Isothermal Amplification Using Temperature-Controlled Frequency Mixing Magnetic Detection-Based Portable Field-Testing Platform
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Frequency Mixing Magnetic Detection
2.3. Pulse Width Modulation Controller
2.4. Recombinase Polymerase Amplification (RPA)
2.5. Lumped Parameter Model
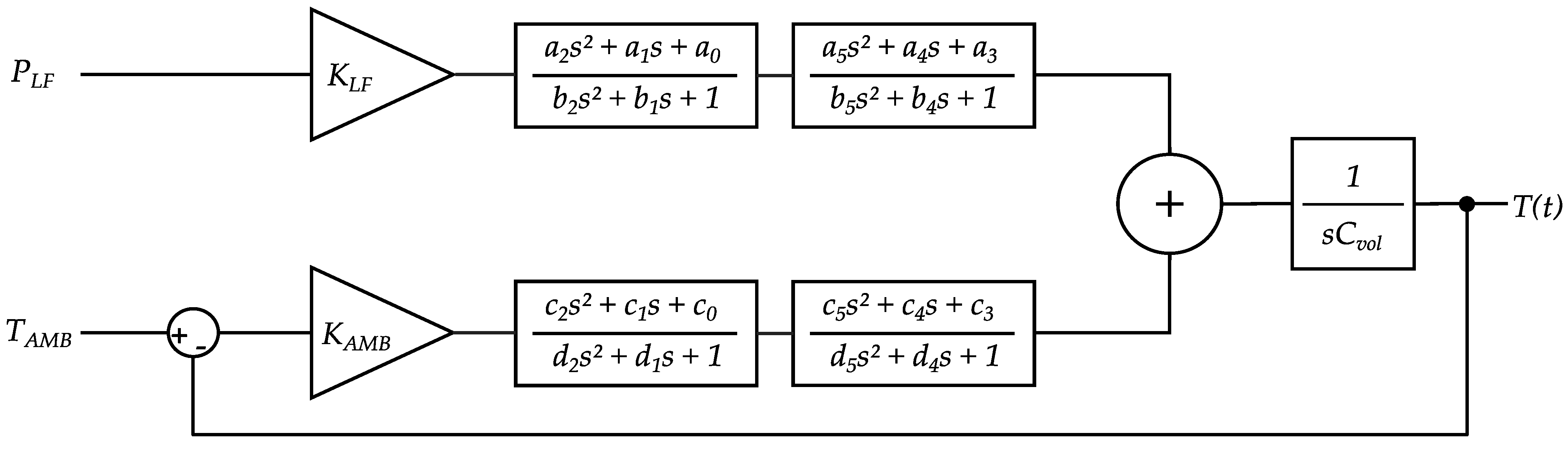
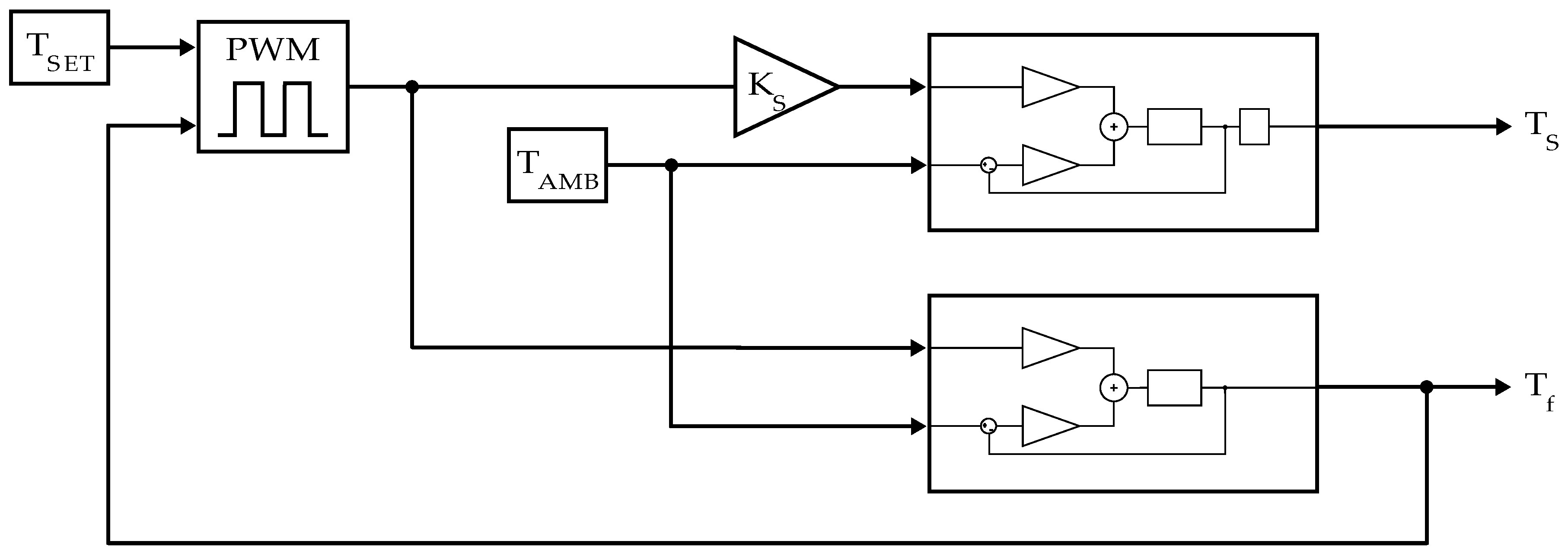
2.5.1. Model Structure Design
- The LF power contributes significantly more to MH heating than the HF-coil power. This assumption is based on the 350-fold difference in power dissipation described in Section 2.2.
- The ambient temperature influences the heating and cooling processes of the MH (heat dissipation is influenced by the temperature difference between the system under test and its surroundings—Newton’s law of cooling, Stefan–Boltzmann law).
- From 1 and 2, it follows that the model has two inputs (ambient temperature and average LF power).
- Heat transfer is a non-integer order process (hence, Padé approximations in the heating and cooling lanes).
- The MH can store a certain amount of heat energy (it has a heat capacity, respectively) denoted by .
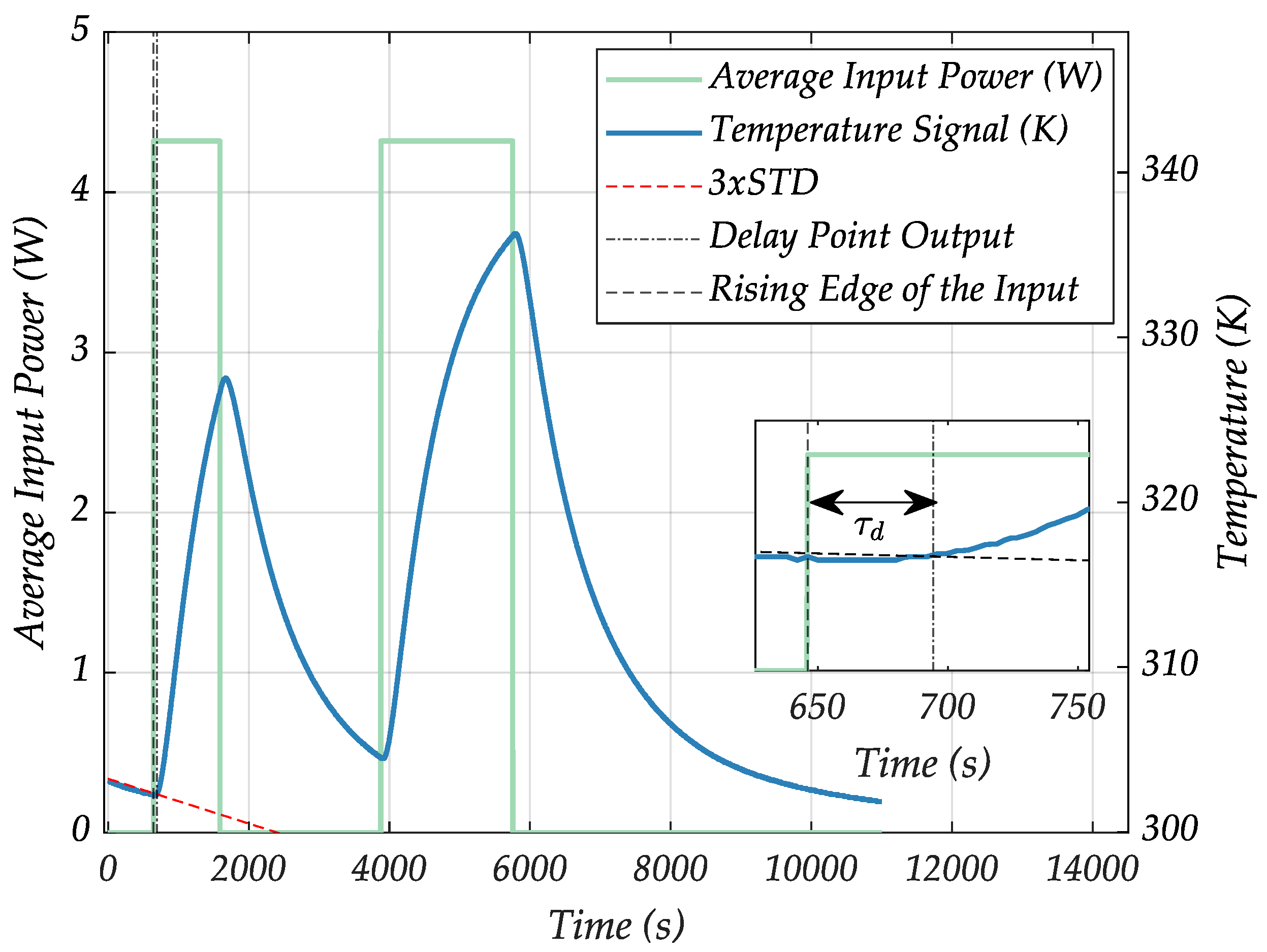
- At the sample position, the system obeys a transport delay term that describes the time the heat needs to travel from the sensor to the sample position.
2.5.2. Model Parameter Estimation (Model Parametrization)
2.6. Model Performance Metrics
3. Results and Discussion
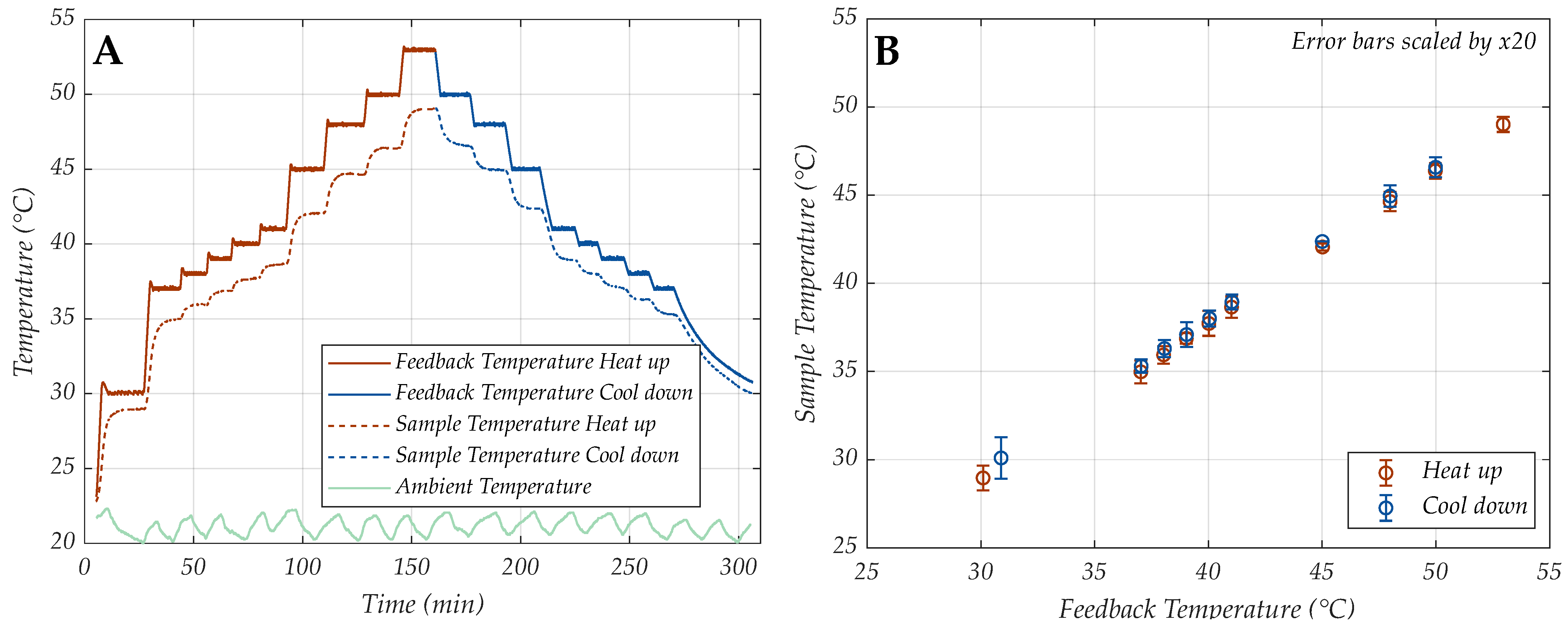
3.1. Controller Performance
- As the temperature difference between the set controller value and the ambient increases, the stability error tends to increase.
- Higher controlled temperatures generally yield larger stability errors.
- For all investigated constant ambient conditions, a stability error of maximally 0.3% was observed.
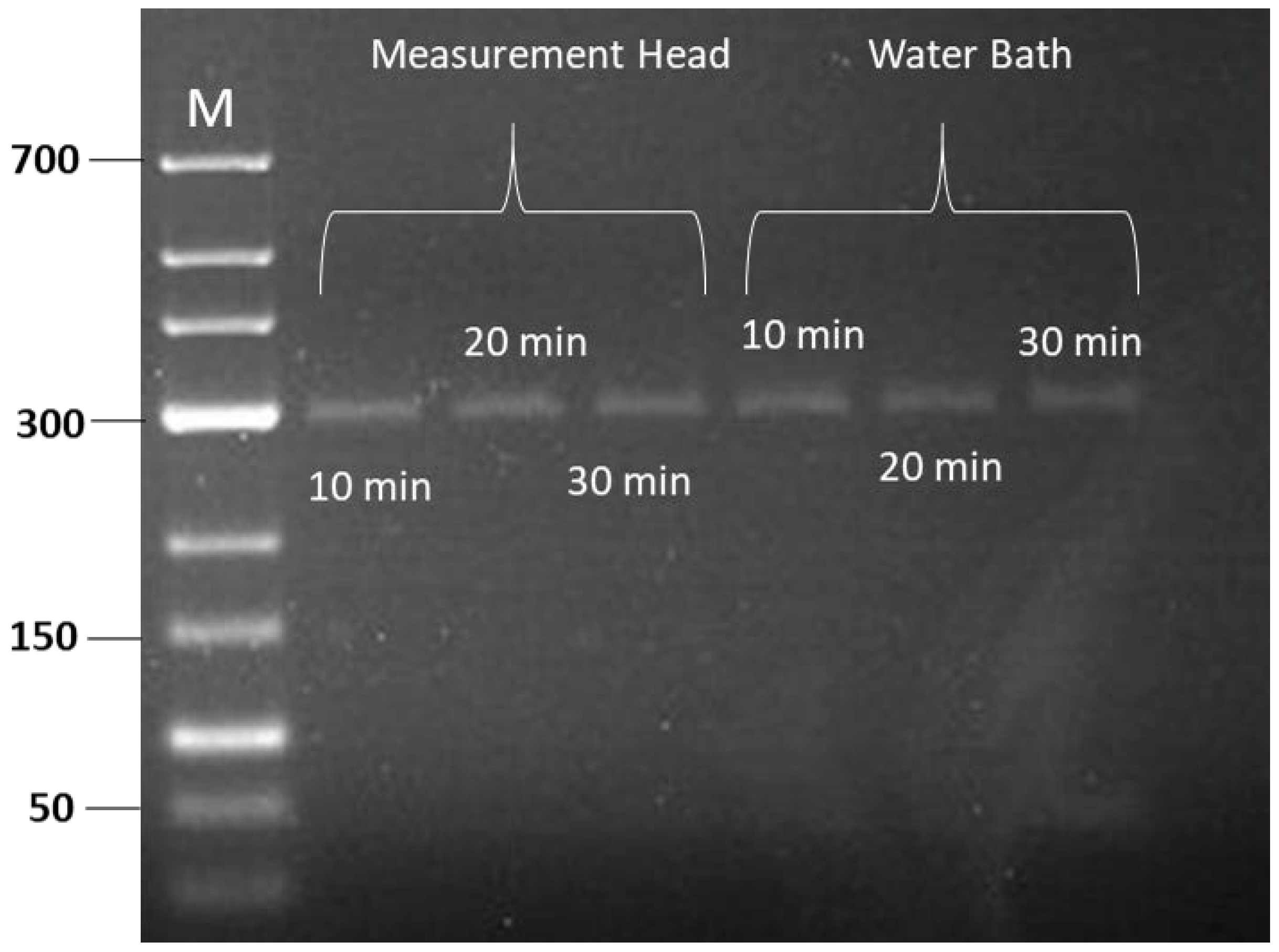
3.2. RPA Amplification
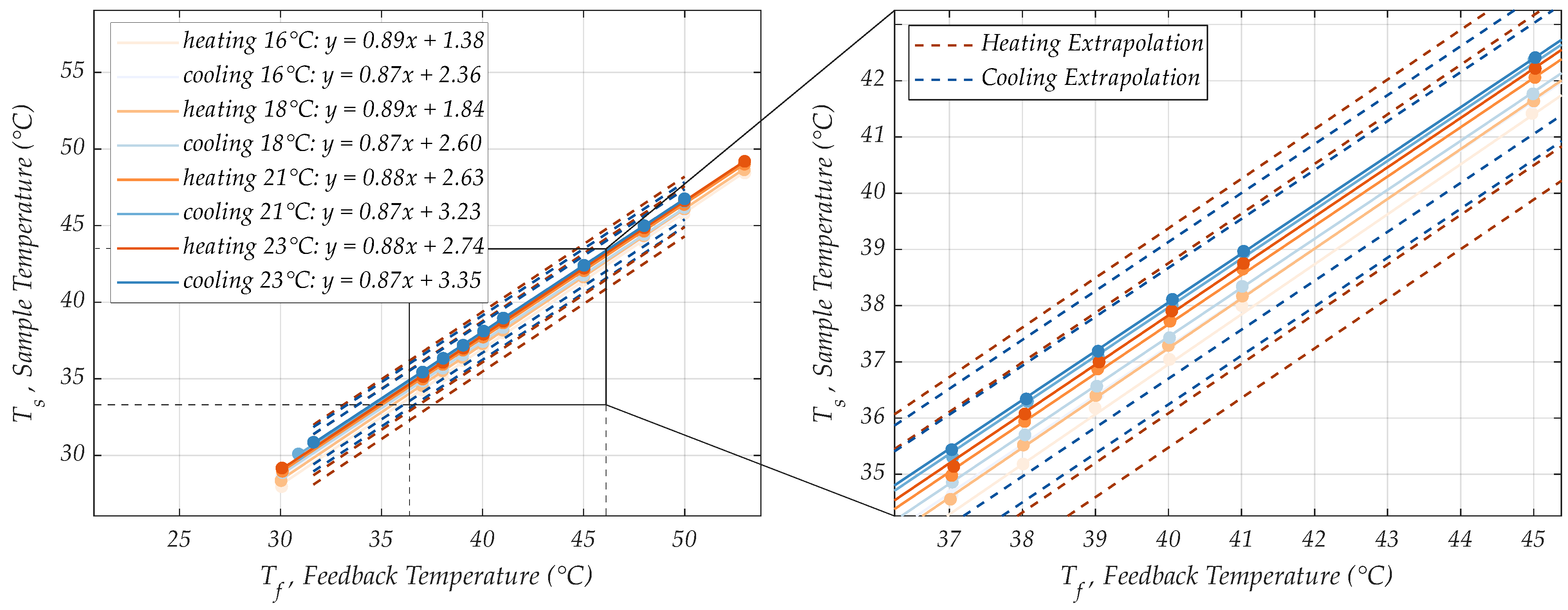
3.3. Linear Extrapolation Model
- The controlled temperature at the sample position cannot exceed 42 °C in any case, as amplification components may denature.
- The PWM controller reached a temperature stability error of less than 0.3% at the sample position. With the successfully executed RPA presented in this section, this stability is exceedingly sufficient.
- A maximum control error of ±1 °C was stipulated at the sample position, as the RPA worked well at 37 °C and 38 °C.
3.4. Thermal Lumped Parameter Model
- It was reported that heat conduction can be more accurately described by a non-integer order process [29]. So, we attempted integer-order polynomial approximations (Padé approximations) to model the non-integer order portion of the problem and enhance the accuracy of the model output compared to measured data. The higher the order of approximation, the more accurately the model output reflects the measured data. In one of the present cases, for example, a fourth order Padé approximation improved model accuracy in terms of R2 value by ~13% over a second order Padé approximation. This improvement diminishes strongly with higher approximation orders. The trade-off involves that a higher number of parameters in the model is needed, and the model requires higher computational parameter estimation effort and therefore more time [30]. Exemplary second order Padé approximations follow the form:
- 2.
- In order to extend the described model structure to be usable as a sample position model instead of determining solely the LF-coil temperature, a transport delay term was multiplied with the system, using a delay term block in MATLAB Simulink. Typically, transport delays occur as nonlinear elements in a first instance. However, the sample position temperature model is consequentially time delayed to the LF-coil temperature model, and hence is nonlinear; both models remain linear relative to the and inputs. The scaled transport delayed temperature output at the sample position can therefore be considered as an output on the LF-coil surface. Linearization of the delay time, which could classically be done by Padé approximations as well, can be omitted for now, which reduces the number of parameters in the model and makes simulations faster.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Liu, K.; Li, Z.; Wang, P. Point of Care Testing for Infectious Diseases. Clin. Chim. Acta 2019, 493, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nehra, M.; Mehta, J.; Dilbaghi, N.; Marrazza, G.; Kaushik, A. Point-of-Care Strategies for Detection of Waterborne Pathogens. Sensors 2019, 19, 4476. [Google Scholar] [CrossRef] [PubMed]
- Niemz, A.; Ferguson, T.M.; Boyle, D.S. Point-of-Care Nucleic Acid Testing for Infectious Diseases. Trends Biotechnol. 2011, 29, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Prasad, D. Isothermal Nucleic Acid Amplification and Its Uses in Modern Diagnostic Technologies. 3 Biotech 2023, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Krause, H.-J.; Wolters, N.; Zhang, Y.; Offenhäusser, A.; Miethe, P.; Meyer, M.H.F.; Hartmann, M.; Keusgen, M. Magnetic Particle Detection by Frequency Mixing for Immunoassay Applications. J. Magn. Magn. Mater. 2007, 311, 436–444. [Google Scholar] [CrossRef]
- Krishna, V.D.; Wu, K.; Su, D.; Cheeran, M.C.J.; Wang, J.-P.; Perez, A. Nanotechnology: Review of Concepts and Potential Application of Sensing Platforms in Food Safety. Food Microbiol. 2018, 75, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Yari, P.; Rezaei, B.; Dey, C.; Chugh, V.K.; Veerla, N.V.R.K.; Wang, J.-P.; Wu, K. Magnetic Particle Spectroscopy for Point-of-Care: A Review on Recent Advances. Sensors 2023, 23, 4411. [Google Scholar] [CrossRef] [PubMed]
- Abuawad, A.; Ashhab, Y.; Offenhäusser, A.; Krause, H.-J. DNA Sensor for the Detection of Brucella Spp. Based on Magnetic Nanoparticle Markers. Int. J. Mol. Sci. 2023, 24, 17272. [Google Scholar] [CrossRef] [PubMed]
- Bikulov, T.; Offenhäusser, A.; Krause, H.-J. Passive Mixer Model for Multi-Contrast Magnetic Particle Spectroscopy. Int. J. Magn. Part. Imaging IJMPI 2023, 9, 2303087. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Saha, R.; Liu, J.; Chugh, V.K.; Wang, J.-P. Magnetic Particle Spectroscopy: A Short Review of Applications Using Magnetic Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 4972–4989. [Google Scholar] [CrossRef]
- Wu, D.Y.; Ugozzoli, L.; Pal, B.K.; Qian, J.; Wallace, R.B. The Effect of Temperature and Oligonucleotide Primer Length on the Specificity and Efficiency of Amplification by the Polymerase Chain Reaction. DNA Cell Biol. 1991, 10, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Milbury, C.A.; Li, J.; Liu, P.; Makrigiorgos, G.M. COLD-PCR: Improving the Sensitivity of Molecular Diagnostics Assays. Expert Rev. Mol. Diagn. 2011, 11, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Quan, P.-L.; Sauzade, M.; Brouzes, E. dPCR: A Technology Review. Sensors 2018, 18, 1271. [Google Scholar] [CrossRef] [PubMed]
- Pohl, G.; Shih, I.-M. Principle and Applications of Digital PCR. Expert Rev. Mol. Diagn. 2004, 4, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Artika, I.M.; Dewi, Y.P.; Nainggolan, I.M.; Siregar, J.E.; Antonjaya, U. Real-Time Polymerase Chain Reaction: Current Techniques, Applications, and Role in COVID-19 Diagnosis. Genes 2022, 13, 2387. [Google Scholar] [CrossRef] [PubMed]
- García-Bernalt Diego, J.; Fernández-Soto, P.; Muro, A. The Future of Point-of-Care Nucleic Acid Amplification Diagnostics after COVID-19: Time to Walk the Walk. Int. J. Mol. Sci. 2022, 23, 14110. [Google Scholar] [CrossRef] [PubMed]
- De Felice, M.; De Falco, M.; Zappi, D.; Antonacci, A.; Scognamiglio, V. Isothermal Amplification-Assisted Diagnostics for COVID-19. Biosens. Bioelectron. 2022, 205, 114101. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.B.; Veigas, B.; Baptista, P.V. Isothermal Amplification of Nucleic Acids: The Race for the Next “Gold Standard”. Front. Sens. 2021, 2, 752600. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, F.; Li, Q.; Wang, L.; Fan, C. Isothermal Amplification of Nucleic Acids. Chem. Rev. 2015, 115, 12491–12545. [Google Scholar] [CrossRef] [PubMed]
- Lobato, I.M.; O’Sullivan, C.K. Recombinase Polymerase Amplification: Basics, Applications and Recent Advances. TrAC 2018, 98, 19–35. [Google Scholar] [CrossRef]
- Zanoli, L.M.; Spoto, G. Isothermal Amplification Methods for the Detection of Nucleic Acids in Microfluidic Devices. Biosensors 2012, 3, 18–43. [Google Scholar] [CrossRef]
- Curtis, K.A.; Rudolph, D.L.; Nejad, I.; Singleton, J.; Beddoe, A.; Weigl, B.; LaBarre, P.; Owen, S.M. Isothermal Amplification Using a Chemical Heating Device for Point-of-Care Detection of HIV-1. PLoS ONE 2012, 7, e31432. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chen, C.-C.; Wei, S.-C.; Lu, H.-H.; Liang, Y.-H.; Lin, C.-W. Diagnostic Devices for Isothermal Nucleic Acid Amplification. Sensors 2012, 12, 8319–8337. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, S.; Toldrà, A.; Campàs, M. Biosensors Based on Isothermal DNA Amplification for Bacterial Detection in Food Safety and Environmental Monitoring. Sensors 2021, 21, 602. [Google Scholar] [CrossRef]
- Achtsnicht, S.; Pourshahidi, A.M.; Offenhäusser, A.; Krause, H.-J. Multiplex Detection of Different Magnetic Beads Using Frequency Scanning in Magnetic Frequency Mixing Technique. Sensors 2019, 19, 2599. [Google Scholar] [CrossRef] [PubMed]
- Wahed, A.A.E.; Patel, P.; Faye, O.; Thaloengsok, S.; Heidenreich, D.; Matangkasombut, P.; Manopwisedjaroen, K.; Sakuntabhai, A.; Sall, A.A.; Hufert, F.T.; et al. Recombinase Polymerase Amplification Assay for Rapid Diagnostics of Dengue Infection. PLoS ONE 2015, 10, e0129682. [Google Scholar] [CrossRef]
- Larrea-Sarmiento, A.; Stack, J.P.; Alvarez, A.M.; Arif, M. Multiplex Recombinase Polymerase Amplification Assay Developed Using Unique Genomic Regions for Rapid On-Site Detection of Genus Clavibacter and C. Nebraskensis. Sci. Rep. 2021, 11, 12017. [Google Scholar] [CrossRef]
- James, A.; Macdonald, J. Recombinase Polymerase Amplification: Emergence as a Critical Molecular Technology for Rapid, Low-Resource Diagnostics. Expert Rev. Mol. Diagn. 2015, 15, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Krzysztof, O. Wojciech Mitkowski Accuracy Analysis for Fractional Order Transfer Function Models with Delay. In Theory and Applications of Non-Integer Order Systems; Lecture Notes in Electrical Engineering (LNEE); Springer International Publishing: New York, NY, USA, 2017; Volume 407. [Google Scholar]
- Deniz, F.N.; Alagoz, B.B.; Tan, N.; Koseoglu, M. Revisiting Four Approximation Methods for Fractional Order Transfer Function Implementations: Stability Preservation, Time and Frequency Response Matching Analyses. Annu. Rev. Control 2020, 49, 239–257. [Google Scholar] [CrossRef]





| Model Parameters | LPM LF-Coil | LPM Sample Pos. |
|---|---|---|
| 0.302 | 0.317 | |
| 0.052 | 0.046 | |
| 36.266 | 45.572 | |
| [1.103, 1.279, 0.001, 1.145, 1.139, 1.000] | [1.142, 1.305, 0.001, 1.048, 1.165, 1.000] | |
| [0.737, 1.011, 0.877, 0.980] | [0.730, 1.011, 0.870, 0.980] | |
| [0.892, 0.999, 0.999, 0.839, 0.996, 1.018] | [0.806, 0.998, 0.999, 0.864, 0.995, 1.018] | |
| [1.007, 1.007, 0.987, 0.997] | [1.010, 1.007, 0.988, 0.997] |
| Metrics | Identification/ Validation | Identification/ Validation |
|---|---|---|
| NRMSE | 0.049/0.075 | 0.047/0.066 |
| 96.91%/96.04% | 95.28%/93.45% | |
| 1.523/1.600 | 1.693/2.257 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jessing, M.P.; Abuawad, A.; Bikulov, T.; Abresch, J.R.; Offenhäusser, A.; Krause, H.-J. Isothermal Amplification Using Temperature-Controlled Frequency Mixing Magnetic Detection-Based Portable Field-Testing Platform. Sensors 2024, 24, 4478. https://doi.org/10.3390/s24144478
Jessing MP, Abuawad A, Bikulov T, Abresch JR, Offenhäusser A, Krause H-J. Isothermal Amplification Using Temperature-Controlled Frequency Mixing Magnetic Detection-Based Portable Field-Testing Platform. Sensors. 2024; 24(14):4478. https://doi.org/10.3390/s24144478
Chicago/Turabian StyleJessing, Max P., Abdalhalim Abuawad, Timur Bikulov, Jan R. Abresch, Andreas Offenhäusser, and Hans-Joachim Krause. 2024. "Isothermal Amplification Using Temperature-Controlled Frequency Mixing Magnetic Detection-Based Portable Field-Testing Platform" Sensors 24, no. 14: 4478. https://doi.org/10.3390/s24144478
APA StyleJessing, M. P., Abuawad, A., Bikulov, T., Abresch, J. R., Offenhäusser, A., & Krause, H.-J. (2024). Isothermal Amplification Using Temperature-Controlled Frequency Mixing Magnetic Detection-Based Portable Field-Testing Platform. Sensors, 24(14), 4478. https://doi.org/10.3390/s24144478






