A Highly Selective Acetone Sensor Based on Coal-Based Carbon/MoO2 Nanohybrid Material
Abstract
:1. Introduction
2. Experiments
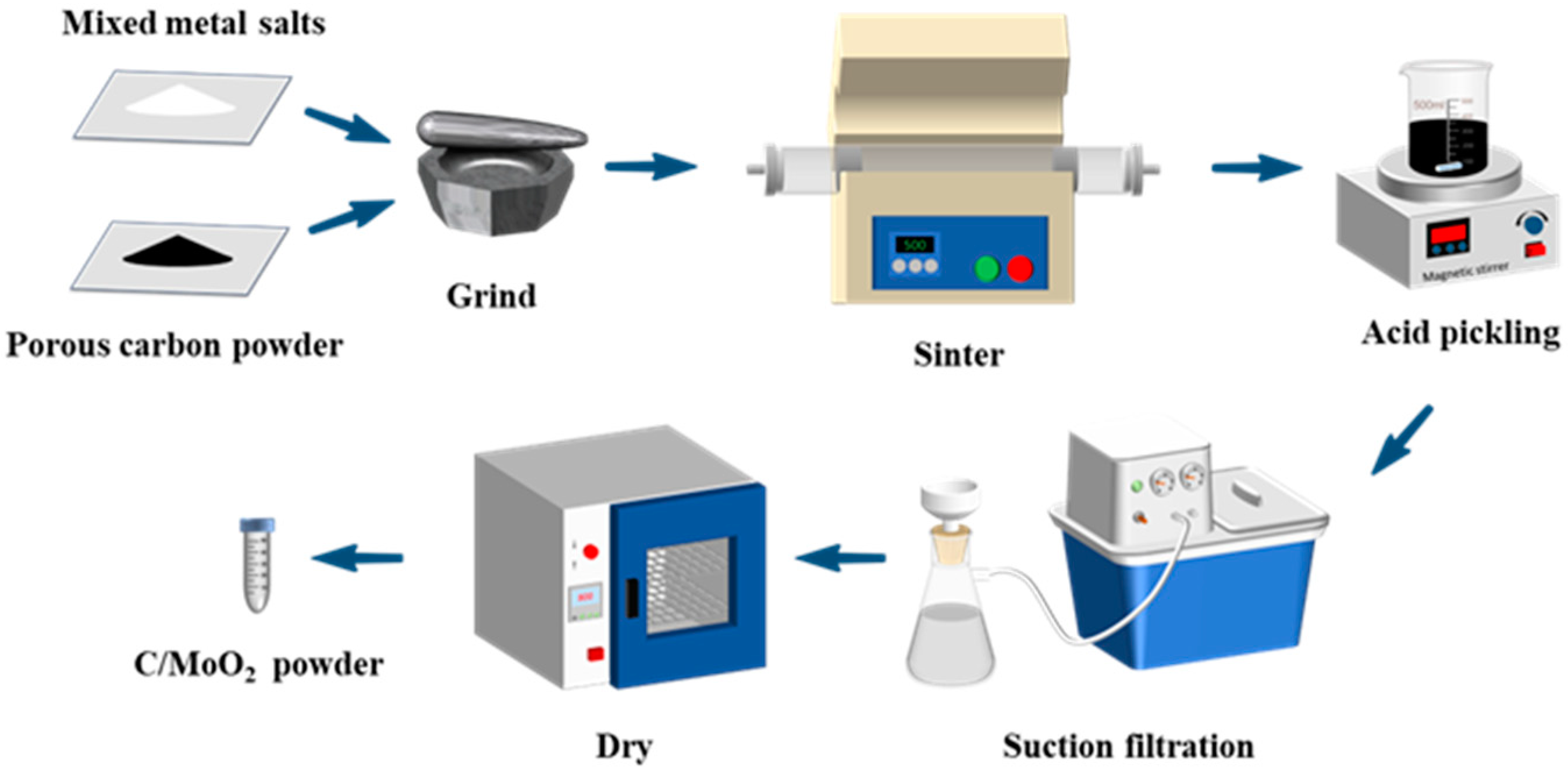
2.1. Materials and Characterization
2.2. Fabrication of Sensors
2.3. Gas-Sensor Testing
3. Results and Discussion
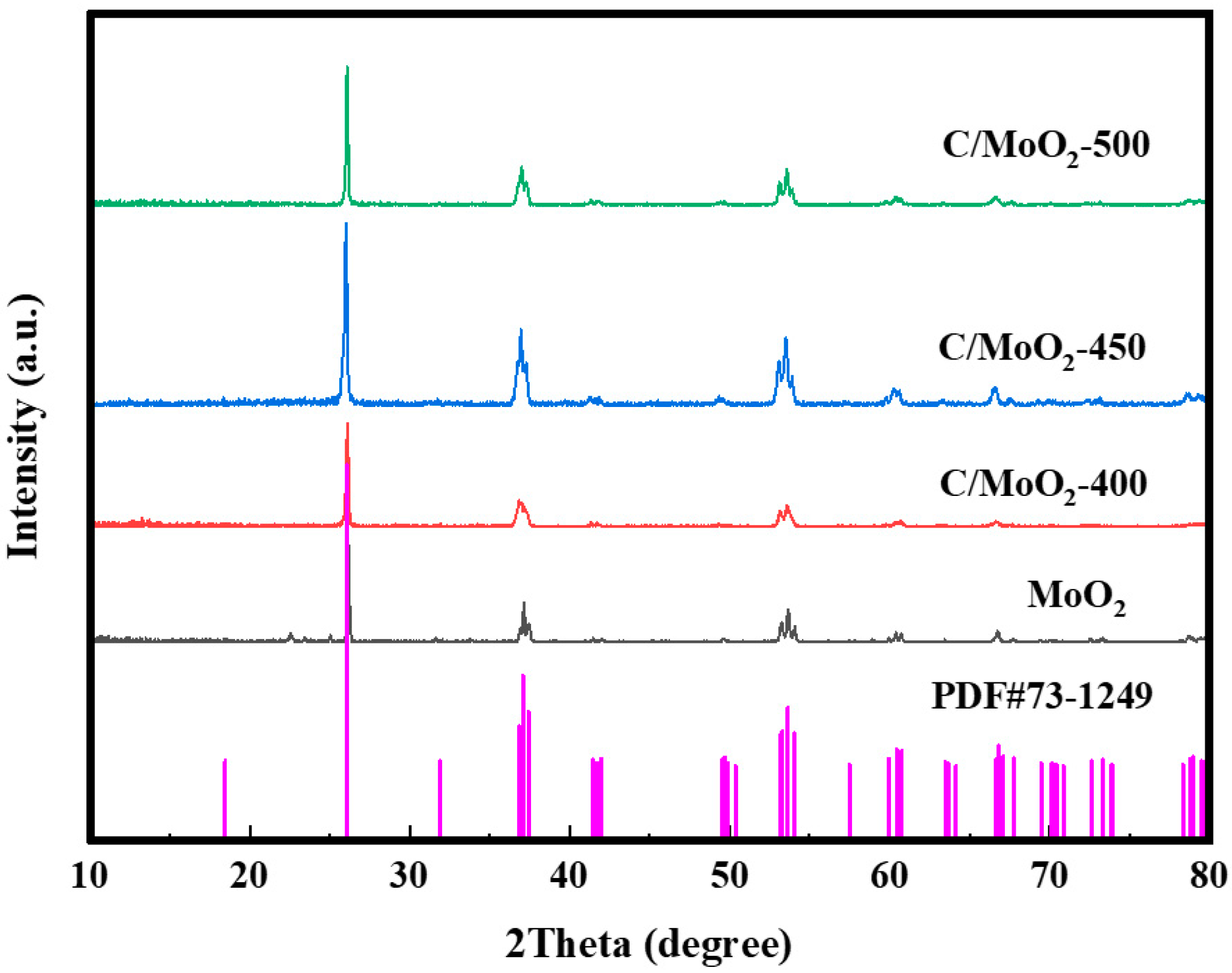
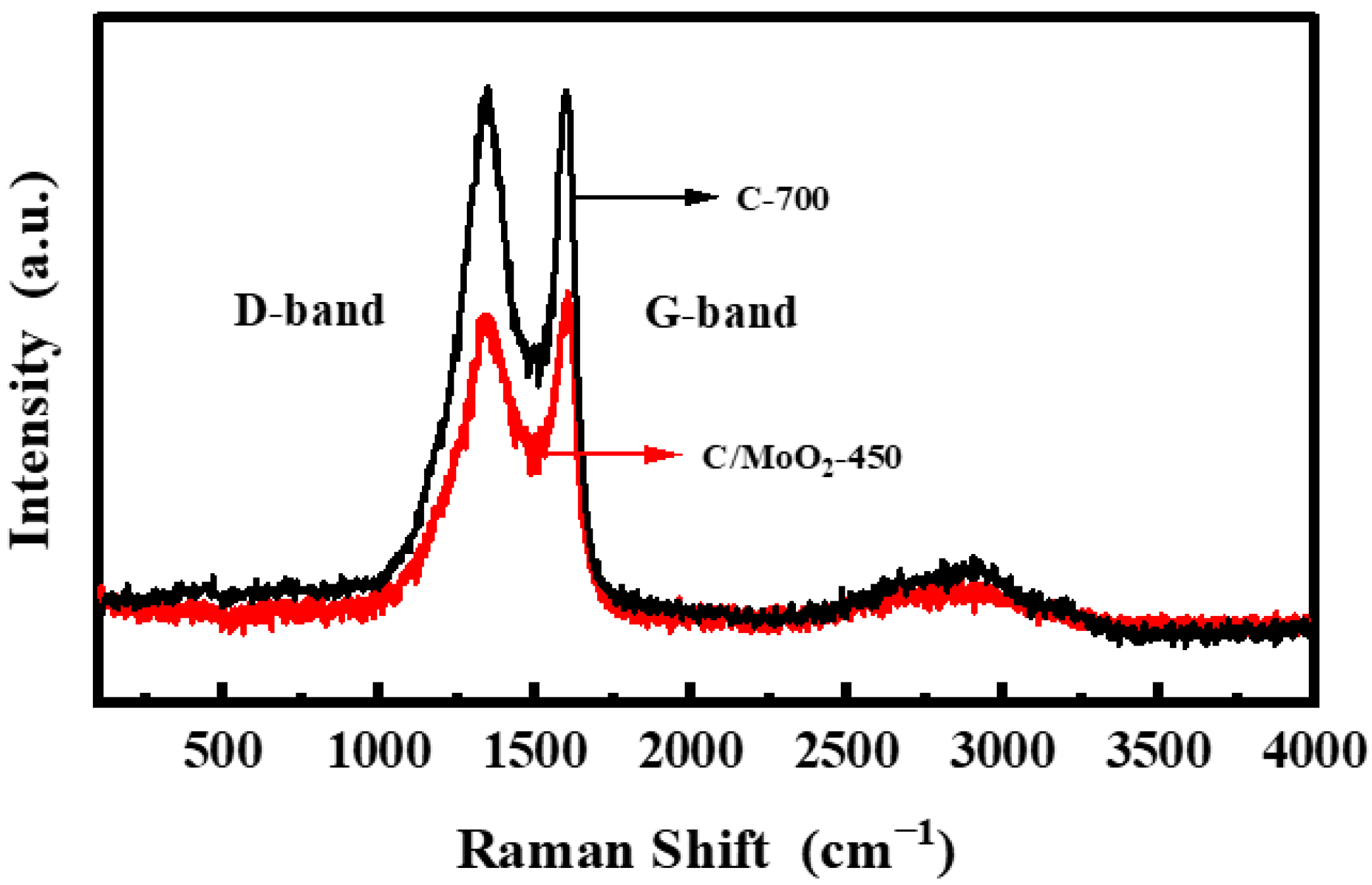
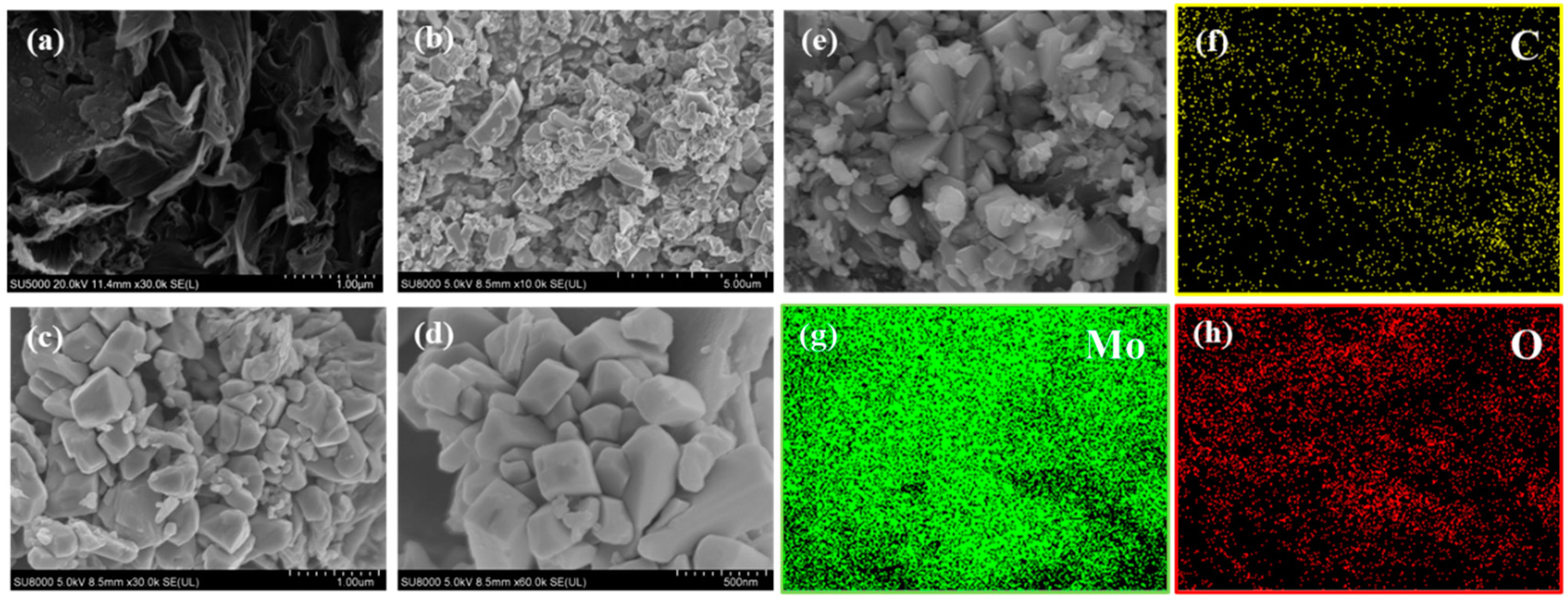
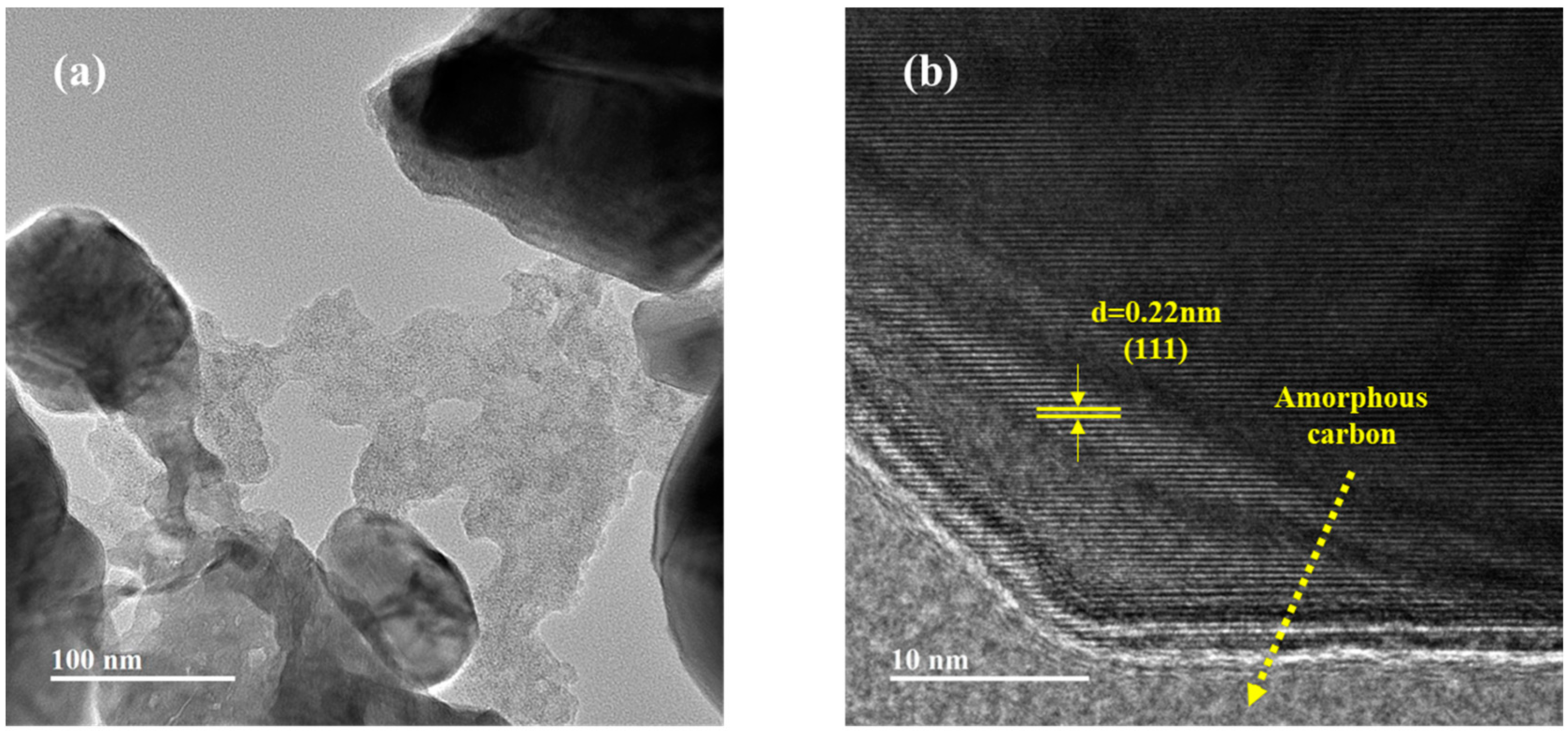
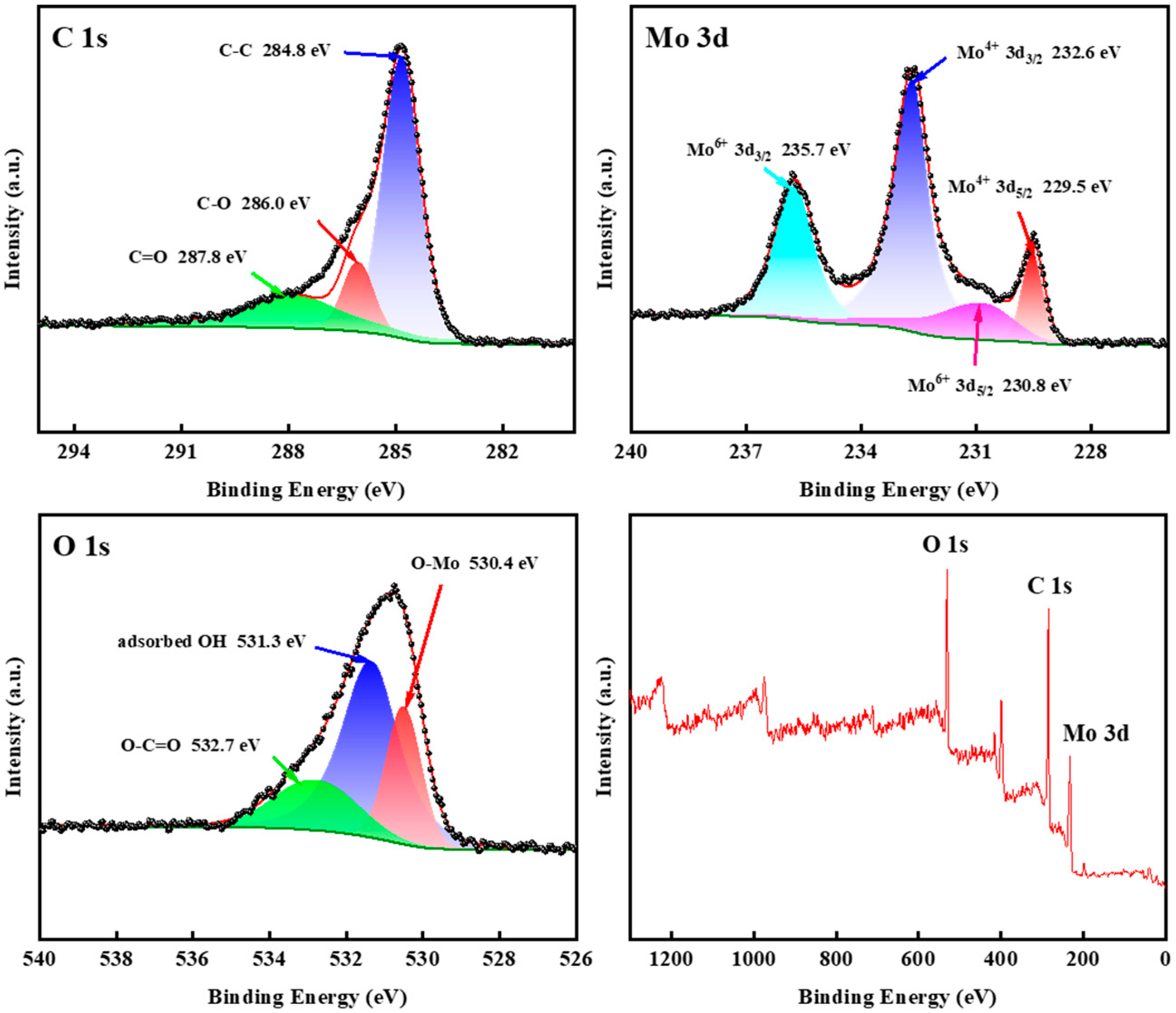
3.1. Characterization of Samples
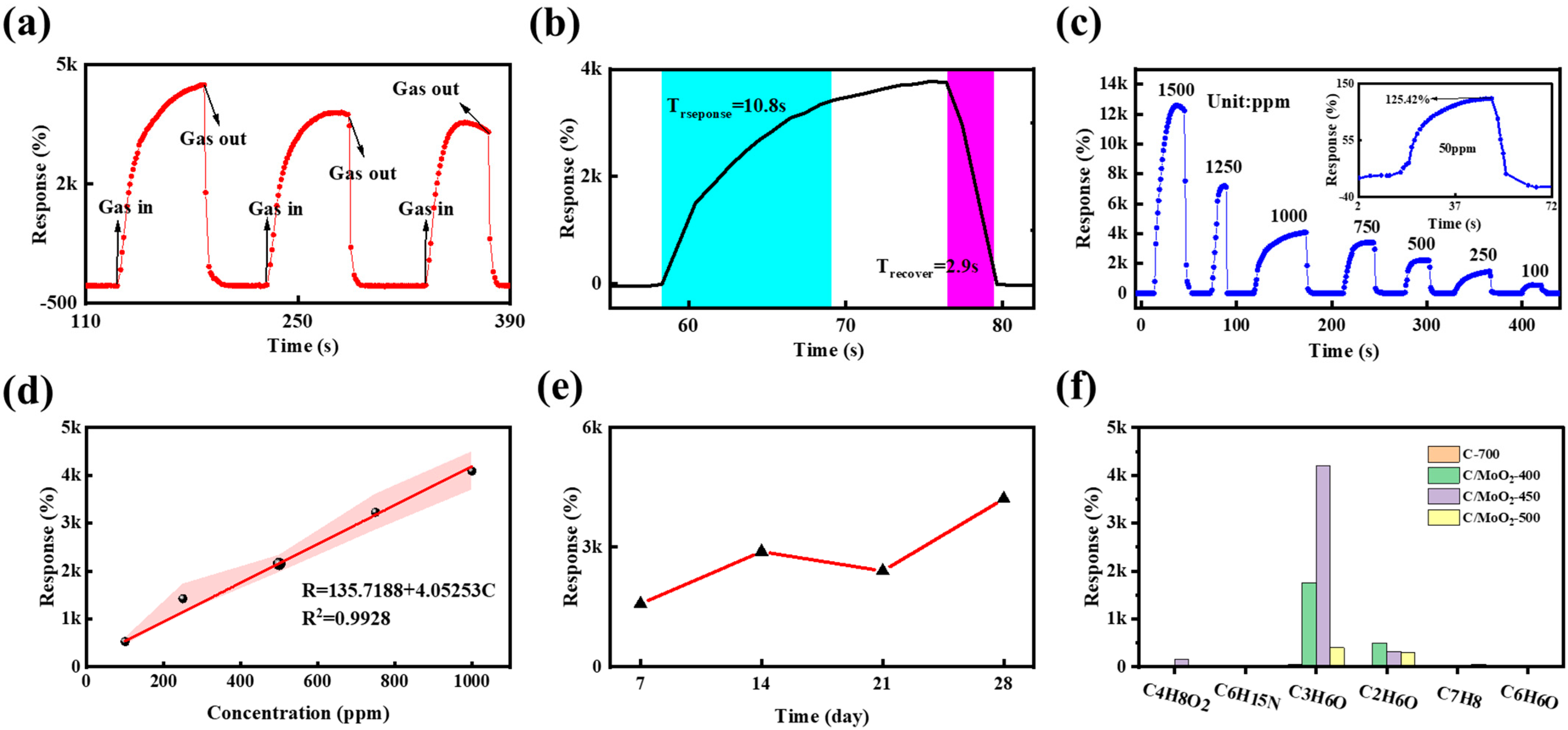
3.2. Gas-Sensing Performances
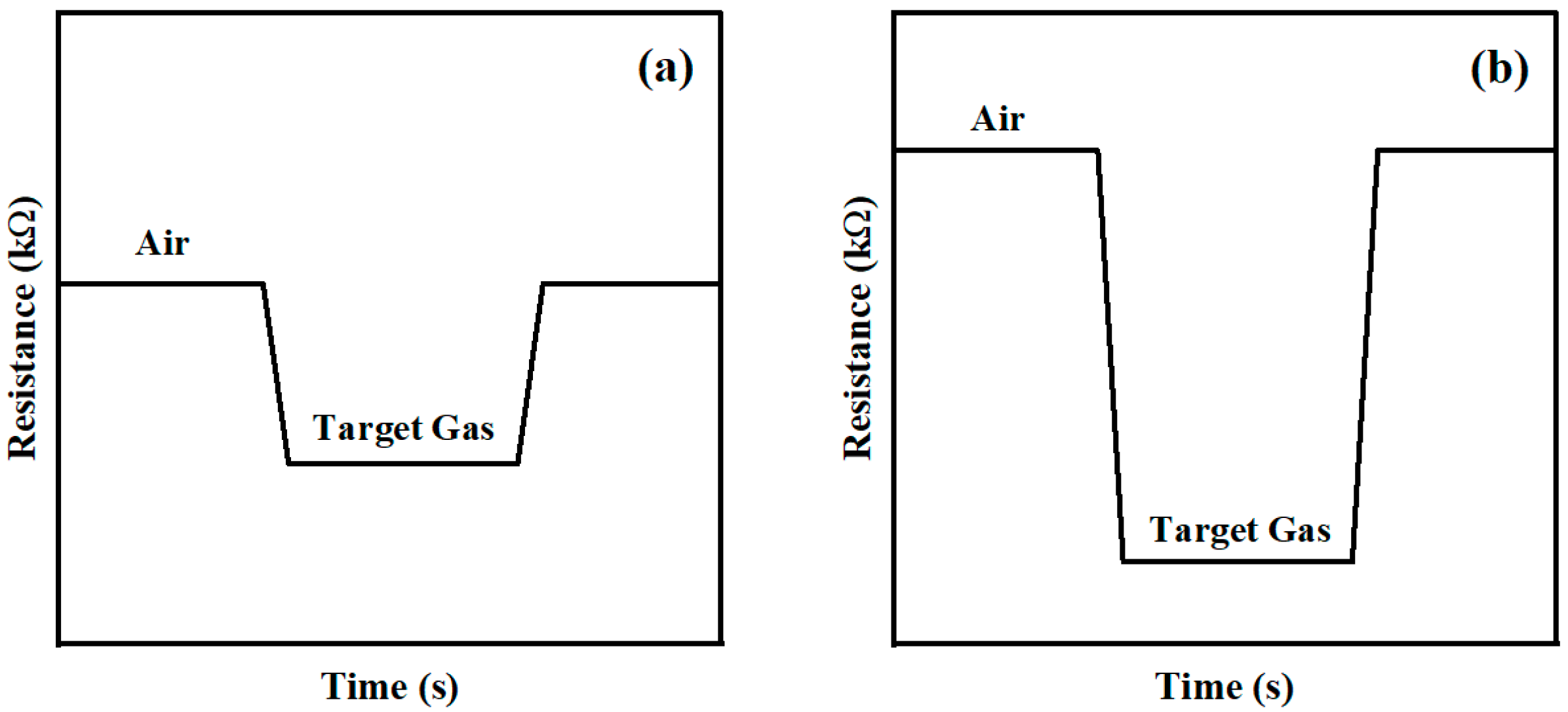
3.3. Gas-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, Q.; Wang, X.; Huang, D.; Wang, Y.; Tan, X.; Chen, Y.; Wang, W.; Yi, M.; Li, H. Highly sensitive and fast response acetone gas sensor based on Co3O4–ZnO heterojunction assembled by porous nanoflowers. J. Mater. Sci. Mater. Electron. 2023, 34, 128. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, C.; Qin, H.; Hu, J. High-performance acetone gas sensor based on ferrite–DyFeO3. J. Mater. Sci. 2020, 55, 16300–16310. [Google Scholar] [CrossRef]
- Durrani, T.; Clapp, R.; Harrison, R.; Shusterman, D. Solvent-based paint and varnish removers: A focused toxicologic review of existing and alternative constituents. J. Appl. Toxicol. 2020, 40, 1325–1341. [Google Scholar] [CrossRef]
- Massart, M.; Dubois, C.; Boileau, M. Mechanical properties and morphology of modified celluloid foams. J. Appl. Polym. Sci. 2021, 138, 50490. [Google Scholar] [CrossRef]
- Mohammed, H.K.; Muklive, A.J.; Zageer, D.S. Characterization of gunpowder component by using analytical chemistry techniques from different ammunition types. Int. J. Med. Toxicol. Leg. Med. 2020, 23, 270–278. [Google Scholar] [CrossRef]
- Wang, J.; Song, H.; Ren, L.; Talukder, M.E.; Chen, S.; Shao, J. Study on the preparation of cellulose acetate separation membrane and new adjusting method of pore size. Membranes 2022, 12, 9. [Google Scholar] [CrossRef]
- Cheng, L.; Ma, S.; Li, X.; Luo, J.; Li, W.; Li, F.; Mao, Y.; Wang, T.; Li, Y. Highly sensitive acetone sensors based on Y-doped SnO2 prismatic hollow nanofibers synthesized by electrospinning. Sens. Actuators B Chem. 2014, 200, 181–190. [Google Scholar] [CrossRef]
- Mathias, M.G.; Okajima, I.; Aoki, Y.; Kong, C.Y.; Itika, A.; Elisante, E.; Sako, T. Extraction of high-quality rice bran oil with CO2-expanded acetone for biofuel production. Fuel 2024, 356, 129491. [Google Scholar] [CrossRef]
- Das, S.; Pal, M. Non-invasive monitoring of human health by exhaled breath analysis: A comprehensive review. J. Electrochem. Soc. 2020, 167, 037562. [Google Scholar] [CrossRef]
- Ali, A.; Greish, Y.E.; Alzard, R.H.; Siddig, L.A.; Alzamly, A.; Qamhieh, N.; Mahmoud, S.T. Bismuth-based metal–organic framework as a chemiresistive sensor for acetone gas detection. Nanomaterials 2023, 13, 3041. [Google Scholar] [CrossRef]
- Ma, M.; Yang, X.; Qiao, J.; Sun, W.; Wang, Z.; Sun, K. Progress and challenges of carbon-fueled solid oxide fuel cells anode. J. Energy Chem. 2021, 56, 209–222. [Google Scholar] [CrossRef]
- Liu, A.; Yan, L.; Zhang, Y.; Ma, R.; Guo, N.; Wang, L.; Zhang, B.; Jia, D.; Sheng, R. Nitrogen-doped coal-based microporous carbon material co-activated by HCOOK and urea for high performance supercapacitors. Surf. Interfaces 2024, 44, 103754. [Google Scholar] [CrossRef]
- Gao, S.; Tang, Y.; Gao, Y.; Liu, L.; Zhao, H.; Li, X.; Wang, X. Highly crystalized Co2Mo3O8 hexagonal nanoplates interconnected by coal-derived carbon via the molten-salt-assisted method for competitive Li-ion battery anodes. ACS Appl. Mater. Interfaces 2019, 11, 7006–7013. [Google Scholar] [CrossRef] [PubMed]
- Edwards, I.A.; Marsh, H.; Menendez, R. Introduction to Carbon Science; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Cai, T.-T.; Liu, B.; Pang, E.-N.; Ren, W.-J.; Li, S.-J.; Hu, S.-L. A review on the preparation and applications of coal-based fluorescent carbon dots. New Carbon Mater. 2020, 35, 646–666. [Google Scholar] [CrossRef]
- Subha, P.; Hasna, K.; Jayaraj, M. Surface modification of TiO2 nanorod arrays by Ag nanoparticles and its enhanced room temperature ethanol sensing properties. Mater. Res. Express 2017, 4, 105037. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Choi, S.K.; Lu, Y. Surface properties and pore structure of anthracite, bituminous coal and lignite. Energies 2018, 11, 1502. [Google Scholar] [CrossRef]
- Xu, L.; Xin, Y.; Ma, Y.; Wang, P. Copper and molybdenum dioxide co-doped octahedral porous carbon framework for high sensitivity electrochemical detection of hydrogen peroxide. Ionics 2022, 28, 919–925. [Google Scholar] [CrossRef]
- Zhu, Y.P.; El-Demellawi, J.K.; Yin, J.; Lopatin, S.; Lei, Y.; Liu, Z.; Miao, X.; Mohammed, O.F.; Alshareef, H.N. Unprecedented surface plasmon modes in monoclinic MoO2 nanostructures. Adv. Mater. 2020, 32, 1908392. [Google Scholar] [CrossRef]
- Sylla, N.F.; Sarr, S.; Ndiaye, N.M.; Mutuma, B.K.; Seck, A.; Ngom, B.D.; Chaker, M.; Manyala, N. Enhanced electrochemical behavior of peanut-shell activated carbon/molybdenum oxide/molybdenum carbide ternary composites. Nanomaterials 2021, 11, 1056. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Luo, W.; Huang, Y. Self-assembled hierarchical MoO2/graphene nanoarchitectures and their application as a high-performance anode material for lithium-ion batteries. ACS Nano 2011, 5, 7100–7107. [Google Scholar] [CrossRef]
- Bhaskar, A.; Deepa, M.; Narasinga Rao, T. MoO2/multiwalled carbon nanotubes (MWCNT) hybrid for use as a Li-ion battery anode. ACS Appl. Mater. Interfaces 2013, 5, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Wang, K.X.; Wu, X.Y.; Jiang, Y.M.; Zhai, Y.B.; Wang, C.; Wei, X.; Chen, J.S. MoO2/Mo2C heteronanotubes function as high-performance Li-ion battery electrode. Adv. Funct. Mater. 2014, 24, 3399–3404. [Google Scholar] [CrossRef]
- Kushwaha, A.; Kumar, R.; Goel, N. Chemiresistive gas sensors beyond metal oxides: Using ultrathin two-dimensional nanomaterials. FlatChem 2024, 43, 100584. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, L.; Jia, D.; Xia, W.; Li, J.; Guo, Z. Coal based activated carbon nanofibers prepared by electrospinning. J. Mater. Chem. A 2014, 2, 9338–9344. [Google Scholar] [CrossRef]
- Cao, S.; Wu, Z.; Sun, Q.; Zhang, W.; Beysen, S.; Wang, S.; Shaymurat, T.; Zhang, M.; Duan, H. Gas sensing properties of cotton-based carbon fibers and ZnO/carbon fibers regulated by changing carbonization temperatures. Sens. Actuators B Chem. 2021, 337, 129818. [Google Scholar] [CrossRef]
- Mun, D.; Vo, A.T.H.; Kim, B.; Shul, Y.-G.; Cho, J.K. Solventless esterification of fatty acids with trimethylolpropane using sulfonated amorphous carbons derived from wood powder. Catal. Commun. 2017, 96, 32–36. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Gao, T.; Zhang, D.; Huang, X.; Pan, Y.; Ye, K.; Cheng, K.; Cao, D.; Wang, G. Synthesis of hierarchically porous sandwich-like carbon materials for high-performance supercapacitors. Chem. Eur. J. 2016, 22, 16863–16871. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Ou, Y.; Tian, W.; Liu, L.; Zhang, Y.; Xiao, P. Bimetallic Co2Mo3O8 suboxides coupled with conductive cobalt nanowires for efficient and durable hydrogen evolution in alkaline electrolyte. J. Mater. Chem. A 2018, 6, 5217–5228. [Google Scholar] [CrossRef]
- Cheng, Y.; Yi, Z.; Wang, C.; Wu, Y.; Wang, L. Controllable fabrication of C/Sn and C/SnO/Sn composites as anode materials for high-performance lithium-ion batteries. Chem. Eng. J. 2017, 330, 1035–1043. [Google Scholar] [CrossRef]
- Liu, J.; Dai, M.; Wang, T.; Sun, P.; Liang, X.; Lu, G.; Shimanoe, K.; Yamazoe, N. Enhanced gas sensing properties of SnO2 hollow spheres decorated with CeO2 nanoparticles heterostructure composite materials. ACS Appl. Mater. Interfaces 2016, 8, 6669–6677. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.H.; Chau, J.L.H.; Yang, C.C.; Shih, H.H. Preparation of amorphous Ga–Sn–Zn–O semiconductor thin films by RF-sputtering method. Mat. Sci. Eng. B 2014, 183, 17–23. [Google Scholar] [CrossRef]
- Quy, C.; Thai, N.; Hoa, N.; Le, D.; Hung, C.; Duy, N.; Hieu, N. C2H5OH and NO2 sensing properties of ZnO nanostructures: Correlation between crystal size, defect level and sensing performance. RSC Adv. 2018, 8, 5629–5639. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zeng, W.; Li, Y. A novel cactus-like WO3-SnO2 nanocomposite and its acetone gas sensing properties. Mater. Lett. 2018, 231, 5–7. [Google Scholar] [CrossRef]
- Qu, F.; Thomas, T.; Zhang, B.; Zhou, X.; Zhang, S.; Ruan, S.; Yang, M. Self-sacrificing templated formation of Co3O4/ZnCo2O4 composite hollow nanostructures for highly sensitive detecting acetone vapor. Sens. Actuators B Chem. 2018, 273, 1202–1210. [Google Scholar] [CrossRef]
- Park, S.; Kheel, H.; Sun, G.-J.; Ko, T.; Lee, W.; Lee, C. Acetone Gas Sensing Properties of a Multiple-Networked Fe2O3-Functionalized CuO Nanorod Sensor. J. Nanomater. 2015, 2015, 830127. [Google Scholar] [CrossRef]
- Wang, S.; Cao, J.; Cui, W.; Fan, L.; Li, X.; Li, D. Facile synthesis of bamboo raft-like Co3O4 with enhanced acetone gas sensing performances. J. Alloys Compd. 2018, 758, 45–53. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, F.; Li, H.; Chen, T.; Wang, Y. Acetone detection properties of single crystalline tungsten oxide plates synthesized by hydrothermal method using cetyltrimethyl ammonium bromide supermolecular template. Sens. Actuators B Chem. 2012, 162, 259–268. [Google Scholar] [CrossRef]
- Vishwakarma, A.K.; Sharma, A.K.; Yadav, N.K.; Yadava, L. Development of CdS-doped TiO2 nanocomposite as acetone gas sensor. Vacuum 2021, 191, 110363. [Google Scholar] [CrossRef]
- Jagtap, S.; Priolkar, K. Evaluation of ZnO nanoparticles and study of ZnO–TiO2 composites for lead free humidity sensors. Sens. Actuators B Chem. 2013, 183, 411–418. [Google Scholar] [CrossRef]
- Tian, X.; Hu, Z.; Wang, T.; Wang, H.; Zhang, Q.; Wei, X. Influence of multi-layer TiO2/SnO2 heterojunctions on fast and sensitive ethanol detection. Vacuum 2023, 207, 111620. [Google Scholar] [CrossRef]
- Rezaeipour, A.; Dehghani, S.; Hoghoghifard, S. Acetone sensor based on porous nickel ferrite nanoparticles. J. Mater. Sci. Mater. Electron. 2022, 33, 26276–26285. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, B.; Corr, S.A.; Shi, Q.; Hu, Y.-S.; Heier, K.R.; Chen, L.; Seshadri, R.; Stucky, G.D. Ordered Mesoporous Metallic MoO2 Materials with Highly Reversible Lithium Storage Capacity. Nano Lett. 2009, 9, 4215–4220. [Google Scholar] [CrossRef]
- Yu, X.; Liu, S.; Lin, G.; Zhu, X.; Zhang, S.; Qu, R.; Zheng, C.; Gao, X. Insight into the significant roles of microstructures and functional groups on carbonaceous surfaces for acetone adsorption. RSC Adv. 2018, 8, 21541–21550. [Google Scholar] [CrossRef]





| Sensing Materials | Acetone | Temperature | Response Time | Recovery Time | Reference |
|---|---|---|---|---|---|
| Cactus-like WO3–SnO2 nanocomposite | 600 ppm | 360 °C | 14 s | 16 s | [35] |
| Co3O4/ZnCo2O4 composite | 100 ppm | 255 °C | 41 s | 47 s | [36] |
| Fe2O3–CuO nanorod | 100 ppm | 240 °C | 149 s | 133 s | [37] |
| Bamboo raft-like Co3O4 | 200 ppm | 180 °C | 32 s | 35 s | [38] |
| WO3 plate | 200 ppm | 307 °C | 10 s | 26 s | [39] |
| CdS-doped TiO2 nanocomposite | 5000 ppm | Room temperature | 55 s | 115 s | [40] |
| C/MoO2 nanohybrid material | 1000 ppm | Room temperature | 10.8 s | 2.9 s | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Han, Y.; Liu, T.; Jia, H. A Highly Selective Acetone Sensor Based on Coal-Based Carbon/MoO2 Nanohybrid Material. Sensors 2024, 24, 4320. https://doi.org/10.3390/s24134320
Zhang M, Han Y, Liu T, Jia H. A Highly Selective Acetone Sensor Based on Coal-Based Carbon/MoO2 Nanohybrid Material. Sensors. 2024; 24(13):4320. https://doi.org/10.3390/s24134320
Chicago/Turabian StyleZhang, Min, Yi Han, Ting Liu, and Hongguang Jia. 2024. "A Highly Selective Acetone Sensor Based on Coal-Based Carbon/MoO2 Nanohybrid Material" Sensors 24, no. 13: 4320. https://doi.org/10.3390/s24134320





