Organic Thermoelectric Materials for Wearable Electronic Devices
Abstract
:1. Introduction
2. Theoretical Principle of Organic Thermoelectrics
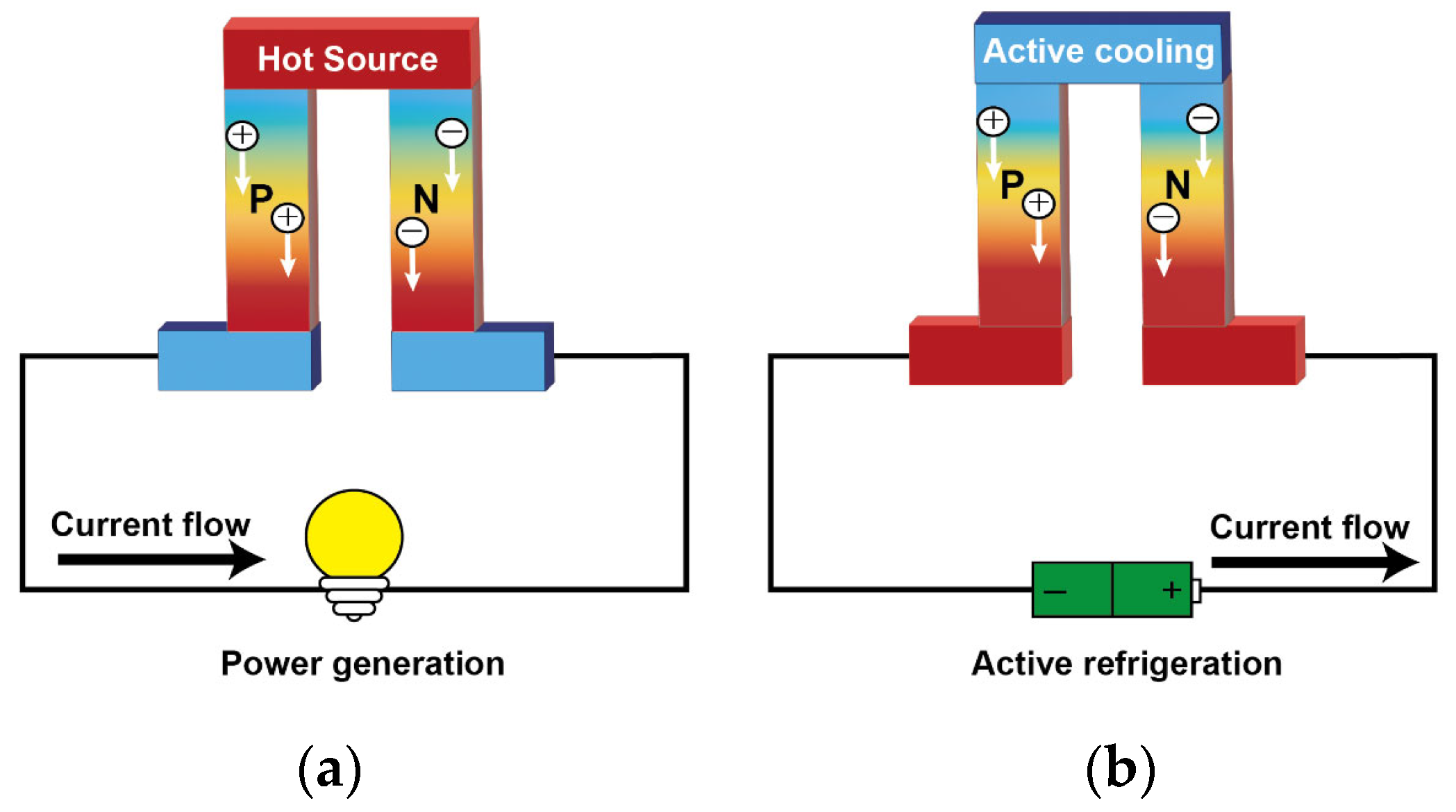
2.1. Basics of Thermoelectric Effect
2.2. Recently Studied Organic Thermoelectric Materials
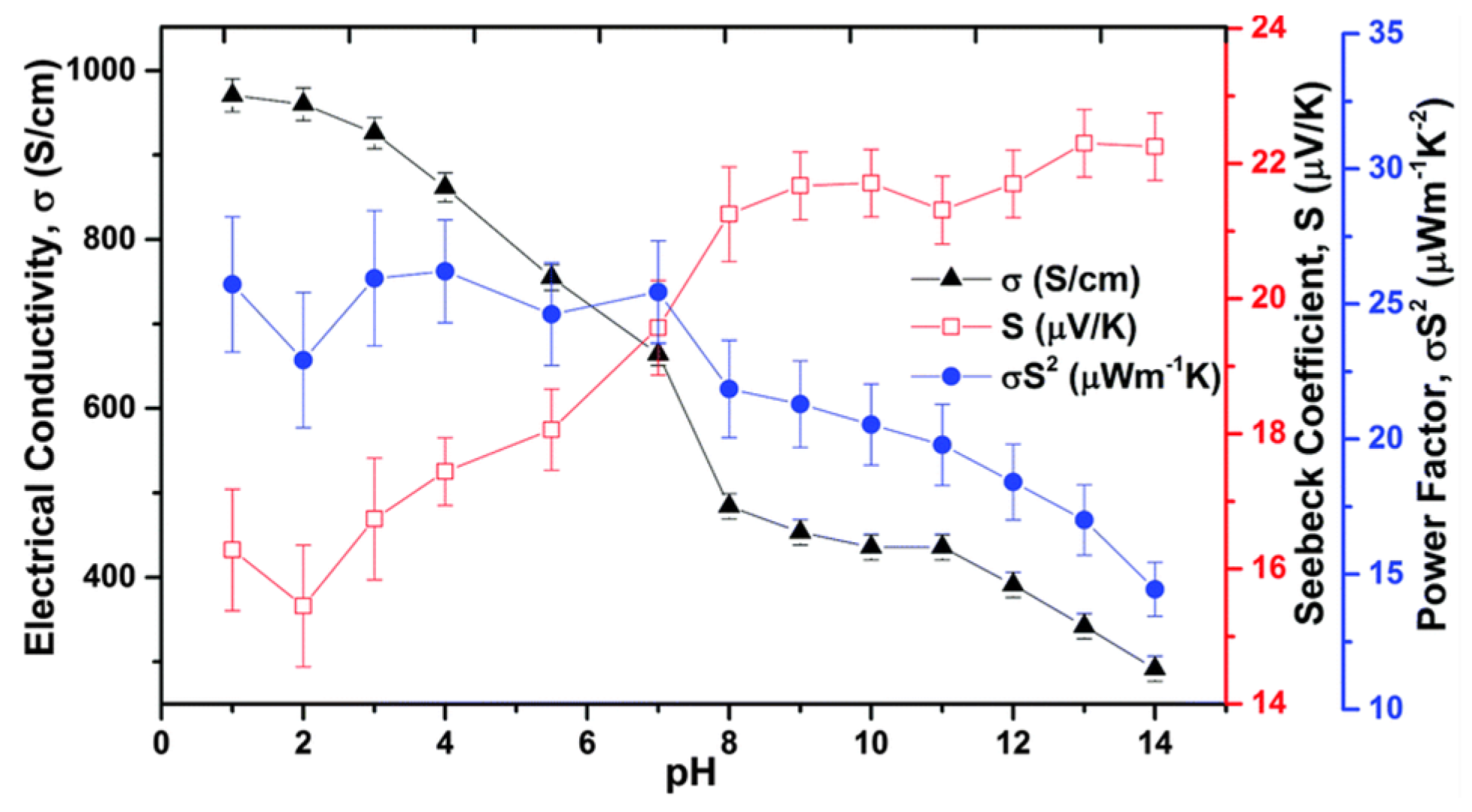
2.2.1. PEDOT
2.2.2. PANI
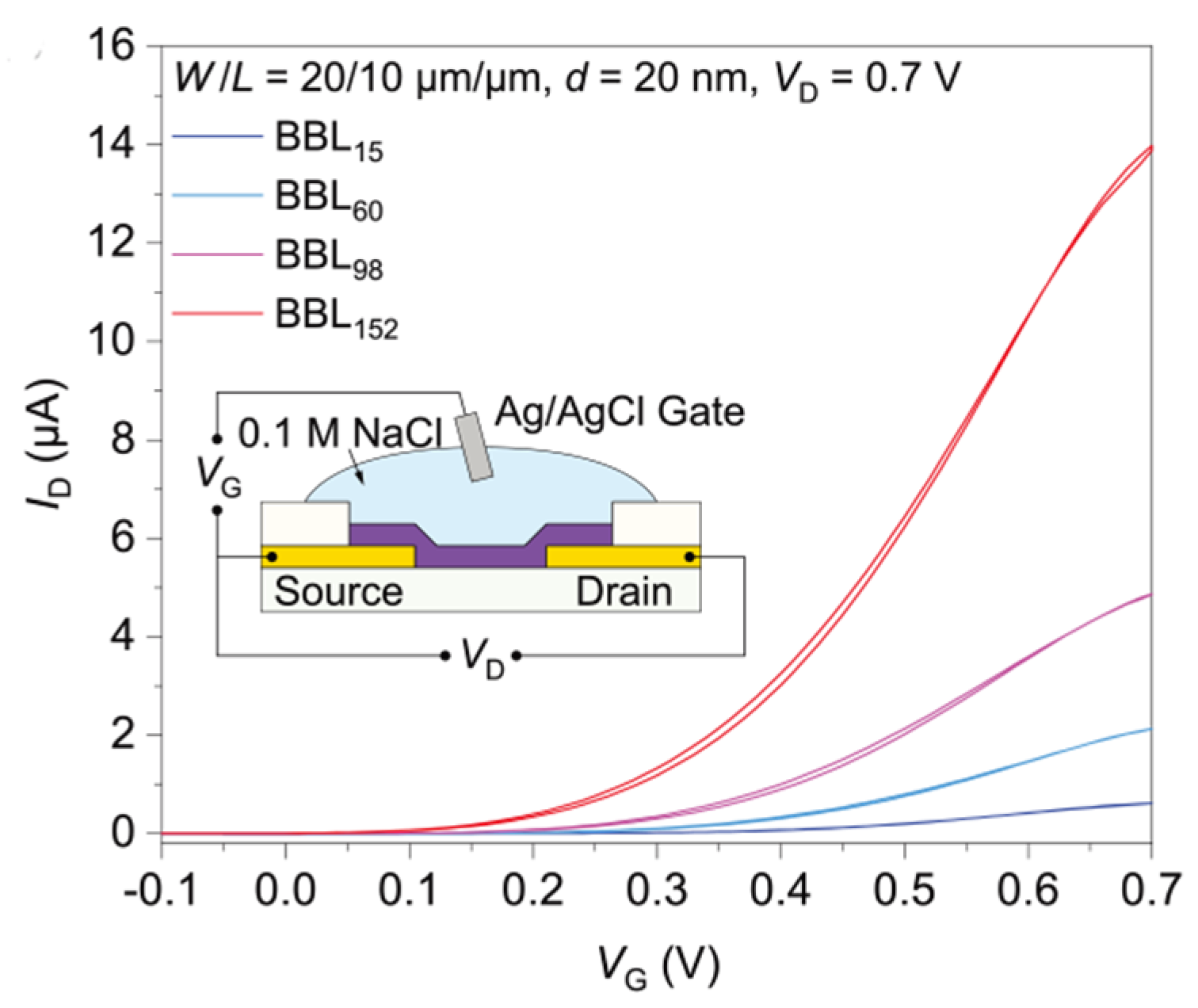
2.2.3. BBL
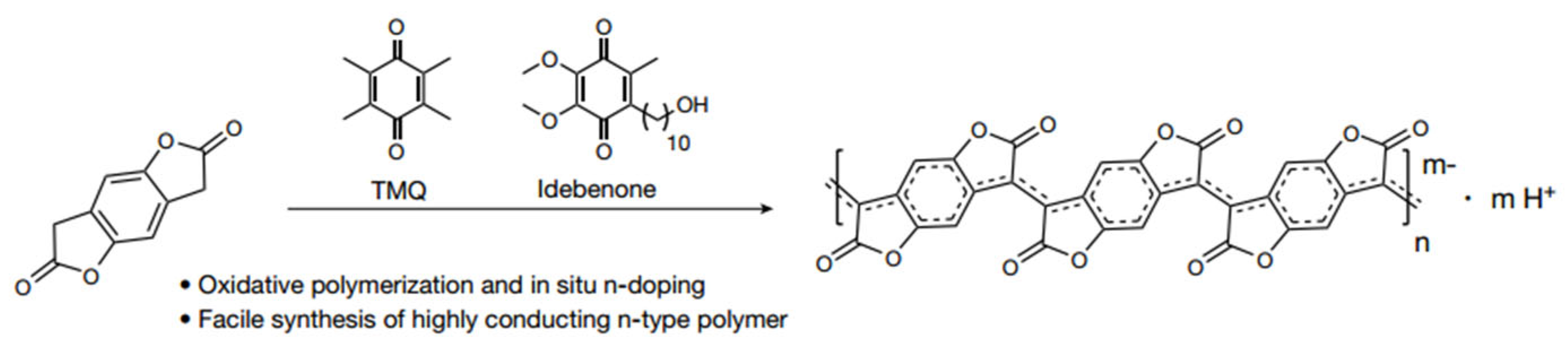
2.2.4. PBFDO
3. Types of Organic Thermoelectric Materials
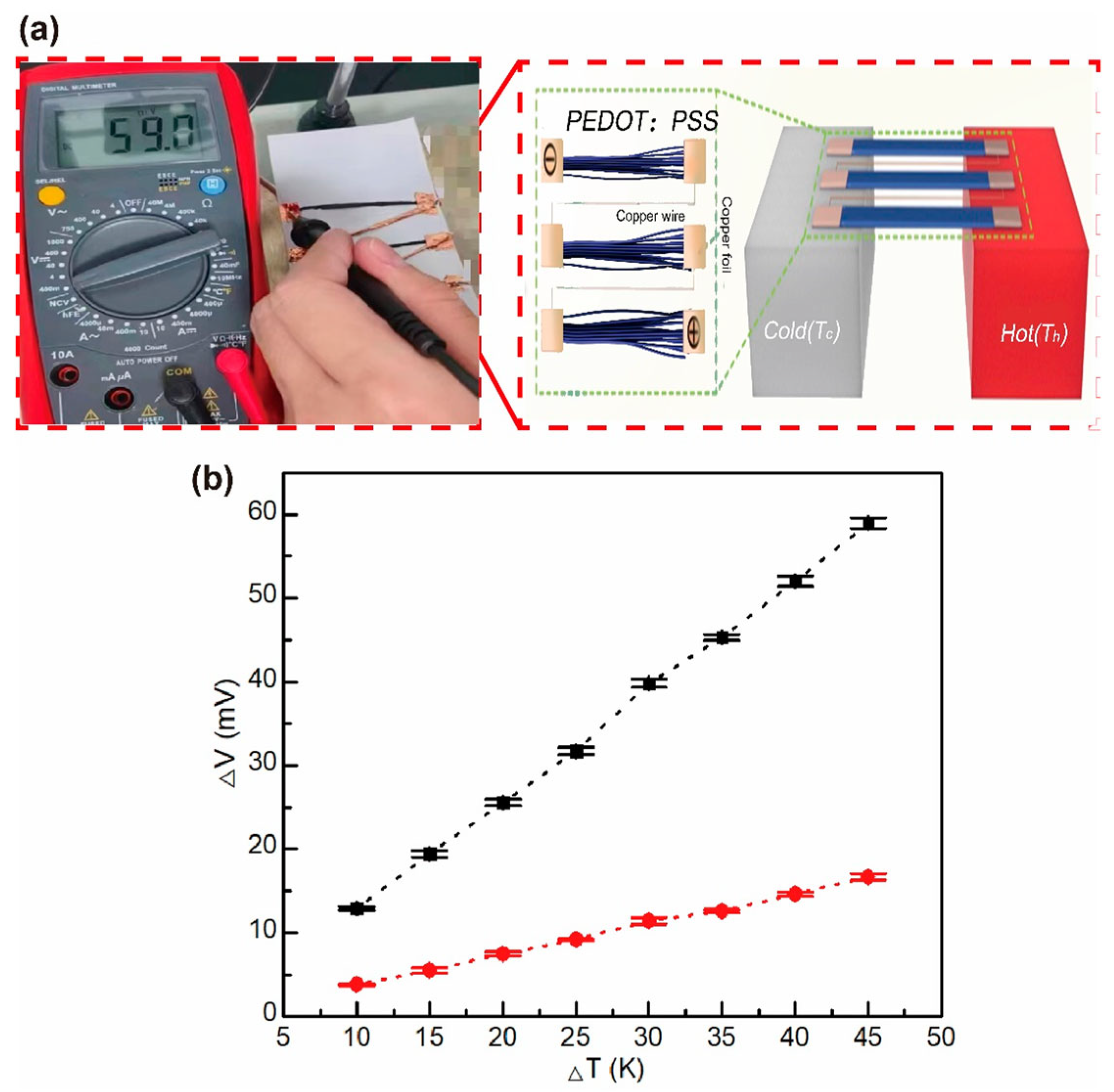
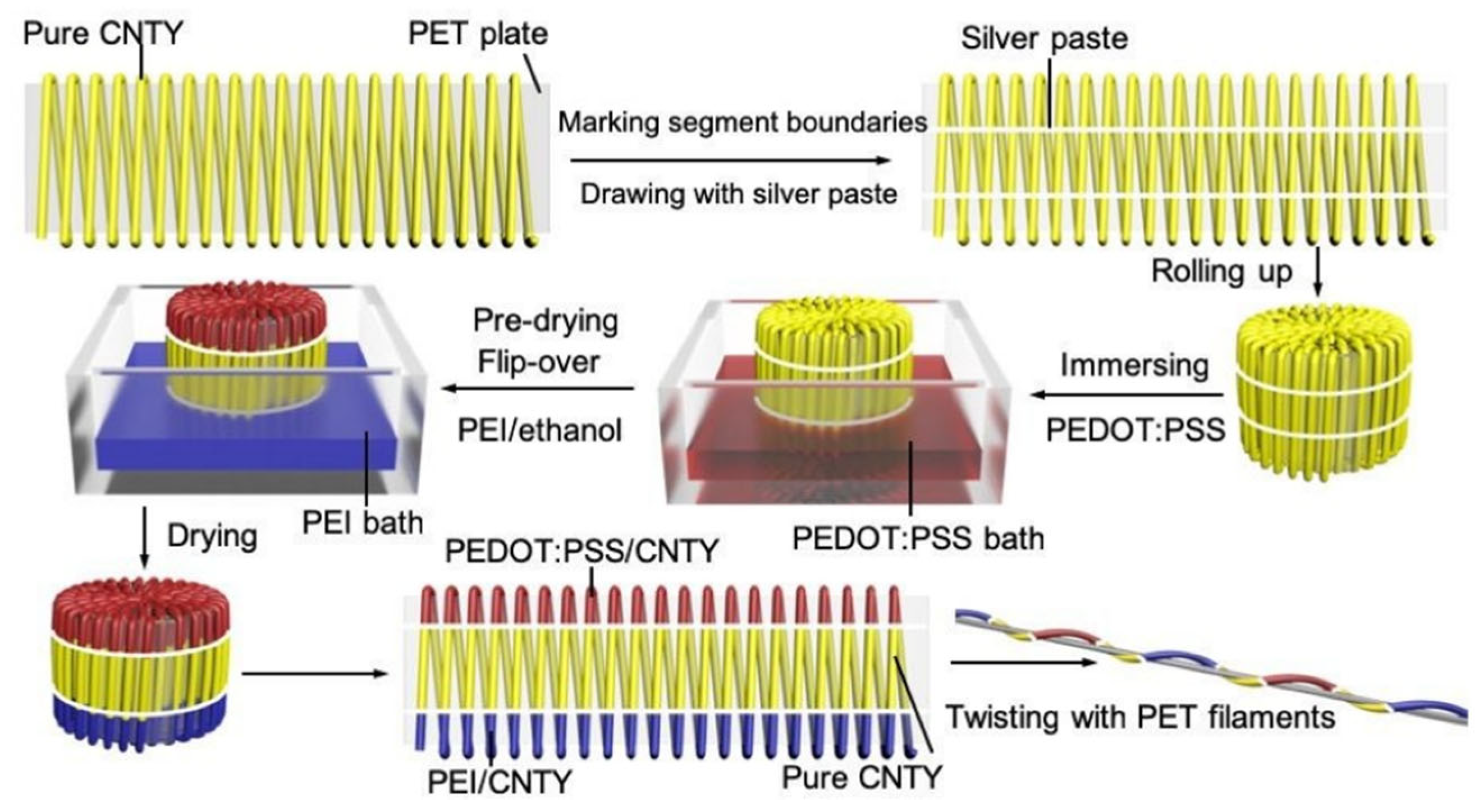
3.1. Organic Thermoelectric Fibers
3.2. Organic Thermoelectric Films
3.3. Bulk Organic Thermoelectric Materials
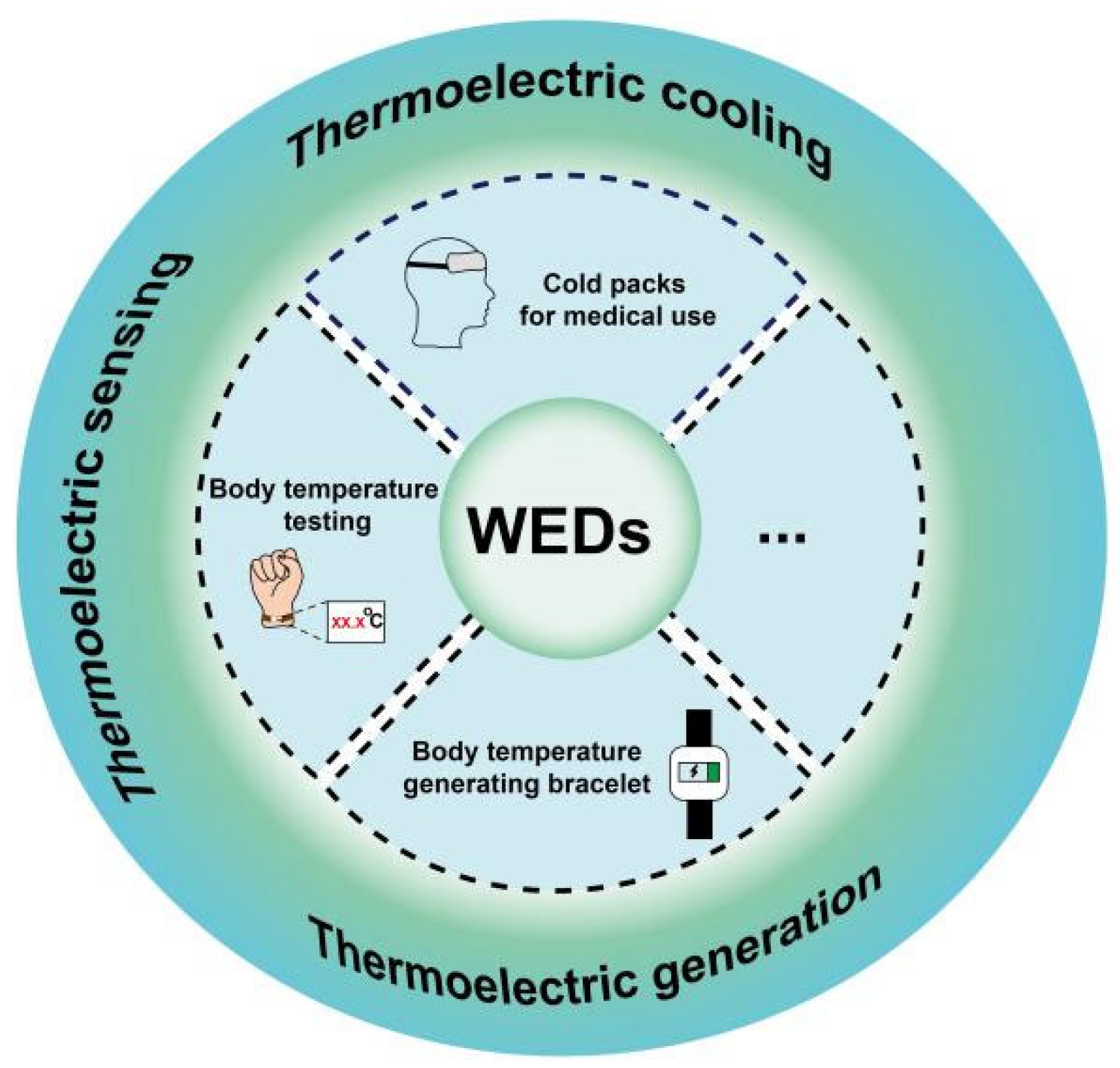
4. Applications of Organic Thermoelectric Materials in Wearable Electronic Devices
4.1. Thermoelectric Generators
4.2. Thermoelectric Sensors
5. Conclusions and Prospects
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yokota, T.; Zalar, P.; Kaltenbrunner, M.; Jinno, H.; Matsuhisa, N.; Kitanosako, H.; Tachibana, Y.; Yukita, W.; Koizumi, M.; Someya, T. Ultraflexible organic photonic skin. Sci. Adv. 2016, 2, e1501856. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cui, B.; Wang, X.; Zheng, M.; Bai, Z.; Yue, O.; Fei, Y.; Jiang, H. Nature-Skin-Derived e-Skin as Versatile “Wound Therapy-Health Monitoring” Bioelectronic Skin-Scaffolds: Skin to Bio-e-Skin. Adv. Healthc. Mater. 2023, 12, e2202971. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhuang, Q.; Ding, Y.; Zhang, C.; Song, X.; Chen, Z.; Zhang, Y.; Mei, Q.; Zhao, X.; Huang, Q.; et al. Wet-Adaptive Electronic Skin. Adv. Mater. 2023, 35, 2305630. [Google Scholar] [CrossRef] [PubMed]
- Bennion, D.M.; Horne, R.; Peel, A.; Reineke, P.; Henslee, A.; Kaufmann, C.; Guymon, C.A.; Hansen, M.R. Zwitterionic Photografted Coatings of Cochlear Implant Biomaterials Reduce Friction and Insertion Forces. Otol. Neurotol. 2021, 42, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Liu, Z.; Li, N.; Shi, B.; Zou, Y.; Xie, F.; Ma, Y.; Li, Z.; Li, H.; Zheng, Q.; et al. Symbiotic cardiac pacemaker. Nat. Commun. 2019, 10, 1821. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Choi, K.; Lee, B.; Kim, Y.; Hong, B.H. Materials for flexible, stretchable electronics: Graphene and 2D materials. Annu. Rev. Mater. Res. 2015, 45, 63–84. [Google Scholar] [CrossRef]
- Kim, T.Y.; Lee, G.H.; Mun, J.; Cheong, S.; Choi, I.; Kim, H.; Hahn, S.K. Smart contact lens systems for ocular drug delivery and therapy. Adv. Drug Deliv. Rev. 2023, 196, 114817. [Google Scholar] [CrossRef]
- Song, H.; Shin, H.; Seo, H.; Park, W.; Joo, B.J.; Kim, J.; Kim, J.; Kim, H.K.; Kim, J.; Park, J. Wireless Non-Invasive Monitoring of Cholesterol Using a Smart Contact Lens. Sci. Adv. 2022, 9, e2203597. [Google Scholar] [CrossRef]
- Ma, D.; Chen, B.; Li, Y.; Pang, X.; Fu, Q.; Xiao, Z.; Shi, Z.; Li, X.; Luo, C.; Zhou, Z.-k.; et al. Au@Ag Nanorods-PDMS Wearable Mouthguard as a Visualized Detection Platform for Screening Dental Caries and Periodontal Diseases. Adv. Healthc. Mater. 2022, 11, 2102682. [Google Scholar] [CrossRef]
- Wang, G.; Poscente, M.D.; Park, S.S.; Andrews, C.N.; Yadid-Pecht, O.; Mintchev, M.P. Wearable microsystem for minimally invasive, pseudo-continuous blood glucose monitoring: The e-mosquito. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 979–987. [Google Scholar] [CrossRef]
- Xiao, J.; Luo, Y.; Su, L.; Lu, J.; Han, W.; Xu, T.; Zhang, X. Hydrophilic metal-organic frameworks integrated uricase for wearable detection of sweat uric acid. Anal. Chim. Acta 2022, 1208, 339843. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, L.; Deng, K.; Liu, Z.; Zhang, Z.; Yang, Y.; Wang, C.; Yang, H.; Jin, A.; Luo, Q.; et al. Individual Water-Filled Single-Walled Carbon Nanotubes as Hydroelectric Power Converters. Adv. Mater. 2008, 20, 1772–1776. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, Z.; Li, X.; Yu, J.; Zhou, J.; Chen, Y.; Guo, W. Waving potential in graphene. Nat. Commun. 2014, 5, 3582. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, X.; Yu, J.; Zhang, Z.; Zhou, J.; Guo, W. Generating electricity by moving a droplet of ionic liquid along graphene. Nat. Nanotechnol. 2014, 9, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Xu, Y.; Ding, T.; Li, J.; Yin, J.; Fei, W.; Cao, Y.; Yu, J.; Yuan, L.; Gong, L.; et al. Water-evaporation-induced electricity with nanostructured carbon materials. Nat. Nanotechnol. 2017, 12, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.S.; Lin, S.; Lee, J.H.; Ryu, H.; Kim, T.Y.; Zhong, H.; Chen, H.; Kim, S.-W. Triboelectrification-Induced Large Electric Power Generation from a Single Moving Droplet on Graphene/Polytetrafluoroethylene. ACS Nano 2016, 10, 7297–7302. [Google Scholar] [CrossRef]
- Huang, S.; Qi, L.; Huang, W.; Shu, L.; Zhou, S.; Jiang, X. Flexoelectricity in dielectrics: Materials, structures and characterizations. J. Adv. Dielectr. 2018, 8, 1830002. [Google Scholar] [CrossRef]
- Chen, B.; Suo, Z. Optoionic sensing. Small 2022, 18, 2103882. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Fan, Z.; Cheng, S.; Hong, L.; Li, Y.; Tian, G.; Chen, D.; Hou, Z.; Qin, M.; Zeng, M.; et al. An Artificial Optoelectronic Synapse Based on a Photoelectric Memcapacitor. Adv. Electron. Mater. 2019, 6, 1900858. [Google Scholar] [CrossRef]
- Choudhry, I.; Khalid, H.R.; Lee, H.K. Flexible piezoelectric transducers for energy harvesting and sensing from human kinematics. ACS Appl. Electron. Mater. 2020, 2, 3346–3357. [Google Scholar] [CrossRef]
- Xu, M.; Wen, Y.; Niu, F.; Yang, Q.; Xiong, C.; Shi, Z. Flexible piezoelectric generator based on PLLA/ZnO oriented fibers for wearable self-powered sensing. Compos. Part A Appl. Sci. Manuf. 2023, 169, 107518. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J.; An, L.; Yang, Y.; Wu, Y.; Liu, R.; Jin, Y.; Liu, Q.; Shi, Q.; Liang, Y. Synergetic effect of MXene/MoS2 heterostructure and gradient multilayer for highly sensitive flexible piezoelectric sensor. Polymer 2023, 286, 126399. [Google Scholar] [CrossRef]
- Lü, C.; Wu, S.; Lu, B.; Zhang, Y.; Du, Y.; Feng, X. Ultrathin flexible piezoelectric sensors for monitoring eye fatigue. J. Micromech. Microeng. 2018, 28, 025010. [Google Scholar] [CrossRef]
- Lu, C.; Wu, S.; Zhang, Y.; Du, Y. Electromechanical modeling of eye fatigue detecting using flexible piezoelectric sensors. Sci. China Inf. Sci. 2018, 61, 1–8. [Google Scholar] [CrossRef]
- Soleimani, Z.; Zoras, S.; Ceranic, B.; Cui, Y.; Shahzad, S. A comprehensive review on the output voltage/power of wearable thermoelectric generators concerning their geometry and thermoelectric materials. Nano Energy 2021, 89, 106325. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Tu, Y.; Zhang, Q.; Peng, F.; Zeng, W.; Zhang, M.; Tao, X. Wearable self-powered human motion sensors based on highly stretchable quasi-solid state hydrogel. Nano Energy 2021, 88, 106272. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, M.; Kong, J.; Li, H.; He, C. Flexible, durable, green thermoelectric composite fabrics for textile-based wearable energy harvesting and self-powered sensing. Compos. Sci. Technol. 2023, 243, 110245. [Google Scholar] [CrossRef]
- Arakawa, K.; Yamaguchi, S. A thermoelectric cooling/heating knife using Bi2Te3-based bulks. ECS Trans. 2010, 25, 75. [Google Scholar] [CrossRef]
- Lyu, S.; He, Y.; Li, X.; Wang, H.; Yao, Y.; Peng, Z.; Ding, Y.; Wang, Y. Skin Thermal Management for Subcutaneous Photoelectric Conversion Reaching 500 mW. Adv. Mater. 2023, 35, 2306903. [Google Scholar] [CrossRef]
- Beretta, D.; Perego, A.; Lanzani, G.; Caironi, M. Organic flexible thermoelectric generators: From modeling, a roadmap towards applications. Sustain. Energy Fuels 2017, 1, 174–190. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, Q.; Wang, L.; Wang, Y.; Sun, J.; Zeng, H.; Jin, Z.; Huang, X.; Chen, L. The synergic regulation of conductivity and Seebeck coefficient in pure polyaniline by chemically changing the ordered degree of molecular chains. J. Mater. Chem. A 2014, 2, 2634–2640. [Google Scholar] [CrossRef]
- Peng, S.; Wang, D.; Lu, J.; He, M.; Xu, C.; Li, Y.; Zhu, S. A review on organic polymer-based thermoelectric materials. J. Polym. Environ. 2017, 25, 1208–1218. [Google Scholar] [CrossRef]
- Tan, G.; Zhao, L.D.; Kanatzidis, M.G. Rationally designing high-performance bulk thermoelectric materials. Chem. Rev. 2016, 116, 12123–12149. [Google Scholar] [CrossRef] [PubMed]
- Zeier, W.G.; Zevalkink, A.; Gibbs, Z.M.; Hautier, G.; Kanatzidis, M.G.; Snyder, G.J. Thinking like a chemist: Intuition in thermoelectric materials. Angew. Chem. Int. Ed. 2016, 55, 6826–6841. [Google Scholar] [CrossRef] [PubMed]
- Kroon, R.; Mengistie, D.A.; Kiefer, D.; Hynynen, J.; Ryan, J.D.; Yu, L.; Müller, C. Thermoelectric plastics: From design to synthesis, processing and structure–property relationships. Chem. Soc. Rev. 2016, 45, 6147–6164. [Google Scholar] [CrossRef] [PubMed]
- Amengual, A.; Isalgue, A.; Marco, F.; Tachoire, H.; Torra, V.; Torra, V.R. Automatic equipment with improved performances (ATD and DSC) in shape memory alloys studies. J. Therm. Anal. Calorim. 1992, 38, 583–592. [Google Scholar] [CrossRef]
- Zogg, A.; Stoessel, F.; Fischer, U.; Hungerbühler, K. Isothermal reaction calorimetry as a tool for kinetic analysis. Thermochim. Acta 2004, 419, 1–17. [Google Scholar] [CrossRef]
- Drebushchak, V.A. The peltier effect. J. Therm. Anal. Calorim. 2008, 91, 311–315. [Google Scholar] [CrossRef]
- Modak, R.; Murata, M.; Hou, D.; Miura, A.; Iguchi, R.; Xu, B.; Guo, R.; Shiomi, J.; Sakuraba, Y.; Uchida, K.-i. Phase-transition-induced giant Thomson effect for thermoelectric cooling. Appl. Phys. Rev. 2022, 9, 011414. [Google Scholar] [CrossRef]
- Thomson, W. 4. on a mechanical theory of thermo-electric currents. Proc. R. Soc. Edinb. 1857, 3, 91–98. [Google Scholar] [CrossRef]
- Thomson, W. IX.—On the dynamical theory of heat. Part V. Thermo-electric currents. Earth Environ. Sci. Trans. R. Soc. Edinb. 1857, 21, 123–171. [Google Scholar] [CrossRef]
- Snyder, G.J.; Snyder, A.H. Figure of merit ZT of a thermoelectric device defined from materials properties. Energy Environ. Sci. 2017, 10, 2280–2283. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Xu, W.; Zhu, D. Organic thermoelectric materials: Emerging green energy materials converting heat to electricity directly and efficiently. Adv. Mater. 2014, 26, 6829–6851. [Google Scholar] [CrossRef] [PubMed]
- Pernstich, K.P.; Rössner, B.; Batlogg, B. Field-effect-modulated Seebeck coefficient in organic semiconductors. Nat. Mater. 2008, 7, 321–325. [Google Scholar] [CrossRef]
- Imae, I.; Akazawa, R.; Ooyama, Y.; Harima, Y. Investigation of organic thermoelectric materials using potential-step chronocoulometry: Effect of polymerization methods on thermoelectric properties of poly (3-hexylthiophene). J. Polym. Sci. 2020, 58, 3004–3008. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Sada, N.; Toshima, N. Thermal transporting properties of electrically conductive polyaniline films as organic thermoelectric materials. J. Therm. Anal. Calorim. 2002, 69, 881–887. [Google Scholar] [CrossRef]
- Moses, D.; Denenstein, A. Experimental determination of the thermal conductivity of a conducting polymer: Pure and heavily doped polyacetylene. Phys. Rev. B 1984, 30, 2090. [Google Scholar] [CrossRef]
- Kim, J.H.; Feldman, A.; Novotny, D. Application of the three omega thermal conductivity measurement method to a film on a substrate of finite thickness. J. Appl. Phys. 1999, 86, 3959–3963. [Google Scholar] [CrossRef]
- Russ, B.; Glaudell, A.; Urban, J.J.; Chabinyc, M.L.; Segalman, R.A. Organic thermoelectric materials for energy harvesting and temperature control. Nat. Rev. Mater. 2016, 1, 16050. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Zhang, F.; Dai, K.; Li, C.; Fan, Y.; Chen, G.; Zheng, Q. Soft organic thermoelectric materials: Principles, current state of the art and applications. Small 2022, 18, 2104922. [Google Scholar] [CrossRef] [PubMed]
- Daoben, Z. Organic Thermoelectrics: From Materials to Devices; Science Press: Beijing, China, 2020. [Google Scholar]
- Khan, Z.U.; Bubnova, O.; Jafari, M.J.; Brooke, R.; Liu, X.; Gabrielsson, R.; Ederth, T.; Evans, D.R.; Andreasen, J.W.; Fahlman, M.; et al. Acido-basic control of the thermoelectric properties of poly(3,4-ethylenedioxythiophene)tosylate (PEDOT-Tos) thin films. J. Mater. Chem. C 2015, 3, 10616–10623. [Google Scholar] [CrossRef] [PubMed]
- Petsagkourakis, I.; Pavlopoulou, E.; Portale, G.; Kuropatwa, B.A.; Dilhaire, S.; Fleury, G.; Hadziioannou, G. Structurally-driven enhancement of thermoelectric properties within poly (3,4-ethylenedioxythiophene) thin films. Sci. Rep. 2016, 6, 30501. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jung, J.H.; Lee, D.E.; Joo, J. Enhancement of electrical conductivity of poly (3,4-ethylenedioxythiophene)/poly (4-styrenesulfonate) by a change of solvents. Synth. Met. 2002, 126, 311–316. [Google Scholar] [CrossRef]
- Jiang, F.; Xiong, J.; Zhou, W.; Liu, C.; Wang, L.; Zhao, F.; Liu, H.; Xu, J. Use of organic solvent-assisted exfoliated MoS 2 for optimizing the thermoelectric performance of flexible PEDOT: PSS thin films. J. Mater. Chem. A 2016, 4, 5265–5273. [Google Scholar] [CrossRef]
- Kim, J.; Jang, J.G.; Hong, J.I.; Kim, S.H.; Kwak, J. Sulfuric acid vapor treatment for enhancing the thermoelectric properties of PEDOT: PSS thin-films. J. Mater. Sci. Mater. Electron. 2016, 27, 6122–6127. [Google Scholar] [CrossRef]
- Saxena, N.; Pretzl, B.; Lamprecht, X.; Bießmann, L.; Yang, D.; Li, N.; Bilko, C.; Bernstorff, S.; Müller-Buschbaum, P. Ionic Liquids as Post-Treatment Agents for Simultaneous Improvement of Seebeck Coefficient and Electrical Conductivity in PEDOT:PSS Films. ACS Appl. Mater. Interfaces 2019, 11, 8060–8071. [Google Scholar] [CrossRef]
- Imae, I.; Uehara, H.; Imato, K.; Ooyama, Y. Thermoelectric Properties of Conductive Freestanding Films Prepared from PEDOT: PSS Aqueous Dispersion and Ionic Liquids. ACS Appl. Mater. Interfaces 2022, 14, 57064–57069. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Fernández, E.; Gabaldón-Saucedo, I.A.; Rodríguez-Rodríguez, Á.; Solano, E.; García-Gutiérrez, M.C.; Nogales, A.; Cirera, A.; Ezquerra, T.A.; Rebollar, E. Laser nanostructuring of thin films of PEDOT:PSS on ITO: Morphology, molecular structure and electrical properties. Appl. Surf. Sci. 2020, 509, 145350. [Google Scholar] [CrossRef]
- Moyen, E.; Hama, A.; Ismailova, E.; Assaud, L.; Malliaras, G.; Hanbücken, M.; Owens, R.M. Nanostructured conducting polymers for stiffness controlled cell adhesion. Nanotechnology 2016, 27, 074001. [Google Scholar] [CrossRef]
- Ohayon, D.; Pitsalidis, C.; Pappa, A.M.; Hama, A.; Zhang, Y.; Gallais, L.; Owens, R.M. Laser Patterning of Self-Assembled Monolayers on Pedot: Pss Films for Controlled Cell Adhesion. Adv. Mater. Interfaces 2017, 4, 1700191. [Google Scholar] [CrossRef]
- Lasagni, A.F.; Hendricks, J.L.; Shaw, C.M.; Yuan, D.; Martin, D.C.; Das, S. Direct laser interference patterning of poly (3,4-ethylene dioxythiophene)-poly (styrene sulfonate)(PEDOT-PSS) thin films. Appl. Surf. Sci. 2009, 255, 9186–9192. [Google Scholar] [CrossRef]
- Radivo, A.; Sovernigo, E.; Caputo, M.; Zilio, S.D.; Endale, T.; Pozzato, A.; Goldoni, A.; Tormen, M. Patterning PEDOT:PSS and tailoring its electronic properties by water-vapour-assisted nanoimprint lithography. RSC Adv. 2014, 4, 34014–34025. [Google Scholar] [CrossRef]
- Choi, J.H.; Choi, H.J.; Shin, J.H.; Kim, H.P.; Jang, J.; Lee, H. Enhancement of organic solar cell efficiency by patterning the PEDOT: PSS hole transport layer using nanoimprint lithography. Org. Electron. 2013, 14, 3180–3185. [Google Scholar] [CrossRef]
- Li, J.Y.; Yu, H.; Wen, J.J.; Li, Z.D.; Xu, Z.C.; Zhang, Y.F.; Yu, H.; Lu, B.R.; Liu, R.; Chen, Y.F. Fabrication of Nano-Strctures on PEDOT:PSS Film by Nanoimprint Lithography. Adv. Mater. Res. 2012, 465, 287–291. [Google Scholar] [CrossRef]
- Li, J.; Chang, X.; Li, S.; Shrestha, P.K.; Tan, E.K.; Chu, D. High-Resolution Electrochemical Transistors Defined by Mold-Guided Drying of PEDOT: PSS Liquid Suspension. ACS Appl. Electron. Mater. 2020, 2, 2611–2618. [Google Scholar] [CrossRef]
- ElMahmoudy, M.; Charrier, A.M.; Malliaras, G.G.; Sanaur, S. Facile nanopatterning of PEDOT: PSS thin films. Adv. Mater. Technol. 2018, 3, 1700344. [Google Scholar] [CrossRef]
- Sanviti, M.; Mester, L.; Hillenbrand, R.; Alegria, A.; Martínez-Tong, D.E. Solvent-structured PEDOT: PSS surfaces: Fabrication strategies and nanoscale properties. Polymer 2022, 246, 124723. [Google Scholar] [CrossRef]
- Imae, I.; Goto, T.; Ooyama, Y.; Harima, Y. Thermoelectric properties of poly (3, 4-ethylenedioxythiophene) with fluorine-containing polyanion as dopant. Polymer 2020, 199, 122538. [Google Scholar] [CrossRef]
- Zhang, L.; Harima, Y.; Imae, I. Highly improved thermoelectric performances of PEDOT: PSS/SWCNT composites by solvent treatment. Org. Electron. 2017, 51, 304–307. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, X.; Wen, M.; Chen, C.; Wen, Q.; Fu, Q.; Deng, H. Highly electrical conductive PEDOT: PSS/SWCNT flexible thermoelectric films fabricated by a high-velocity non-solvent turbulent secondary doping approach. ACS Appl. Mater. Interfaces 2023, 15, 10947–10957. [Google Scholar] [CrossRef] [PubMed]
- Jun, L.; Lian-meng, Z.; Li, H.; Xin-feng, T. Synthesis and thermoelectric properties of polyaniline. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2003, 18, 53–55. [Google Scholar] [CrossRef]
- Yan, H.; Toshima, N. Thermoelectric properties of alternatively layered films of polyaniline and (±)-10-camphorsulfonic acid-doped polyaniline. Chem. Lett. 1999, 28, 1217–1218. [Google Scholar] [CrossRef]
- Wang, L.; Yao, Q.; Xiao, J.; Zeng, K.; Shi, W.; Qu, S.; Chen, L. Enhanced Thermoelectric Properties of Polyaniline Nanofilms Induced by Self-Assembled Supramolecules. Chem. –Asian J. 2016, 11, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, Y.; Xu, W.; Zhang, Q. Investigating thermoelectric properties of doped polyaniline nanowires. Synth. Met. 2014, 189, 177–182. [Google Scholar] [CrossRef]
- Wu, H.Y.; Yang, C.Y.; Li, Q.; Kolhe, N.B.; Strakosas, X.; Stoeckel, M.A.; Wu, Z.; Jin, W.; Savvakis, M.; Kroon, R.; et al. Influence of Molecular Weight on the Organic Electrochemical Transistor Performance of Ladder-Type Conjugated Polymers. Adv. Mater. 2021, 34, 2106235. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Dexter Tam, T.L.; Lin, T.; Chien, S.W.; Lin, M.; Meng, H.; Huang, W.; Xu, J. π-Extended Poly(benzimidazoanthradiisoquinolinedione) Ladder-type Conjugated Polymer. ACS Macro Lett. 2022, 11, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Ruoko, T.P.; Shokrani, M.; Scheunemann, D.; Abdalla, H.; Sun, H.; Yang, C.Y.; Puttisong, Y.; Kolhe, N.B.; Figueroa, J.S.M.; et al. On the Origin of Seebeck Coefficient Inversion in Highly Doped Conducting Polymers. Adv. Funct. Mater. 2022, 32, 2112276. [Google Scholar] [CrossRef]
- Tang, H.; Liang, Y.; Liu, C.; Hu, Z.; Deng, Y.; Guo, H.; Yu, Z.; Song, A.; Zhao, H.; Zhao, D.; et al. A solution-processed n-type conducting polymer with ultrahigh conductivity. Nature 2022, 611, 271–277. [Google Scholar] [CrossRef]
- Liang, Y.; Che, C.; Tang, H.; Zhang, K.; Lan, L.; Zhou, C.; Ma, Y.; Huang, F. Influence of Interaction between Electrolyte with Side-Chain Free Conjugated Polymer on the Performance of Organic Electrochemical Transistors. ACS Appl. Mater. Interfaces 2023, 16, 19977–19986. [Google Scholar] [CrossRef]
- Park, T.; Park, C.; Kim, B.; Shina, H.; Kim, E. Flexible PEDOT electrodes with large thermoelectric power factors to generate electricity by the touch of fingertips. Energy Environ. Sci. 2013, 6, 788–792. [Google Scholar] [CrossRef]
- Kim, G.H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered doping of organic semiconductors for enhanced thermoelectric efficiency. Nat. Mater. 2013, 12, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Li, P.; Du, D.; Ouyang, J. Significantly enhanced thermoelectric properties of PEDOT: PSS films through sequential post-treatments with common acids and bases. Adv. Energy Mater. 2017, 7, 1602116. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, Z.; Wang, X.; Zhang, Z.; Ouyang, J. Enhancement of the thermoelectric properties of PEDOT: PSS via one-step treatment with cosolvents or their solutions of organic salts. J. Mater. Chem. A 2018, 6, 7080–7087. [Google Scholar] [CrossRef]
- Mazaheripour, A.; Majumdar, S.; Hanemann-Rawlings, D.; Thomas, E.M.; McGuiness, C.; d’Alencon, L.; Chabinyc, M.L.; Segalman, R.A. Tailoring the Seebeck Coefficient of PEDOT:PSS by Controlling Ion Stoichiometry in Ionic Liquid Additives. Chem. Mater. 2018, 30, 4816–4822. [Google Scholar] [CrossRef]
- Fan, Z.; Du, D.; Guan, X.; Ouyang, J. Polymer films with ultrahigh thermoelectric properties arising from significant seebeck coefficient enhancement by ion accumulation on surface. Nano Energy 2018, 51, 481–488. [Google Scholar] [CrossRef]
- Yao, H.; Fan, Z.; Li, P.; Li, B.; Guan, X.; Du, D.; Ouyang, J. Solution processed intrinsically conductive polymer films with high thermoelectric properties and good air stability. J. Mater. Chem. A 2018, 6, 24496–24502. [Google Scholar] [CrossRef]
- Ni, D.; Song, H.; Chen, Y.; Cai, K. Free-standing highly conducting PEDOT films for flexible thermoelectric generator. Energy 2019, 170, 53–61. [Google Scholar] [CrossRef]
- Li, P.; Zhao, Y.; Li, H.; Liu, S.; Liang, Y.; Cheng, X.; He, C. Facile green strategy for improving thermoelectric performance of carbon nanotube/polyaniline composites by ethanol treatment. Compos. Sci. Technol. 2020, 189, 108023. [Google Scholar] [CrossRef]
- Zhou, W.; Fan, Q.; Zhang, Q.; Cai, L.; Li, K.; Gu, X.; Yang, F.; Zhang, N.; Wang, Y.; Liu, H.; et al. High-performance and compact-designed flexible thermoelectric modules enabled by a reticulate carbon nanotube architecture. Nat. Commun. 2017, 8, 14886. [Google Scholar] [CrossRef]
- Liang, L.; Chen, G.; Guo, C.Y. Polypyrrole nanostructures and their thermoelectric performance. Mater. Chem. Front. 2017, 1, 380–386. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, Q.; Du, K.; Mo, S.; Yin, Q. Thermoelectric properties of polypyrrole nanotubes. Macromol. Res. 2020, 28, 973–978. [Google Scholar] [CrossRef]
- Han, S.; Zhai, W.; Chen, G.; Wang, X. Morphology and thermoelectric properties of graphene nanosheets enwrapped with polypyrrole. RSC Adv. 2014, 4, 29281–29285. [Google Scholar] [CrossRef]
- Song, H.; Cai, K.; Wang, J.; Shen, S. Influence of polymerization method on the thermoelectric properties of multi-walled carbon nanotubes/polypyrrole composites. Synth. Met. 2016, 211, 58–65. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, J.; Song, H.; Pan, Y.; Song, Y.; Zhu, Y.; Yao, Y.; Huang, F.; Zuo, C. High performance polypyrrole/SWCNTs composite film as a promising organic thermoelectric material. RSC Adv. 2021, 11, 17704–17709. [Google Scholar] [CrossRef] [PubMed]
- Ran, G.; Wu, Q.; Lei, L.; Zhong, D.; Hai, J.; Lu, Z. Research progress on doping modification of n-type organic thermoelectric materials. Mater. Rep. 2022, 36, 200–210. [Google Scholar]
- Wen, N.; Fan, Z.; Yang, S.; Zhao, Y.; Cong, T.; Xu, S.; Zhang, H.; Wang, J.; Huang, H.; Li, C.; et al. Highly conductive, ultra-flexible and continuously processable PEDOT:PSS fibers with high thermoelectric properties for wearable energy harvesting. Nano Energy 2020, 78, 105361. [Google Scholar] [CrossRef]
- Chen, H.; Xu, H.; Luo, M.; Wang, W.; Qing, X.; Lu, Y.; Liu, Q.; Yang, L.; Zhong, W.; Li, M.; et al. Highly Conductive, Ultrastrong, and Flexible Wet-Spun PEDOT:PSS/Ionic Liquid Fibers for Wearable Electronics. ACS Appl. Mater. Interfaces 2023, 15, 20346–20357. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhang, X.; Tan, P.; Zhang, W.; Sun, H.; Sun, F.; Li, D.; Jia, J.; Chen, T. Thermoelectric Energy Conversion Using Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) Fibers Based on Low-Temperature In Situ Polymerization and the Freeze–Thaw Method. ACS Appl. Polym. Mater. 2024, 6, 1772–1780. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Q.; Jin, W.; Jing, Y.; Chen, X.; Han, X.; Bao, Q.; Liu, Y.; Wang, X.; Wang, S.; et al. Carbon nanotube yarn based thermoelectric textiles for harvesting thermal energy and powering electronics. J. Mater. Chem. A 2020, 8, 2984–2994. [Google Scholar] [CrossRef]
- Li, P.; Guo, Y.; Mu, J.; Wang, H.; Zhang, Q.; Li, Y. Single-walled carbon nanotubes/polyaniline-coated polyester thermoelectric textile with good interface stability prepared by ultrasonic induction. RSC Adv. 2016, 6, 90347–90353. [Google Scholar] [CrossRef]
- Lee, W.; Lee, S.; Kim, H.; Kim, Y. Organic thermoelectric devices with PEDOT: PSS/ZnO hybrid composites. Chem. Eng. J. 2021, 415, 128935. [Google Scholar] [CrossRef]
- Liu, D.; Yan, Z.; Zhao, Y.; Zhang, Z.; Zhang, B.; Shi, P.; Xue, C. Facile self-supporting and flexible Cu2S/PEDOT: PSS composite thermoelectric film with high thermoelectric properties for body energy harvesting. Results Phys. 2021, 31, 105061. [Google Scholar] [CrossRef]
- Paulraj, I.; Liang, T.F.; Yang, T.S.; Wang, C.H.; Chen, J.L.; Wang, Y.W.; Liu, C.J. High performance of post-treated PEDOT: PSS thin films for thermoelectric power generation applications. ACS Appl. Mater. Interfaces 2021, 13, 42977–42990. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Yuan, H.; Liu, C.; Lan, J.L.; Yang, X.; Lin, Y.H. Flexible PANI/SWCNT thermoelectric films with ultrahigh electrical conductivity. RSC Adv. 2018, 8, 26011–26019. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, W.; Ravichandran, D.; Jambhulkar, S.; Song, K. A gill-mimicking thermoelectric generator (TEG) for waste heat recovery and self-powering wearable devices. J. Mater. Chem. A 2021, 9, 8514–8526. [Google Scholar] [CrossRef]
- Li, Y.; Lou, Q.; Yang, J.; Cai, K.; Liu, Y.; Lu, Y.; Qiu, Y.; Lu, Y.; Wang, Z.; Wu, M.; et al. Exceptionally High Power Factor Ag2Se/Se/Polypyrrole Composite Films for Flexible Thermoelectric Generators. Adv. Funct. Mater. 2021, 32, 2106902. [Google Scholar] [CrossRef]
- Su, H.; Lin, P.; Lu, H.; Zhao, X.; Sheng, X.; Chen, Y. Janus-type hydroxyapatite-incorporated Kevlar aerogel@ Kevlar aerogel supported phase-change material gel toward wearable personal thermal management. ACS Appl. Mater. Interfaces 2022, 14, 12617–12629. [Google Scholar] [CrossRef]
- Han, S.; Jiao, F.; Khan, Z.U.; Edberg, J.; Fabiano, S.; Crispin, X. Thermoelectric polymer aerogels for pressure–temperature sensing applications. Adv. Funct. Mater. 2017, 27, 1703549. [Google Scholar] [CrossRef]
- Lee, C.Y.; Lin, Y.T.; Hong, S.H.; Wang, C.H.; Jeng, U.S.; Tung, S.H.; Liu, C.L. Mixed Ionic–Electronic Conducting Hydrogels with Carboxylated Carbon Nanotubes for High Performance Wearable Thermoelectric Harvesters. ACS Appl. Mater. Interfaces 2023, 15, 56072–56083. [Google Scholar] [CrossRef]
- He, Y.; Wei, Y.; Qian, Y.; Wang, C.; Liu, Y.; Ye, Z.; Chen, G. A flexible phase change organohydrogel created using Pickering emulsion technology for thermoelectric conversion and temperature sensing. J. Mater. Chem. A 2022, 10, 18856–18865. [Google Scholar] [CrossRef]
- Wu, B.; Geng, J.; Lin, Y.; Hou, C.; Zhang, Q.; Li, Y.; Wang, H. Hydrogel-based printing strategy for high-performance flexible thermoelectric generators. Nanoscale 2022, 14, 16857–16864. [Google Scholar] [CrossRef]
- Li, M.; Zeng, F.; Luo, M.; Qing, X.; Wang, W.; Lu, Y.; Zhong, W.; Yang, L.; Liu, Q.; Wang, Y.; et al. Synergistically Improving Flexibility and Thermoelectric Performance of Composite Yarn by Continuous Ultrathin PEDOT:PSS/DMSO/Ionic Liquid Coating. ACS Appl. Mater. Interfaces 2021, 13, 50430–50440. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Hu, J. Waterborne polyurethane based thermoelectric composites and their application potential in wearable thermoelectric textiles. Compos. Part B Eng. 2016, 107, 59–66. [Google Scholar] [CrossRef]
- Liu, J.; Jia, Y.; Jiang, Q.; Jiang, F.; Li, C.; Wang, X.; Liu, P.; Liu, P.; Hu, F.; Du, Y.; et al. Highly Conductive Hydrogel Polymer Fibers toward Promising Wearable Thermoelectric Energy Harvesting. ACS Appl. Mater. Interfaces 2018, 10, 44033–44040. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Lee, T.C.; Liu, C.L. All-solution-processed polythiophene/carbon nanotube nanocomposites integrated on biocompatible silk fibroin substrates for wearable thermoelectric generators. ACS Appl. Energy Mater. 2023, 6, 2602–2610. [Google Scholar] [CrossRef]
- Zhang, X.; Li, T.-T.; Ren, H.-T.; Peng, H.-K.; Shiu, B.-C.; Wang, Y.; Lou, C.-W.; Lin, J.-H. Dual-Shell Photothermoelectric Textile Based on a PPy Photothermal Layer for Solar Thermal Energy Harvesting. ACS Appl. Mater. Interfaces 2020, 12, 55072–55082. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.D.; Lund, A.; Hofmann, A.I.; Kroon, R.; Sarabia-Riquelme, R.; Weisenberger, M.C.; Müller, C. All-organic textile thermoelectrics with carbon-nanotube-coated n-type yarns. ACS Appl. Energy Mater. 2018, 1, 2934–2941. [Google Scholar] [CrossRef] [PubMed]
- Darabi, S.; Yang, C.Y.; Li, Z.; Huang, J.D.; Hummel, M.; Sixta, H.; Fabiano, S.; Müller, C. Polymer-Based n-Type Yarn for Organic Thermoelectric Textiles. Adv. Electron. Mater. 2023, 9, 2201235. [Google Scholar] [CrossRef]
- Tsai, C.H.; Tung, S.H.; Lin, J.M.; Liu, C.L. A PEDOT: PSS nanocomposite film doped with black phosphorus modified with silver nanoparticles for wearable photothermoelectric generators. J. Mater. Chem. A 2023, 11, 24890–24901. [Google Scholar] [CrossRef]
- Jiang, K.; Hong, S.H.; Tung, S.H.; Liu, C.L. Effects of cation size on thermoelectricity of PEDOT: PSS/ionic liquid hybrid films for wearable thermoelectric generator application. J. Mater. Chem. A 2022, 10, 18792–18802. [Google Scholar] [CrossRef]
- Hasan, M.N.; Ahmad Asri, M.I.; Saleh, T.; Muthalif, A.G.; Mohamed Ali, M.S. Wearable thermoelectric generator with vertically aligned PEDOT: PSS and carbon nanotubes thermoelements for energy harvesting. Int. J. Energy Res. 2022, 46, 15824–15836. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.P.; Gao, C.Y.; Li, H.P.; Fan, X.H.; Cao, X.; Yang, L.M. Electrochemical assembly of flexible PPy/Bi–Te alloy/PPy thermoelectric composite films with a sandwich-type structure. New J. Chem. 2024, 48, 1634–1641. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Zhou, Y.; Buckingham, M.A.; Aldous, L.; Sherrell, P.C.; Wallace, G.G.; Ryder, G.; Faisal, S.; Officer, D.L.; et al. Advanced wearable thermocells for body heat harvesting. Adv. Energy Mater. 2020, 10, 2002539. [Google Scholar] [CrossRef]
- Chen, C.; Wang, R.; Li, X.-L.; Zhao, B.; Wang, H.; Zhou, Z.; Zhu, J.; Liu, J.-W. Structural Design of Nanowire Wearable Stretchable Thermoelectric Generator. Nano Lett. 2022, 22, 4131–4136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Z.; Zhou, J.; Shao, L.; Wang, Y.; Deng, Y. High-performance Stretchable Organic Thermoelectric Generator via Rational Thermal Interface Design for Wearable Electronics. Adv. Energy Mater. 2022, 12, 2102835. [Google Scholar] [CrossRef]
- Liang, L.; Wang, M.; Wang, X.; Peng, P.; Liu, Z.; Chen, G.; Sun, G. Initiating a stretchable, compressible, and wearable thermoelectric generator by a spiral architecture with ternary nanocomposites for efficient heat harvesting. Adv. Funct. Mater. 2022, 32, 2111435. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, B.; Zheng, Q.; Wang, L.; Jiang, W.; Snyder, G.J. Stretchable fabric generates electric power from woven thermoelectric fibers. Nat. Commun. 2020, 11, 572. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, Y.; Sheng, M.; Wang, Y.; Yu, Y.; Deng, Y. A flexible active dual-parameter sensor for sensitive temperature and physiological signal monitoring via integrating thermoelectric and piezoelectric conversion. J. Mater. Chem. A 2019, 7, 8258–8267. [Google Scholar] [CrossRef]
- Liu, H.; Sun, K.; Guo, X.-L.; Liu, Z.-L.; Wang, Y.-H.; Yang, Y.; Yu, D.; Li, Y.-T.; Ren, T.-L. An Ultrahigh Linear Sensitive Temperature Sensor Based on PANI:Graphene and PDMS Hybrid with Negative Temperature Compensation. ACS Nano 2022, 16, 21527–21535. [Google Scholar] [CrossRef]
- Wei, H.; Li, A.; Kong, D.; Li, Z.; Cui, D.; Li, T.; Dong, B.; Guo, Z. Polypyrrole/reduced graphene aerogel film for wearable piezoresisitic sensors with high sensing performances. Adv. Compos. Hybrid Mater. 2021, 4, 86–95. [Google Scholar] [CrossRef]
- Jang, G.N.; Hong, S.Y.; Park, H.; Lee, Y.H.; Park, H.; Lee, H.; Jeong, Y.R.; Jin, S.W.; Keum, K.; Ha, J.S. Highly sensitive pressure and temperature sensors fabricated with poly(3-hexylthiophene-2,5-diyl)-coated elastic carbon foam for bio-signal monitoring. Chem. Eng. J. 2021, 423, 130197. [Google Scholar] [CrossRef]
- Han, Y.; Sun, L.; Wen, C.; Wang, Z.; Dai, J.; Shi, L. Flexible conductive silk-PPy hydrogel toward wearable electronic strain sensors. Biomed. Mater. 2022, 17, 024107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lai, J.; Zheng, B.; Jin, X.; Zhao, G.; Liu, H.; Chen, W.; Ma, A.; Li, X.; Wu, Y. From Glutinous-Rice-Inspired Adhesive Organohydrogels to Flexible Electronic Devices Toward Wearable Sensing, Power Supply, and Energy Storage. Adv. Funct. Mater. 2021, 32, 2108423. [Google Scholar] [CrossRef]
- Fan, W.; An, Z.; Liu, F.; Gao, Z.; Zhang, M.; Fu, C.; Zhu, T.; Liu, Q.; Zhao, X. High-Performance Stretchable Thermoelectric Generator for Self-Powered Wearable Electronics. Sci. Adv. 2023, 10, 2206397. [Google Scholar] [CrossRef]

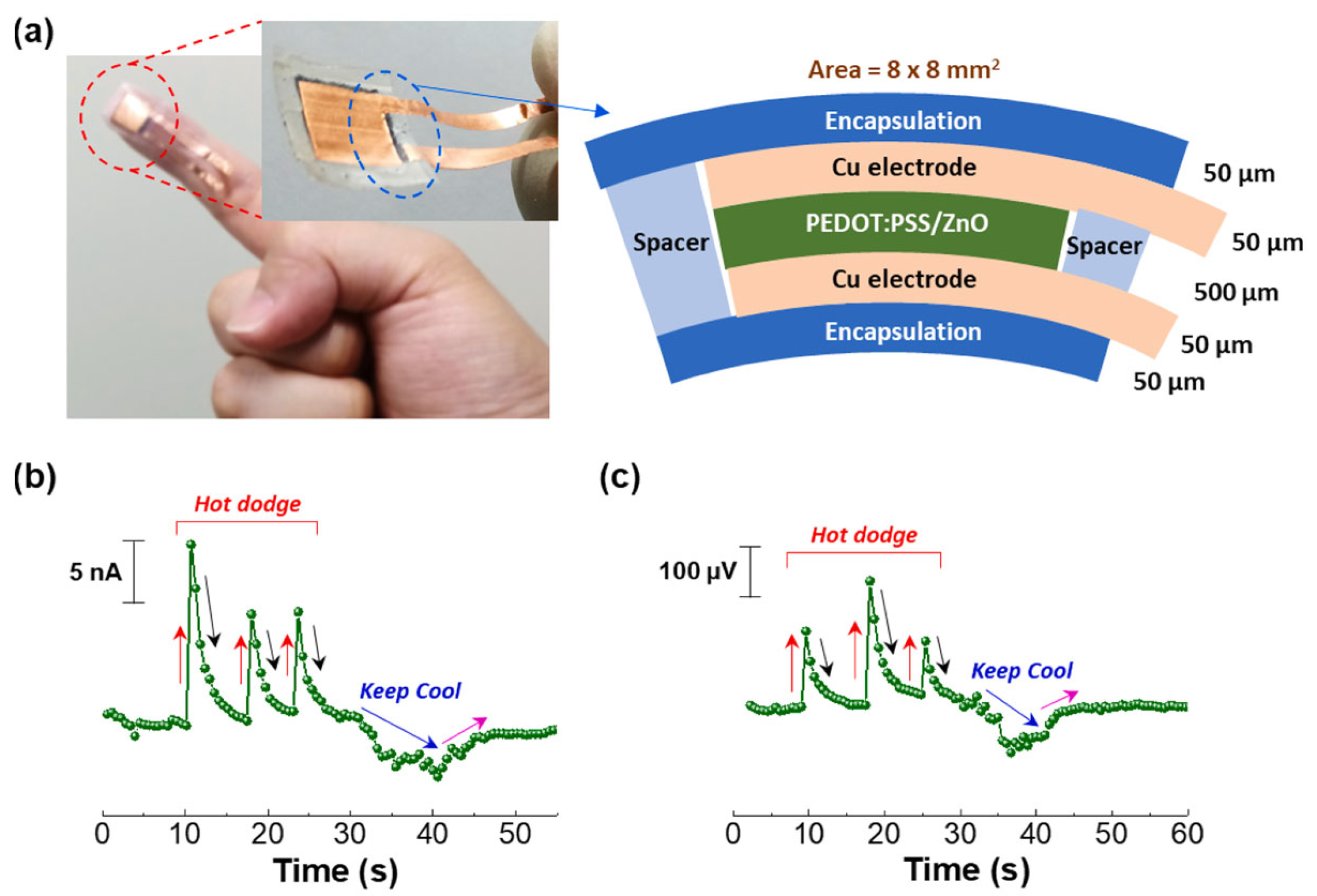

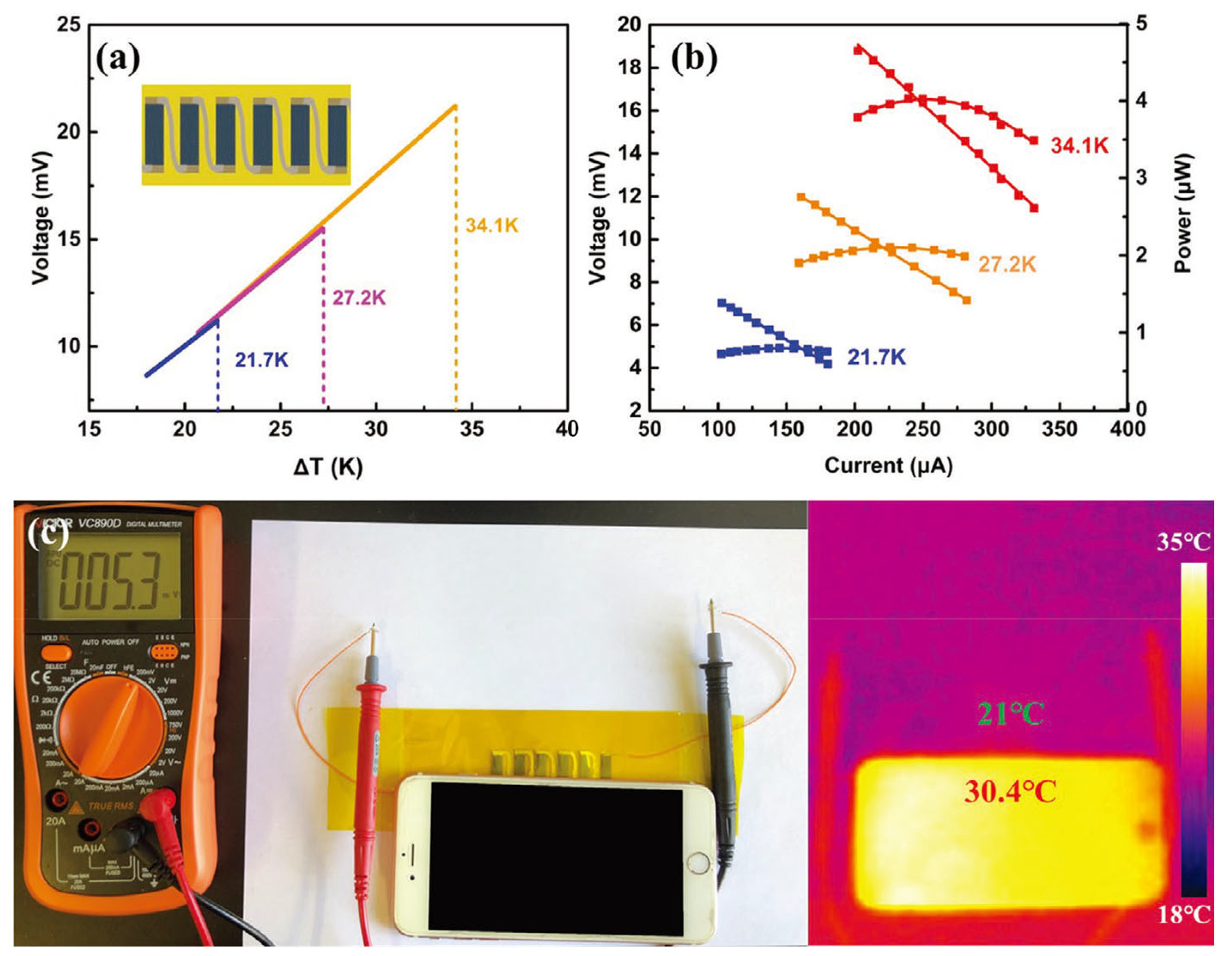

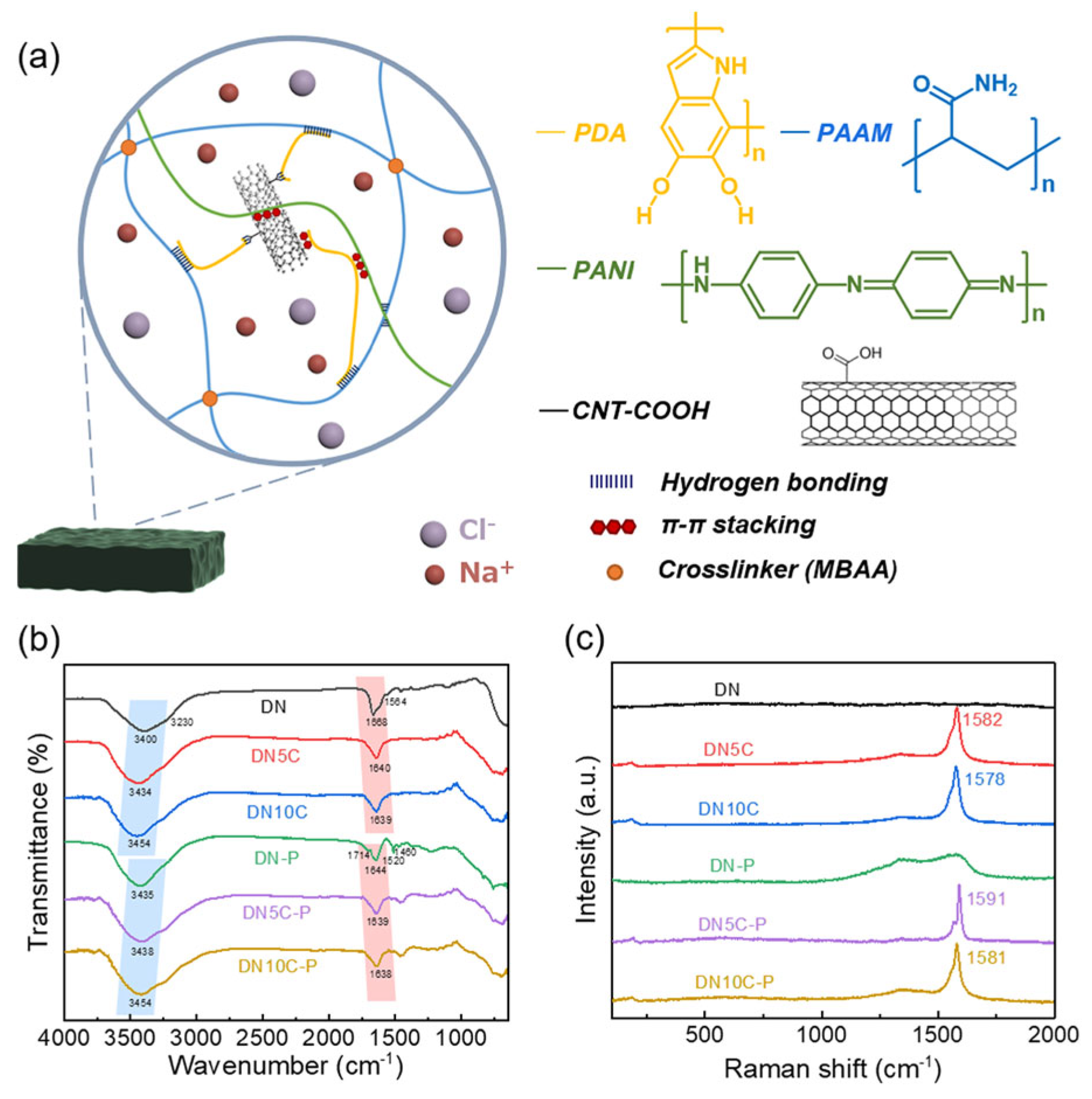
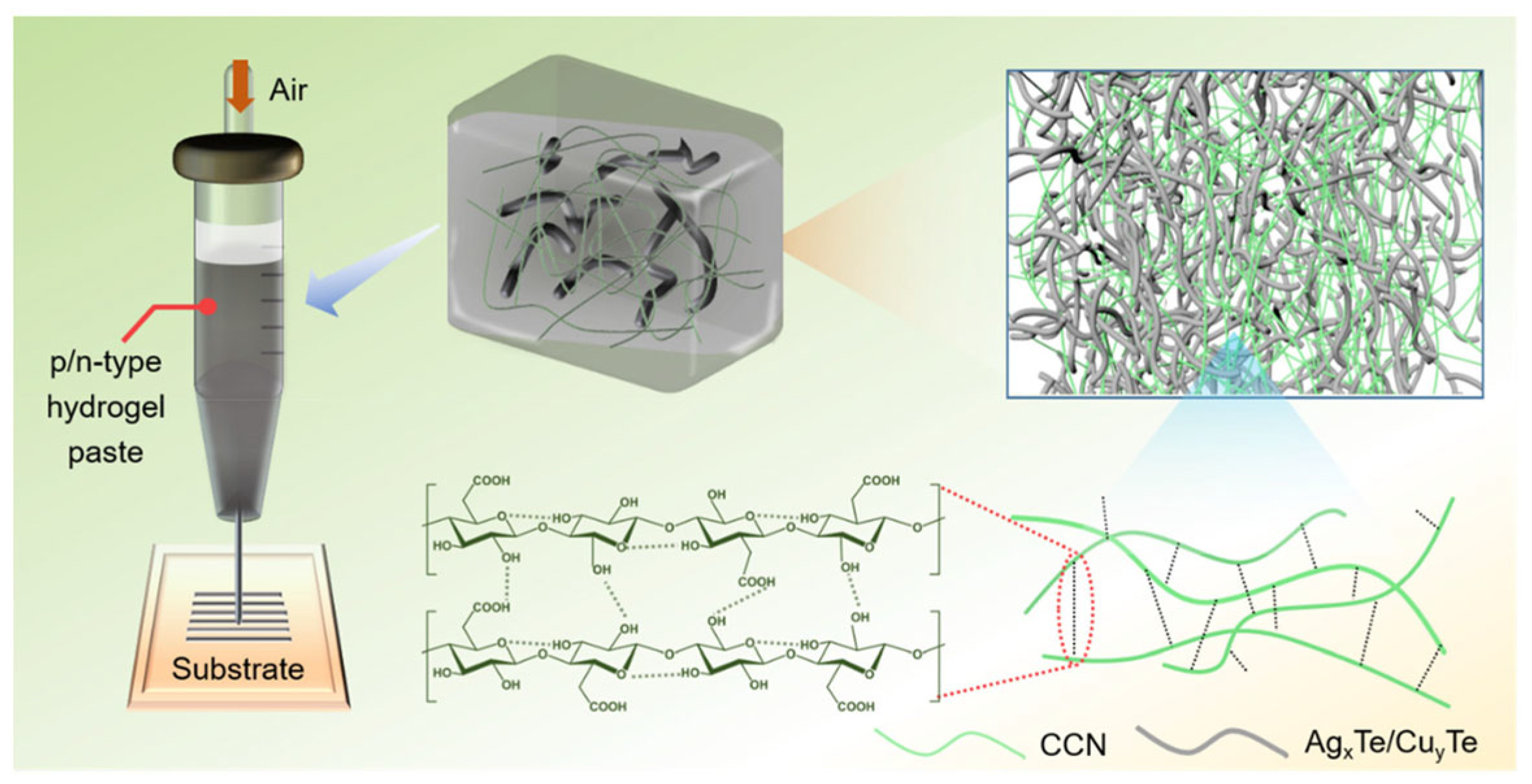
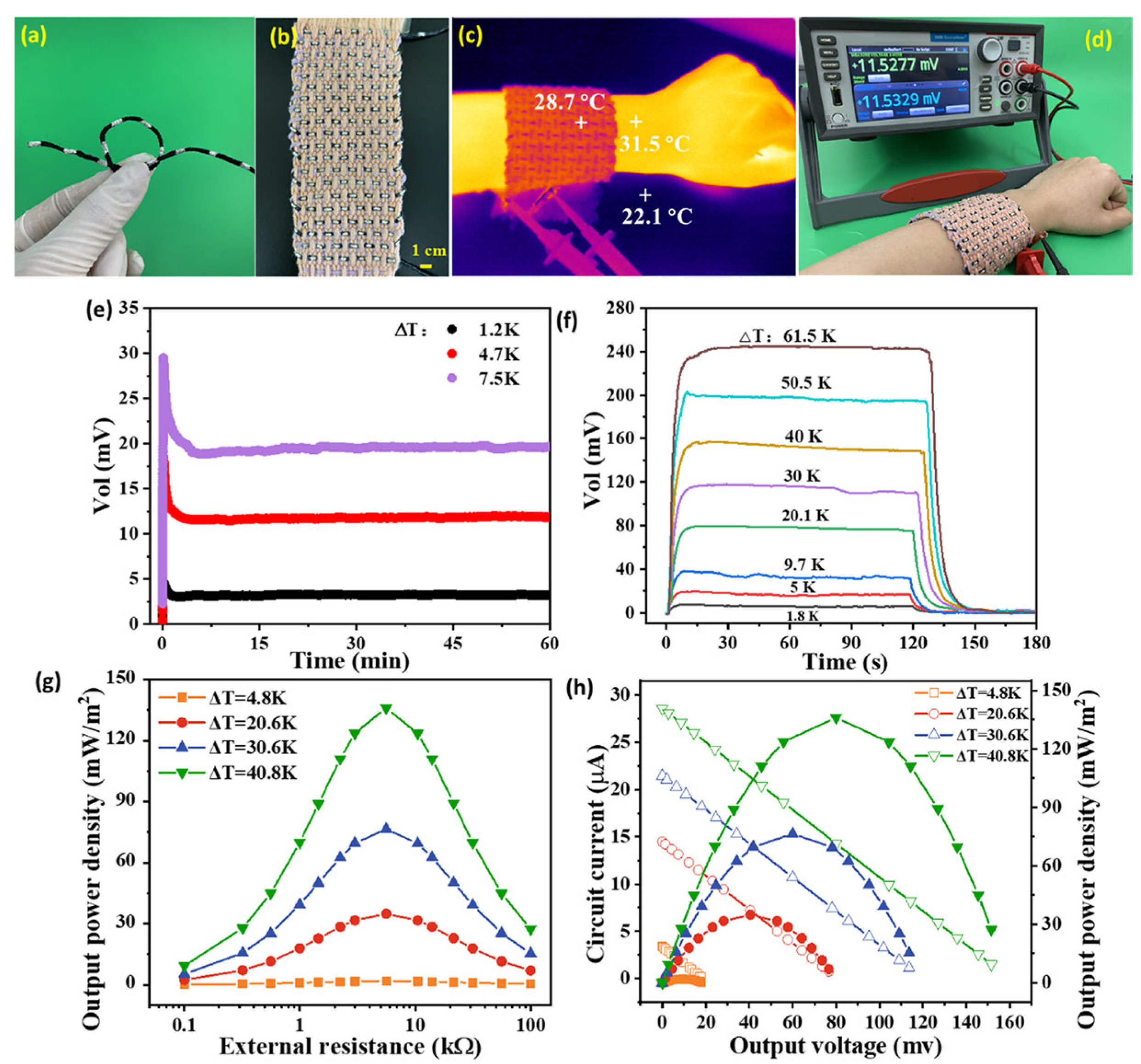
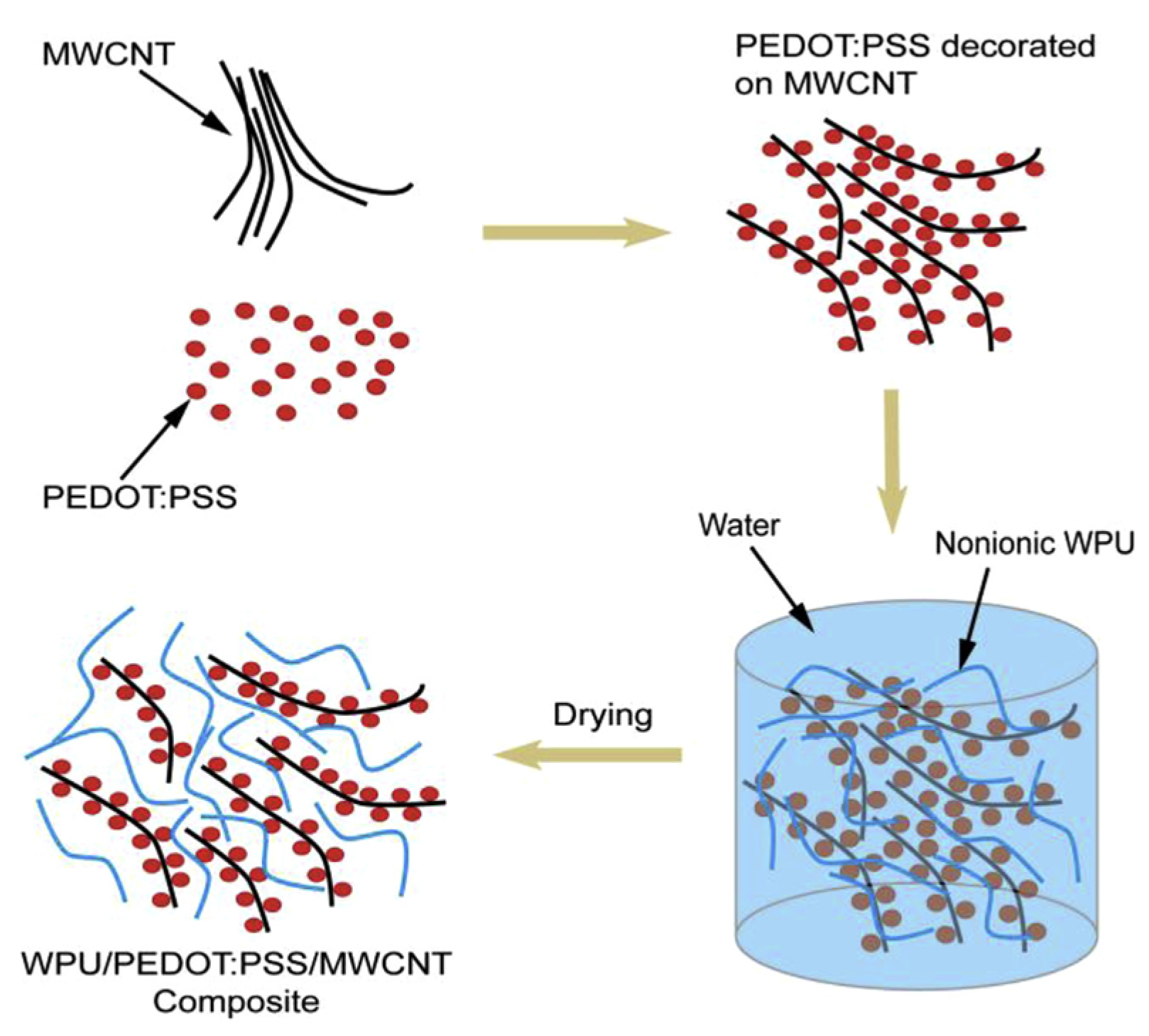

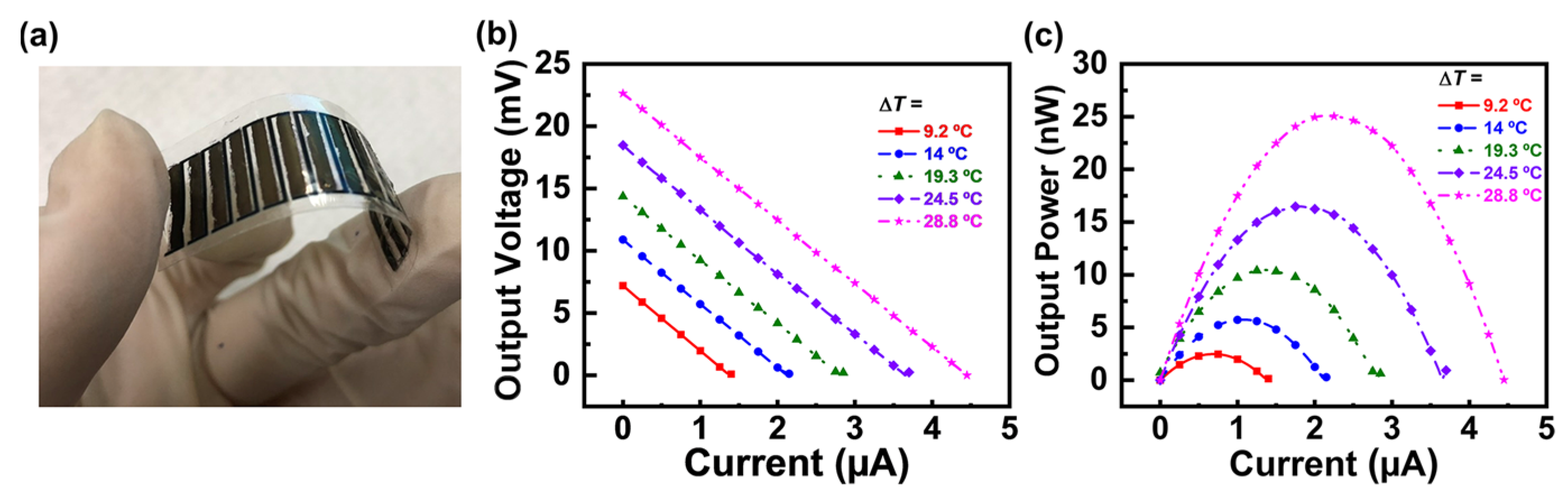
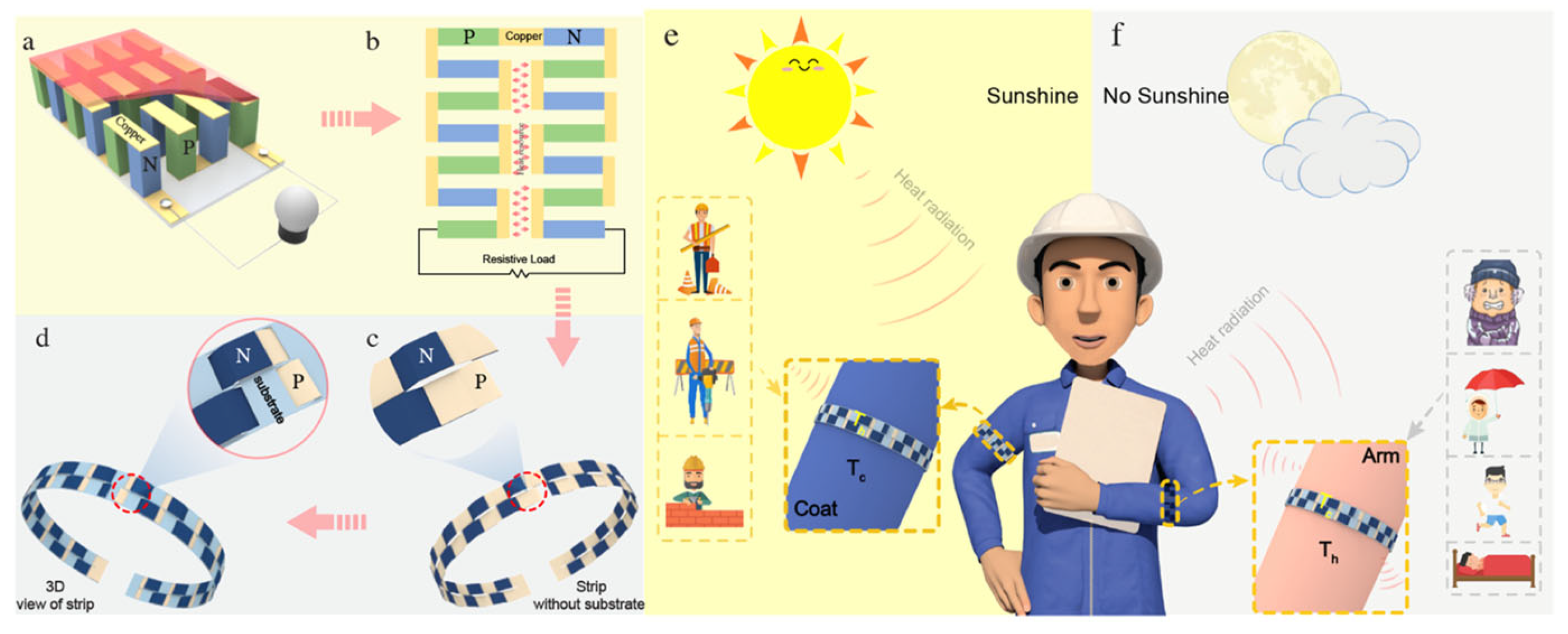
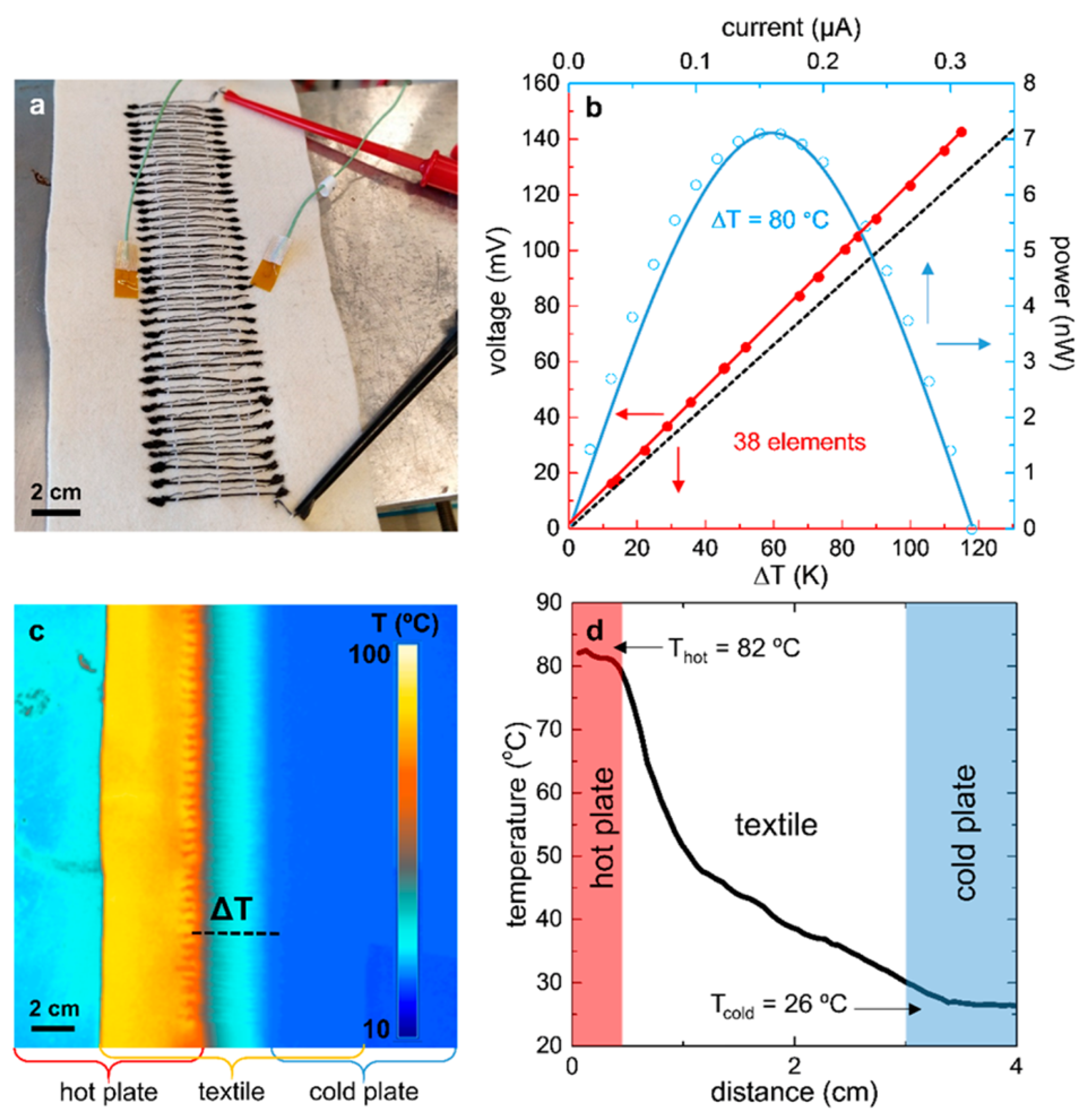
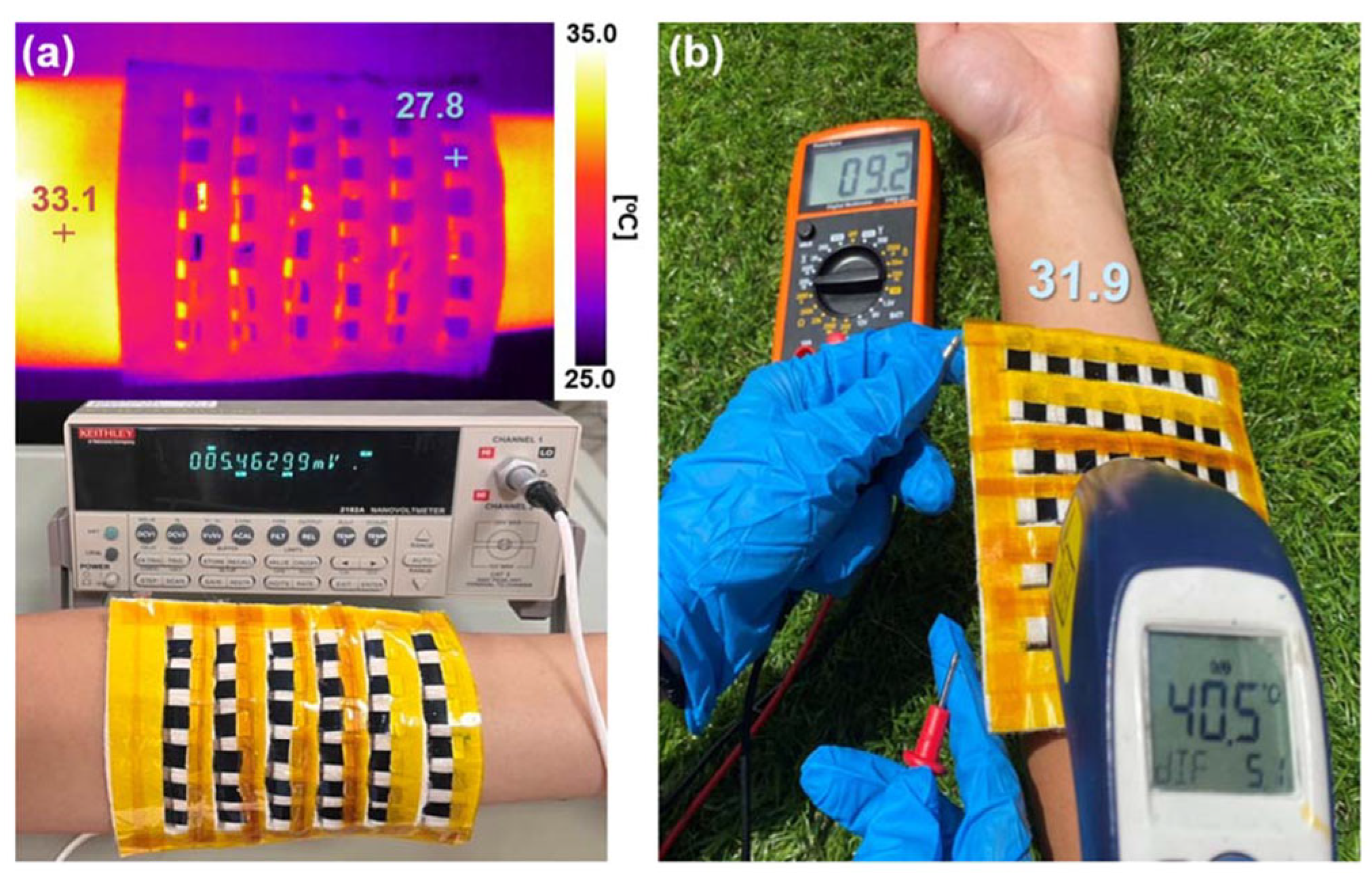
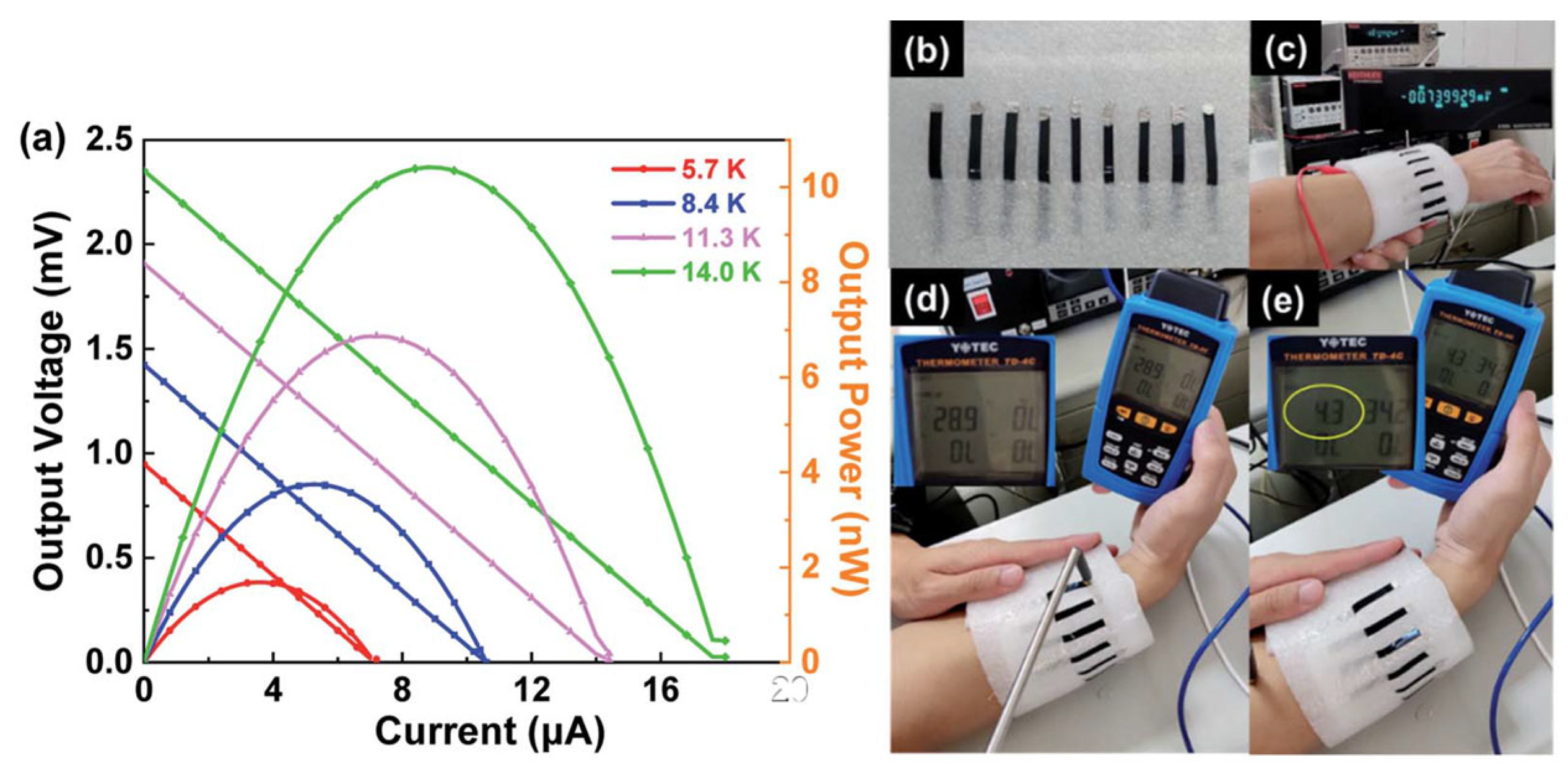
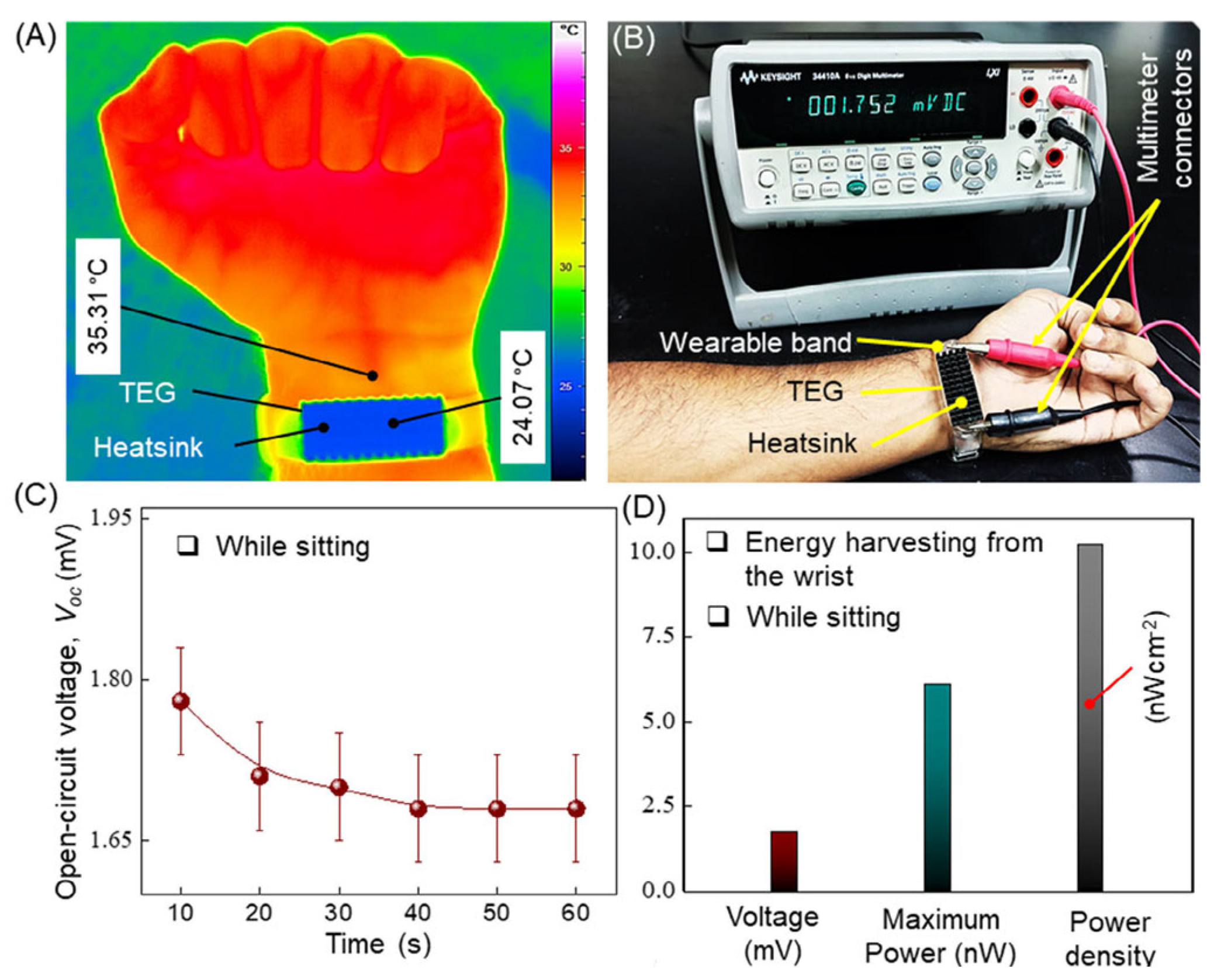

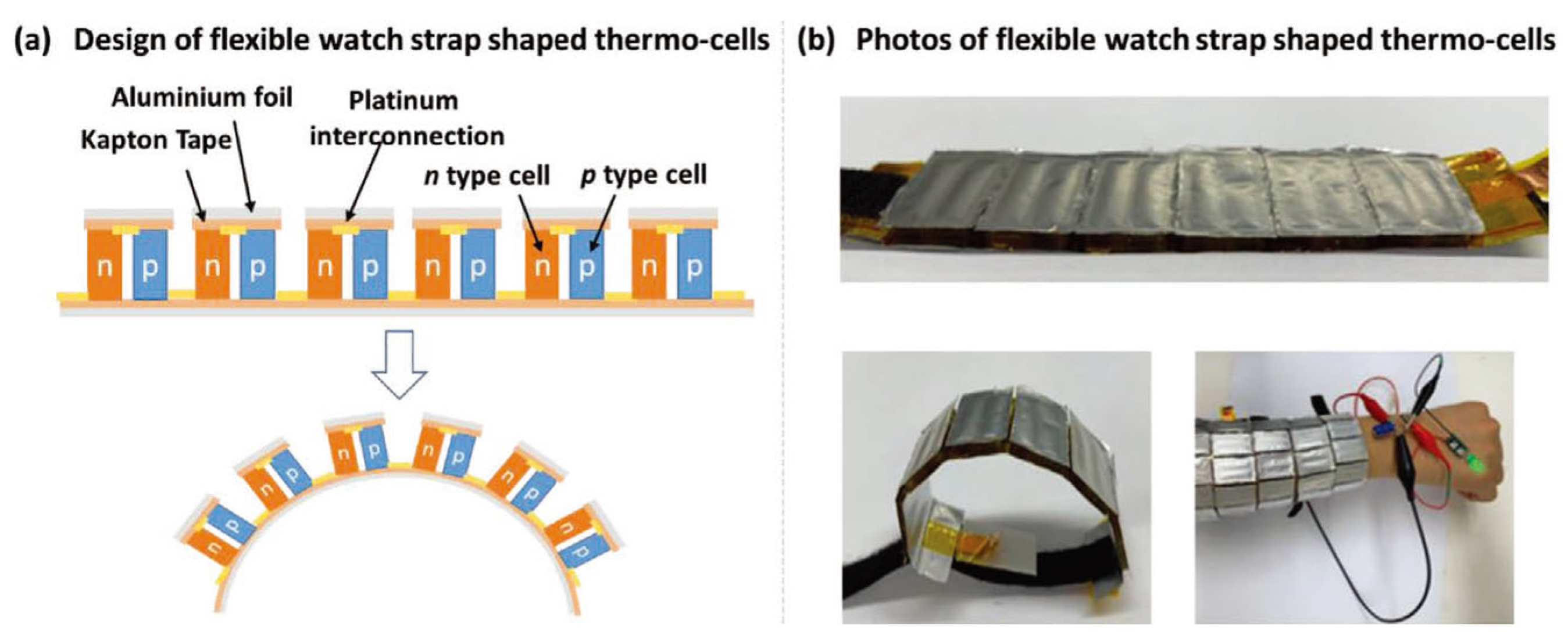

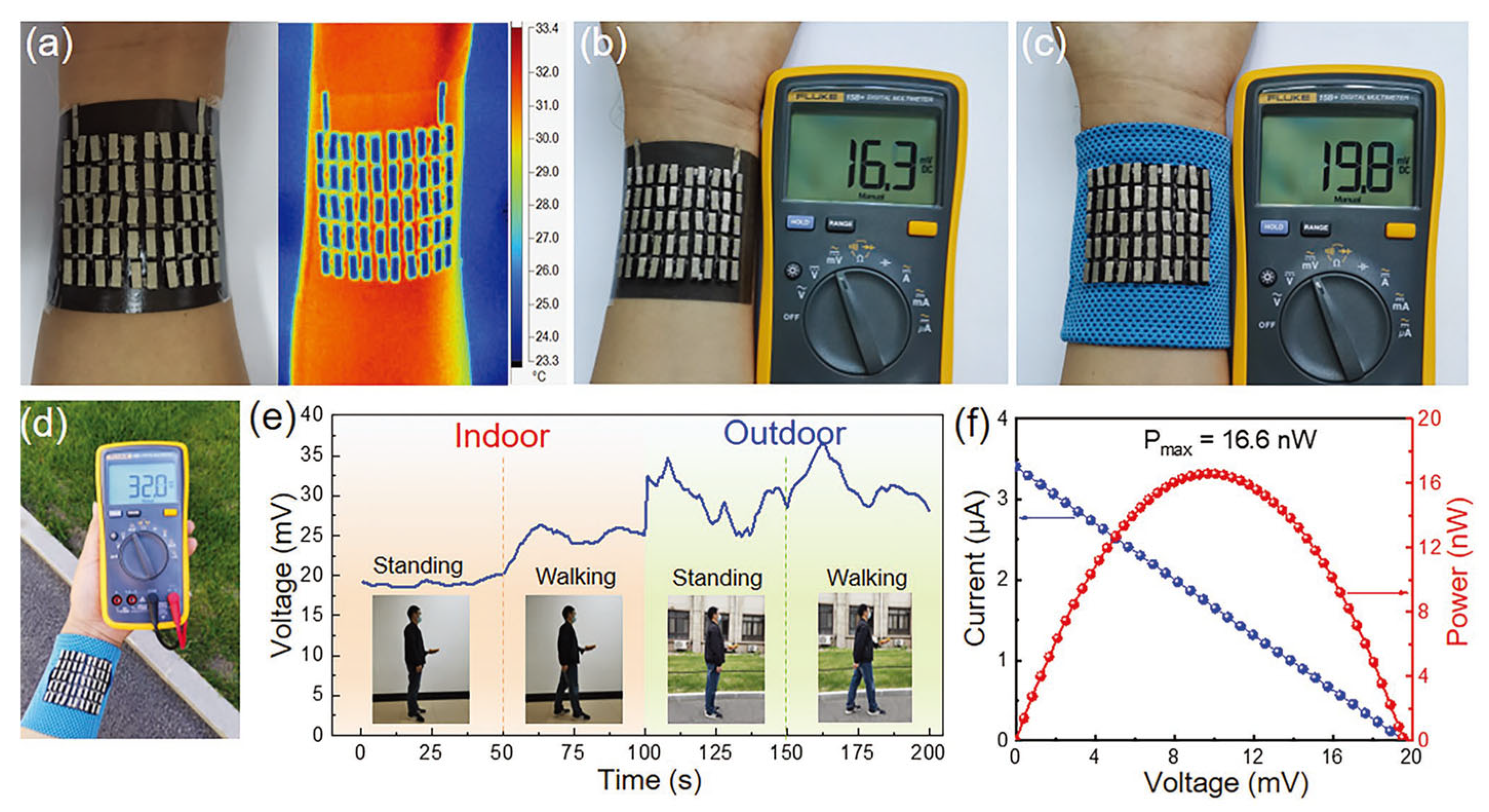


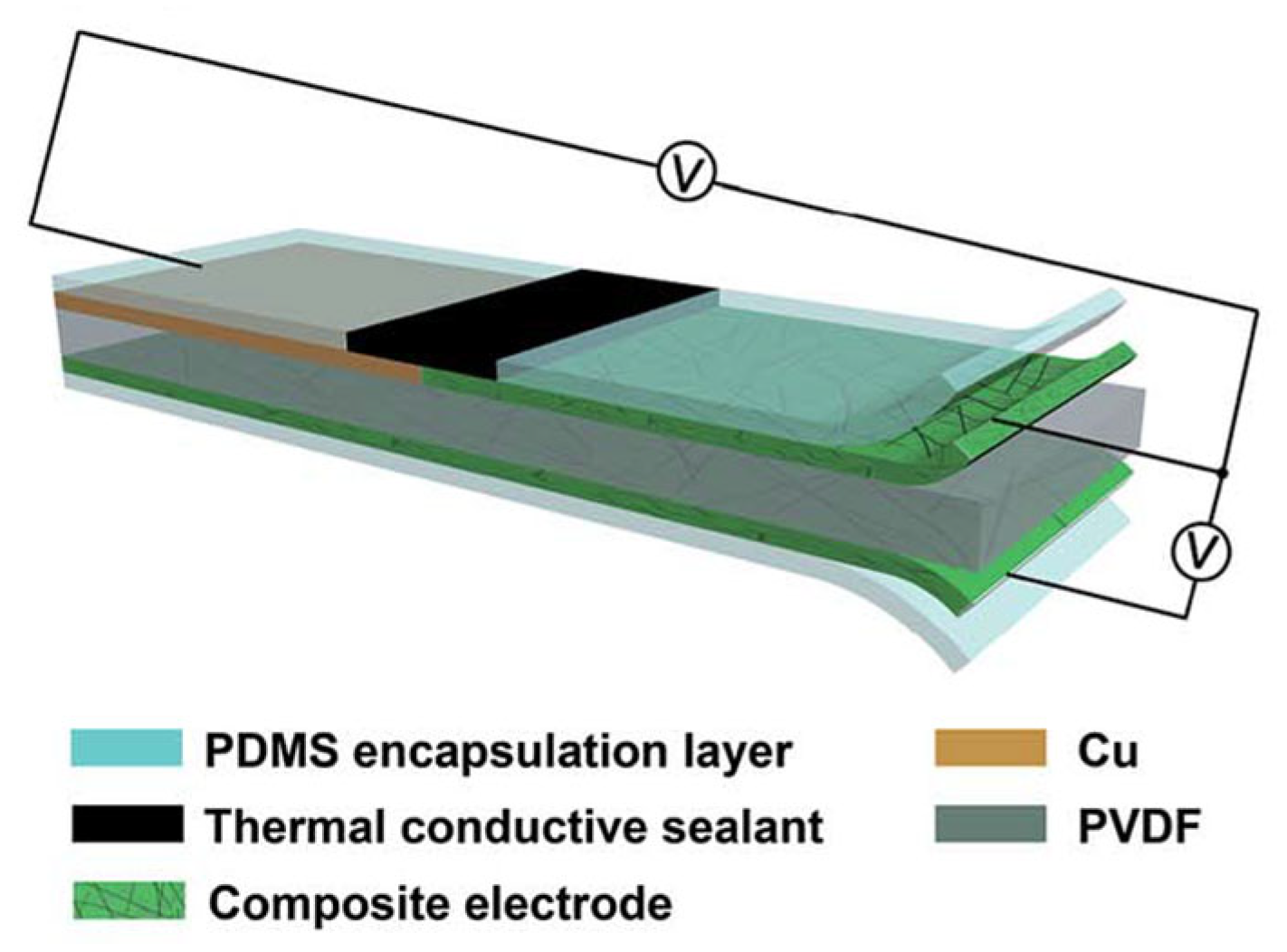
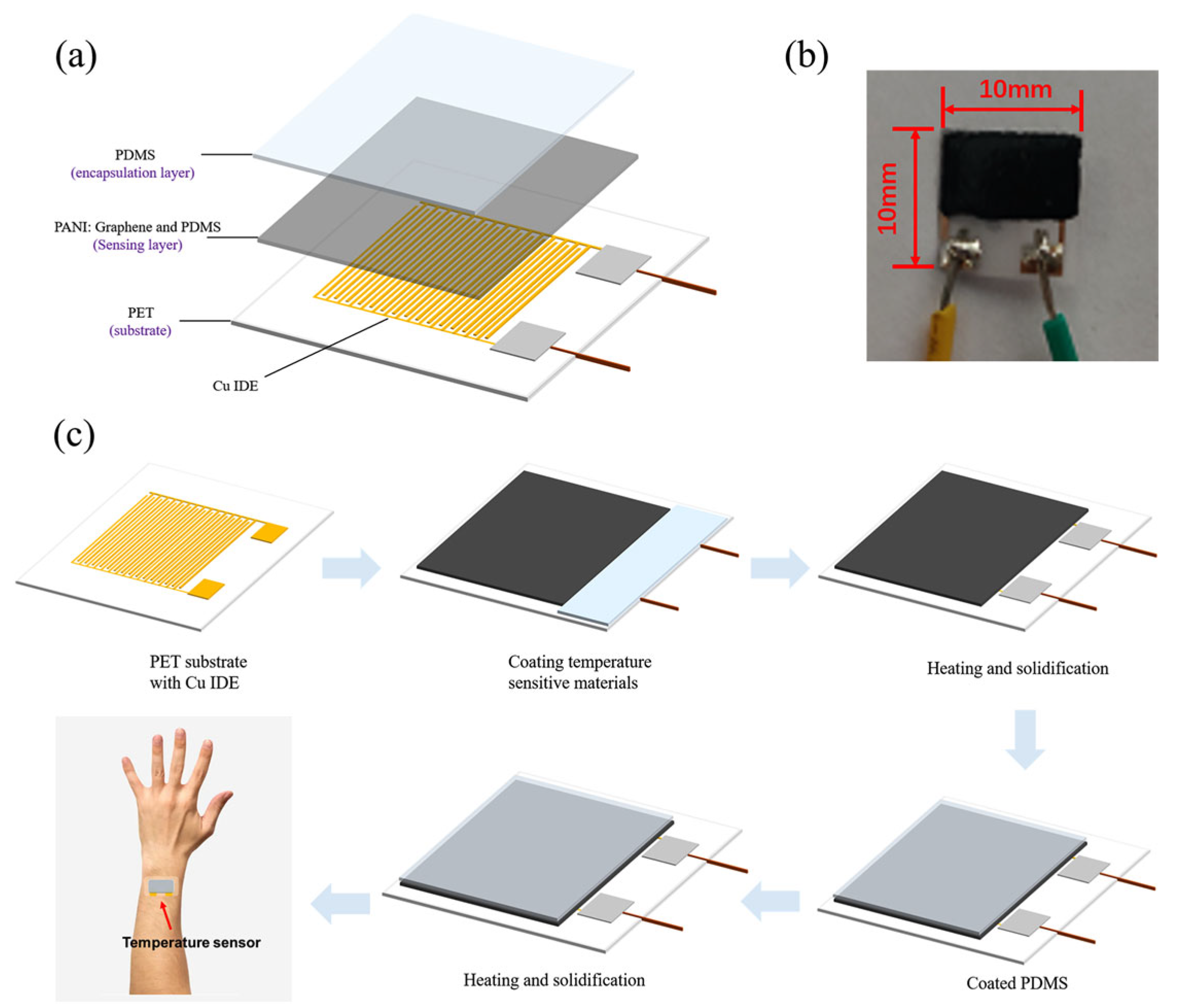
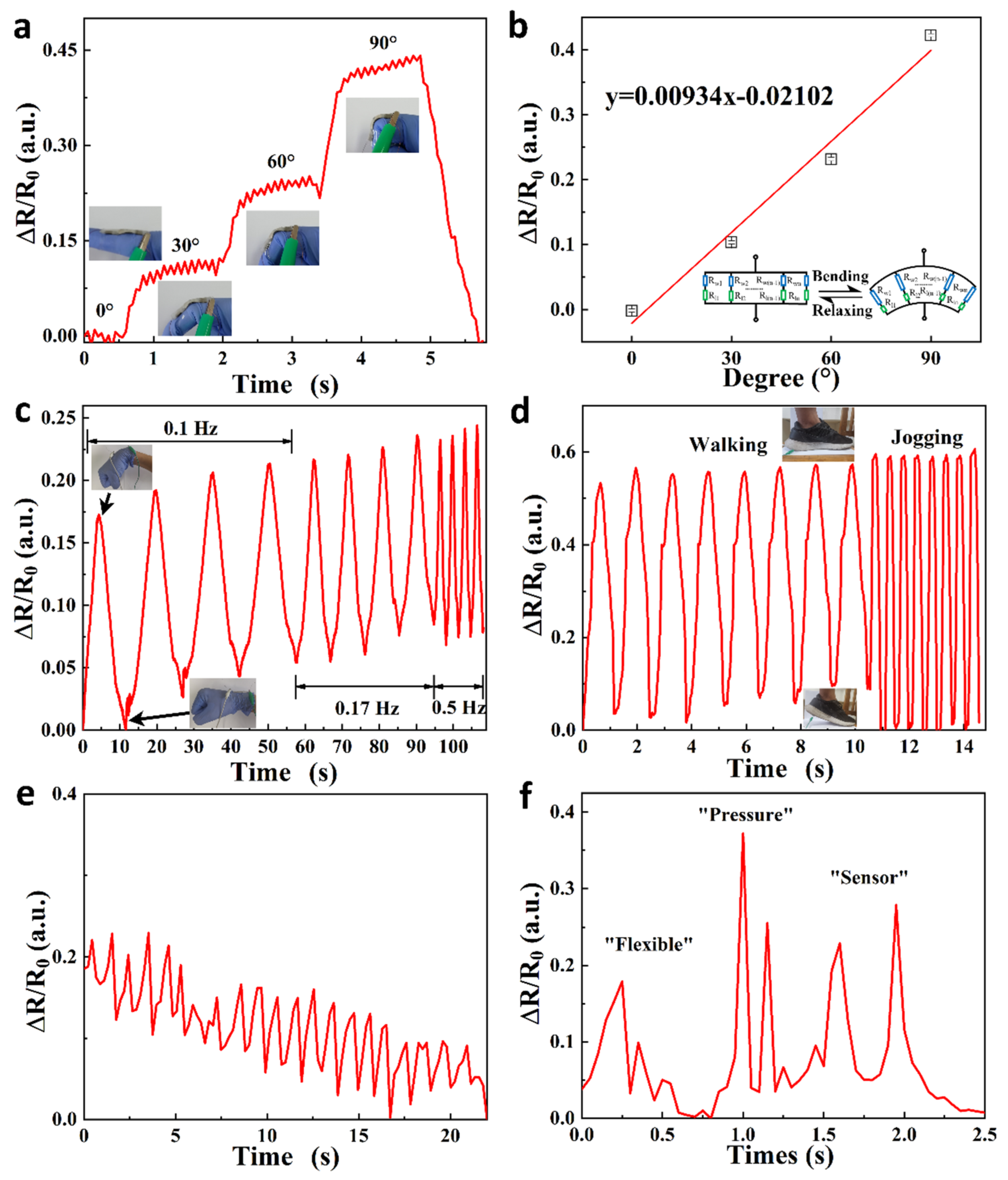
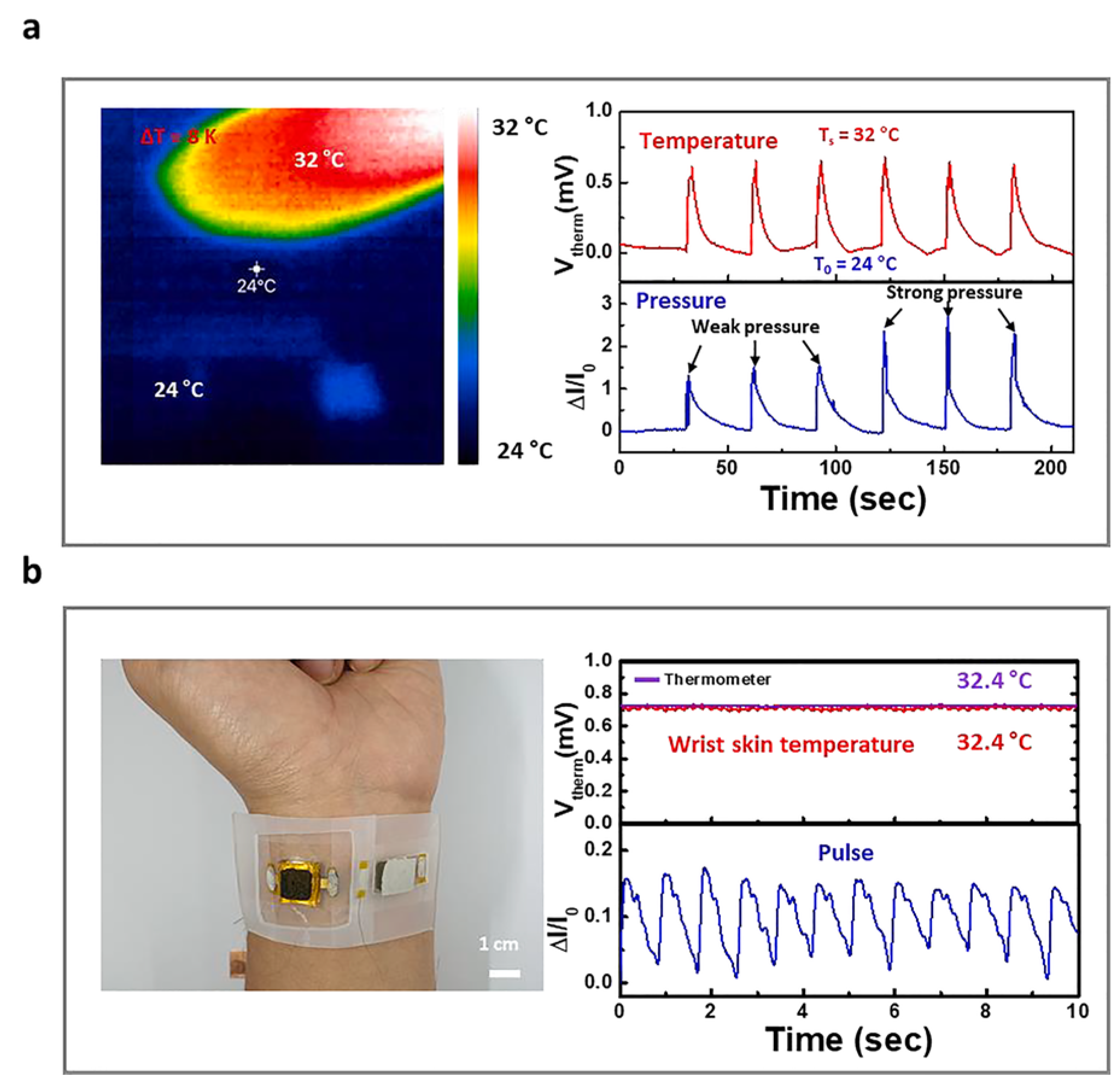

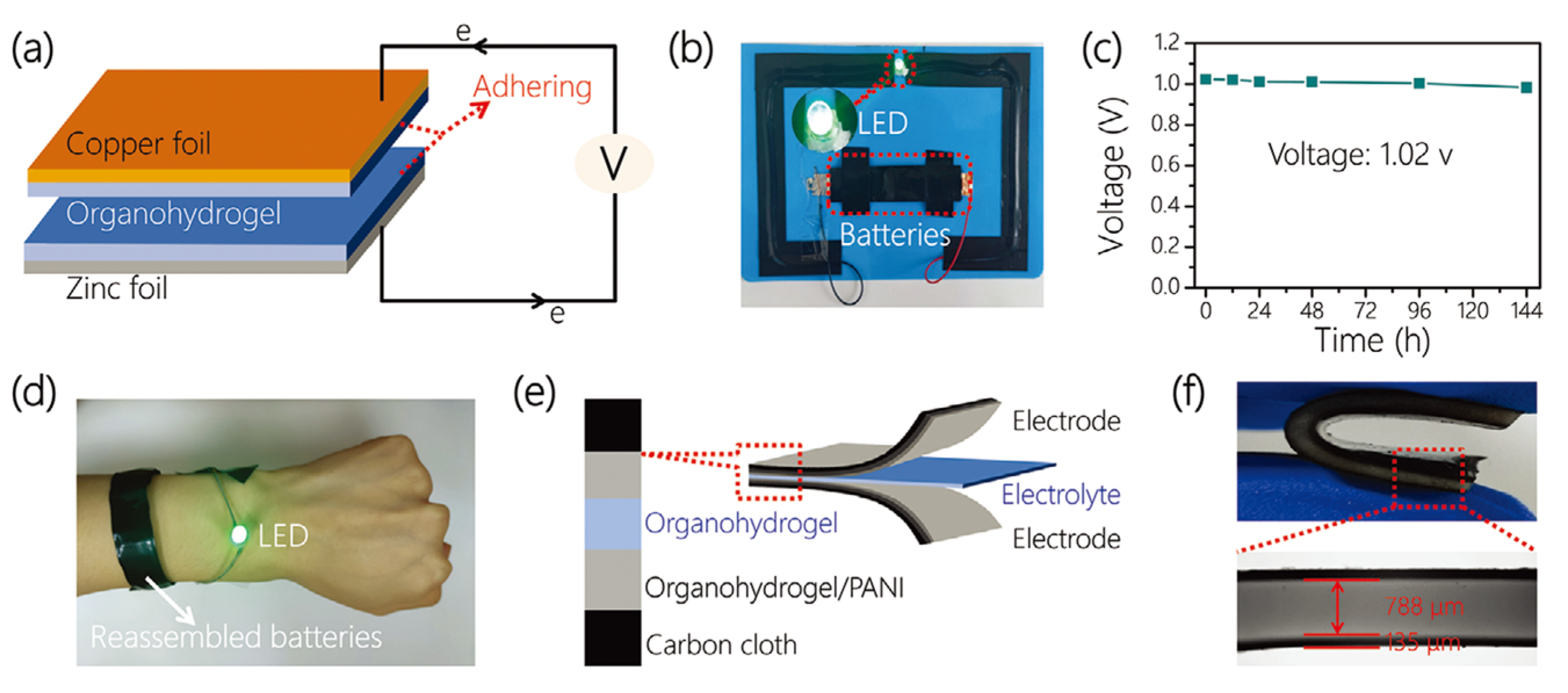

| Material | S | σ | PF | Ref. |
|---|---|---|---|---|
| µV/K | S/cm | µW/m K2 | ||
| PBFDO | −21 | 2000 | 90 | [80] |
| PEDOT:Tos | 117 | 923 | 1270 | [82] |
| PEDOT:PSS | 72.6 | 890 | 469 | [83] |
| PEDOT:PSS (H2SO4 and NaOH post treatments) | 39.2 | 2170 | 334 | [84] |
| PEDOT:PSS (a solution of 0.1 M MAl in 80% DMF/20% water) | 28 | 1831 | 144 | [85] |
| PEDOT:PSS (IL treatment) | 17 | 520 | 15 | [86] |
| PEDOT:PSS (IL and acid-treated, then base-treated) | 1594.8 | 63.3 | 754 | [87] |
| PEDOT:OTf (NaOH treatment) | 2342 ± 98 | 49.2 | 568 ± 64 | [88] |
| PEDOT:NWs (H2SO4 and NaOH treatments) | 715.3 | 25.5 | 46.51 | [89] |
| SWCNT/PANI (ethanol treatment) | 2356 | 39.2 | 362 | [90] |
| P3HT | 39.5 | 2 | 31 | [91] |
| PPy nanowires (chemical oxidation polymerization) | 2.22 ± 0.3 | 10.1 ± 0.1 | 2.26 ± 0.36 | [92] |
| PPy nanotube (chemical oxidation polymerization) | 33.82 | 12.76 | 0.55 | [93] |
| PPy/rGO (template-directed in situ polymerization) | 41.6 | 26.9 | 3.01 | [94] |
| PPy/MWCNTs (68 wt%) (in situ polymerization) | 35–40 | 24.4 | 2.2 | [95] |
| PPy/SWCNTs (interfacial polymerization) | 47 ± 34.2 | 33.2 ± 0.7 | 37.6 ± 2.3 | [96] |
| PDPF | −235 | 1.35 | 4.65 | [97] |
| PDPH | −87 | 1.01 × 10−4 | 5.11 × 10−4 | [97] |
| BBL | −101 | 0.42 | 0.43 | [97] |
| FBDPPV | −210 | 14 | 25.5 | [97] |
| BDPPV | −320 | 0.26 | 1.6 | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, R.; Zhou, X.; Zhang, C.; Liu, X.; Han, S.; Che, C. Organic Thermoelectric Materials for Wearable Electronic Devices. Sensors 2024, 24, 4600. https://doi.org/10.3390/s24144600
Xiao R, Zhou X, Zhang C, Liu X, Han S, Che C. Organic Thermoelectric Materials for Wearable Electronic Devices. Sensors. 2024; 24(14):4600. https://doi.org/10.3390/s24144600
Chicago/Turabian StyleXiao, Runfeng, Xiaoyan Zhou, Chan Zhang, Xi Liu, Shaobo Han, and Canyan Che. 2024. "Organic Thermoelectric Materials for Wearable Electronic Devices" Sensors 24, no. 14: 4600. https://doi.org/10.3390/s24144600





