Cautious Gait during Navigational Tasks in People with Hemiparesis: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Participants
2.3. Materials
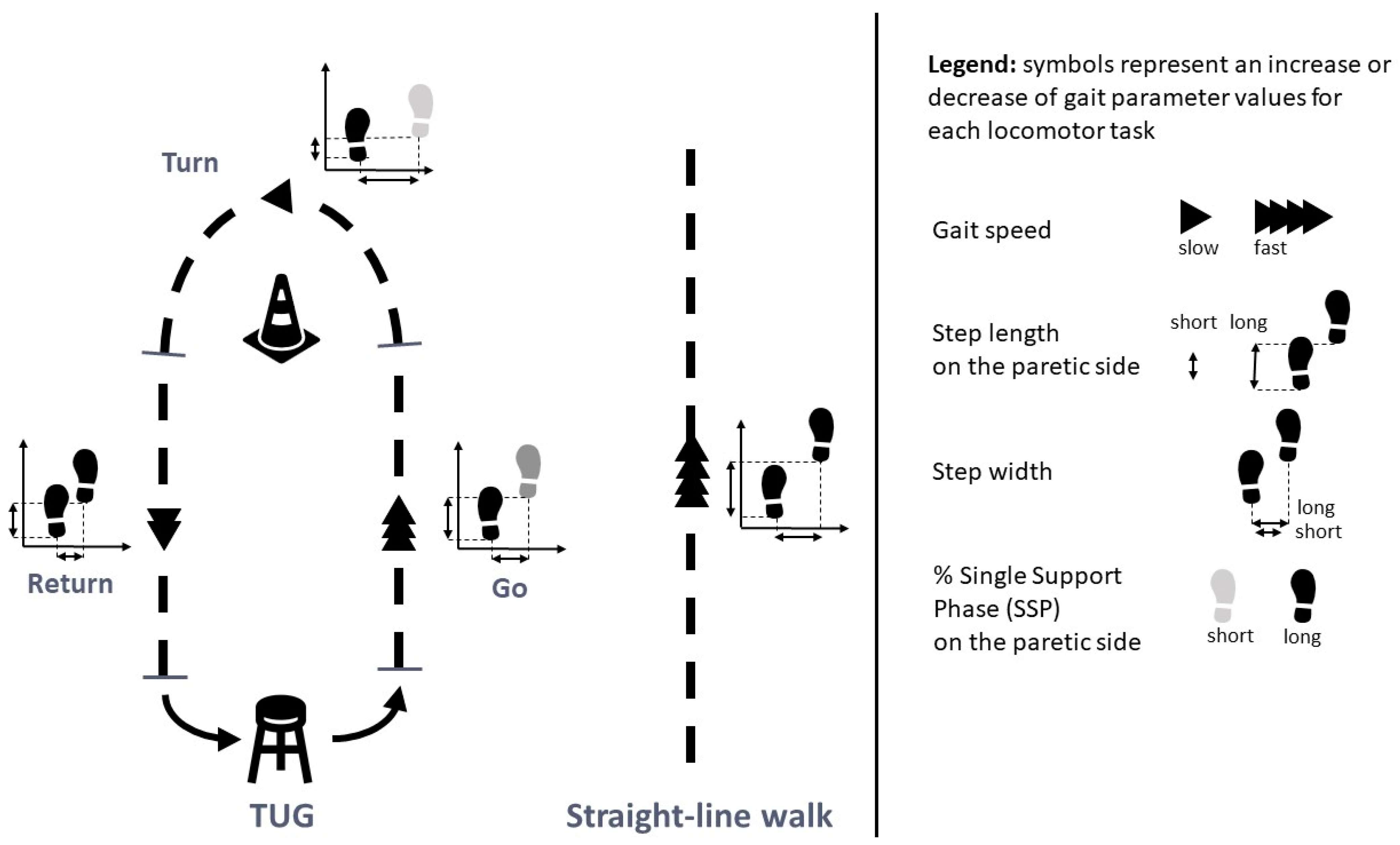
2.4. Experimental Procedure
2.5. Data Processing
2.6. Parameters Analyzed
2.7. Statistical Analysis
3. Results
3.1. Comparisons between Tasks
3.2. Correlations between STPs and Clinical Variables and Differences between Fallers and Non-Fallers
3.3. Determinants of TUG Performance
3.3.1. Biomechanical Determinants
3.3.2. Determinant Subtasks
4. Discussion
4.1. Gait Adaptations according to the Nature of the Task
4.2. Gait Adaptations Differ between Fallers and Non-Fallers
4.3. Cautious Gait Associated with the Complexity of the Task
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, K.; Ellis, P.; Bernhardt, J.; Maggs, P.; Hull, S. Balance and mobility outcomes for stroke patients: A comprehensive audit. Aust. J. Physiother. 1997, 43, 173–180. [Google Scholar] [CrossRef] [PubMed]
- de Peretti C. Grimaud, O.; Tuppin, P.; Chin, F.; Woimant, F. Prévalence des accidents vasculaires cérébraux et de leurs séquelles et impact sur les activités de la vie quotidienne: Apports des enquêtes déclaratives Handicap-santé-ménages et Handicap-santé-institution, 2008–2009. Santé publique France. Bulletin Epidémiologique Hebdomadaire. Prévalence 2012, 10, 1–6. [Google Scholar]
- Mayo, N.E.; Wood-Dauphinee, S.; Ahmed, S.; Carron, G.; Higgins, J.; Mcewen, S.; Salbach, N. Disablement following stroke. Disabil. Rehabil. 1999, 21, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Norrving, B.; Barrick, J.; Davalos, A.; Dichgans, M.; Cordonnier, C.; Guekht, A.; Kutluk, K.; Mikulik, R.; Wardlaw, J.; Richard, E.; et al. Action Plan for Stroke in Europe 2018–2030. Eur. Stroke J. 2018, 3, 309–336. [Google Scholar] [CrossRef]
- Yavuzer, G.; Öken, Ö.; Elhan, A.; Stam, H.J. Repeatability of lower limb three-dimensional kinematics in patients with stroke. Gait Posture 2008, 27, 31–35. [Google Scholar] [CrossRef] [PubMed]
- McGinley, J.L.; Baker, R.; Wolfe, R.; Morris, M.E. The reliability of three-dimensional kinematic gait measurements: A systematic review. Gait Posture 2009, 29, 360–369. [Google Scholar] [CrossRef]
- Woollacott, M.H.; Tang, P.-F. Balance Control during Walking in the Older Adult: Research and Its Implications. Phys. Ther. 1997, 77, 646–660. [Google Scholar] [CrossRef]
- Glaister, B.C.; Bernatz, G.C.; Klute, G.K.; Orendurff, M.S. Video task analysis of turning during activities of daily living. Gait Posture 2007, 25, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Berthoz, A.; Viaud-Delmon, I. Multisensory integration in spatial orientation. Curr. Opin. Neurobiol. 1999, 9, 708–712. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Ng, S.S.; Hui-Chan, C.W. The Timed Up & Go Test: Its Reliability and Association with Lower-Limb Impairments and Locomotor Capacities in People with Chronic Stroke. Arch. Phys. Med. Rehabil. 2005, 86, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Owings, T.M.; Pavol, M.J.; Foley, K.T.; Grabiner, M.D. Measures of Postural Stability Are Not Predictors of Recovery from Large Postural Disturbances in Healthy Older Adults. J. Am. Geriatr. Soc. 2000, 48, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Manaf, H.; Justine, M.; Omar, M.; Md Isa, K.A.; Salleh, Z. Turning Ability in Stroke Survivors: A Review of Literature. ISRN Rehabil. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Bonnyaud, C.; Pradon, D.; Bensmail, D.; Roche, N. Dynamic Stability and Risk of Tripping during the Timed Up and Go Test in Hemiparetic and Healthy Subjects. Baron J-C, éditeur. PLoS ONE 2015, 10, e0140317. [Google Scholar] [CrossRef] [PubMed]
- Hyndman, D.; Ashburn, A.; Stack, E. Fall events among people with stroke living in the community: Circumstances of falls and characteristics of fallers. Arch. Phys. Med. Rehabil. 2002, 83, 165–170. [Google Scholar] [CrossRef]
- Young, J. Incidence and consequences of falls due to stroke: A systematic inquiry. BMJ 1995, 311, 83–86. [Google Scholar] [CrossRef]
- Harris, J.E.; Eng, J.J.; Marigold, D.S.; Tokuno, C.D.; Louis, C.L. Relationship of Balance and Mobility to Fall Incidence in People with Chronic Stroke. Phys. Ther. 2005, 85, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.A.; Miller, W.C.; Eng, J.J. Effect of Stroke on Fall Rate, Location and Predictors: A Prospective Comparison of Older Adults with and without Stroke. Cifu D, éditeur. PLoS ONE 2011, 6, e19431. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the Probability for Falls in Community-Dwelling Older Adults Using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar]
- Andersson, Å.; Kamwendo, K.; Seiger, Å.; Appelros, P. How to identify potential fallers in a stroke unit: Validity indexes of 4 test methods. J. Rehabil. Med. 2006, 38, 186–191. [Google Scholar] [CrossRef]
- Persson, C.; Hansson, P.; Sunnerhagen, K. Clinical tests performed in acute stroke identify the risk of falling during the first year: Postural stroke study in Gothenburg (POSTGOT). J. Rehabil. Med. 2011, 43, 348–353. [Google Scholar] [CrossRef]
- Bower, K.; Thilarajah, S.; Pua, Y.-H.; Williams, G.; Tan, D.; Mentiplay, B.; Denehy, L.; Clark, R. Dynamic balance and instrumented gait variables are independent predictors of falls following stroke. J. NeuroEng. Rehabil. 2019, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.-S.; Liu, P.-T.; Chang, L.-W.; Liu, S.-Y. Gait asymmetry, ankle spasticity, and depression as independent predictors of falls in ambulatory stroke patients. Martines F, éditeur. PLoS ONE 2017, 12, e0177136. [Google Scholar] [CrossRef]
- Bonnyaud, C.; Roche, N.; Van Hamme, A.; Bensmail, D.; Pradon, D. Locomotor Trajectories of Stroke Patients during Oriented Gait and Turning. Baron J-C, éditeur. PLoS ONE 2016, 11, e0149757. [Google Scholar] [CrossRef]
- Patterson, M.R.; Whelan, D.; Reginatto, B.; Caprani, N.; Walsh, L.; Smeaton, A.F.; Inomata, A.; Caulfield, B. Does external walking environment affect gait patterns? In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 2981–2984. [Google Scholar] [CrossRef]
- Bueno, G.A.S.; Gervásio, F.M.; Ribeiro, D.M.; Martins, A.C.; Lemos, T.V.; de Menezes, R.L. Fear of Falling Contributing to Cautious Gait Pattern in Women Exposed to a Fictional Disturbing Factor: A Non-randomized Clinical Trial. Front. Neurol. 2019, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-J.; Lin, S.-I. Older adults adopted more cautious gait patterns when walking in socks than barefoot. Gait Posture 2013, 37, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Sudarsky, L.; Ronthal, M. Gait disorders in the elderly: Assessing the risk for falls. In Falls, Balance and Gait Disorders in the Elderly; Vellas, B., Toupet, M., Rubenstein, L., Albarede, J., Christen, Y., Eds.; Elsevier: Paris, France, 1992; pp. 117–127. [Google Scholar]
- Winter, D.A.; Patla, A.E.; Frank, J.S.; Walt, S.E. Biomechanical walking pattern changes in the fit and healthy elderly. Phys. Ther. 1990, 70, 340–347. [Google Scholar] [CrossRef]
- Nutt, J.G. Classification of gait and balance disorders. Adv. Neurol. 2001, 87, 135–141. [Google Scholar]
- Menz, H.B. Age-related differences in walking stability. Age Ageing 2003, 32, 137–142. [Google Scholar] [CrossRef]
- Terrier, P.; Reynard, F. Effect of age on the variability and stability of gait: A cross-sectional treadmill study in healthy individuals between 20 and 69 years of age. Gait Posture 2015, 41, 170–174. [Google Scholar] [CrossRef]
- Allali, G.; Ayers, E.I.; Holtzer, R.; Verghese, J. The role of postural instability/gait difficulty and fear of falling in predicting falls in non-demented older adults. Arch. Gerontol. Geriatr. 2017, 69, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.B.; Rodacki, A.L.F.; Pereira, G.; Bento, P.C.B. Does functional capacity, fall risk awareness and physical activity level predict falls in older adults in different age groups? Arch. Gerontol. Geriatr. 2018, 77, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Visuomotor Control of Human Adaptive Locomotion: Understanding the Anticipatory Nature. Front. Psychol. 2013, 4, 277. [Google Scholar] [CrossRef] [PubMed]
- Turcato, A.M.; Godi, M.; Giardini, M.; Arcolin, I.; Nardone, A.; Giordano, A.; Schieppati, M. Abnormal gait pattern emerges during curved trajectories in high-functioning Parkinsonian patients walking in line at normal speed. Jan Y-K, éditeur. PLoS ONE 2018, 13, e0197264. [Google Scholar] [CrossRef] [PubMed]
- Mari, S.; Serrao, M.; Casali, C.; Conte, C.; Ranavolo, A.; Padua, L.; Draicchio, F.; Iavicoli, S.; Monamì, S.; Sandrini, G.; et al. Turning strategies in patients with cerebellar ataxia. Exp. Brain Res. 2012, 222, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Hak, L.; Houdijk, H.; van der Wurff, P.; Prins, M.R.; Mert, A.; Beek, P.J.; van Dieën, J.H. Stepping strategies used by post-stroke individuals to maintain margins of stability during walking. Clin. Biomech. 2013, 28, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Cromwell, R.L.; Newton, R.A. Relationship between Balance and Gait Stability in Healthy Older Adults. J. Aging Phys. Act. 2004, 12, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Belluscio, V.; Bergamini, E.; Tramontano, M.; Formisano, R.; Buzzi, M.G.; Vannozzi, G. Does Curved Walking Sharpen the Assessment of Gait Disorders? An Instrumented Approach Based on Wearable Inertial Sensors. Sensors 2020, 20, 5244. [Google Scholar] [CrossRef]
- Maki, B.E. Gait Changes in Older Adults: Predictors of Falls or Indicators of Fear? J. Am. Geriatr. Soc. 1997, 45, 313–320. [Google Scholar] [CrossRef]
- Caetano, M.J.D.; Lord, S.R.; Brodie, M.A.; Schoene, D.; Pelicioni, P.H.S.; Sturnieks, D.L.; Menant, J.C. Executive functioning, concern about falling and quadriceps strength mediate the relationship between impaired gait adaptability and fall risk in older people. Gait Posture 2018, 59, 188–192. [Google Scholar] [CrossRef]
- Geerse, D.J.; Roerdink, M.; Marinus, J.; van Hilten, J.J. Walking adaptability for targeted fall-risk assessments. Gait Posture 2019, 70, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Patten, C.; Kothari, D.H.; Zajac, F.E. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture 2005, 22, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.K.; Parafianowicz, I.; Danells, C.J.; Closson, V.; Verrier, M.C.; Staines, W.R.; Black, S.E.; McIlroy, W.E. Gait Asymmetry in Community-Ambulating Stroke Survivors. Arch. Phys. Med. Rehabil. 2008, 89, 304–310. [Google Scholar] [CrossRef]
- Cho, K.H.; Lee, W.H. Changes of spatio-temporal gait parameters according to experience falls in post-stroke patients. Phys. Ther. Rehabil. Sci. 2012, 1, 22–27. [Google Scholar]
- Hollands, K.L.; Hollands, M.A.; Zietz, D.; Wing, A.M.; Wright, C. Kinematics of Turning 180° during the Timed Up and Go in Stroke Survivors with and without Falls History. Neurorehabil. Neural Repair 2010, 24, 358–367. [Google Scholar] [CrossRef]
- Lam, T.; Luttmann, K. Turning Capacity in Ambulatory Individuals Poststroke. Am. J. Phys. Med. Rehabil. 2009, 88, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.L.; Ng, S.S.M. The Timed 180° Turn Test for Assessing People with Hemiplegia from Chronic Stroke. BioMed Res. Int. 2018, 2018, 9629230. [Google Scholar] [CrossRef] [PubMed]
- Lewallen, L.K.; Srivastava, S.; Kautz, S.A.; Neptune, R.R. Assessment of turning performance and muscle coordination in individuals post-stroke. J. Biomech. 2021, 114, 110113. [Google Scholar] [CrossRef] [PubMed]
- Faria, C.D.C.d.M.; Paula de Carvalho-Pinto, B.; Nadeau, S.; Teixeira-Salmela, L.F. 180° turn while walking: Characterization and comparisons between subjects with and without stroke. J. Phys. Ther. Sci. 2016, 28, 2694–2699. [Google Scholar] [CrossRef]
- Barrois, R.P.-M.; Ricard, D.; Oudre, L.; Tlili, L.; Provost, C.; Vienne, A.; Vidal, P.-P.; Buffat, S.; Yelnik, A.P. Observational Study of 180° Turning Strategies Using Inertial Measurement Units and Fall Risk in Poststroke Hemiparetic Patients. Front. Neurol. 2017, 8, 194. [Google Scholar] [CrossRef]
- Chisholm, A.E.; Qaiser, T.; Lam, T. Neuromuscular control of curved walking in people with stroke: Case report. J. Rehabil. Res. Dev. 2015, 52, 775–784. [Google Scholar] [CrossRef]
- Nardone, A.; Godi, M.; Schieppati, M. Curved walking in hemiparetic patients. J. Rehabil. Med. 2010, 42, 858–865. [Google Scholar] [CrossRef]
- Wall, J.C. The timed get-up-and-go test revisited: Measurement of the component tasks. J. Rehabil. Res. Dev. 2000, 37, 109–113. [Google Scholar]
- Kadaba, M.P.; Ramakrishnan, H.K.; Wootten, M.E. Measurement of lower extremity kinematics during level walking. J. Orthop. Res. 1990, 8, 383–392. [Google Scholar] [CrossRef]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 1992, 83 (Suppl. S2), S7–S11. [Google Scholar]
- Mao, H.-F.; Hsueh, I.-P.; Tang, P.-F.; Sheu, C.-F.; Hsieh, C.-L. Analysis and Comparison of the Psychometric Properties of Three Balance Measures for Stroke Patients. Stroke 2002, 33, 1022–1027. [Google Scholar] [CrossRef]
- Powell, L.E.; Myers, A.M. The Activities-specific Balance Confidence (ABC) Scale. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50A, M28–M34. [Google Scholar] [CrossRef]
- Salbach, N.M.; Mayo, N.E.; Hanley, J.A.; Richards, C.L.; Wood-Dauphinee, S. Psychometric Evaluation of the Original and Canadian French Version of the Activities-Specific Balance Confidence Scale Among People with Stroke. Arch. Phys. Med. Rehabil. 2006, 87, 1597–1604. [Google Scholar] [CrossRef]
- Perry, J. Gait Analysis: Normal and Pathological Function; SLACK: Thorofare, NJ, USA, 1992. [Google Scholar]
- Faria, C.D.; Teixeira-Salmela, L.F.; Silva, E.B.; Nadeau, S. Expanded Timed Up and Go Test with Subjects with Stroke: Reliability and Comparisons with Matched Healthy Controls. Arch. Phys. Med. Rehabil. 2012, 93, 1034–1038. [Google Scholar] [CrossRef]
- Frykberg, G.E.; Åberg, A.C.; Halvorsen, K.; Borg, J.; Hirschfeld, H. Temporal Coordination of the Sit-to-Walk Task in Subjects with Stroke and in Controls. Arch. Phys. Med. Rehabil. 2009, 90, 1009–1017. [Google Scholar] [CrossRef]
- Dempster, W.T. The anthropometry of body action. In Space Requirements of the Seated Operator; WADC Tech Rep Wright-Patterson Air Force Base: Dayt, OH, USA, 1955. [Google Scholar] [CrossRef]
- Detrembleur, C.; Dierick, F.; Stoquart, G.; Chantraine, F.; Lejeune, T. Energy cost, mechanical work, and efficiency of hemiparetic walking. Gait Posture 2003, 18, 47–55. [Google Scholar] [CrossRef]
- MacLellan, M.J.; Patla, A.E. Adaptations of walking pattern on a compliant surface to regulate dynamic stability. Exp. Brain Res. 2006, 173, 521–530. [Google Scholar] [CrossRef]
- Laxhammar, R.; Falkman, G. Sequential Conformal Anomaly Detection in trajectories based on Hausdorff distance. In Proceedings of the 14th International Conference on Information Fusion 1–8, Chicago, IL, USA, 5–8 July 2011. [Google Scholar]
- Psarrou, A.; Gong, S.; Walter, M. Recognition of human gestures and behaviour based on motion trajectories. Image Vis. Comput. 2002, 20, 349–358. [Google Scholar] [CrossRef]
- Domholdt, E. Physical Therapy Research—Principles and Application, 2nd ed.; WB Saunders Co.: Philadelphia, PA, USA, 2000. [Google Scholar]
- Adusumilli, G.; Lancia, S.; Levasseur, V.A.; Amblee, V.; Orchard, M.; Wagner, J.M.; Naismith, R.T. Turning is an important marker of balance confidence and walking limitation in persons with multiple sclerosis. Bayer A, éditeur. PLoS ONE 2018, 13, e0198178. [Google Scholar] [CrossRef]
- Nakamura, R.; Handa, T.; Watanabe, S.; Morohashi, I. Walking cycle after stroke. Tohoku J. Exp. Med. 1988, 154, 241–244. [Google Scholar] [CrossRef]
- Lamontagne, A.; Fung, J. Faster Is Better: Implications for Speed-Intensive Gait Training After Stroke. Stroke 2004, 35, 2543–2548. [Google Scholar] [CrossRef]
- Kim, C.M.; Eng, J.J. Magnitude and pattern of 3D kinematic and kinetic gait profiles in persons with stroke: Relationship to walking speed. Gait Posture 2004, 20, 140–146. [Google Scholar] [CrossRef]
- Tyrell, C.M.; Roos, M.A.; Rudolph, K.S.; Reisman, D.S. Influence of Systematic Increases in Treadmill Walking Speed on Gait Kinematics After Stroke. Phys. Ther. 2011, 91, 392–403. [Google Scholar] [CrossRef]
- Olney, S.J.; Griffin, M.P.; McBride, I.D. Temporal, Kinematic, and Kinetic Variables Related to Gait Speed in Subjects with Hemiplegia: A Regression Approach. Phys. Ther. 1994, 74, 872–885. [Google Scholar] [CrossRef]
- Gimunová, M.; Sebera, M.; Kasović, M.; Svobodová, L.; Vespalec, T. Spatio-Temporal Gait Parameters in Association with Medications and Risk of Falls in the Elderly. Clin. Interv. Aging 2022, 17, 873–883. [Google Scholar] [CrossRef]
- Bourgarel, E.; Risser, C.; Blanc, F.; Vogel, T.; Kaltenbach, G.; Meyer, M.; Schmitt, E. Spatio-Temporal Gait Parameters of Hospitalized Older Patients: Comparison of Fallers and Non-Fallers. Int. J. Environ. Res. Public Health 2023, 20, 4563. [Google Scholar] [CrossRef]
- Said, C.M.; Goldie, P.A.; Patla, A.E.; Sparrow, W.A. Effect of stroke on step characteristics of obstacle crossing. Arch. Phys. Med. Rehabil. 2001, 82, 1712–1719. [Google Scholar] [CrossRef]
- Lord, S.E.; Rochester, L.; Weatherall, M.; McPherson, K.M.; McNaughton, H.K. The Effect of Environment and Task on Gait Parameters After Stroke: A Randomized Comparison of Measurement Conditions. Arch. Phys. Med. Rehabil. 2006, 87, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Donovan, K.; Lord, S.E.; McNaughton, H.K.; Weatherall, M. Mobility beyond the clinic: The effect of environment on gait and its measurement in community-ambulant stroke survivors. Clin. Rehabil. 2008, 22, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Hicheur, H.; Pham, Q.-C.; Arechavaleta, G.; Laumond, J.-P.; Berthoz, A. The formation of trajectories during goal-oriented locomotion in humans. I. A stereotyped behaviour. Eur. J. Neurosci. 2007, 26, 2376–2390. [Google Scholar] [CrossRef] [PubMed]
- Gerin-Lajoie, M.; Richards, C.L.; McFadyen, B.J. The circumvention of obstacles during walking in different environmental contexts: A comparison between older and younger adults. Gait Posture 2006, 24, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Marigold, D.S.; Patla, A.E. Strategies for Dynamic Stability during Locomotion on a Slippery Surface: Effects of Prior Experience and Knowledge. J. Neurophysiol. 2002, 88, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Hollands, K.L.; van Vliet, P.; Zietz, D.; Wing, A.; Wright, C.; Hollands, M.A. Stroke-related differences in axial body segment coordination during preplanned and reactive changes in walking direction. Exp. Brain Res. 2010, 202, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Kadaba, M.P.; Ramakrishnan, H.K.; Wootten, M.E.; Gainey, J.; Gorton, G.; Cochran, G.V.B. Repeatability of kinematic, kinetic, and electromyographic data in normal adult gait. J. Orthop. Res. 1989, 7, 849–860. [Google Scholar] [CrossRef]
- Brach, J.S.; Studenski, S.A.; Perera, S.; VanSwearingen, J.M.; Newman, A.B. Gait Variability and the Risk of Incident Mobility Disability in Community-Dwelling Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 6, 983–988. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Nelson, M.E.; Kaliton, D.; Layne, J.E.; Bernstein, M.J.; Nuernberger, A.; Singh, M.A.F. Etiology and modification of gait instability in older adults: A randomized controlled trial of exercise. J. Appl. Physiol. 2001, 9, 2117–2129. [Google Scholar] [CrossRef]


| Sex (M/F) | Age (years) | Time Since Stroke (years) | BMI (kg/m2) | Hemiparetic Side (Right/Left) | BBS (0–56) | ABC Scale (0–100%) | Fear of Falling VAS (0–10) | Number of Falls within the Last 3 Months |
|---|---|---|---|---|---|---|---|---|
| 18 M/11 F | 54.2 ± 12.2 | 8.3 ± 6.1 | 25.6 ± 4.3 | 12 R/17 L | 50.5 ± 2.3 | 76.3 ± 12.9 | 3.1 ± 3.2 | 0.65 ± 0.67 |
| TUG Subtasks | Straight-Line Walk | p-Value (η2) | |||
|---|---|---|---|---|---|
| Kinematic Criteria | Go | Turn | Return | ||
| Gait speed (m/s) | 0.41 ± 0.08 | 0.29 ± 0.6 a,d | 0.40 ± 0.07 | 0.77 ± 0.02 c,e,f | <0.001 * (0.91) |
| Cadence (steps/min) | 93.48 ± 11.13 | 92.60 ± 11.82 | 92.99 ± 10.49 | 91.23 ± 11.75 | 0.155 (0.06) |
| Step length P (m) | 0.45 ± 0.08 | 0.28 ± 0.09 a,d | 0.44 ± 0.07 | 0.51 ± 0.09 c,e,f | <0.001 * (0.68) |
| Step length NP (m) | 0.42 ± 0.09 | 0.32 ± 0.09 a,d | 0.43 ± 0.09 | 0.45 ± 0.01 e | <0.001 * (0.34) |
| Step width (m) | 0.17 ± 0.05 | 0.22 ± 0.05 a,d | 0.16 ± 0.05 | 0.19 ± 0.04 c,e,f | <0.001 * (0.63) |
| % SSP P (%) | 28.45 ± 3.98 | 26.80 ± 4.30 a,d | 29.20 ± 3.69 | 30.04 ± 4.05 c,e | <0.001 * (0.45) |
| % SSP NP (%) | 39.90 ± 3.36 | 36.52 ± 4.35 a,d | 39.15 ± 2.83 | 40.76 e,f ± 3.71 | <0.001 * (0.42) |
| % DSP (%) | 31.83 ± 5.76 | 36.69 ± 7.20 a,d | 31.84 ± 5.00 | 29.20 ± 5.15 c,e,f | <0.001 * (0.59) |
| Hip flexion P (°) | 40.57 ± 10.59 | 35.93 ± 9.64 a | 36.42 ± 9.60 b | 35.75 ± 9.63 c | <0.001 * (0.41) |
| Hip extension P (°) | −2.83 ± 8.54 | −5.47 ± 9.34 a,d | −1.15 ± 8.32 b | −0.13 ± 8.60 c,e | <0.001 * (0.59) |
| Knee flexion P (°) | 44.13 ± 8.58 | 40.15 ± 8.43 a,d | 44.28 ± 10.36 | 44.25 ± 12.12 e | <0.001 * (0.26) |
| Knee extension P (°) | −2.01 ± 7.07 | −2.62 ± 7.46 | −1.14 ± 6.27 | −0.90 ± 5.96 e | 0.024 * (0.11) |
| Ankle dorsiflexion P (°) | 16.86 ± 6.62 | 14.86 ± 6.28 a,d | 16.40 ± 6.76 | 15.97 ± 7.22 | <0.001 * (0.20) |
| Ankle dorsiflexion swing phase P (°) | 1.26 ± 7.28 | 0.18 ± 8.67 | 0.63 ± 7.39 | 1.75 ± 5.98 e | 0.033 * (0.10) |
| Ankle plantar flexion P (°) | 10.37 ± 7.79 | 9.85 ± 9.54 | 10.82 ± 8.60 | 10.24 ± 7.30 | 0.436 (0.03) |
| Hip flexion NP (°) | 47.10 ± 8.42 | 43.80 ± 8.25 a | 44.80 ± 8.13 b | 45.50 ± 8.10 c,e | <0.001 * (0.34) |
| Hip extension NP (°) | 4.50 ± 8.77 | 3.10 ± 8.61 a,d | 5.60 ± 8.66 b | 6.30 ± 8.54 c,e | <0.001 * (0.49) |
| Knee flexion NP (°) | 70.50 ± 5.03 | 69.40 ± 5.61 a | 69.90 ± 5.14 | 69.00 ± 4.64 c,f | <0.001 * (0.19) |
| Knee extension NP (°) | −5.70 ± 5.23 | 5.10 ± 5.14 | −5.10 ± 5.56 | −6.00 ± 5.95 | 0.091 (0.07) |
| Ankle dorsiflexion NP (°) | 23.70 ± 3.71 | 20.00 ± 3.58 a,d | 22.00 ± 3.49 b | 21.40 ± 3.90 c,e | <0.001 * (0.51) |
| Ankle dorsiflexion swing phase NP (°) | 16.30 ± 6.17 | 13.80 ± 6.09 | 15.00 ± 6.89 | 7.60 ± 3.26 c,e,f | <0.001 * (0.45) |
| Ankle plantar flexion NP (°) | 11.30 ± 6.16 | 9.90 ± 5.61 | 10.70 ± 6.21 | 9.70 ± 5.63 | 0.079 (0.08) |
| Berg Balance Scale | Fear of Falling VAS | ABC Scale | Number of Falls | |
|---|---|---|---|---|
| Gait speed Go (m/s) | 0.41 (0.025) | −0.40 (0.033) | 0.42 (0.022) | 0.38 (0.044) |
| Step length P Go (m) | 0.45 (0.013) | −0.30 (0.113) | 0.35 (0.064) | 0.27 (0.149) |
| Step width Go (m) | −0.35 (0.060) | 0.37 (0.051) | −0.08 (0.696) | 0.05 (0.810) |
| % SSP P Go (%) | 0.38 (0.042) | −0.27 (0.157) | 0.21 (0.278) | 0.33 (0.083) |
| % DSP Go (%) | −0.34 (0.073) | 0.22 (0.253) | −0.26 (0.180) | −0.43 (0.021) |
| Gait speed Turn (s) | 0.34 (0.071) | −0.34 (0.072) | 0.18 (0.340) | 0.49 (0.007) |
| Step length P Turn (m) | 0.45 (0.014) | −0.26 (0.172) | 0.26 (0.164) | 0.25 (0.195) |
| Step width Turn (m) | −0.35 (0.059) | 0.15 (0.421) | 0.01 (0.947) | −0.08 (0.695) |
| % SSP P Turn (%) | 0.38 (0.045) | −0.40 (0.033) | −0.24 (0.215) | 0.38 (0.042) |
| % DSP Turn (%) | −0.31 (0.099) | 0.28 (0.138) | −0.26 (0.169) | −0.46 (0.011) |
| Gait speed Return (s) | 0.39 (0.037) | −0.45 (0.013) | 0.42 (0.023) | 0.44 (0.016) |
| Step length P Return (m) | 0.35 (0.063) | −0.26 (0.180) | 0.35 (0.065) | 0.42 (0.023) |
| Step width Return (m) | −0.29 (0.118) | 0.32 (0.091) | −0.02 (0.917) | −0.01 (0.969) |
| % SSP P Return (%) | 0.33 (0.075) | −0.34 (0.071) | 0.27 (0.160) | 0.36 (0.056) |
| % DSP Return (%) | −0.24 (0.202) | 0.22 (0.247) | −0.28 (0.141) | −0.46 (0.011) |
| Fallers | Non-Fallers | p-Value (Effect Size) | |
|---|---|---|---|
| Gait speed Go (m/s) | 0.44 (0.09) | 0.38 (0.07) | 0.063 (0.72) |
| Gait speed Turn (m/s) | 0.31 (0.07) | 0.25 (0.04) | 0.012 (1.0) * |
| Gait speed Return (m/s) | 0.43 (0.08) | 0.37 (0.05) | 0.013 (0.99) * |
| Gait speed Straight-line (m/s) | 0.81 (0.02) | 0.72 (0.02) | 0.202 (0.49) |
| Step length P Go (m) | 0.47 (0.09) | 0.43 (0.07) | 0.208 (0.48) |
| Step length P Turn (m) | 0.29 (0.11) | 0.25 (0.07) | 0.264 (0.43) |
| Step length P Return (m) | 0.47 (0.07) | 0.40 (0.05) | 0.017 (0.95) * |
| Step length P Straight-line (m) | 0.51 (0.12) | 0.52 (0.05) | 0.840 (−0.08) |
| Cadence Go (steps/min) | 95.1 (11.9) | 91.5 (10.2) | 0.401 (0.32) |
| Cadence Turn (steps/min) | 94.5 (11.8) | 90.3 (11.9) | 0.348 (0.36) |
| Cadence Return (steps/min) | 94.5 (11.2) | 91.1 (9.6) | 0.396 (0.32) |
| Cadence Straigth-line (steps/min) | 92.1 (14.2) | 90.2 (8.3) | 0.679 (0.16) |
| Step width Go (m) | 0.18 (0.05) | 0.16 (0.05) | 0.583 (0.21) |
| Step width Turn (m) | 0.23 (0.05) | 0.22 (0.04) | 0.666 (0.16) |
| Step width Return (m) | 0.16 (0.05) | 0.15 (0.04) | 0.605 (0.19) |
| Step width Straigth-line (m) | 0.20 (0.06) | 0.19 (0.02) | 0.508 (0.25) |
| % SSP P Go (%) | 29.4 (4.3) | 27.2 (3.23) | 0.135 (0.57) |
| % SSP P Turn (%) | 28.3 (4.5) | 24.9 (28.3) | 0.046 (0.78) * |
| % SSP P Return (%) | 30.3 (3.9) | 27.8 (2.9) | 0.072 (0.69) |
| % SSP P Straigth-line (%) | 30.7 (4.3) | 29.2 (3.7) | 0.323 (0.38) |
| % DSP Go (%) | 30.3 (5.8) | 34.6 (5.3) | 0.052 (−0.76) |
| % DSP Turn (%) | 33.9 (6.2) | 40.1 (6.2) | 0.013 (−0.99) * |
| % DSP Return (%) | 30.3 (5.2) | 34.5 (3.8) | 0.023 (−0.90) * |
| % DSP Straight-line (%) | 27.7 (4.2) | 31.1 (5.7) | 0.075 (−0.69) |
| Navigational Subtasks of the TUG | |||
|---|---|---|---|
| TUG Performance, Stability, and Trajectory Criteria | Go | Turn | Return |
| TUG performance (s) | 4.6 ± 1.0 | 3.2 ± 0.8 | 3.8 ± 0.9 |
| Vert-COM (cm) | 4.5 ± 1.1 | 3.6 ± 0.8 | 4.6 ± 0.9 |
| Vert-V (cm/s) | 18.9 ± 4.7 | 15.5 ± 3.6 | 20.5 ± 4.3 |
| ML-COM (cm) | 8.9 ± 1.8 | 19.0 ± 4.4 | 9.2 ± 2.0 |
| ML-V (cm/s) | 23.0 ± 4.4 | 30.1 ± 5.9 | 22.6 ± 4.6 |
| DTW | 5894.5 ± 4699.8 | 7074.1 ± 4301.6 | 7131.6 ± 6970.3 |
| Model | Adjusted R2 | Standard Error | p |
|---|---|---|---|
| DTW Turn | 0.54 | 8.33 | <0.001 |
| DTW Turn + %SSP P Go | 0.64 | 10.92 | <0.001 |
| DTW Turn + %SSP P Go + Vert-V Go | 0.71 | 10.16 | <0.001 |
| Model | Adjusted R2 | Standard Error | p |
|---|---|---|---|
| Turn performance time | 0.63 | 1.90 | <0.001 |
| Turn performance + Go performance time | 0.82 | 1.66 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Roy, A.; Dubois, F.; Roche, N.; Brunel, H.; Bonnyaud, C. Cautious Gait during Navigational Tasks in People with Hemiparesis: An Observational Study. Sensors 2024, 24, 4241. https://doi.org/10.3390/s24134241
Le Roy A, Dubois F, Roche N, Brunel H, Bonnyaud C. Cautious Gait during Navigational Tasks in People with Hemiparesis: An Observational Study. Sensors. 2024; 24(13):4241. https://doi.org/10.3390/s24134241
Chicago/Turabian StyleLe Roy, Albane, Fabien Dubois, Nicolas Roche, Helena Brunel, and Céline Bonnyaud. 2024. "Cautious Gait during Navigational Tasks in People with Hemiparesis: An Observational Study" Sensors 24, no. 13: 4241. https://doi.org/10.3390/s24134241
APA StyleLe Roy, A., Dubois, F., Roche, N., Brunel, H., & Bonnyaud, C. (2024). Cautious Gait during Navigational Tasks in People with Hemiparesis: An Observational Study. Sensors, 24(13), 4241. https://doi.org/10.3390/s24134241






