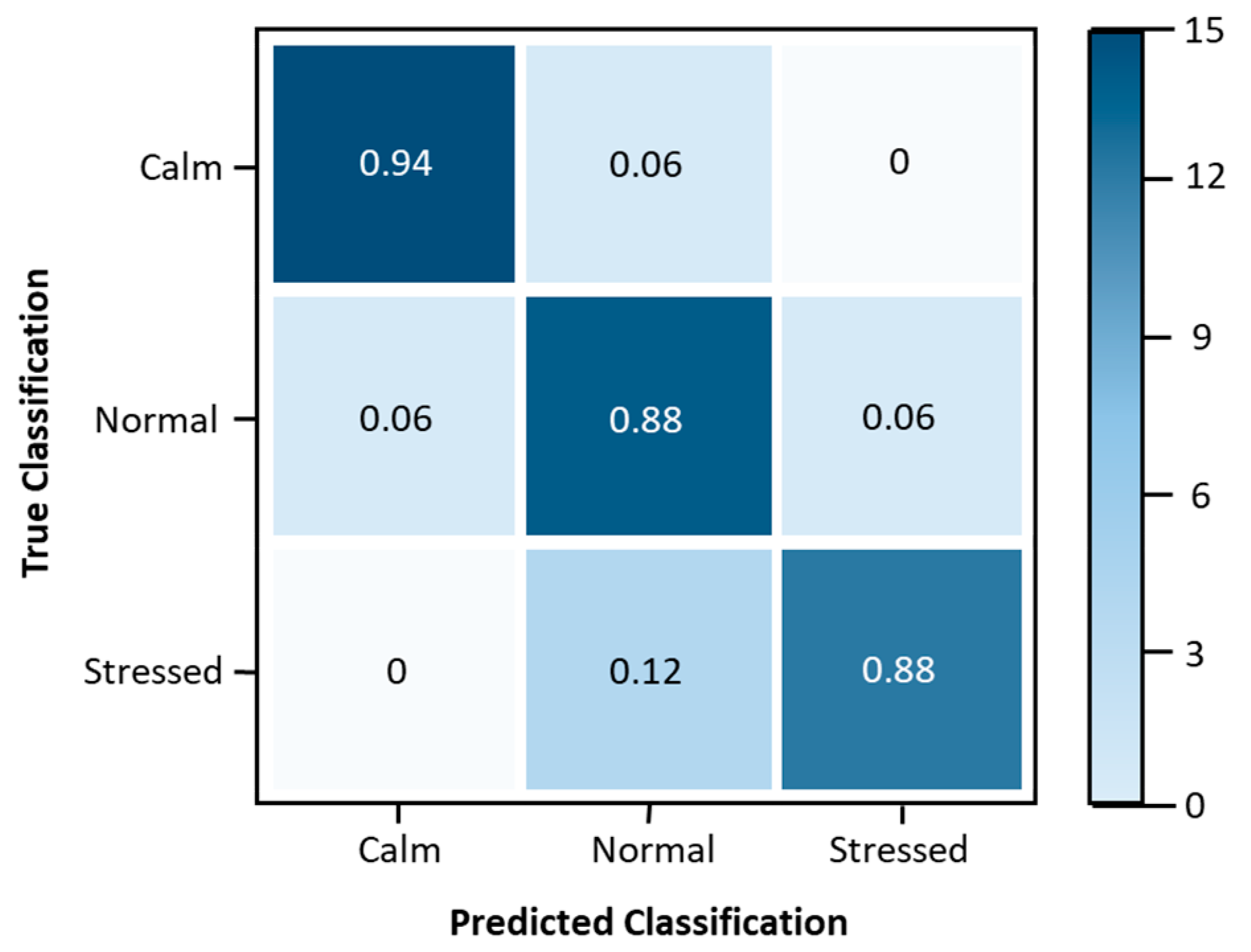
4.1. Stress Level Classification Model Update
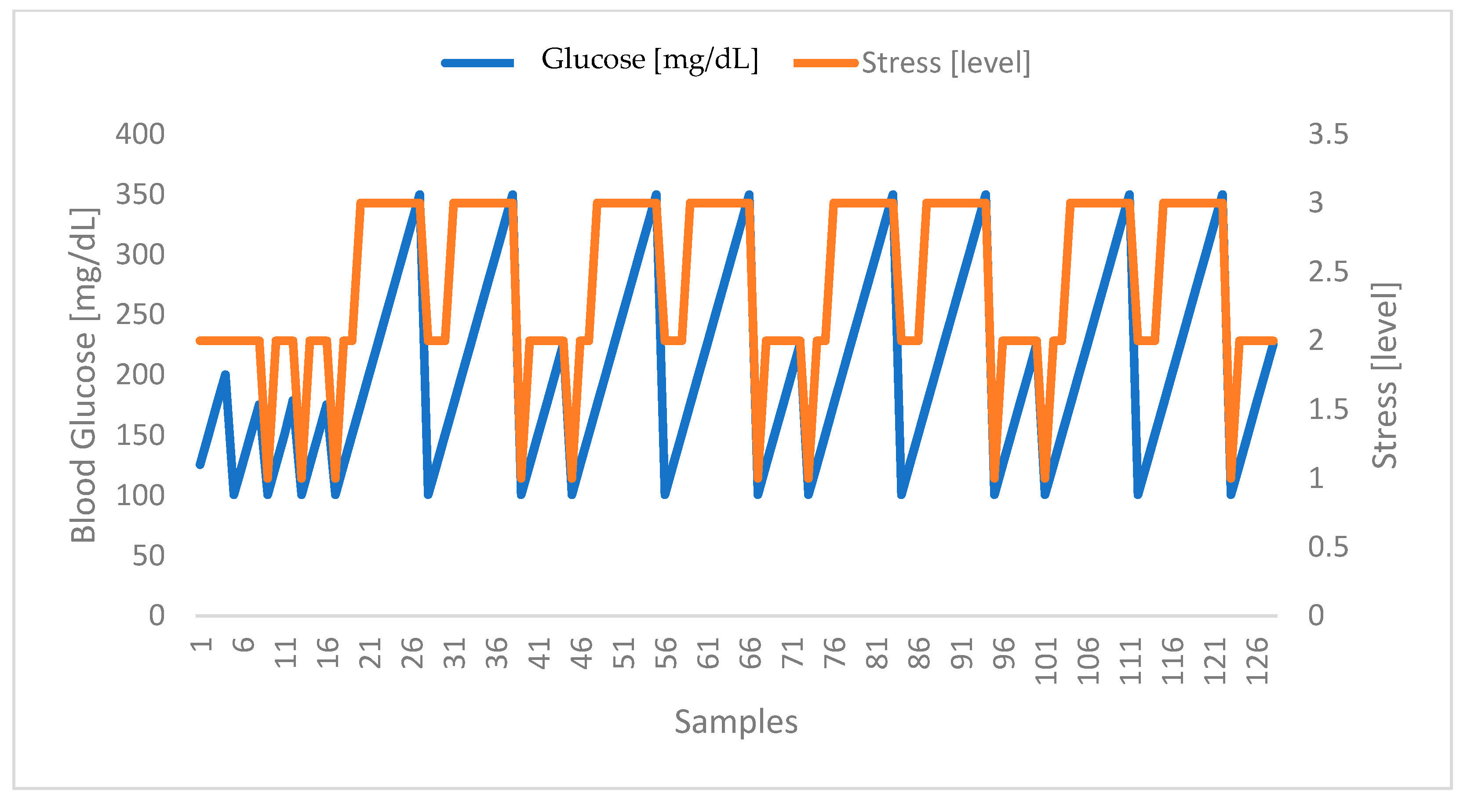
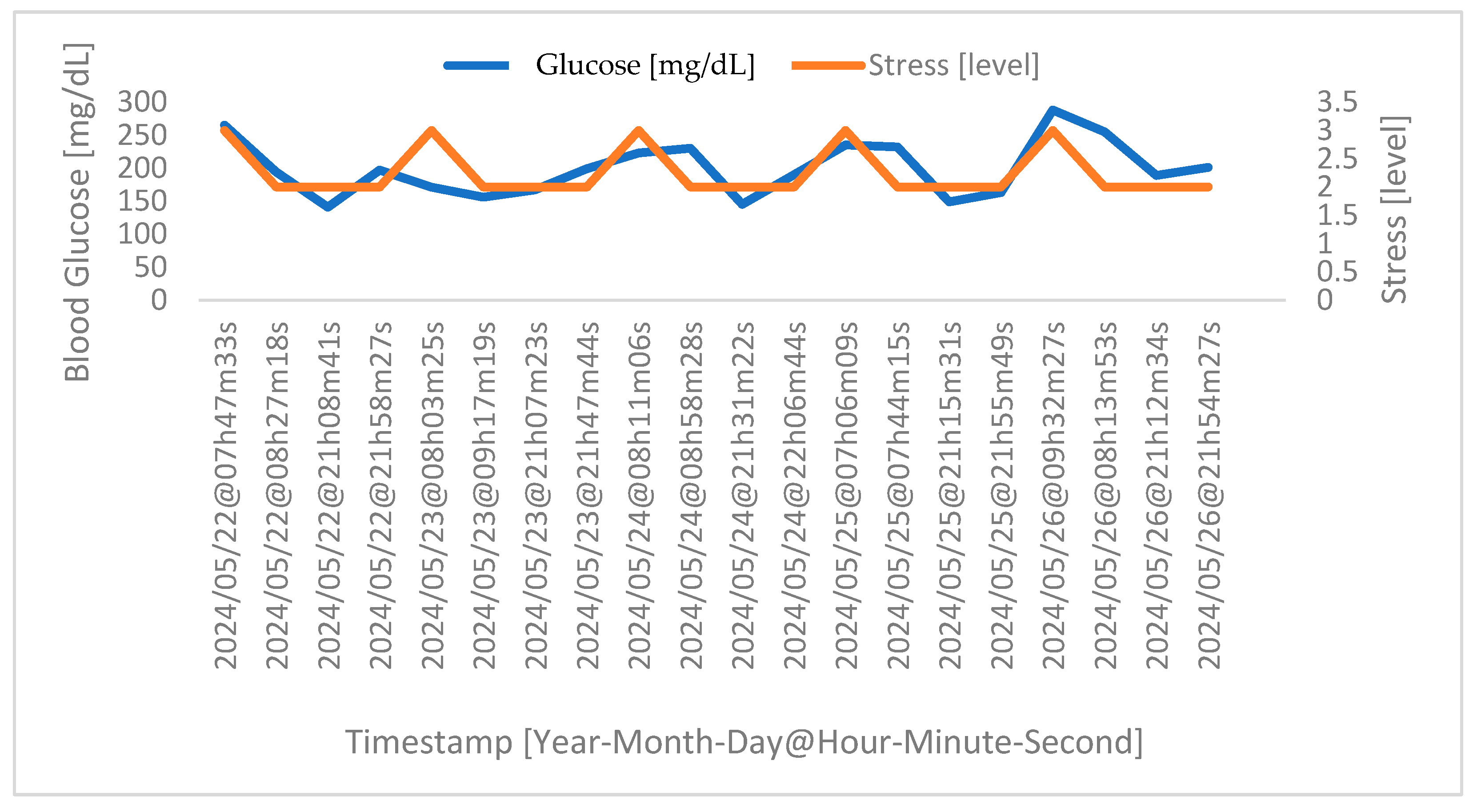
4.2. Correlation between Stress and Glucose Levels
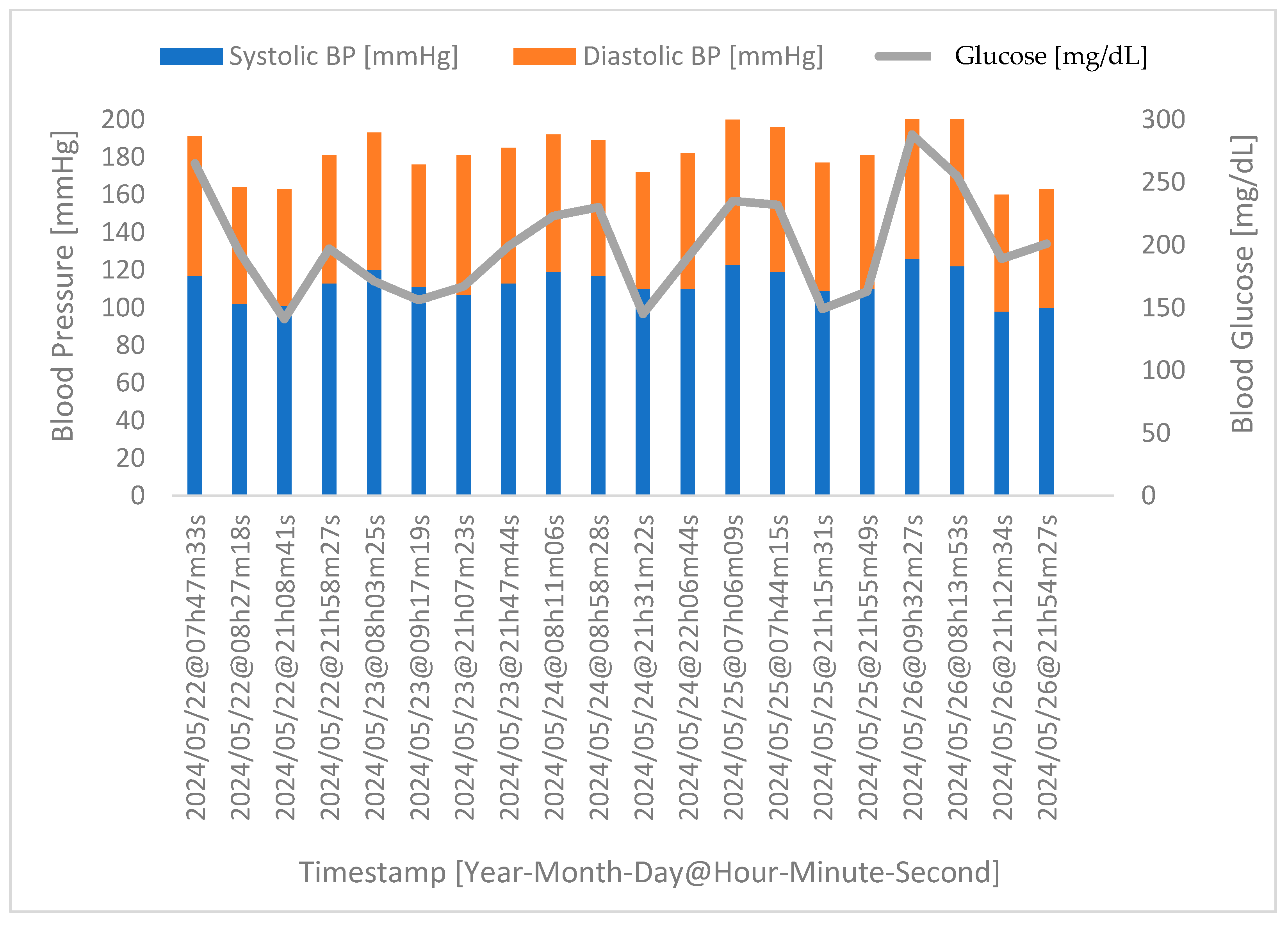
4.3. Correlation between Glucose Levels and Blood Pressure
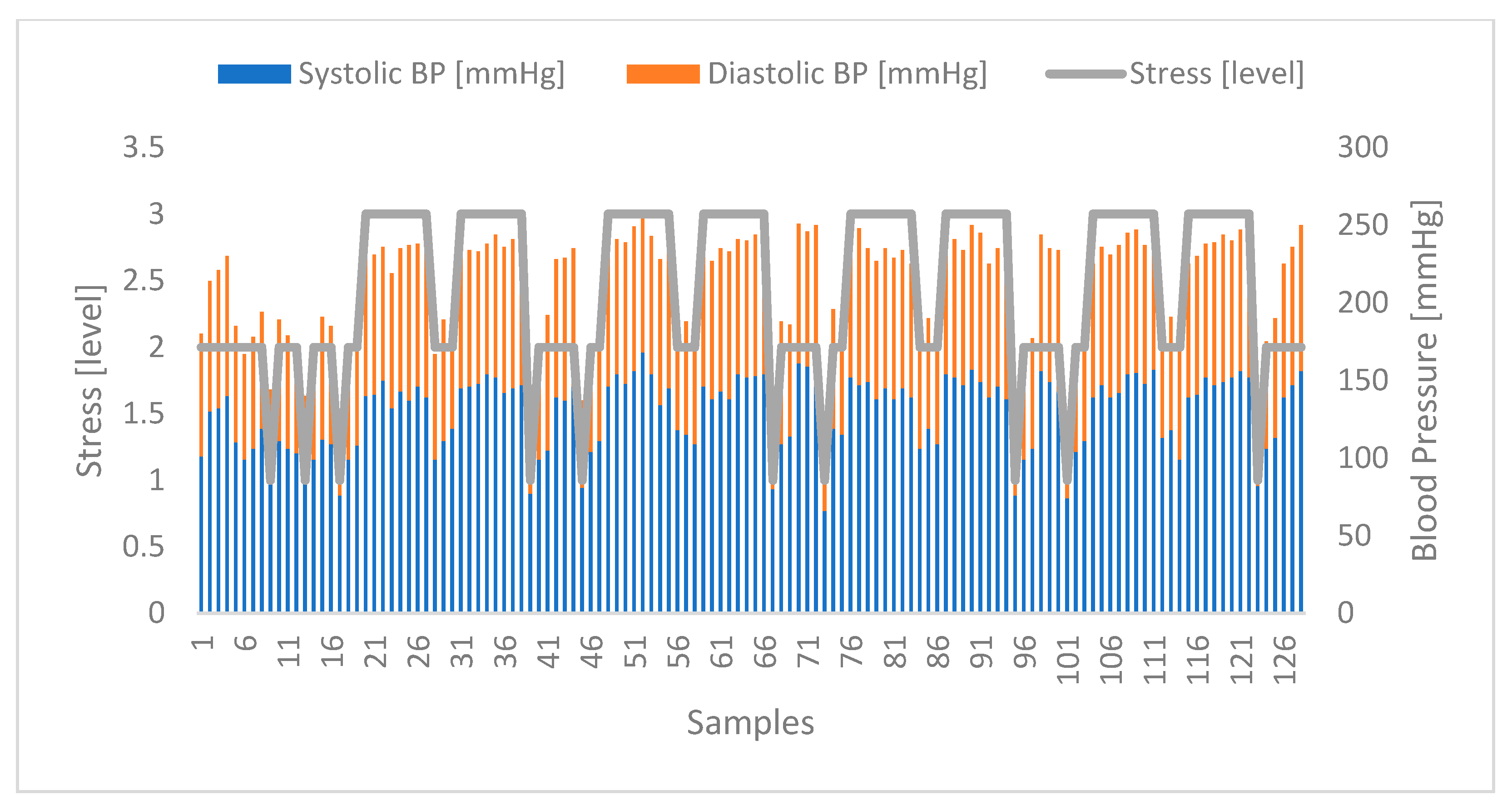
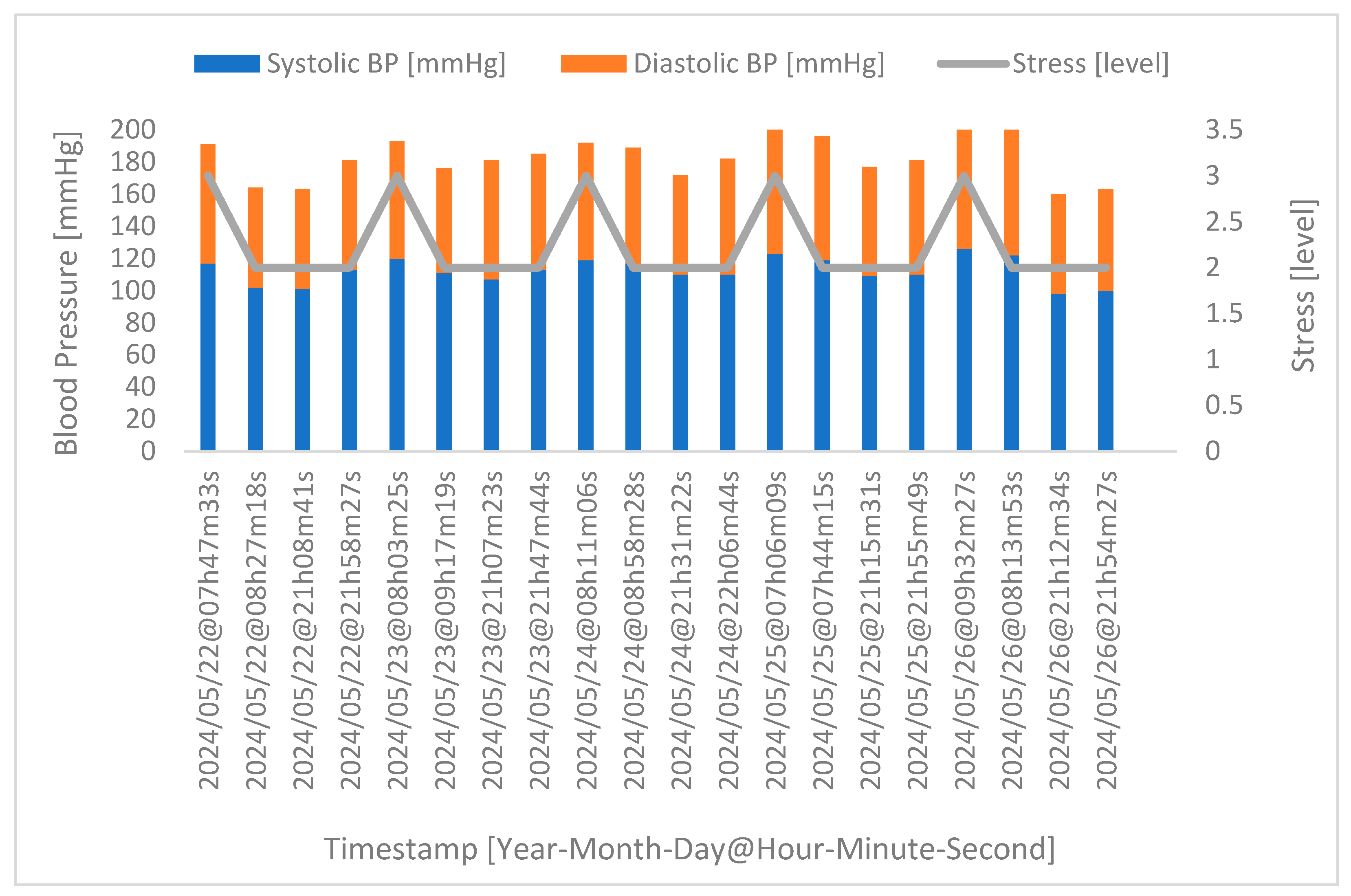
4.4. Correlation between Stress and Blood Pressure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuo, T.; McQueen, A.; Chen, T.C.; Wang, J.C. Regulation of glucose homeostasis by glucocorticoids. Adv. Exp. Med. Biol. 2015, 872, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Kiran, R.; Singla, H.K.; Sah, A.N. Stress management through regulation of blood pressure among college students. Work 2016, 54, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Kavya, R.; Nayana, N.; Karangale, K.B.; Madhura, H.; Sheela, S.J. Photoplethysmography—A modern approach and applications. In Proceedings of the 2020 International Conference for Emerging Technology INCET 2020, Belgaum, India, 5–7 June 2020; pp. 10–13. [Google Scholar] [CrossRef]
- Ribeiro, G.; Postolache, O.; Ferrero, F. A New Intelligent Approach for Automatic Stress Level Assessment Based on Multiple Physiological Parameters Monitoring. IEEE Trans. Instrum. Meas. 2024, 73, 1–14. [Google Scholar] [CrossRef]
- Griffin, R.M. 10 Health Problems Related to Stress That You Can Fix. Available online: https://www.webmd.com/balance/stress-management/features/10-fixable-stress-related-health-problems (accessed on 1 June 2024).
- Sharma, K.; Akre, S.; Chakole, S.; Wanjari, M.B. Stress-Induced Diabetes: A Review. Cureus 2022, 14, e29142. [Google Scholar] [CrossRef] [PubMed]
- Rozanski, A.; Blumenthal, J.A.; Kaplan, J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 1999, 99, 2192–2217. [Google Scholar] [CrossRef] [PubMed]
- Soeters, M.R.; Soeters, P.B. The evolutionary benefit of insulin resistance. Clin. Nutr. 2012, 31, 1002–1007. [Google Scholar] [CrossRef]
- Eter, P.; Hepherd, R.S.; Arbara, B.; Ahn, B.K. Glucose Transporters and Insulin Action Implications for Insulin Resistance and Diabetes Mellitus. N. Engl. J. Med. 1999, 341, 248–257. [Google Scholar]
- Oddo, M.; Schmidt, J.M.; Carrera, E.; Badjatia, N.; Connolly, E.S.; Presciutti, M.; Ostapkovich, N.D.; Levine, J.M.; Le Roux, P.; Mayer, S.A. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: A microdialysis study. Crit. Care Med. 2008, 36, 3233–3238. [Google Scholar] [CrossRef]
- Duning, T.; van den Heuvel, I.; Dickmann, A.; Volkert, T.; Wempe, C.; Reinholz, J.; Lohmann, H.; Freise, H.; Ellger, B. Hypoglycemia aggravates critical illness-induced neurocognitive dysfunction. Diabetes Care 2010, 33, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Randall, O.; Esler, M.; Culp, B.; Julius, S.; Zweifler, A. Determinants of baroreflex sensitivity in man. J. Lab. Clin. Med. 1978, 91, 514–519. Available online: https://pubmed.ncbi.nlm.nih.gov/627752/ (accessed on 5 April 2024).
- Malhotra, V. A Study on the Effect of Individual Asanas on Blood Pressure. 2005. Available online: https://www.researchgate.net/publication/328214060 (accessed on 1 June 2024).
- Patel, D.M.; Bose, M.; Cooper, M.E. Glucose and blood pressure-dependent pathways–the progression of diabetic kidney disease. Int. J. Mol. Sci. 2020, 21, 2218. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Cooper, M.E. Mechanisms of Diabetic Complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zeng, F.; Wang, X.; Wang, L. Prevalence, risk factors, and prognostic significance of masked hypertension in diabetic patients. Medicine 2017, 96, e8363. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Yao, Y.; Ye, J.; Guo, X.; Dou, J.; Shen, L.; Zhang, A.; Xue, Z.; Yu, Y.; Jin, L. Association of Blood Pressure with Fasting Blood Glucose Levels in Northeast China: A Cross-Sectional Study. Sci. Rep. 2018, 8, 7917. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, M. Hypertension with diabetes mellitus: Physiology and pathology review-article. Hypertens. Res. 2018, 41, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Osawa, H.; Doi, Y.; Makino, H.; Ninomiya, T.; Yonemoto, K.; Kawamura, R.; Hata, J.; Tanizaki, Y.; Iida, M.; Kiyohara, Y. Diabetes and hypertension markedly increased the risk of ischemic stroke associated with high serum resistin concentration in a general Japanese population: The Hisayama Study. Cardiovasc. Diabetol. 2009, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Shin, D.W.; Yun, J.M.; Kim, S.H.; Nam, Y.S.; Cho, B.; Lim, J.S.; Jeong, H.Y.; Kwon, H.M.; Park, J.H. Insulin Resistance Is a Risk Factor for Silent Lacunar Infarction. Stroke 2016, 47, 2938–2944. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, G.; Postolache, O. Sensors and Mobile Interfaces for Stress level Monitoring in People with Diabetes. In Proceedings of the 12th International Symposium on Advanced Topics in Electrical Engineering, ATEE 2021, Bucharest, Romania, 25–27 March 2021; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Ribeiro, G.; Postolache, O. New Approach for Stress Assessment Based on Healthcare Ecosystems. In Proceedings of the 2023 IEEE Sensors Applications Symposium, SAS 2023—Proceedings, Ottawa, ON, Canada, 18–20 July 2023; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2023. [Google Scholar] [CrossRef]
- Ribeiro, G.; Postolache, O. New Approaches to Monitoring Respiratory Activity as Part of an Intelligent Model for Stress Assessment. In Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer Science and Business Media Deutschland GmbH: Cham, Switzerland, 2023; pp. 726–740. [Google Scholar] [CrossRef]
- Tivatansakul, S.; Ohkura, M. Improvement of emotional healthcare system with stress detection from ECG signal. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 6792–6795. [Google Scholar] [CrossRef]
- Giannakakis, G.; Pediaditis, M.; Manousos, D.; Kazantzaki, E.; Chiarugi, F.; Simos, P.G.; Marias, K.; Tsiknakis, M. Stress and anxiety detection using facial cues from videos. Biomed. Signal Process. Control 2017, 31, 89–101. [Google Scholar] [CrossRef]
- Can, Y.S.; Chalabianloo, N.; Ekiz, D.; Fernandez-Alvarez, J.; Riva, G.; Ersoy, C. Personal stress-level clustering and decision-level smoothing to enhance the performance of ambulatory stress detection with smartwatches. IEEE Access 2020, 8, 38146–38163. [Google Scholar] [CrossRef]
- Ciman, M.; Wac, K. Individuals’ stress assessment using humansmartphone interaction analysis. IEEE Trans. Affect. Comput. 2018, 9, 51–65. [Google Scholar] [CrossRef]
- Element Diabetes. Element NEO. Available online: https://www.elementdiabetes.com/language/en-US/Os-nossos-Produtos/Gluc%C3%B3metros/Element-NEO (accessed on 3 April 2024).
- iHealth. iHealth Track. ihealthlabs.eu/en. Available online: https://ihealthlabs.eu/en/blood-pressure-monitors/33-ihealth-track-6930251800835.html (accessed on 3 April 2024).
- Firebase. App Development Solutions. firebase.google.com. ‘Use Cases’. Available online: https://firebase.google.com/solutions (accessed on 3 April 2024).
- Firebase. Privacy and Security in Firebase. firebase.google.com. Available online: https://firebase.google.com/support/privacy (accessed on 3 April 2024).
- Fitness, H.L. What’s a Normal Resting Heart Rate? Available online: https://www.mayoclinic.org/healthy-lifestyle/fitness/expertanswers/heart-rate/faq-20057979 (accessed on 3 April 2024).
- EHRV. Normative HRV Scores by Age and Gender [Heart Rate Variability Chart]. elitehrv.com. Available online: https://elitehrv.com/normal-heart-rate-variability-age-gender (accessed on 3 April 2024).
- Healthline. What Is a Normal Respiratory Rate for Adults and Children? Available online: https://www.healthline.com/health/normal-respiratory-rate (accessed on 3 April 2024).
- NCOA Adviser, How to Use a Pulse Oximeter. Available online: https://www.ncoa.org/adviser/oxygen-machines/how-to-read-a-pulse-oximeter/ (accessed on 26 June 2024).
- Villarejo, M.V.; Zapirain, B.G.; Zorrilla, A.M. A stress sensor based on galvanic skin response (GSR) controlled by ZigBee. Sensors 2012, 12, 6075–6101. [Google Scholar] [CrossRef] [PubMed]
- Kuht, J.; Farmery, A.D. Body temperature and its regulation. Anaesth. Intensive Care Med. 2018, 19, 507–512. [Google Scholar] [CrossRef]
- heart.org. Understanding Blood Pressure Readings. Available online: https://www.heart.org/en/health-topics/high-blood-pressure/understanding-blood-pressure-readings (accessed on 3 April 2024).
- Health, C.M. What Your Blood Glucose Test Mean. Available online: https://www.cmihealth.com/blogs/news/what-your-blood-glucose-test-results-mean-cmi-health-blog (accessed on 3 April 2024).
- Mandala, S.K. Easiest Diabetes Classification Dataset [Data Set]; Kaggle: San Francisco, CA, USA, 2023. [Google Scholar] [CrossRef]


| Parameter | Classification Based on Parameter Reference Values | ||
|---|---|---|---|
| Low | Normal | High | |
| HR [beat-per-minute] | <60 | 60–90 | >90 |
| PRV 1 [ms] | <32 | 32–77 | >77 |
| RR [breath-per-minute] | <12 | 12–18 | >18 |
| SpO2 [%] | <97 | 97–99 | >99 |
| GSR [kOhm] | <30 | 30–50 | >50 |
| BT [°C] | <36.5 | 36.0–37.5 | >37.5 |
| Systolic BP [mmHg] | <90 | 90–120 | >120 |
| Diastolic BP [mmHg] | <60 | 60–80 | >80 |
| Status | Rules (R) |
|---|---|
| Calm | R1 = Low(HR) Λ Low(HRV) Λ Low(RR) Λ High(SpO2) Λ High(GSR) Λ High(BT) Λ Low(Systolic BP) Λ Low(Diastolic BP) |
| Normal | R2 = Normal(HR) Λ Normal(HRV) Λ Normal(RR) Λ Normal(SpO2) Λ Normal(GSR) Λ Normal(BT) Λ Normal(Systolic BP) Λ Normal(Diastolic BP) |
| Stressed | R3 = High(HR) Λ High(HRV) Λ High(RR) Λ Low(SpO2) Λ Low(GSR) Λ Low(BT) Λ High(Systolic BP) Λ High(Diastolic BP) |
| Glucose Levels in Fasting [mg/dL] | Glucose Levels after Meals [mg/dL] | Blood Glucose Category |
|---|---|---|
| <70 | <70 | Low blood glucose levels associated with hypoglycemia. |
| 70–99 | 70–139 | Normal blood glucose levels. |
| 100–125 | 140–199 | Pre-diabetes status. |
| >125 | >199 | Diabetes diagnosis. |
| Male Gender | Female Gender | Total | |
|---|---|---|---|
| Volunteers | 68 | 60 | 128 |
| Age Range | 12–75 | 17–75 | 12–75 |
| Average Age | 41 | 43 | 42 |
| Standard Deviation of Ages | 17 | 16 | 17 |
| Metric | Stress Levels Classification | ||
|---|---|---|---|
| Calm | Normal | Stressed | |
| Sensitivity | 0.94 | 0.82 | 0.93 |
| Specificity | 0.97 | 0.94 | 0.94 |
| Precision | 0.94 | 0.88 | 0.88 |
| Accuracy | 0.96 | 0.90 | 0.94 |
| F1 Score | 0.94 | 0.85 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, G.; Monge, J.; Postolache, O.; Pereira, J.M.D. A Novel AI Approach for Assessing Stress Levels in Patients with Type 2 Diabetes Mellitus Based on the Acquisition of Physiological Parameters Acquired during Daily Life. Sensors 2024, 24, 4175. https://doi.org/10.3390/s24134175
Ribeiro G, Monge J, Postolache O, Pereira JMD. A Novel AI Approach for Assessing Stress Levels in Patients with Type 2 Diabetes Mellitus Based on the Acquisition of Physiological Parameters Acquired during Daily Life. Sensors. 2024; 24(13):4175. https://doi.org/10.3390/s24134175
Chicago/Turabian StyleRibeiro, Gonçalo, João Monge, Octavian Postolache, and José Miguel Dias Pereira. 2024. "A Novel AI Approach for Assessing Stress Levels in Patients with Type 2 Diabetes Mellitus Based on the Acquisition of Physiological Parameters Acquired during Daily Life" Sensors 24, no. 13: 4175. https://doi.org/10.3390/s24134175
APA StyleRibeiro, G., Monge, J., Postolache, O., & Pereira, J. M. D. (2024). A Novel AI Approach for Assessing Stress Levels in Patients with Type 2 Diabetes Mellitus Based on the Acquisition of Physiological Parameters Acquired during Daily Life. Sensors, 24(13), 4175. https://doi.org/10.3390/s24134175










