In Situ Growth of COF/PVA-Carrageenan Hydrogel Using the Impregnation Method for the Purpose of Highly Sensitive Ammonia Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Bare COF Film and Particles
2.2.1. COF Film
2.2.2. COF Particles
2.3. Synthesis of Bare κPVA Hydrogel
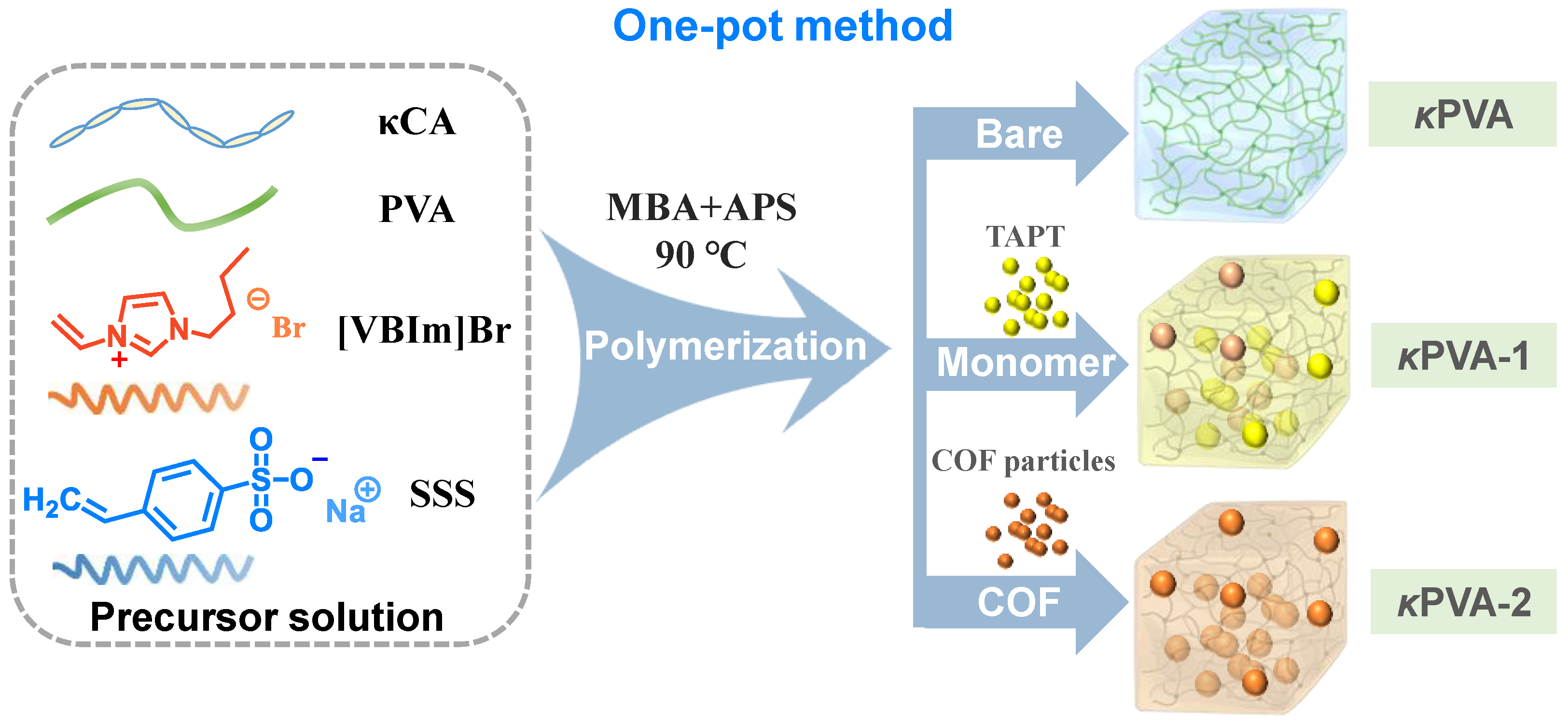
2.4. Synthesis of COF/κPVA Composite Hydrogels
2.4.1. One-POT Method
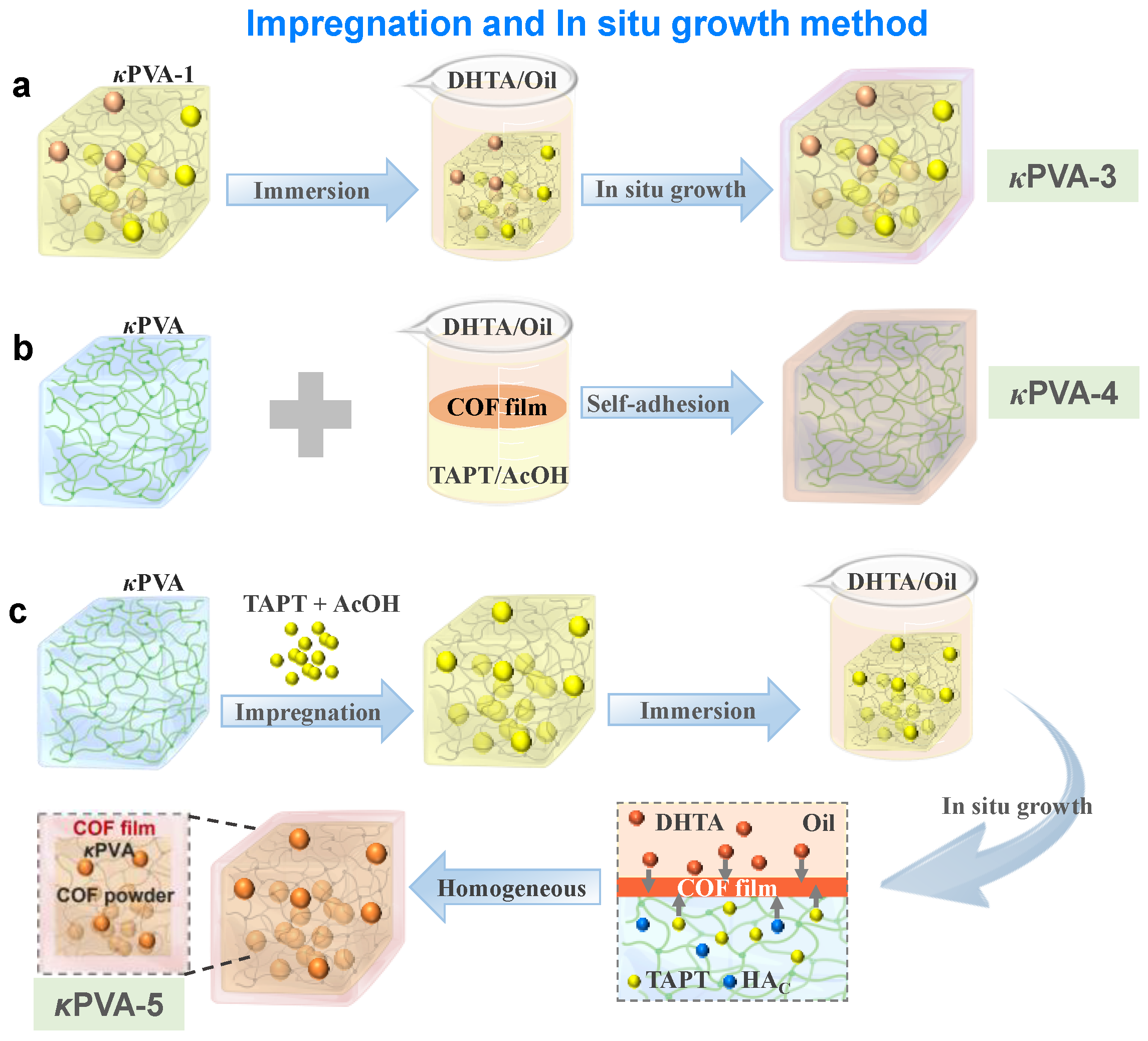
2.4.2. Impregnation and In Situ Growth Method
2.5. Characterization
2.6. Sensing Performance Test of the COF/κPVA Hydrogel
2.7. Mechanical Characterization
2.8. Water Content and Swelling Ratio Measurement
3. Results
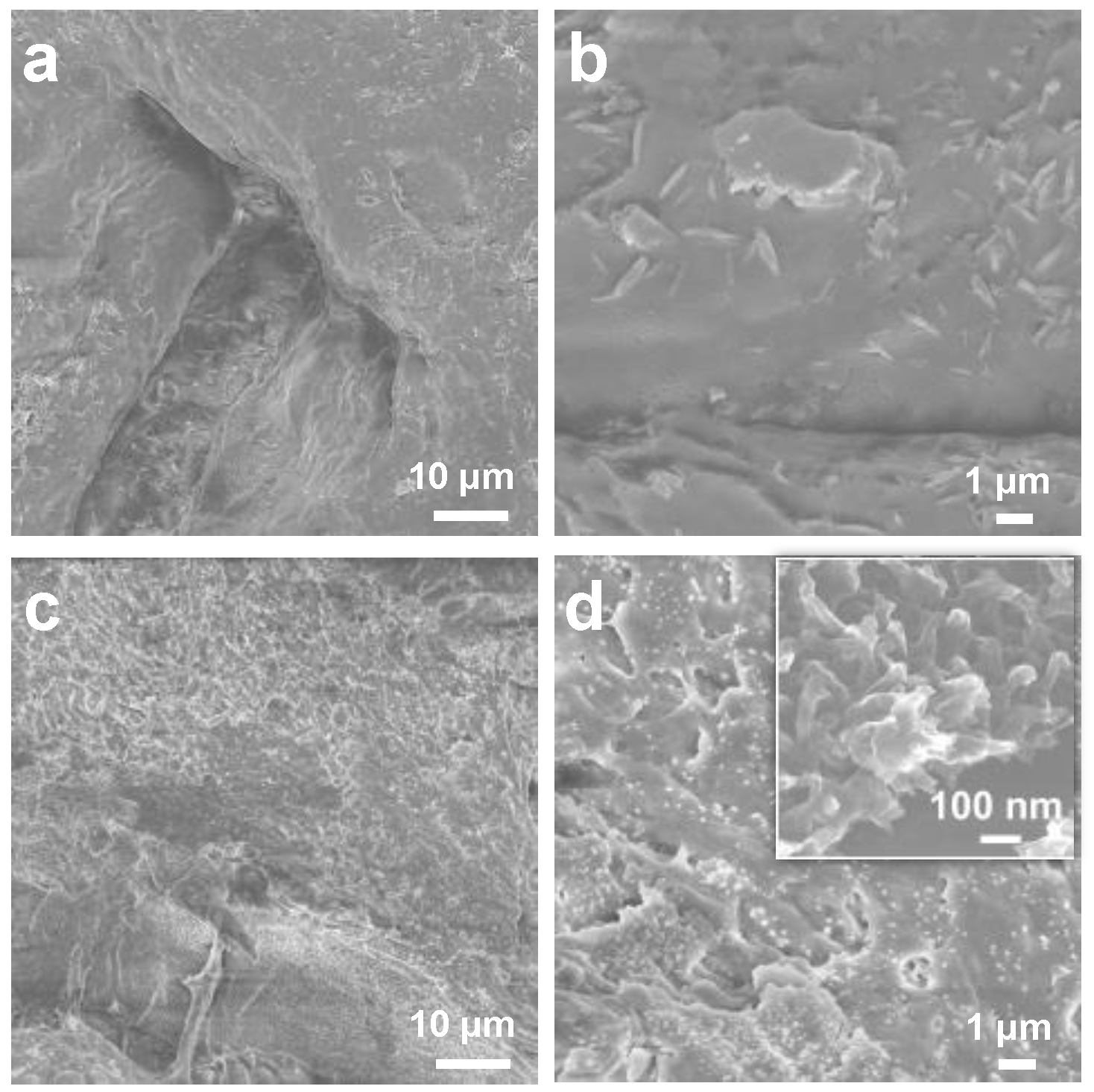
3.1. The Design and Characterization of the COF/κPVA Hydrogel
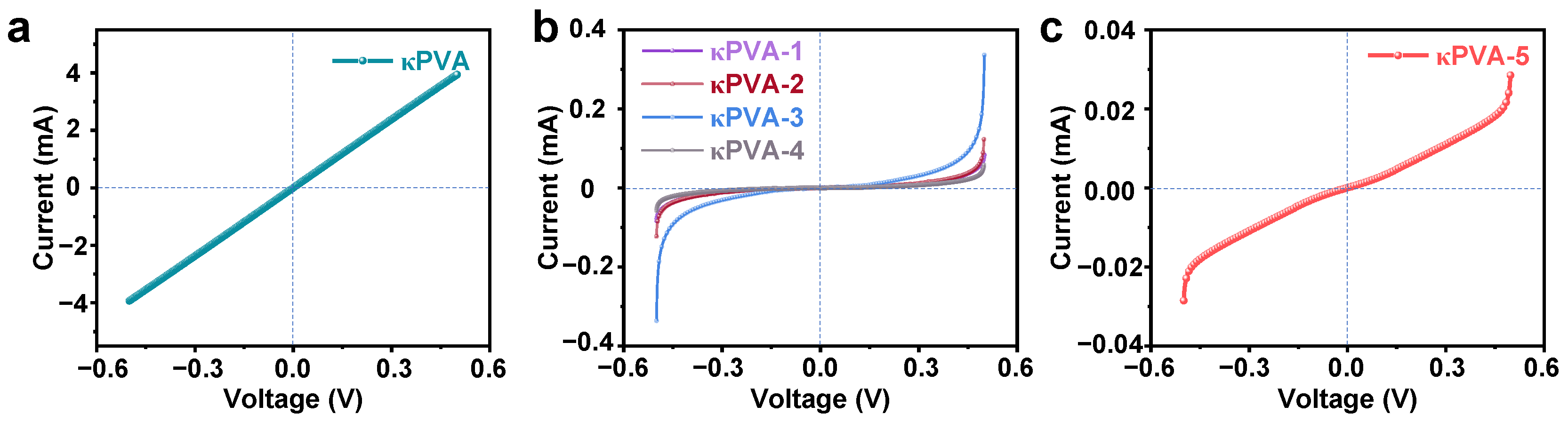
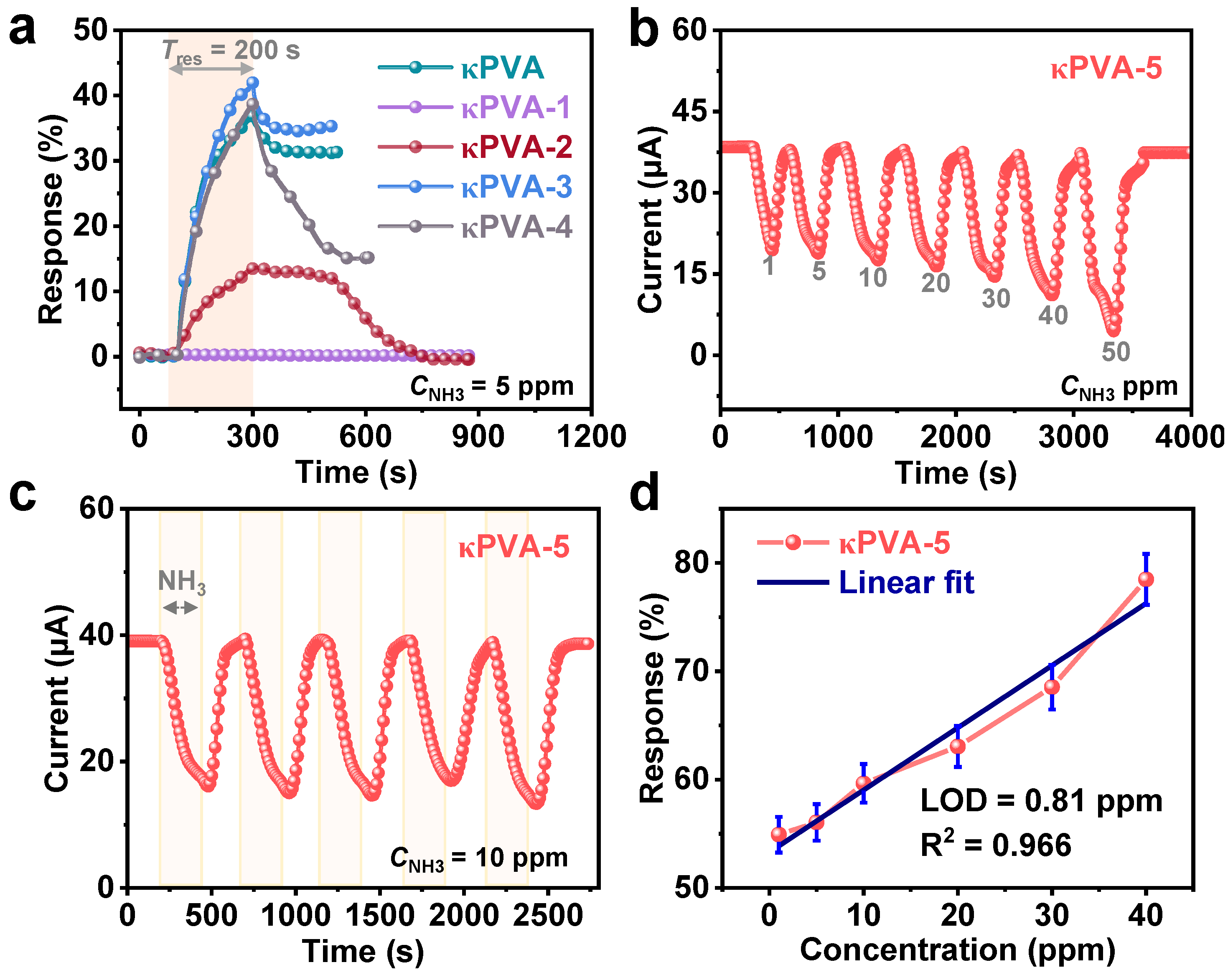
3.2. Gas Sensing Properties of the Hydrogel-Based Devices
3.3. Water Content, Swelling Ratio, and Water Retention Capacity of Hydrogels
3.4. Sensing Mechanism of the Hydrogel-Based Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, W.; Chen, Z.; Song, Z.; Wang, C.; Wan, Z.A.; Chan, C.L.J.; Chen, Z.; Ye, W.; Fan, Z. Microheater integrated nanotube array gas sensor for parts-per-trillion level gas detection and single sensor-based gas discrimination. ACS Nano 2022, 16, 10968–10978. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, J.; Ding, Q.; Wang, H.; Liu, C.; Wu, J. Functionalized hydrogel-based wearable gas and humidity sensors. Nano-Micro Lett. 2023, 15, 136. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, C.; Sun, X.; Huang, W. Current development of materials science and engineering towards epidermal sensors. Prog. Mater. Sci. 2022, 128, 100962. [Google Scholar] [CrossRef]
- Chen, B.; Qian, Z.; Song, G.; Zhuang, Z.; Sun, X.; Ma, S.; Liang, Y.; Ren, L.; Ren, L. Gas-permeable and stretchable on-skin electronics based on a gradient porous elastomer and self-assembled silver nanowires. Chem. Eng. J. 2023, 463, 142350. [Google Scholar] [CrossRef]
- Dutta, T.; Chaturvedi, P.; Llamas-Garro, I.; Velázquez-González, J.S.; Dubey, R.; Mishra, S.K. Smart materials for flexible electronics and devices: Hydrogel. RSC Adv. 2024, 14, 12984–13004. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, F.; Abdiryim, T.; Liu, X. An overview of conductive composite hydrogels for flexible electronic devices. Adv. Compos. Hybrid Mater. 2024, 7, 35. [Google Scholar] [CrossRef]
- Duan, G.; Huang, S.; Feng, Z.; Xie, P.; Zhang, F.; Zhou, Y.; Han, S.-T. Three-terminal artificial olfactory sensors based on emerging materials: Mechanism and application. Adv. Funct. Mater. 2023, 33, 202209969. [Google Scholar] [CrossRef]
- Zaim, O.; Bouchikhi, B.; Motia, S.; Abelló, S.; Llobet, E.; El Bari, N. Discrimination of diabetes mellitus patients and healthy individuals based on volatile organic compounds (VOCs): Analysis of exhaled breath and urine samples by using e-nose and VE-tongue. Chemosensors 2023, 11, 350. [Google Scholar] [CrossRef]
- Xing, X.; Du, L.; Feng, D.; Wang, C.; Tian, Y.; Li, Z.; Liu, H.; Yang, D. Twistable and tailorable V2O5/PANI/GO nanocomposites textile for wearable ammonia sensing. Sens. Actuators B Chem. 2022, 351, 130944. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, W.; Wu, K.; Liu, T. Constructing ultrathin defective Co3O4/MoS2 nanosheets based 2D/2D heterojunction toward room temperature NH3 detection. J. Alloys Compd. 2022, 927, 166962. [Google Scholar] [CrossRef]
- Liu, W.D.; Xiong, Y.; Shen, A.; Wang, X.Z.; Chang, X.; Lu, W.B.; Tian, J. SnS2 nanosheets decorated SnO2 hollow multishelled nanostructures for enhanced sensing of triethylamine gas. Rare Met. 2024, 43, 2339–2348. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Zhang, Z.; Li, J.; Wang, H.; Xu, G.; Wang, X.; Tian, J. Synthesis of ZnO nanosheets @In2O3 hollow micro-rods heterostructures for enhanced ethanol gas sensing performance. Sens. Actuators B Chem. 2024, 404, 135271. [Google Scholar] [CrossRef]
- Nadekar, B.; Khollam, Y.B.; Shaikh, S.F.; Wavhal, D.; Varshney, P.; Pandit, B.; More, P.S. Enhanced ammonia/amines sensitivity at room temperature using plasma polymerized polyvinyl acetate-reduced graphene oxide composite film sensors. Surf. Interfaces 2023, 42, 103453. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Sysoev, V.V.; Glukhova, O.E.; Brzhezinskaya, M.; Stolyarova, D.Y.; Varezhnikov, A.S.; Solomatin, M.A.; Barkov, P.V.; Kirilenko, D.A.; Pavlov, S.I.; et al. Guiding graphene derivatization for the on-chip multisensor arrays: From the synthesis to the theoretical background. Adv. Technol. 2022, 7, 2101250. [Google Scholar] [CrossRef]
- Lee, S.H.; Eom, W.; Shin, H.; Ambade, R.B.; Bang, J.H.; Kim, H.W.; Han, T.H. Room-temperature, highly durable Ti3C2Tx MXene/graphene hybrid fibers for NH3 gas sensing. ACS Appl. Mater. Interfaces 2020, 12, 10434–10442. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chang, J.; Zhi, H.; Li, C.; Feng, L. A PDA functionalized CNT/PANI self-powered sensing system for meat spoilage biomarker NH3 monitoring. Sens. Actuators B Chem. 2022, 356, 131292. [Google Scholar] [CrossRef]
- Ulloa-Gomez, A.M.; Lucas, A.; Koneru, A.; Barui, A.; Stanciu, L. Simultaneous colorimetric and electrochemical detection of trace mercury (Hg2+) using a portable and miniaturized aptasensor. Biosens. Bioelectron. 2023, 221, 114419. [Google Scholar] [CrossRef]
- Yuan, H.; Li, N.; Linghu, J.; Dong, J.; Wang, Y.; Karmakar, A.; Yuan, J.; Li, M.; Buenconsejo, P.J.S.; Liu, G.; et al. Chip-level integration of covalent organic frameworks for trace benzene sensing. ACS Sens. 2020, 5, 1474–1481. [Google Scholar] [CrossRef]
- Zhai, L.; Han, D.; Dong, J.; Jiang, W.; Nie, R.; Yang, X.; Luo, X.; Li, Z. Constructing stable and porous covalent organic frameworks for efficient iodine vapor capture. Macromol. Rapid Commun. 2021, 42, 2100032. [Google Scholar] [CrossRef]
- Chen, X.; Zeng, M.; Yang, J.; Hu, N.; Duan, X.; Cai, W.; Su, Y.; Yang, Z. Two-dimensional bimetallic phthalocyanine covalent-organic-framework-based chemiresistive gas sensor for ppb-level NO2 detection. Nanomaterials 2023, 13, 1660. [Google Scholar] [CrossRef]
- He, Y.; An, N.; Meng, C.; Xiao, L.; Wei, Q.; Zhou, Y.; Yang, Y.; Li, Z.; Hu, Z. COF-based electrodes with vertically supported tentacle array for ultrahigh stability flexible energy storage. ACS Appl. Mater. Interfaces 2022, 14, 57328–57339. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Liu, P.; Qi, Q.; Zhang, F.; Lu, G.; Zhao, X.; Huang, X. Few layer covalent organic frameworks with graphene sheets as cathode materials for lithium-ion batteries. Nanoscale 2019, 11, 5330–5335. [Google Scholar] [CrossRef]
- Shirokura, T.; Hirohata, T.; Sato, K.; Villani, E.; Sekiya, K.; Chien, Y.-A.; Kurioka, T.; Hifumi, R.; Hattori, Y.; Sone, M.; et al. Site-selective synthesis and concurrent immobilization of imine-based covalent organic frameworks on electrodes using an electrogenerated acid. Angew. Chem. Int. Ed. 2023, 62, e202307343. [Google Scholar] [CrossRef]
- Yan, H.; Kou, Z.; Li, S.; Zhang, T. Synthesis of sp2 carbon-conjugated covalent organic framework thin-films via copper-surface-mediated knoevenagel polycondensation. Small 2023, 19, 2207972. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Gannett, C.N.; Carpenter, K.L.; Shen, L.; Abruna, H.D.; Dichtel, W.R. Phenazine-based covalent organic framework cathode materials with high energy and power dsdfsfensities. J. Am. Chem. Soc. 2020, 142, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, S.; Li, W.; Zhang, Z.; Wang, Y.; Yang, Y.; Zhang, H.; Liu, P.; Xie, L.; Ying, Y. Pesticide detection with covalent-organic-framework nanofilms at terahertz band. Biosens. Bioelectron. 2022, 209, 114274. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Li, Z.-J.; Lu, C.; Sun, B.; Zhong, Y.-W.; Wan, L.-J.; Wang, D. Oriented two-dimensional covalent organic framework films for near-infrared electrochromic application. J. Am. Chem. Soc. 2019, 141, 19831–19838. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yin, L.; Cheng, D.; Zhao, S.; Zang, H.-Y.; Zhang, N.; Zhu, G. Surface-mediated construction of an ultrathin free-standing covalent organic framework membrane for efficient proton conduction. Angew. Chem. Int. Ed. 2021, 60, 14875–14880. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Hu, Z.; Rogers, T.K.; Barnes, M.; Tseng, C.-P.; Mei, H.; Sassi, L.M.; Zhang, Z.; Rahman, M.M.; Ajayan, P.M.; et al. Patterning, transfer, and tensile testing of covalent organic framework films with nanoscale thickness. Chem. Mater. 2021, 33, 6724–6730. [Google Scholar] [CrossRef]
- Han, X.; Huang, J.; Yuan, C.; Liu, Y.; Cui, Y. Chiral 3D covalent organic frameworks for high performance liquid chromatographic enantioseparation. J. Am. Chem. Soc. 2018, 140, 892–895. [Google Scholar] [CrossRef]
- Evans, A.M.; Bradshaw, N.P.; Litchfield, B.; Strauss, M.J.; Seckman, B.; Ryder, M.R.; Castano, I.; Gilmore, C.; Gianneschi, N.C.; Mulzer, C.R.; et al. High-sensitivity acoustic molecular sensors based on large-area, spray-coated 2D covalent organic frameworks. Adv. Mater. 2020, 32, 2004205. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Gui, B.; Lin, G.; Fu, Q.; Yin, S.; Liu, X.; Sun, J.; Wang, C. A crystalline three-dimensional covalent organic framework with flexible building blocks. J. Am. Chem. Soc. 2021, 143, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.-H.; Chang, H.; Choi, D.; Kim, S.; Moon, J.; Lim, J.A.; Lee, K.-Y.; Yi, H. Hydrogel-templated transfer-printing of conductive nanonetworks for wearable sensors on topographic flexible substrates. Nano Lett. 2019, 19, 3684–3691. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kong, L.; Mehrez, J.A.-A.; Fan, C.; Quan, W.; Zhang, Y.; Zeng, M.; Yang, J.; Hu, N.; Su, Y.; et al. Outstanding humidity chemiresistors based on imine-linked covalent organic framework films for human respiration monitoring. Nano-Micro Lett. 2023, 15, 149. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Zhao, C.; Sun, B.; Lu, C.; Liu, J.; Liu, M.; Wan, L.-J.; Wang, D. Confined synthesis of two-dimensional covalent organic framework thin films within superspreading water layer. J. Am. Chem. Soc. 2018, 140, 12152–12158. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jia, X.; Liu, R.; Chen, K.; Wang, Z.; Lyu, T.; Cui, X.; Zhao, Y.; Tian, Y. Multifunctional gradient hydrogel with ultrafast thermo-responsive actuation and ultrahigh conductivity. J. Mater. Chem. A 2022, 10, 21874–21883. [Google Scholar] [CrossRef]
- Guo, R.; Han, Y.; Su, C.; Chen, X.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; Wei, H.; Yang, Z. Ultrasensitive room temperature NO2 sensors based on liquid phase exfoliated WSe2 nanosheets. Sens. Actuators B Chem. 2019, 300, 127013. [Google Scholar] [CrossRef]
- Wang, W.; Hu, J.; Zhang, R.; Yan, C.; Cui, L.; Zhu, J. A pH-responsive carboxymethyl cellulose/chitosan hydrogel for adsorption and desorption of anionic and cationic dyes. Cellulose 2021, 28, 897–909. [Google Scholar] [CrossRef]
- Wu, X.; Wang, B.; Yang, Z.; Chen, L. Novel imine-linked covalent organic frameworks: Preparation, characterization and application. J. Mater. Chem. A 2019, 7, 5650–5655. [Google Scholar] [CrossRef]
- Afshari, M.; Dinari, M. Synthesis of new imine-linked covalent organic framework as high efficient absorbent and monitoring the removal of direct fast scarlet 4BS textile dye based on mobile phone colorimetric platform. J. Hazard. Mater. 2020, 385, 121514. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Radinekiyan, F.; Aliabadi, H.A.M.; Sukhtezari, S.; Tahmasebi, B.; Maleki, A.; Madanchi, H. Chitosan hydrogel/silk fibroin/Mg(OH)2 nanobiocomposite as a novel scaffold with antimicrobial activity and improved mechanical properties. Sci. Rep. 2021, 11, 650. [Google Scholar] [CrossRef] [PubMed]
- Zepon, K.M.; Martins, M.M.; Marques, M.S.; Heckler, J.M.; Dal Pont Morisso, F.; Moreira, M.G.; Ziulkoski, A.L.; Kanis, L.A. Smart wound dressing based on κ–carrageenan/locust bean gum/cranberry extract for monitoring bacterial infections. Carbohydr. Polym. 2019, 206, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.L.; Xia, H.R.; Wang, X.Q.; Ling, Z.C.; Xu, J.; Wei, Y.L.; Liu, Y.K.; Han, H. Spectroscopic manifestation for the isotopic substitution in potassium dihydrogen phosphate. J. Alloys Compd. 2007, 430, 226–231. [Google Scholar] [CrossRef]
- Ayyappa, B.; Suvardhan, K.; Rajasekhar, C.; Reddy, P.; Mulpuri, R.K. Recent trends in the electrochemical sensors on β- and calcium channel blockers for hypertension and angina pectoris: A comprehensive review. Microchem. J. 2013, 192, 108930. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, Y.; Feng, X.; Yu, X.; Jin, X.; Qiu, L.; Zhao, G. Theoretical modeling of the hydrated serotonin in solution: Insight into intermolecular hydrogen bonding dynamics and spectral shift in the electronic excited states. J. Mol. Liq. 2019, 288, 111093. [Google Scholar] [CrossRef]
- Li, K.; Yang, X.; Dong, X.; Cao, H.; Zhuang, S.; Gu, X. Easy regulation of chitosan-based hydrogel microstructure with citric acid as an efficient buffer. Carbohydr. Polym. 2023, 300, 120258. [Google Scholar] [CrossRef]
- Kim, S.; Choi, H.C. Light-promoted synthesis of highly-conjugated crystalline covalent organic framework. Commun. Chem. 2019, 2, 60. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Wang, S.; Wang, L.; Wu, M. Neuron-inspired multifunctional conductive hydrogels for flexible wearable sensors. J. Mater. Chem. C 2022, 10, 4327–4335. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, J.; Sun, D.; Ren, W.; Shao, C.; Sun, R. Solvent-induced in-situ self-assembly lignin nanoparticles to reinforce conductive nanocomposite organogels as anti-freezing and anti-dehydration flexible strain sensors. Chem. Eng. J. 2022, 433, 133202. [Google Scholar] [CrossRef]
- Jin, X.; Wei, C.; Wu, C.; Zhang, W. Gastric fluid-induced double network hydrogel with high swelling ratio and long-term mechanical stability. Compos. Part B Eng. 2022, 236, 109816. [Google Scholar] [CrossRef]
- Zhi, H.; Gao, J.; Feng, L. Hydrogel-based gas sensors for NO2 and NH3. ACS Sens. 2020, 5, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhuang, X.; Shi, W.; Yang, X.; Li, L.; Yu, J. Poly(3-hexylthiophene)/polystyrene (P3HT/PS) blends based organic field-effect transistor ammonia gas sensor. Sens. Actuators B Chem. 2016, 225, 10–15. [Google Scholar] [CrossRef]
- Xie, T.; Xie, G.Z.; Su, Y.J.; Du, H.F.; Ye, Z.B.; Jiang, Y.D. Ammonia gas sensors based on poly(3-hexylthiophene)-molybdenum disulfide film transistors. Nanotechnology 2016, 27, 065502. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tao, K.; Chen, D.; Miao, J.; Norford, L.K. 3D sulfonated graphene hydrogel for enhanced chemical sensing. In Proceedings of the 2017 IEEE 30th International Conference on Micro Electro Mechanical Systems, Las Vegas, NV, USA, 22–26 January 2017; pp. 350–353. [Google Scholar] [CrossRef]
- Liu, L.; Fei, T.; Guan, X.; Lin, X.; Zhao, H.; Zhang, T. Room temperature ammonia gas sensor based on ionic conductive biomass hydrogels. Sens. Actuators B Chem. 2020, 320, 128318. [Google Scholar] [CrossRef]
- Wu, J.; Tao, K.; Miao, J.; Norford, L.K. Enhanced gas sensing by 3D water steamed graphene hydrogel. Solid State Electron. 2017, 138, 101–107. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Xing, X.-L.; Liao, Q.-B.; Li, Z.-Q.; Li, C.-Y.; Xi, K.; Wang, K.; Xia, X.-H. Study on ammonia content and distribution in the microenvironment based on covalent organic framework nanochannels. Anal. Chem. 2022, 94, 11224–11229. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Shao, Z.-W.; Zhu, J.-L.; Tao, L.-M.; Ding, Y. Structural evolution of imine-linked covalent organic frameworks and their NH3 sensing performance. J. Mater. Chem. C 2021, 9, 8562–8569. [Google Scholar] [CrossRef]
- Gilli, P.; Bertolasi, V.; Ferretti, V.; Gilli, G. Evidence for resonance-assisted hydrogen bonding. 4. Covalent nature of the strong homonuclear hydrogen bond. Study of the O-H-O system by crystal structure correlation methods. J. Am. Chem. Soc. 1994, 116, 909–915. [Google Scholar] [CrossRef]
- Grabowski, S.J. Hydrogen bonding strength—Measures based on geometric and topological parameters. J. Phys. Org. Chem. 2004, 17, 18–31. [Google Scholar] [CrossRef]
- Tang, S.; Cao, Z. Adsorption and dissociation of ammonia on graphene oxides: A first-principles study. J. Phys. Chem. C 2012, 116, 8778–8791. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zeng, M.; Wang, T.; Ni, W.; Yang, J.; Hu, N.; Zhang, T.; Yang, Z. In Situ Growth of COF/PVA-Carrageenan Hydrogel Using the Impregnation Method for the Purpose of Highly Sensitive Ammonia Detection. Sensors 2024, 24, 4324. https://doi.org/10.3390/s24134324
Chen X, Zeng M, Wang T, Ni W, Yang J, Hu N, Zhang T, Yang Z. In Situ Growth of COF/PVA-Carrageenan Hydrogel Using the Impregnation Method for the Purpose of Highly Sensitive Ammonia Detection. Sensors. 2024; 24(13):4324. https://doi.org/10.3390/s24134324
Chicago/Turabian StyleChen, Xiyu, Min Zeng, Tao Wang, Wangze Ni, Jianhua Yang, Nantao Hu, Tong Zhang, and Zhi Yang. 2024. "In Situ Growth of COF/PVA-Carrageenan Hydrogel Using the Impregnation Method for the Purpose of Highly Sensitive Ammonia Detection" Sensors 24, no. 13: 4324. https://doi.org/10.3390/s24134324
APA StyleChen, X., Zeng, M., Wang, T., Ni, W., Yang, J., Hu, N., Zhang, T., & Yang, Z. (2024). In Situ Growth of COF/PVA-Carrageenan Hydrogel Using the Impregnation Method for the Purpose of Highly Sensitive Ammonia Detection. Sensors, 24(13), 4324. https://doi.org/10.3390/s24134324









