Sensor-Assisted Analysis of Autonomic and Cerebrovascular Dysregulation following Concussion in an Individual with a History of Ten Concussions: A Case Study
Abstract
:1. Introduction
2. Case and Methods
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patricios, J.S.; Schneider, K.J.; Dvorak, J.; Ahmed, O.H.; Blauwet, C.; Cantu, R.C.; Davis, G.A.; Echemendia, R.J.; Makdissi, M.; McNamee, M.; et al. Consensus statement on concussion in sport: The 6th International Conference on Concussion in Sport-Amsterdam, October 2022. Br. J. Sports Med. 2023, 57, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Giza, C.C.; Hovda, D.A. The new neurometabolic cascade of concussion. Neurosurgery 2014, 75 (Suppl. S4), S24–S33. [Google Scholar] [CrossRef] [PubMed]
- Giza, C.C.; Hovda, D.A. The neurometabolic cascade of concussion. J. Athl. Train. 2001, 36, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Kamins, J.; Bigler, E.; Covassin, T.; Henry, L.; Kemp, S.; Leddy, J.J.; Mayer, A.; McCrea, M.; Prins, M.; Schneider, K.J.; et al. What is the physiological time to recovery after concussion? A systematic review. Br. J. Sports Med. 2017, 51, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; Smirl, J.D.; Bryk, K.; Fraser, S.; Jakovac, M.; van Donkelaar, P. Sport-related concussion alters indices of dynamic cerebral autoregulation. Front. Neurol. 2018, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Zemek, R.; Barrowman, N.; Freedman, S.B.; Gravel, J.; Gagnon, I.; McGahern, C.; Aglipay, M.; Sangha, G.; Boutis, K.; Beer, D.; et al. Clinical Risk Score for Persistent Postconcussion Symptoms Among Children with Acute Concussion in the ED. JAMA 2016, 315, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Yeates, K.O.; Räisänen, A.M.; Premji, Z.; Debert, C.T.; Frémont, P.; Hinds, S.; Smirl, J.D.; Barlow, K.; Davis, G.A.; Echemendia, R.J.; et al. What tests and measures accurately diagnose persisting post-concussive symptoms in children, adolescents and adults following sport-related concussion? A systematic review. Br. J. Sports Med. 2023, 57, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Leddy, J.J.; Kozlowski, K.; Fung, M.; Pendergast, D.R.; Willer, B. Regulatory and autoregulatory physiological dysfunction as a primary characteristic of post concussion syndrome: Implications for treatment. NeuroRehabilitation 2007, 22, 199–205. [Google Scholar] [CrossRef]
- Toth, P.; Szarka, N.; Farkas, E.; Ezer, E.; Czeiter, E.; Amrein, K.; Ungvari, Z.; Hartings, J.A.; Buki, A.; Koller, A. Traumatic brain injury-induced autoregulatory dysfunction and spreading depression-related neurovascular uncoupling: Pathomechanisms, perspectives, and therapeutic implications. Am. J. Physiol.-Heart Circ. Physiol. 2016, 311, H1118–H1131. [Google Scholar] [CrossRef]
- Tan, C.O.; Meehan, W.P., 3rd; Iverson, G.L.; Taylor, J.A. Cerebrovascular regulation, exercise, and mild traumatic brain injury. Neurology 2014, 83, 1665–1672. [Google Scholar] [CrossRef]
- Roby, P.R.; Mozel, A.E.; Grady, M.F.; Master, C.L.; Arbogast, K.B. Neurovascular Coupling in Acutely Concussed Adolescent Patients. J. Neurotrauma 2024. [Google Scholar] [CrossRef] [PubMed]
- Forcione, M.; Chiarelli, A.M.; Perpetuini, D.; Davies, D.J.; O’Halloran, P.; Hacker, D.; Merla, A.; Belli, A. Tomographic Task-Related Functional Near-Infrared Spectroscopy in Acute Sport-Related Concussion: An Observational Case Study. Int. J. Mol. Sci. 2020, 21, 6273. [Google Scholar] [CrossRef] [PubMed]
- Pertab, J.L.; Merkley, T.L.; Cramond, A.J.; Cramond, K.; Paxton, H.; Wu, T. Concussion and the autonomic nervous system: An introduction to the field and the results of a systematic review. NeuroRehabilitation 2018, 42, 397–427. [Google Scholar] [CrossRef] [PubMed]
- Abaji, J.P.; Curnier, D.; Moore, R.D.; Ellemberg, D. Persisting Effects of Concussion on Heart Rate Variability during Physical Exertion. J. Neurotrauma 2016, 33, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Gall, B.; Parkhouse, W.; Goodman, D. Heart rate variability of recently concussed athletes at rest and exercise. Med. Sci. Sports Exerc. 2004, 36, 1269–1274. [Google Scholar] [CrossRef]
- Balestrini, C.S.; Moir, M.E.; Abbott, K.C.; Klassen, S.A.; Fischer, L.K.; Fraser, D.D.; Shoemaker, J.K. Autonomic Dysregulation in Adolescent Concussion Is Sex- and Posture-Dependent. Clin. J. Sport. Med. 2019, 31, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J.L.; Yarbrough, M.B.; Perez, J.; Evans, K.; Buckley, T. Sport-related concussion induces transient cardiovascular autonomic dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R575–R584. [Google Scholar] [CrossRef]
- Paniccia, M.; Verweel, L.; Thomas, S.G.; Taha, T.; Keightley, M.; Wilson, K.E.; Reed, N. Heart rate variability following youth concussion: How do autonomic regulation and concussion symptoms differ over time postinjury? BMJ Open Sport. Exerc. Med. 2018, 4, e000355. [Google Scholar] [CrossRef] [PubMed]
- Leddy, J.J.; Haider, M.N.; Ellis, M.J.; Mannix, R.; Darling, S.R.; Freitas, M.S.; Suffoletto, H.N.; Leiter, J.; Cordingley, D.M.; Willer, B. Early Subthreshold Aerobic Exercise for Sport-Related Concussion: A Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 319–325. [Google Scholar] [CrossRef]
- Iaccarino, M.A.; Fitzgerald, M.; Pulli, A.; Woodworth, K.Y.; Spencer, T.J.; Zafonte, R.; Biederman, J. Sport concussion and attention deficit hyperactivity disorder in student athletes: A cohort study. Neurol. Clin. Pract. 2018, 8, 403–411. [Google Scholar] [CrossRef]
- Iverson, G.L.; Gardner, A.J.; Terry, D.P.; Ponsford, J.L.; Sills, A.K.; Broshek, D.K.; Solomon, G.S. Predictors of clinical recovery from concussion: A systematic review. Br. J. Sports Med. 2017, 51, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Dreher, N.; Hannah, T.; Li, A.; Asghar, N.; Spiera, Z.; Marayati, N.F.; Durbin, J.; Gometz, A.; Lovell, M.; et al. Concussion Incidence and Recovery Among Youth Athletes With ADHD Taking Stimulant-Based Therapy. Orthop. J. Sports Med. 2021, 9, 23259671211032564. [Google Scholar] [CrossRef] [PubMed]
- Koerte, I.K.; Schultz, V.; Sydnor, V.J.; Howell, D.R.; Guenette, J.P.; Dennis, E.; Kochsiek, J.; Kaufmann, D.; Sollmann, N.; Mondello, S.; et al. Sex-Related Differences in the Effects of Sports-Related Concussion: A Review. J. Neuroimaging 2020, 30, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Putukian, M.; Purcell, L.; Schneider, K.J.; Black, A.M.; Burma, J.S.; Chandran, A.; Boltz, A.; Master, C.L.; Register-Mihalik, J.K.; Anderson, V.; et al. Clinical recovery from concussion-return to school and sport: A systematic review and meta-analysis. Br. J. Sports Med. 2023, 57, 798–809. [Google Scholar] [CrossRef]
- Putukian, M.; Riegler, K.; Amalfe, S.; Bruce, J.; Echemendia, R. Preinjury and Postinjury Factors That Predict Sports-Related Concussion and Clinical Recovery Time. Clin. J. Sport Med. 2021, 31, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Langer, L.K.; Alavinia, S.M.; Lawrence, D.W.; Munce, S.E.P.; Kam, A.; Tam, A.; Ruttan, L.; Comper, P.; Bayley, M.T. Prediction of risk of prolonged post-concussion symptoms: Derivation and validation of the TRICORDRR (Toronto Rehabilitation Institute Concussion Outcome Determination and Rehab Recommendations) score. PLoS Med. 2021, 18, e1003652. [Google Scholar] [CrossRef]
- Osmond, M.H.; Legace, E.; Gill, P.J.; Correll, R.; Cowan, K.; Dawson, J.E.; Duncan, R.; Fox, E.; Gupta, K.; Kolstad, A.T.; et al. Partnering With Patients, Caregivers, and Clinicians to Determine Research Priorities for Concussion. JAMA Netw. Open 2023, 6, e2316383. [Google Scholar] [CrossRef] [PubMed]
- Mild Traumatic Brain Injury Committee, A. Definition of mild traumatic brain injury. J. Head. Trauma. Rehabil. 1993, 8, 86–87. [Google Scholar]
- Willie, C.K.; Tzeng, Y.C.; Fisher, J.A.; Ainslie, P.N. Integrative regulation of human brain blood flow. J. Physiol. 2014, 592, 841–859. [Google Scholar] [CrossRef]
- Kennedy, C.M.; Burma, J.S.; Newel, K.T.; Brassard, P.; Smirl, J.D. Time-course recovery of cerebral blood velocity metrics post aerobic exercise: A systematic review. J. Appl. Physiol. 2022, 133, 471–489. [Google Scholar] [CrossRef]
- Echemendia, R.J.; Meeuwisse, W.; McCrory, P.; Davis, G.A.; Putukian, M.; Leddy, J.; Makdissi, M.; Sullivan, S.J.; Broglio, S.P.; Raftery, M.; et al. The Sport Concussion Assessment Tool 5th Edition (SCAT5): Background and rationale. Br. J. Sports Med. 2017, 51, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Leddy, J.J.; Hinds, A.L.; Miecznikowski, J.; Darling, S.; Matuszak, J.; Baker, J.G.; Picano, J.; Willer, B. Safety and Prognostic Utility of Provocative Exercise Testing in Acutely Concussed Adolescents: A Randomized Trial. Clin. J. Sport Med. 2018, 28, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Burma, J.S.; Graver, S.; Miutz, L.N.; Macaulay, A.; Copeland, P.V.; Smirl, J.D. The validity and reliability of ultra-short-term heart rate variability parameters and the influence of physiological covariates. J. Appl. Physiol. 2021, 130, 1848–1867. [Google Scholar] [CrossRef]
- Burma, J.S.; Van Roessel, R.K.; Oni, I.K.; Dunn, J.F.; Smirl, J.D. Neurovascular coupling on trial: How the number of trials completed impacts the accuracy and precision of temporally derived neurovascular coupling estimates. J. Cereb. Blood Flow. Metab. 2022, 42, 1478–1492. [Google Scholar] [CrossRef] [PubMed]
- Burma, J.S.; Wassmuth, R.M.; Kennedy, C.M.; Miutz, L.N.; Newel, K.T.; Carere, J.; Smirl, J.D. Does task complexity impact the neurovascular coupling response similarly between males and females? Physiol. Rep. 2021, 9, e15020. [Google Scholar] [CrossRef]
- Smirl, J.D.; Wright, A.D.; Bryk, K.; van Donkelaar, P. Where’s Waldo? The utility of a complicated visual search paradigm for transcranial Doppler-based assessments of neurovascular coupling. J. Neurosci. Methods 2016, 270, 92–101. [Google Scholar] [CrossRef]
- Handford, M.; Little, B. Where’s Waldo? Candlewick Press: Cambridge, MA, USA, 1987; ISBN 9780763695798. [Google Scholar]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Claassen, J.A.; Meel-van den Abeelen, A.S.; Simpson, D.M.; Panerai, R.B. Transfer function analysis of dynamic cerebral autoregulation: A white paper from the International Cerebral Autoregulation Research Network. J. Cereb. Blood Flow. Metab. 2016, 36, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Panerai, R.B.; Brassard, P.; Burma, J.S.; Castro, P.; Claassen, J.A.; van Lieshout, J.J.; Liu, J.; Lucas, S.J.; Minhas, J.S.; Mitsis, G.D.; et al. Transfer function analysis of dynamic cerebral autoregulation: A CARNet white paper 2022 update. J. Cereb. Blood Flow Metab. 2023, 43, 3–25. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar]
- Rajendra Acharya, U.; Paul Joseph, K.; Kannathal, N.; Lim, C.M.; Suri, J.S. Heart rate variability: A review. Med. Biol. Eng. Comput. 2006, 44, 1031–1051. [Google Scholar] [CrossRef] [PubMed]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T., Jr. Heart rate variability: Measurement and clinical utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Skow, R.J.; Brothers, R.M.; Claassen, J.; Day, T.A.; Rickards, C.A.; Smirl, J.D.; Brassard, P. On the use and misuse of cerebral hemodynamics terminology using transcranial Doppler ultrasound: A call for standardization. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H350–H357. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nelson, L.D.; LaRoche, A.A.; Pfaller, A.Y.; Nencka, A.S.; Koch, K.M.; McCrea, M.A. Cerebral Blood Flow Alterations in Acute Sport-Related Concussion. J. Neurotrauma 2016, 33, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, S.; Williams, B.; Murphy, M.; Lyng, S.; Sabo, T.; Bell, K.R. Reduced heart rate variability and lower cerebral blood flow associated with poor cognition during recovery following concussion. Auton. Neurosci. 2019, 220, 102548. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nencka, A.S.; Meier, T.B.; Guskiewicz, K.; Mihalik, J.P.; Alison Brooks, M.; Saykin, A.J.; Koch, K.M.; Wu, Y.C.; Nelson, L.D.; et al. Cerebral blood flow in acute concussion: Preliminary ASL findings from the NCAA-DoD CARE consortium. Brain Imaging Behav. 2019, 13, 1375–1385. [Google Scholar] [CrossRef]
- Churchill, N.W.; Hutchison, M.G.; Richards, D.; Leung, G.; Graham, S.J.; Schweizer, T.A. The first week after concussion: Blood flow, brain function and white matter microstructure. NeuroImage Clin. 2017, 14, 480–489. [Google Scholar] [CrossRef]
- Burma, J.S.; Rattana, S.; Johnson, N.E.; Smirl, J.D. Do mean values tell the full story? Cardiac cycle and biological sex comparisons in temporally derived neurovascular coupling metrics. J. Appl. Physiol. 2023, 134, 426–443. [Google Scholar] [CrossRef]
- Tabor, J.; La, P.; Kline, G.; Wang, M.; Bonfield, S.; Machan, M.; Wynne-Edwards, K.; Emery, C.; Debert, C. Saliva Cortisol as a Biomarker of Injury in Youth Sport-Related Concussion. J. Neurotrauma 2023, 40, 296–308. [Google Scholar] [CrossRef] [PubMed]
- McCorry, L.K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 2007, 71, 78. [Google Scholar] [CrossRef]
- Bishop, S.; Dech, R.; Baker, T.; Butz, M.; Aravinthan, K.; Neary, J.P. Parasympathetic baroreflexes and heart rate variability during acute stage of sport concussion recovery. Brain Inj. 2017, 31, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.A.; Dech, R.T.; Guzik, P.; Neary, J.P. Heart rate variability and implication for sport concussion. Clin. Physiol. Funct. Imaging 2018, 38, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Senthinathan, A.; Mainwaring, L.M.; Hutchison, M. Heart Rate Variability of Athletes Across Concussion Recovery Milestones: A Preliminary Study. Clin. J. Sport. Med. 2017, 27, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Karemaker, J.M. An introduction into autonomic nervous function. Physiol. Meas. 2017, 38, R89–R118. [Google Scholar] [CrossRef]
- Panerai, R.B.; Batterham, A.; Robinson, T.G.; Haunton, V.J. Determinants of cerebral blood flow velocity change during squat-stand maneuvers. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R452–R466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zuckerman, J.H.; Giller, C.A.; Levine, B.D. Transfer function analysis of dynamic cerebral autoregulation in humans. Am. J. Physiol. 1998, 274 Pt 2, H233–H241. [Google Scholar] [CrossRef] [PubMed]
- Patricios, J.S.; Schneider, G.M.; van Ierssel, J.; Purcell, L.K.; Davis, G.A.; Echemendia, R.J.; Fremont, P.; Fuller, G.W.; Herring, S.A.; Harmon, K.G.; et al. Beyond acute concussion assessment to office management: A systematic review informing the development of a Sport Concussion Office Assessment Tool (SCOAT6) for adults and children. Br. J. Sports Med. 2023, 57, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Patricios, J.S.; Davis, G.A.; Ahmed, O.H.; Blauwet, C.; Schneider, G.M.; Purcell, L.K.; Echemendia, R.J.; Fremont, P.; Fuller, G.W.; Herring, S.A.; et al. Introducing the Sport Concussion Office Assessment Tool 6 (SCOAT6). Br. J. Sports Med. 2023, 57, 648–650. [Google Scholar] [CrossRef] [PubMed]
- Danzer, S.C. Valproic Acid Leads New Neurons Down the Wrong Path. Epilepsy Curr. 2019, 19, 132–133. [Google Scholar] [CrossRef]
- Lewis, C.A.; Mueller, K.; Zsido, R.G.; Reinelt, J.; Regenthal, R.; Okon-Singer, H.; Forbes, E.E.; Villringer, A.; Sacher, J. A single dose of escitalopram blunts the neural response in the thalamus and caudate during monetary loss. J. Psychiatry Neurosci. 2021, 46, E319–E327. [Google Scholar] [CrossRef]
- Cook, N.E.; Sapigao, R.G.; Silverberg, N.D.; Maxwell, B.A.; Zafonte, R.; Berkner, P.D.; Iverson, G.L. Attention-Deficit/Hyperactivity Disorder Mimics the Post-concussion Syndrome in Adolescents. Front. Pediatr. 2020, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Iverson, G.L.; Silverberg, N.D.; Mannix, R.; Maxwell, B.A.; Atkins, J.E.; Zafonte, R.; Berkner, P.D. Factors Associated With Concussion-like Symptom Reporting in High School Athletes. JAMA Pediatr. 2015, 169, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Register-Mihalik, J.K.; DeFreese, J.D.; Callahan, C.E.; Carneiro, K. Utilizing the Biopsychosocial Model in Concussion Treatment: Post-Traumatic Headache and beyond. Curr. Pain. Headache Rep. 2020, 24, 44. [Google Scholar] [CrossRef] [PubMed]
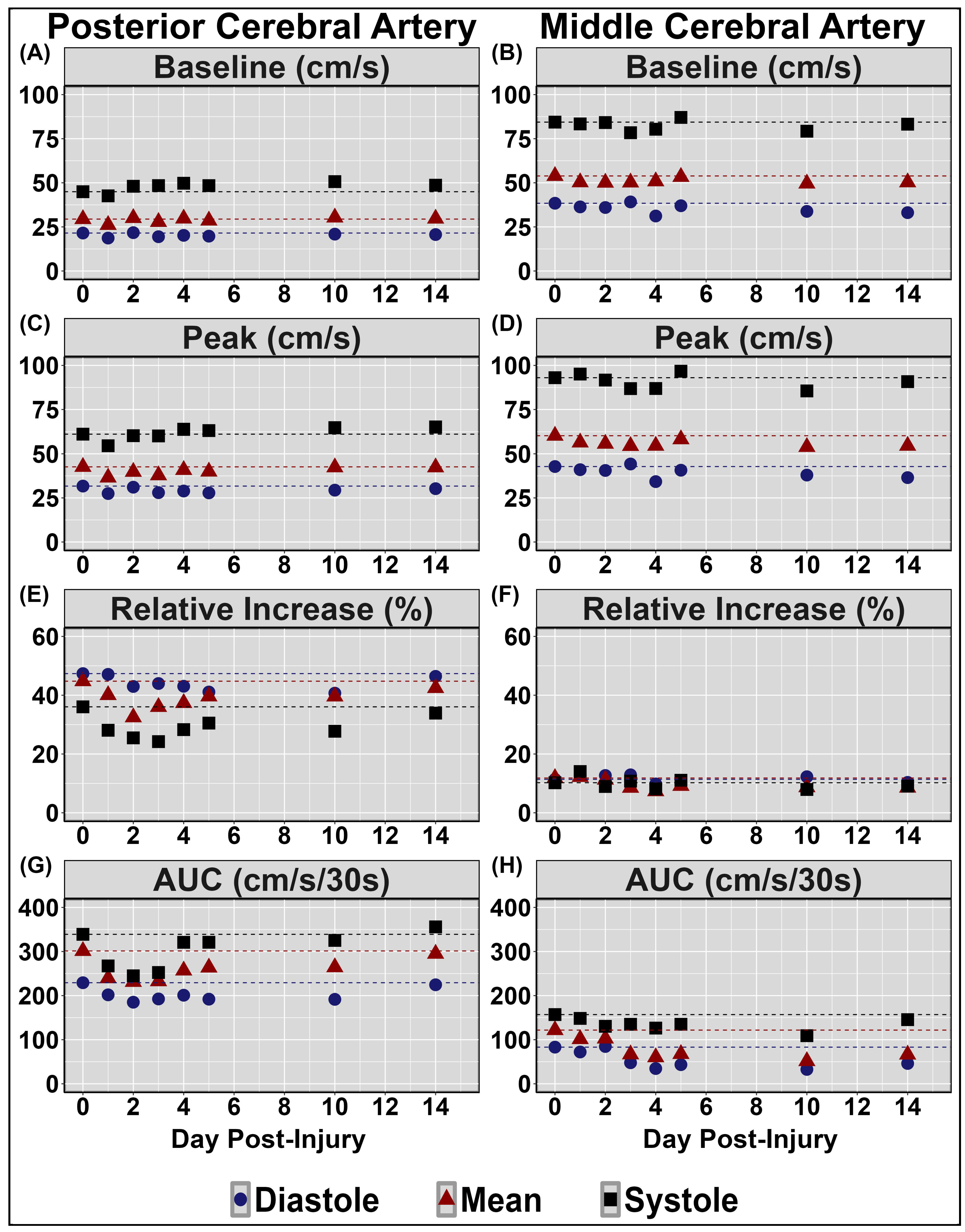
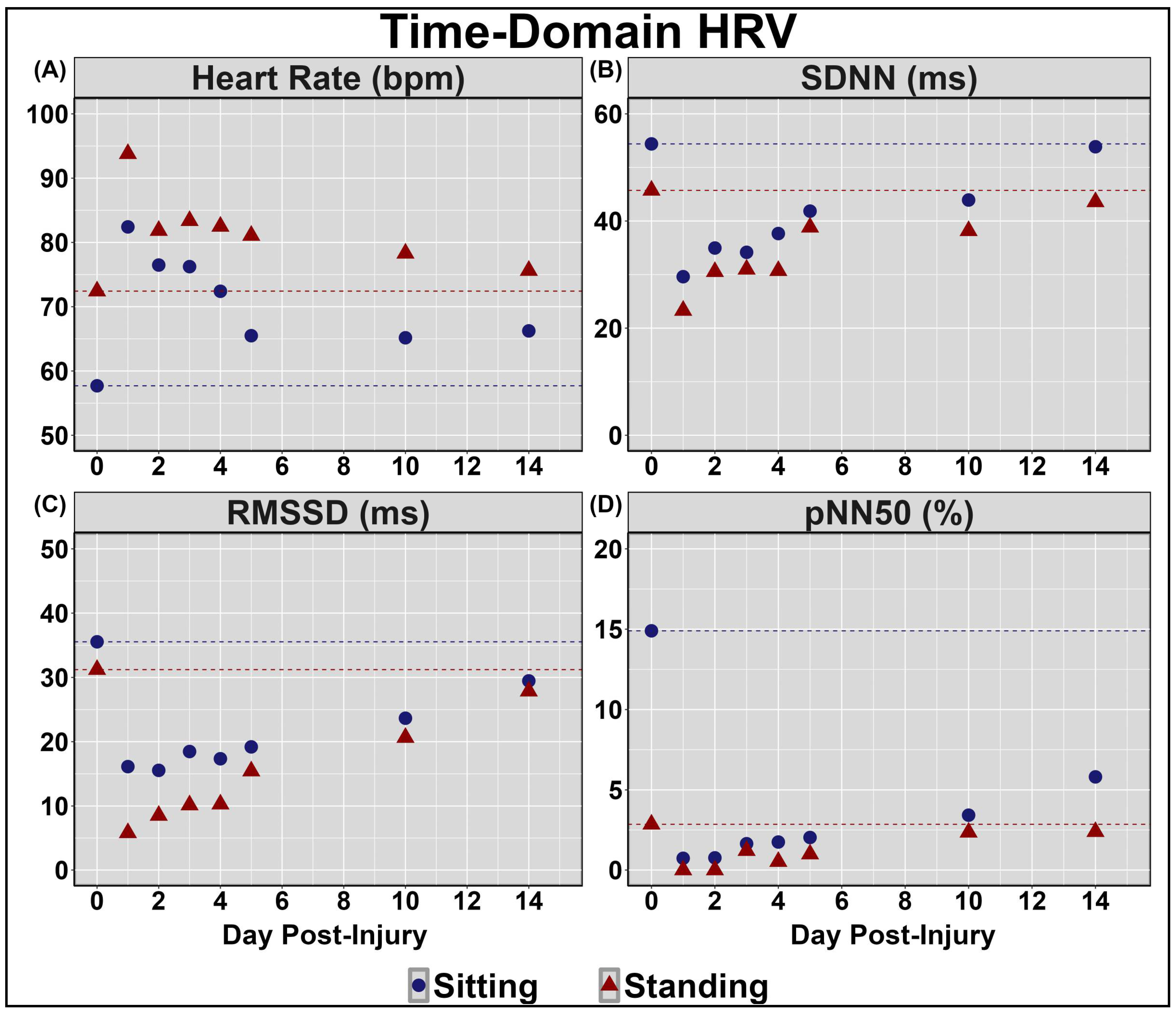


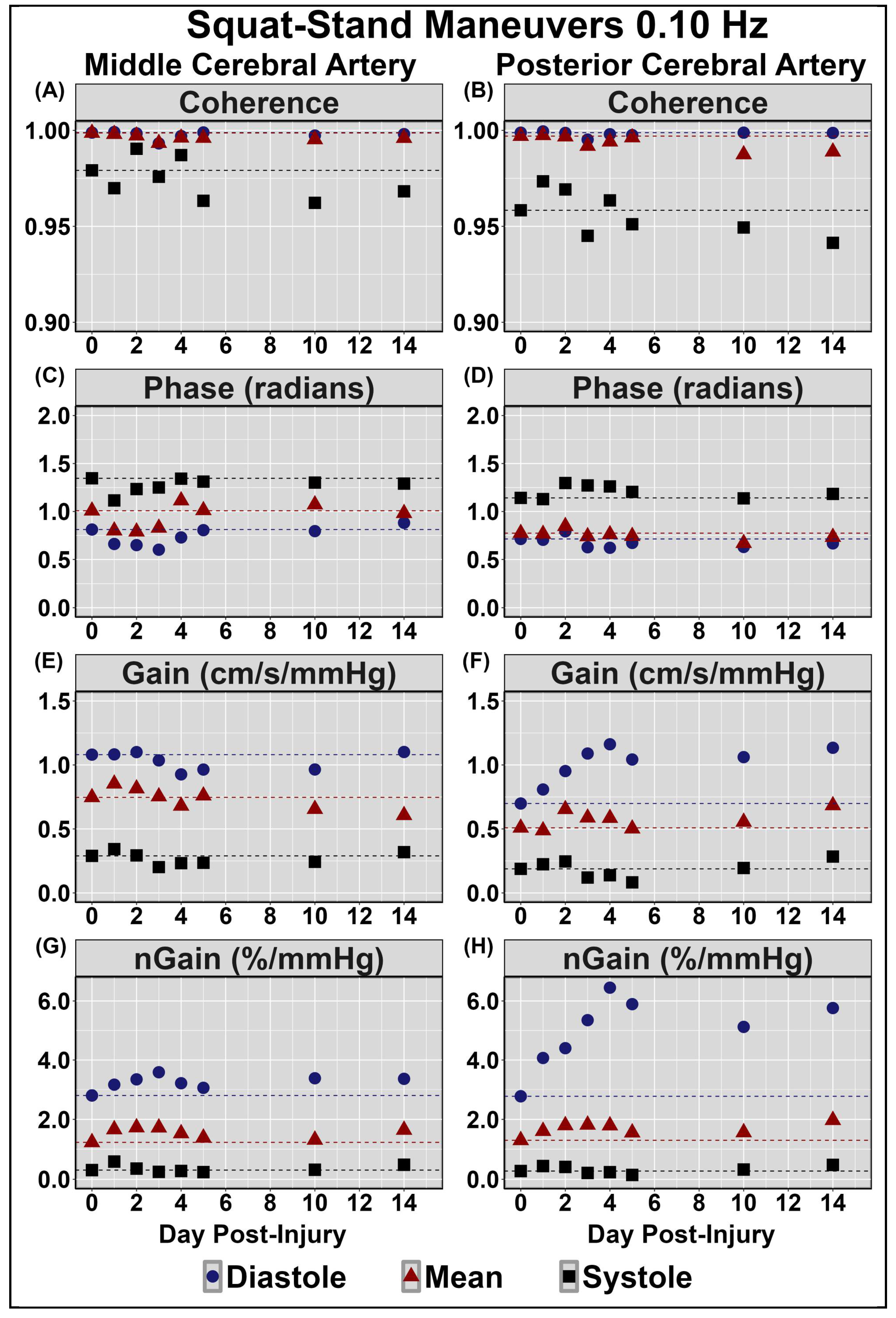
| Concussion Number | Date of Injury | Age at Injury | Mechanism of Injury | Duration of Recovery |
|---|---|---|---|---|
| 1 | January 1996 | 7 months | Fall—downstairs | Unknown |
| 2 | August 2008 | 13 years | Fall—longboard | Two days |
| 3 | November 2011 | 16 years | SRC—basketball | Three days |
| 4 | January 2012 | 16 years | SRC—basketball | Two days |
| 5 | September 2013 | 18 years | SRC—basketball | One month |
| 6 | October 2013 | 18 years | SRC—basketball | Seven months |
| 7 | June 2014 | 19 years | MVC—whiplash | Two months |
| 8 | September 2016 | 21 years | Struck to head—weightlifting | One month |
| 9 | March 2018 | 23 years | SRC—basketball | Two days |
| 10 | August 2019 | 24 years | SRC—wakeboarding | One month |
| Baseline | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 10 | Day 14 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||
| Headaches | 1 | 4 | 5 | 3 | 3 | 3 | 4 | 2 | 3 | 2 | 3 | 1 | 1 | 1 | 1 |
| Pressure in Head | 1 | 4 | 5 | 3 | 3 | 3 | 4 | 2 | 3 | 2 | 3 | 1 | 1 | 1 | 1 |
| Neck Pain | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nausea or Vomiting | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dizziness | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blurred or Double Vision | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Balance Problems | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sensitivity to Light | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 |
| Sensitivity to Noise | 0 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 |
| Feeling Slowed Down | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 1 | 0 |
| Feeling “in a Fog” | 0 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Don’t Feel Right | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Difficulty Concentrating | 0 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Difficulty Remembering | 0 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatigue or Low Energy | 1 | 2 | 1 | 1 | 2 | 3 | 3 | 2 | 1 | 2 | 2 | 1 | 1 | 0 | 1 |
| Confusion | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Drowsiness | 0 | 2 | 2 | 0 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 |
| More Emotional | 1 | 3 | 2 | 2 | 2 | 3 | 2 | 3 | 3 | 3 | 1 | 1 | 1 | 0 | 0 |
| Irritability | 0 | 3 | 4 | 3 | 2 | 3 | 2 | 3 | 3 | 3 | 2 | 1 | 1 | 0 | 0 |
| Sadness | 1 | 2 | 1 | 2 | 2 | 3 | 2 | 2 | 3 | 3 | 1 | 1 | 0 | 0 | 0 |
| Nervous or Anxious | 0 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 |
| Trouble Falling Asleep | 0 | 0 | 0 | 2 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 1 |
| Total Symptom Score | 7 | 14 | 13 | 16 | 17 | 17 | 16 | 16 | 13 | 15 | 14 | 9 | 7 | 7 | 5 |
| Symptom Severity Score | 7 | 31 | 30 | 27 | 26 | 34 | 31 | 24 | 24 | 27 | 21 | 8 | 7 | 7 | 5 |
| Overall Condition | 0 | 4 | 7 | 4 | 7 | 5 | 8 | 4 | 7 | 3 | 5 | 2 | 2 | 1 | 1 |
| Post 30 Overall Condition | 5 | 5 | 7 | 2 | 2 | 1 | 1 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kennedy, C.M.; Burma, J.S.; Smirl, J.D. Sensor-Assisted Analysis of Autonomic and Cerebrovascular Dysregulation following Concussion in an Individual with a History of Ten Concussions: A Case Study. Sensors 2024, 24, 4404. https://doi.org/10.3390/s24134404
Kennedy CM, Burma JS, Smirl JD. Sensor-Assisted Analysis of Autonomic and Cerebrovascular Dysregulation following Concussion in an Individual with a History of Ten Concussions: A Case Study. Sensors. 2024; 24(13):4404. https://doi.org/10.3390/s24134404
Chicago/Turabian StyleKennedy, Courtney M., Joel S. Burma, and Jonathan D. Smirl. 2024. "Sensor-Assisted Analysis of Autonomic and Cerebrovascular Dysregulation following Concussion in an Individual with a History of Ten Concussions: A Case Study" Sensors 24, no. 13: 4404. https://doi.org/10.3390/s24134404





