The Use of Imaging to Quantify the Impact of Seed Aging on Lettuce Seed Germination and Seedling Vigor
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Environmental Conditions
2.2. Seed Aging Procedure
2.3. Experimental Setup, Image Acquisition, and Image Analysis
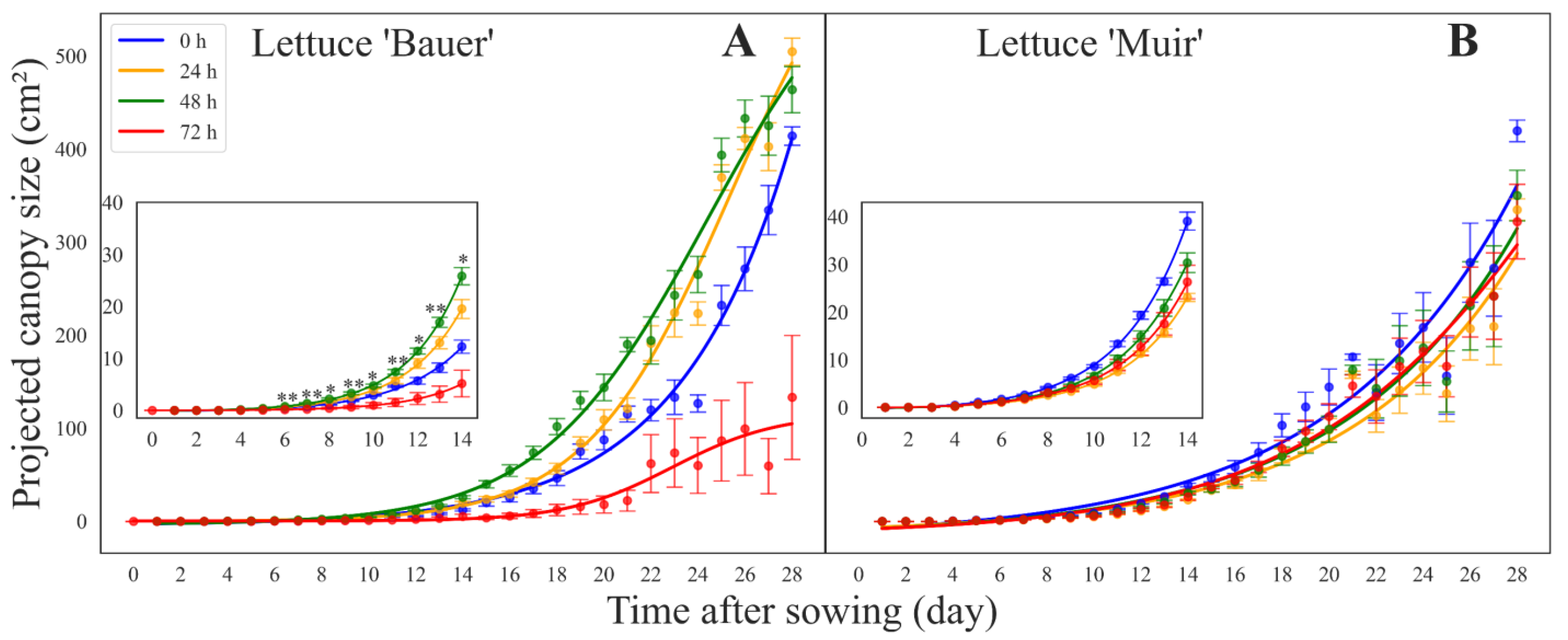
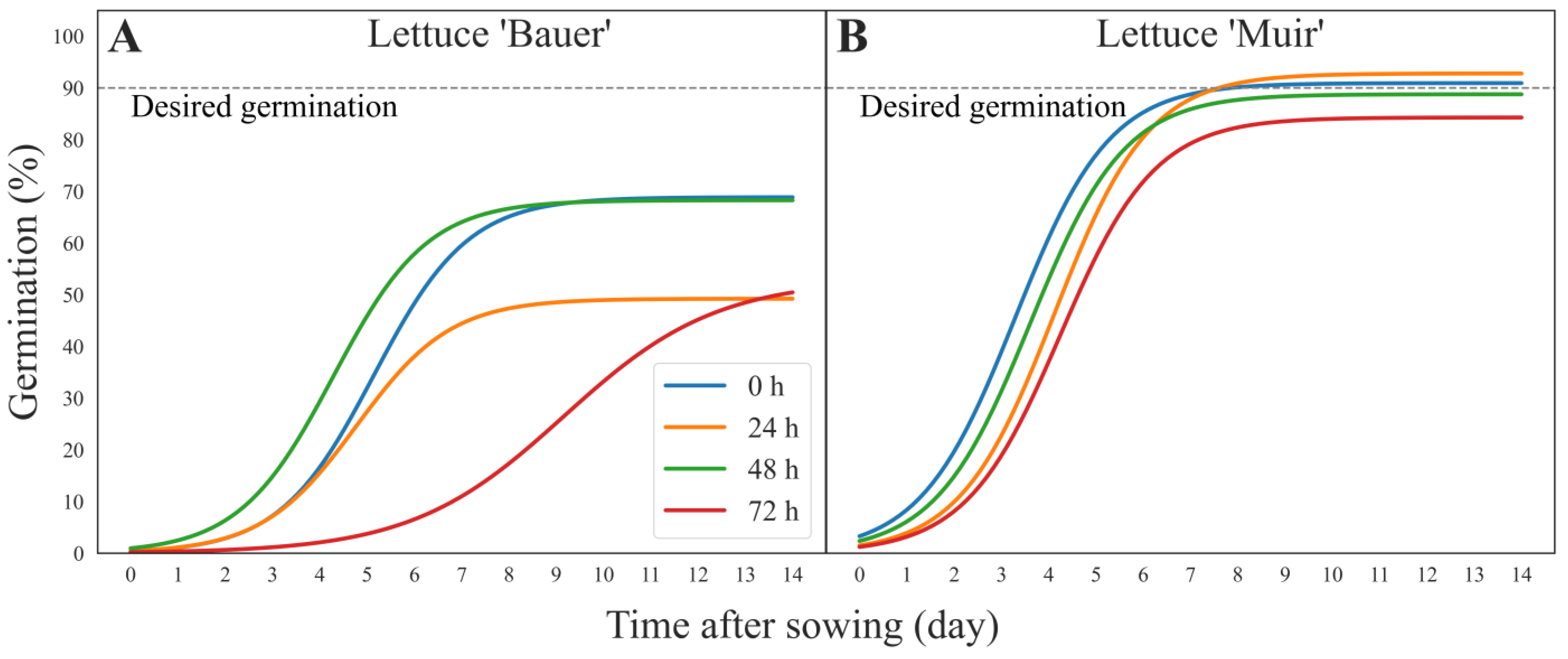
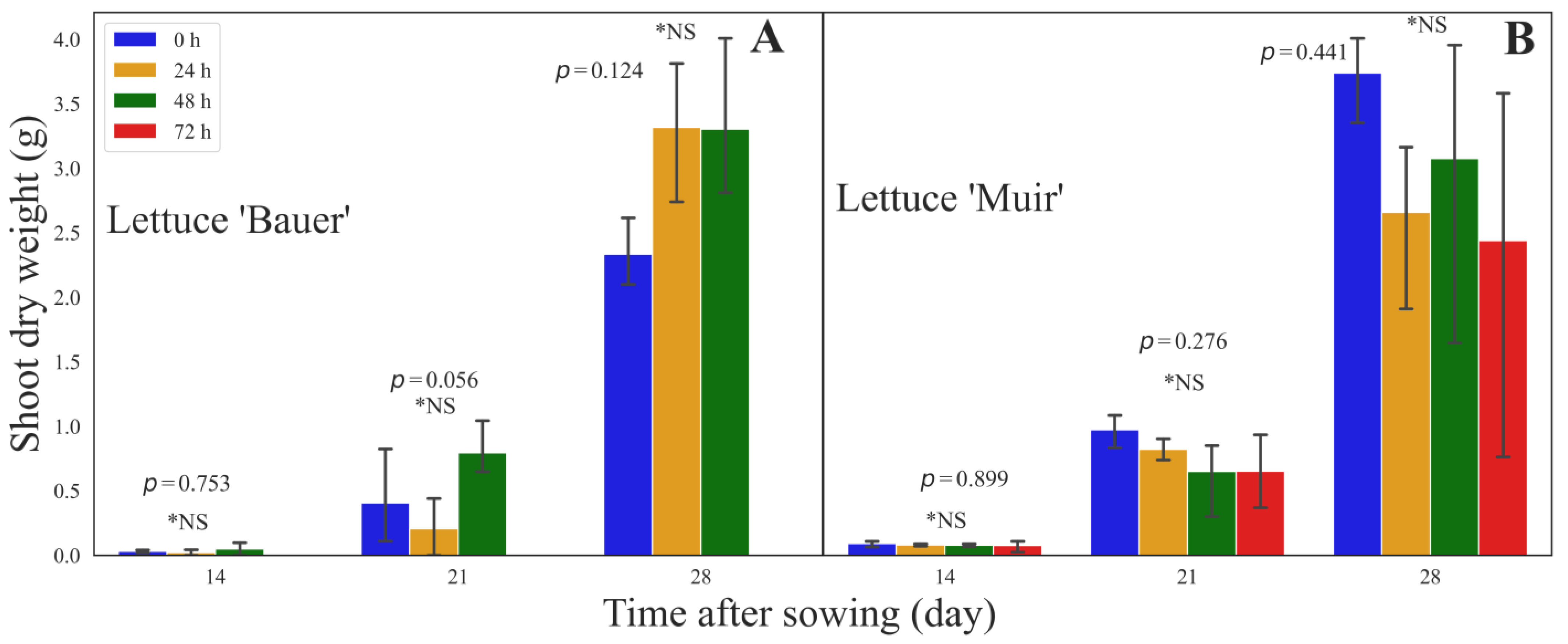
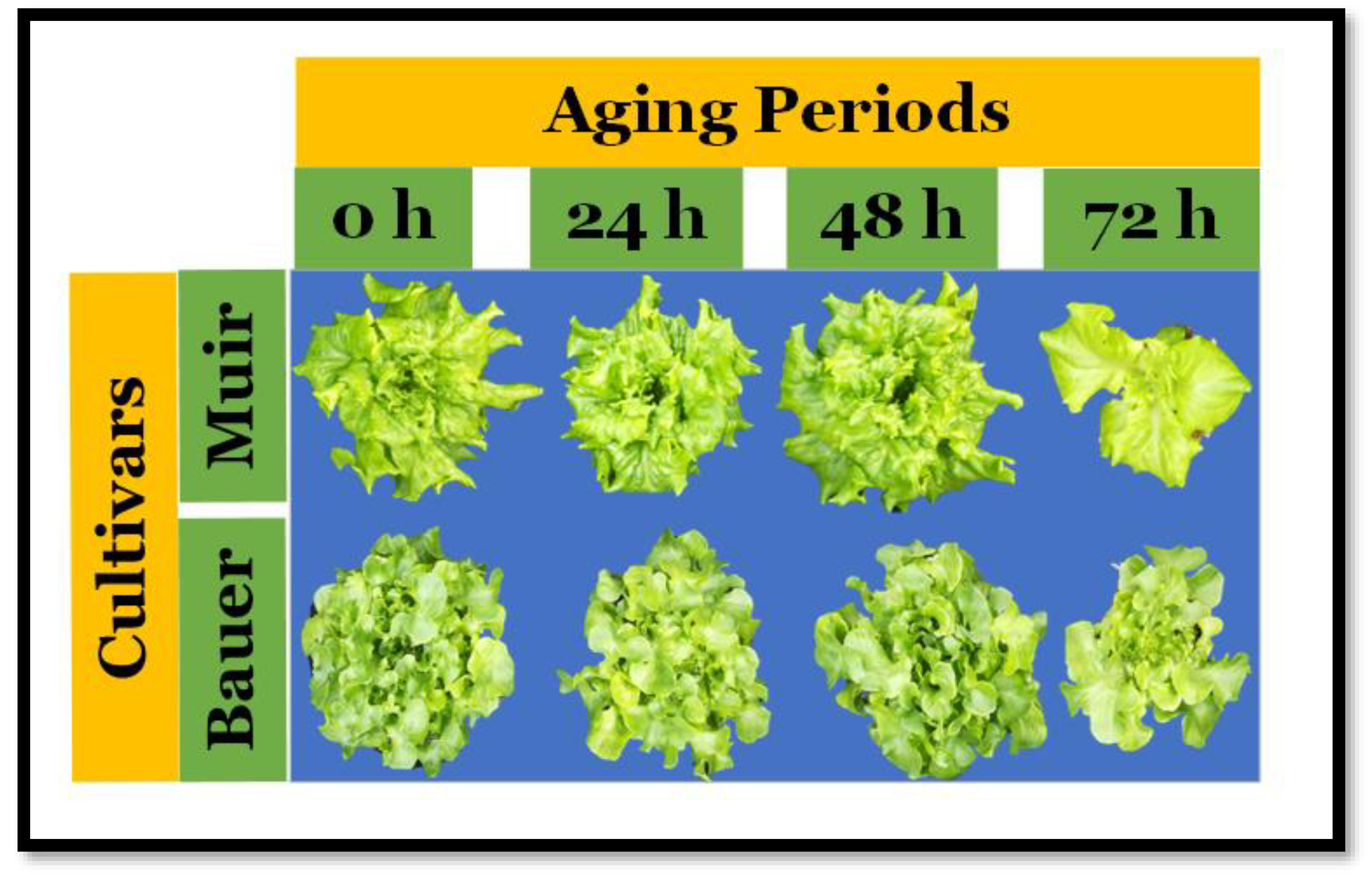
3. Results
3.1. Projected Canopy Size over Time
3.2. Germination Curves
3.3. Shoot Dry Weight
3.4. PCS and Shoot Dry Weight
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, Y.; Xu, Y.; Li, J.; Zhang, C.; Fan, S. Recent advances in emerging techniques for non-destructive detection of seed viability: A review. Artif. Intell. Agric. 2019, 1, 35–47. [Google Scholar] [CrossRef]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–540. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Solberg, S.Ø.; Yndgaard, F.; Andreasen, C.; von Bothmer, R.; Loskutov, I.G.; Asdal, Å. Long-term storage and longevity of orthodox seeds: A Systematic review. Front. Plant Sci. 2020, 11, 1007. [Google Scholar] [CrossRef] [PubMed]
- Snider, J.L.; Pilon, C.; Virk, G. Seed Characteristics and Seedling Vigor. Cotton Seed and Seedlings; Chastain, D.R., Kaur, G., Reddy, K.R., Oosterhuis, D.M., Eds.; The Cotton Foundation: Cordova, TN, USA, 2020; pp. 9–22. Available online: https://www.cotton.org/foundation/upload/Cotton-Seed-and-Seedlings-FULL-2.pdf (accessed on 23 June 2024).
- Demir, I.; Mavi, K. Controlled deterioration and accelerated aging tests to estimate the relative storage potential of cucurbit seed lots. HortScience 2008, 43, 1544–1548. [Google Scholar] [CrossRef]
- Marcos-Filho, J. Seed vigor testing: An overview of the past, present and future perspective. Sci. Agric. 2015, 72, 363–374. [Google Scholar] [CrossRef]
- Delouche, J.C.; Baskin, C.C. Accelerated aging techniques for predicting the relative storability of seed lots. Seed Technol. Pap. 2021, 10. Available online: https://scholarsjunction.msstate.edu/seedtechpapers/10/ (accessed on 10 March 2022).
- Bennett, M.A. Saturated Salt accelerated aging (SSAA) and other vigor tests for vegetable seeds. Seed Technol. 2004, 26, 67–74. [Google Scholar]
- Zhang, J.; Fang, W.; Xu, C.; Xiong, A.; Zhang, M.; Goebel, R.; Bo, G. Current optical sensing applications in seeds vigor determination. Agronomy 2023, 13, 1167. [Google Scholar] [CrossRef]
- Chen, C.; Bai, M.; Wang, T.; Zhang, W.; Yu, H.; Pang, T.; Wu, J.; Li, Z.; Wang, X. An RGB image dataset for seed germination prediction and vigor detection-maize. Front Plant Sci. 2024, 15, 1341335. [Google Scholar] [CrossRef]
- de Medeiros, A.D.; Capobiango, N.P.; da Silva, J.M.; da Silva, L.J.; da Silva, C.B.; dos Santos Dias, D.C.F. Interactive machine learning for soybean seed and seedling quality classification. Sci. Rep. 2020, 10, 11267. [Google Scholar] [CrossRef]
- Iradukunda, M.; van Iersel, M.W.; Seymour, L.; Lu, G.; Ferrarezi, R.S. Low-cost imaging to quantify germination rate and seedling vigor across lettuce cultivars. Sensors 2024, 24. accepted. [Google Scholar]
- Mcdonald, M.B. The saturated salt accelerated aging test of pansy and impatiens seeds. Seed Technol. 1997, 19, 103–109. [Google Scholar]
- Peñaloza, P.; Ramirez-Rosales, G.; Mcdonald, M.B.; Bennett, M.A. Lettuce (Lactuca sativa L.) seed quality evaluation using seed physical attributes, saturated salt accelerated aging and the seed vigour imaging system. Electron. J. Biotechnol. 2005, 8, 717–3458. [Google Scholar] [CrossRef][Green Version]
- Park, J.I.; Cho, D.M.; Oh, J.H.; Cho, J.S.; Kang, N.J. Improvement of germinability of lettuce seeds with drum-priming under high-temperature conditions. Hortic. Environ. Biotechnol. 2022, 63, 477–487. [Google Scholar] [CrossRef]
- Basak, O.; Demir, I.; Mavi, K.; Matthews, S. Controlled deterioration test for predicting seedling emergence and longevity of pepper (Capsicum annuum L.) seed lots. Seed Sci. Technol. 2006, 34, 701–712. [Google Scholar] [CrossRef]
- Joukhadar, I.; Tonnessen, B.; Coon, D.; Walker, S. Performance of heat-tolerant lettuce cultivars in southern New Mexico in 2020—21. HortTechnology 2023, 33, 313–316. [Google Scholar] [CrossRef]
- Christensen, S.; Goudriaan, J. Deriving light interception and biomass from spectral reflectance ratio. Remote Sens. Environ. 1993, 43, 87–95. [Google Scholar] [CrossRef]
- Kim, C.; van Iersel, M.W. Morphological and physiological screening to predict lettuce biomass production in controlled environment agriculture. Remote Sens. 2022, 14, 316. [Google Scholar] [CrossRef]
- Hoshino, R.; Yoshida, Y.; Tsukaya, H. Multiple steps of leaf thickening during sun-leaf formation in Arabidopsis. Plant J. 2019, 100, 738–753. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front Plant Sci. 2015, 6, 167. [Google Scholar] [CrossRef]
- Kim, M.H.; Choi, E.G.; Baek, G.Y.; Kim, C.H.; Jink, B.O.; Moon, B.E.; Kim, D.E.; Kim, H.T. Lettuce growth prediction in plant factory using image processing technology. IFAC Proc. Vol. 2013, 46, 156–159. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iradukunda, M.; van Iersel, M.W.; Seymour, L.; Lu, G.; Ferrarezi, R.S. The Use of Imaging to Quantify the Impact of Seed Aging on Lettuce Seed Germination and Seedling Vigor. Sensors 2024, 24, 4235. https://doi.org/10.3390/s24134235
Iradukunda M, van Iersel MW, Seymour L, Lu G, Ferrarezi RS. The Use of Imaging to Quantify the Impact of Seed Aging on Lettuce Seed Germination and Seedling Vigor. Sensors. 2024; 24(13):4235. https://doi.org/10.3390/s24134235
Chicago/Turabian StyleIradukunda, Mark, Marc W. van Iersel, Lynne Seymour, Guoyu Lu, and Rhuanito Soranz Ferrarezi. 2024. "The Use of Imaging to Quantify the Impact of Seed Aging on Lettuce Seed Germination and Seedling Vigor" Sensors 24, no. 13: 4235. https://doi.org/10.3390/s24134235
APA StyleIradukunda, M., van Iersel, M. W., Seymour, L., Lu, G., & Ferrarezi, R. S. (2024). The Use of Imaging to Quantify the Impact of Seed Aging on Lettuce Seed Germination and Seedling Vigor. Sensors, 24(13), 4235. https://doi.org/10.3390/s24134235






