Effect of Sampling Rate, Filtering, and Torque Onset Detection on Quadriceps Rate of Torque Development and Torque Steadiness
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Isometric Strength
2.3. Signal Processing
2.4. Statistical Analyses
3. Results
3.1. Early Rate of Torque Development: Torque Onset to 100 ms
3.2. Late Rate of Torque Development: Torque Onset to 200 ms
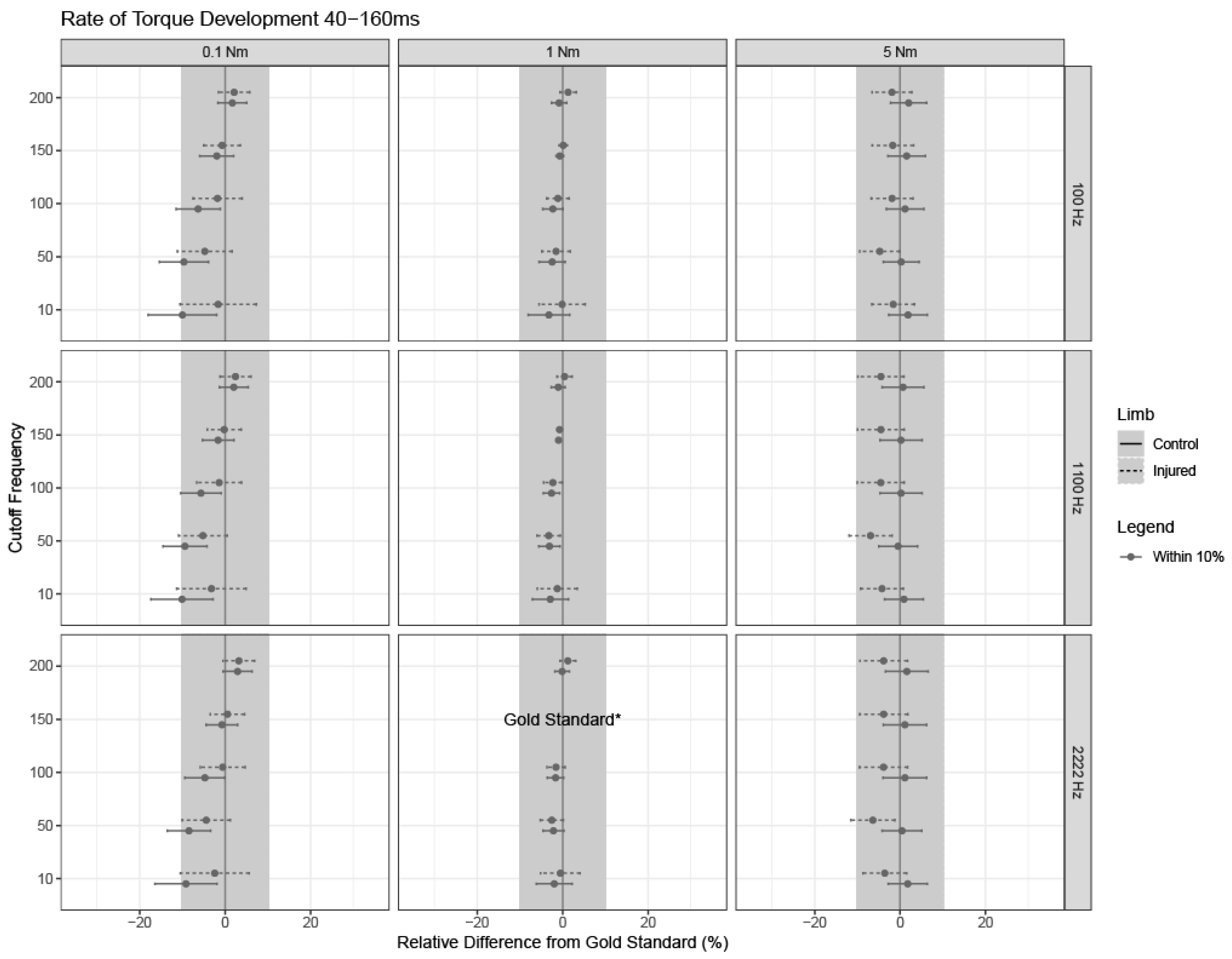
3.3. Rate of Torque Development between 40 and 160 ms
3.4. Torque Steadiness
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reid, K.F.; Fielding, R.A. Skeletal muscle power: A critical determinant of physical functioning in older adults. Exerc. Sport Sci. Rev. 2012, 40, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Angelozzi, M.; Madama, M.; Corsica, C.; Calvisi, V.; Properzi, G.; McCaw, S.T.; Cacchio, A. Rate of force development as an adjunctive outcome measure for return-to-sport decisions after anterior cruciate ligament reconstruction. J. Orthop. Sports Phys. Ther. 2012, 42, 772–780. [Google Scholar] [CrossRef]
- Aagaard, P.; Simonsen, E.B.; Andersen, J.L.; Magnusson, P.; Dyhre-Poulsen, P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J. Appl. Physiol. (1985) 2002, 93, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, C.J.; Kooistra, R.D.; Paalman, M.I.; de Haan, A. Initial phase of maximal voluntary and electrically stimulated knee extension torque development at different knee angles. J. Appl. Physiol. (1985) 2004, 97, 1693–1701. [Google Scholar] [CrossRef]
- Maffiuletti, N.A.; Aagaard, P.; Blazevich, A.J.; Folland, J.; Tillin, N.; Duchateau, J. Rate of force development: Physiological and methodological considerations. Eur. J. Appl. Physiol. 2016, 116, 1091–1116. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.L.; Aagaard, P. Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. Eur. J. Appl. Physiol. 2006, 96, 46–52. [Google Scholar] [CrossRef]
- Graham, M.C.; Thompson, K.L.; Hawk, G.S.; Fry, C.S.; Noehren, B. Muscle Fiber Cross-Sectional Area Is Associated with Quadriceps Strength and Rate of Torque Development After ACL Injury. J. Strength Cond. Res. 2024, 38, e273–e279. [Google Scholar] [CrossRef] [PubMed]
- Harridge, S.D.; Bottinelli, R.; Canepari, M.; Pellegrino, M.A.; Reggiani, C.; Esbjornsson, M.; Saltin, B. Whole-muscle and single-fibre contractile properties and myosin heavy chain isoforms in humans. Pflugers Arch. 1996, 432, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Kline, P.W.; Jacobs, C.A.; Duncan, S.T.; Noehren, B. Rate of torque development is the primary contributor to quadriceps avoidance gait following total knee arthroplasty. Gait Posture 2019, 68, 397–402. [Google Scholar] [CrossRef]
- Knurr, K.A.; Cobian, D.G.; Kliethermes, S.A.; Stiffler-Joachim, M.R.; Heiderscheit, B.C. The Influence of Quadriceps Strength and Rate of Torque Development on the Recovery of Knee Biomechanics During Running after Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2023, 51, 3171–3178. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Chang, E.; Johnson, S.T.; Pollard, C.D.; Hoffman, M.A.; Norcross, M.F. Explosive Quadriceps Strength and Landing Mechanics in Females with and without Anterior Cruciate Ligament Reconstruction. Int. J. Environ. Res. Public Health 2020, 17, 7431. [Google Scholar] [CrossRef]
- Balshaw, T.G.; Massey, G.J.; Maden-Wilkinson, T.M.; Tillin, N.A.; Folland, J.P. Training-specific functional, neural, and hypertrophic adaptations to explosive- vs. sustained-contraction strength training. J. Appl. Physiol. (1985) 2016, 120, 1364–1373. [Google Scholar] [CrossRef]
- Palmer, T.B.; Akehi, K. Rate of torque development as a discriminator of playing level in collegiate female soccer players. J. Musculoskelet. Neuronal Interact. 2022, 22, 326–335. [Google Scholar] [PubMed]
- Thompson, B.J.; Ryan, E.D.; Sobolewski, E.J.; Smith, D.B.; Akehi, K.; Conchola, E.C.; Buckminster, T. Relationships between rapid isometric torque characteristics and vertical jump performance in division I collegiate American football players: Influence of body mass normalization. J. Strength Cond. Res. 2013, 27, 2737–2742. [Google Scholar] [CrossRef] [PubMed]
- Tayfur, B.; Keneen Johnson, A.; Palmieri-Smith, R. Changes in Quadriceps Rate of Torque Development after Anterior Cruciate Ligament Reconstruction and Association to Single-Leg Hop Distance. Sports Health 2023, 19417381231205295. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; Lund, C.J.; Gingrich, M.D.; Schtul, K.L.; Wray, M.E.; Herda, T.J. The effect of rate of torque development on motor unit recruitment and firing rates during isometric voluntary trapezoidal contractions. Exp. Brain Res. 2019, 237, 2653–2664. [Google Scholar] [CrossRef]
- Quinlan, J.I.; Maganaris, C.N.; Franchi, M.V.; Smith, K.; Atherton, P.J.; Szewczyk, N.J.; Greenhaff, P.L.; Phillips, B.E.; Blackwell, J.I.; Boereboom, C.; et al. Muscle and Tendon Contributions to Reduced Rate of Torque Development in Healthy Older Males. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 539–545. [Google Scholar] [CrossRef]
- Tavoian, D.; Clark, B.C.; Clark, L.A.; Wages, N.P.; Russ, D.W. Comparison of strategies for assessment of rate of torque development in older and younger adults. Eur. J. Appl. Physiol. 2024, 124, 551–560. [Google Scholar] [CrossRef]
- Ventura, A.; Muendle, B.; Friesenbichler, B.; Casartelli, N.C.; Kramers, I.; Maffiuletti, N.A. Deficits in rate of torque development are accompanied by activation failure in patients with knee osteoarthritis. J. Electromyogr. Kinesiol. 2019, 44, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.; Davis, K.; Jacobs, C.; Johnson, D.; Ireland, M.L.; Noehren, B. Decreased quadriceps force steadiness following anterior cruciate ligament reconstruction is associated with altered running kinematics. Clin. Biomech. 2020, 72, 58–62. [Google Scholar] [CrossRef]
- De la Fuente, C.; Stoelben, K.J.V.; Silvestre, R.; Yanez, R.; Cheyre, J.; Guadagnin, E.C.; Carpes, F.P. Steadiness training improves the quadriceps strength and self-reported outcomes in persistent quadriceps weakness following nine months of anterior cruciate ligament reconstruction and failed conventional physiotherapy. Clin. Biomech. 2022, 92, 105585. [Google Scholar] [CrossRef] [PubMed]
- Osawa, Y.; Studenski, S.A.; Ferrucci, L. Knee extension rate of torque development and peak torque: Associations with lower extremity function. J. Cachexia Sarcopenia Muscle 2018, 9, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Tillin, N.A.; Jimenez-Reyes, P.; Pain, M.T.; Folland, J.P. Neuromuscular performance of explosive power athletes versus untrained individuals. Med. Sci. Sports Exerc. 2010, 42, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Mirkov, D.M.; Nedeljkovic, A.; Milanovic, S.; Jaric, S. Muscle strength testing: Evaluation of tests of explosive force production. Eur. J. Appl. Physiol. 2004, 91, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Morcelli, M.H.; LaRoche, D.P.; Crozara, L.F.; Marques, N.R.; Hallal, C.Z.; Rossi, D.M.; Goncalves, M.; Navega, M.T. Neuromuscular performance in the hip joint of elderly fallers and non-fallers. Aging Clin. Exp. Res. 2016, 28, 443–450. [Google Scholar] [CrossRef]
- Tillin, N.A.; Pain, M.T.; Folland, J. Explosive force production during isometric squats correlates with athletic performance in rugby union players. J. Sports Sci. 2013, 31, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.J. Influence of signal filtering and sample rate on isometric torque—Time parameters using a traditional isokinetic dynamometer. J. Biomech. 2019, 83, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.J.; Ryan, E.D.; Herda, T.J.; Costa, P.B.; Walter, A.A.; Sobolewski, E.J.; Cramer, J.T. Consistency of rapid muscle force characteristics: Influence of muscle contraction onset detection methodology. J. Electromyogr. Kinesiol. 2012, 22, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Conchola, E.C.; Thiele, R.M.; Palmer, T.B.; Smith, D.B.; Thompson, B.J. Effects of neuromuscular fatigue on electromechanical delay of the leg extensors and flexors in young men and women. Muscle Nerve 2015, 52, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Del Balso, C.; Cafarelli, E. Adaptations in the activation of human skeletal muscle induced by short-term isometric resistance training. J. Appl. Physiol. (1985) 2007, 103, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Tillin, N.A.; Pain, M.T.; Folland, J.P. Short-term training for explosive strength causes neural and mechanical adaptations. Exp. Physiol. 2012, 97, 630–641. [Google Scholar] [CrossRef]
- Gokeler, A.; Welling, W.; Zaffagnini, S.; Seil, R.; Padua, D. Development of a test battery to enhance safe return to sports after anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 192–199. [Google Scholar] [CrossRef]
- Thomee, R.; Kaplan, Y.; Kvist, J.; Myklebust, G.; Risberg, M.A.; Theisen, D.; Tsepis, E.; Werner, S.; Wondrasch, B.; Witvrouw, E. Muscle strength and hop performance criteria prior to return to sports after ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1798–1805. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, G.M.; Laird, N.M.; Ware, J. Applied Longitudinal Analysis, 2nd ed.; Wiley: New York, NY, USA, 2011. [Google Scholar]
- Kenward, M.G.; Roger, J.H. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997, 53, 983–997. [Google Scholar] [CrossRef]
- Lewis, F.; Butler, A.; Gilbert, L. A unified approach to model selection using the likelihood ratio test. Methods Ecol. Evol. 2011, 2, 155–162. [Google Scholar] [CrossRef]
- Littell, R.C.; Pendergast, J.; Natarajan, R. Modelling covariance structure in the analysis of repeated measures data. Stat. Med. 2000, 19, 1793–1819. [Google Scholar] [CrossRef] [PubMed]
- Folland, J.P.; Buckthorpe, M.W.; Hannah, R. Human capacity for explosive force production: Neural and contractile determinants. Scand. J. Med. Sci. Sports 2014, 24, 894–906. [Google Scholar] [CrossRef]
- Morel, B.; Rouffet, D.M.; Saboul, D.; Rota, S.; Clemencon, M.; Hautier, C.A. Peak torque and rate of torque development influence on repeated maximal exercise performance: Contractile and neural contributions. PLoS ONE 2015, 10, e0119719. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, M.S.; Graham, M.C.; Janatova, T.; Hawk, G.S.; Thompson, K.L.; Noehren, B. Effect of Sampling Rate, Filtering, and Torque Onset Detection on Quadriceps Rate of Torque Development and Torque Steadiness. Sensors 2024, 24, 4250. https://doi.org/10.3390/s24134250
White MS, Graham MC, Janatova T, Hawk GS, Thompson KL, Noehren B. Effect of Sampling Rate, Filtering, and Torque Onset Detection on Quadriceps Rate of Torque Development and Torque Steadiness. Sensors. 2024; 24(13):4250. https://doi.org/10.3390/s24134250
Chicago/Turabian StyleWhite, McKenzie S., Megan C. Graham, Tereza Janatova, Gregory S. Hawk, Katherine L. Thompson, and Brian Noehren. 2024. "Effect of Sampling Rate, Filtering, and Torque Onset Detection on Quadriceps Rate of Torque Development and Torque Steadiness" Sensors 24, no. 13: 4250. https://doi.org/10.3390/s24134250
APA StyleWhite, M. S., Graham, M. C., Janatova, T., Hawk, G. S., Thompson, K. L., & Noehren, B. (2024). Effect of Sampling Rate, Filtering, and Torque Onset Detection on Quadriceps Rate of Torque Development and Torque Steadiness. Sensors, 24(13), 4250. https://doi.org/10.3390/s24134250






