Smart Bioimpedance Device for the Assessment of Peripheral Muscles in Patients with COPD
Abstract
:1. Introduction
2. Materials and Methods
- Study population: Patients diagnosed with COPD following the criteria established by the American Thoracic Society (ATS) and the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) who presented moderate-severe airflow obstruction (forced expiratory volume in the first second ( ) < 80%) and with a clinical impact of their disease (criteria for inclusion in the respiratory rehabilitation program of the Virgen del Rocío University Hospital in Seville, Spain). All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Research Ethics Committee of the Virgen Macarena and Virgen del Rocío University Hospitals (Project identification code: PI-0041-2014). The patients were stable and with appropriate therapy, and had not suffered exacerbations of the disease in a period of three months prior to the study, and without treatment with oral corticosteroids for at least the same period.
- Measurements: The reference measurements of muscle dimensions were carried out by ultrasound using an Aloka SSD-900 Ultrasound Machine (ALOKA Co. Ltd., Tokyo, Japan). Procedure: The patient was placed in a supine position, with a rolled towel under the popliteal fossa to relax the thigh. The transducer was placed with the long axis perpendicular to the longitudinal axis of the rectus femoris muscle, at the midpoint between the anterior superior iliac spine and the superior edge of the patella. Using the device’s software, the area and dimensions of different muscle sections were estimated, recording the data for subsequent analysis. The following measurements were obtained, referring to the image in Figure 1: thickness of the vastus intermedius (), length from the femur to the upper limit of the rectus femoris (), length from the femur to the skin (), transverse thickness of the rectus femoris () and area of the rectus femoris (). Isometric strength is defined as a static contraction without a change in muscle length. To measure this strength, the subject was seated and with a knee flexion of 90°. The knee was extended against static resistance and the isometric quadriceps strength was established using an isometric dynamometer. The isotonic force was evaluated through the 1RM test, defined in this case as the maximum weight that an individual sitting and with knee flexion of 90º can lift in a single repetition. For bioimpedance measurements, the adapted device described in the present work was used following the measurement protocol described in Section 2.1.
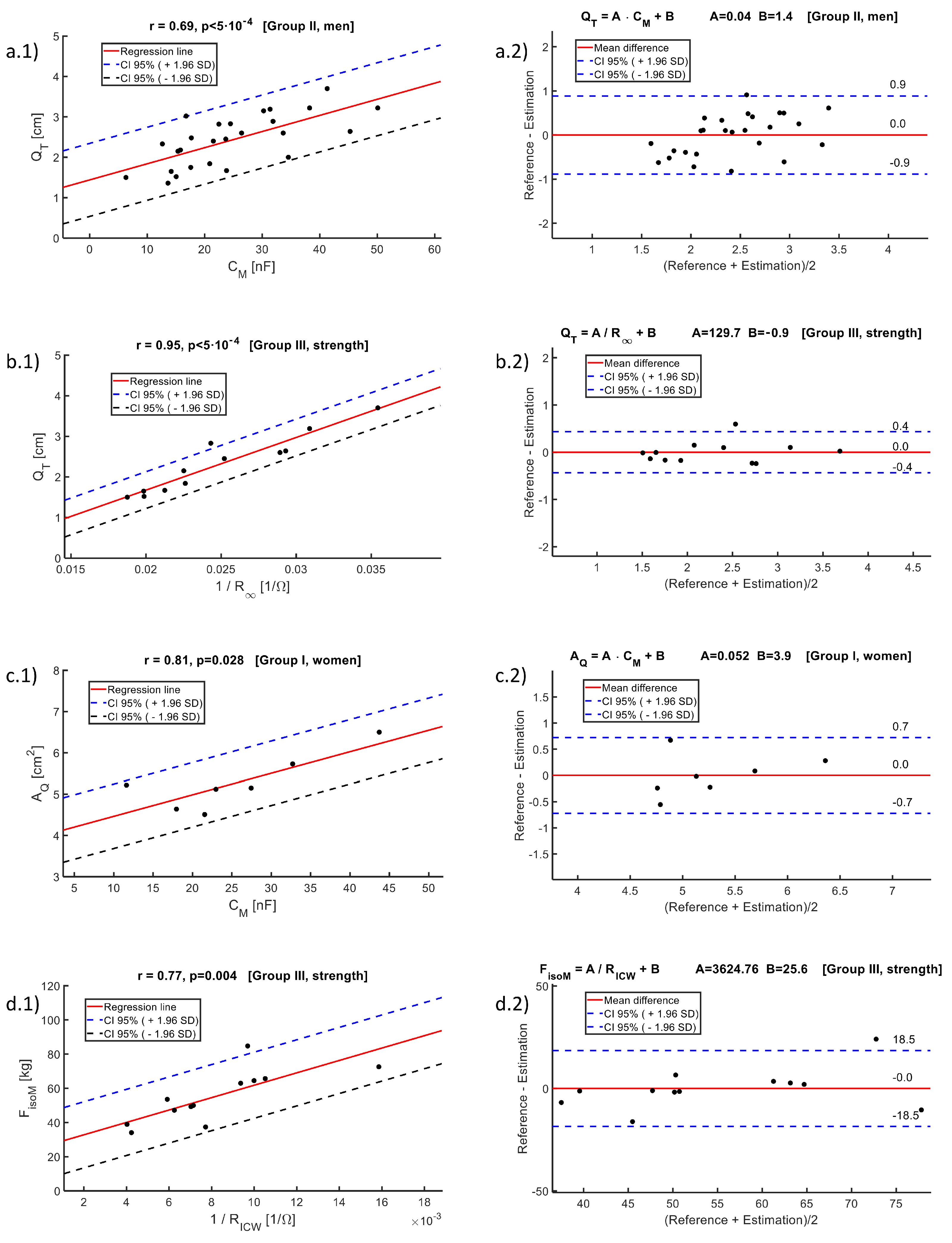
- Data analysis: For the comparative analysis of the groups, the mean value and standard deviation (SD) were used. To compare the means between groups, the one-factor analysis of variance (ANOVA) method was used. Normality was established by the D’Agostino and Pearson test. The reference values used in the correlation analysis were estimations of quadriceps dimensions performed by ultrasound and measurements of muscle strength (isometric and isotonic). The reference values were compared with the parameters of the Cole bioimpedance model using the Pearson coefficient (or the Spearman coefficient in case the data did not have a normal distribution), considering results statistically significant when . The regression line that minimized the mean square error was estimated, performing a Bland–Altman agreement analysis, calculating the average difference between the measurements and the predictions of the regression line, with its corresponding SD. According to the Bland–Altman method, the 95% confidence intervals of the differences were calculated from the upper and lower agreement limits, ( times the SD). The Matlab desktop environment (version R2022a) for Windows was used to analyze the data.
2.1. Experimental Setup for Bioimpedance Measurements
- Before carrying out the measurements, the patient remained on a stretcher in a supine position for 5 min to promote homogeneous distribution of water throughout the body.
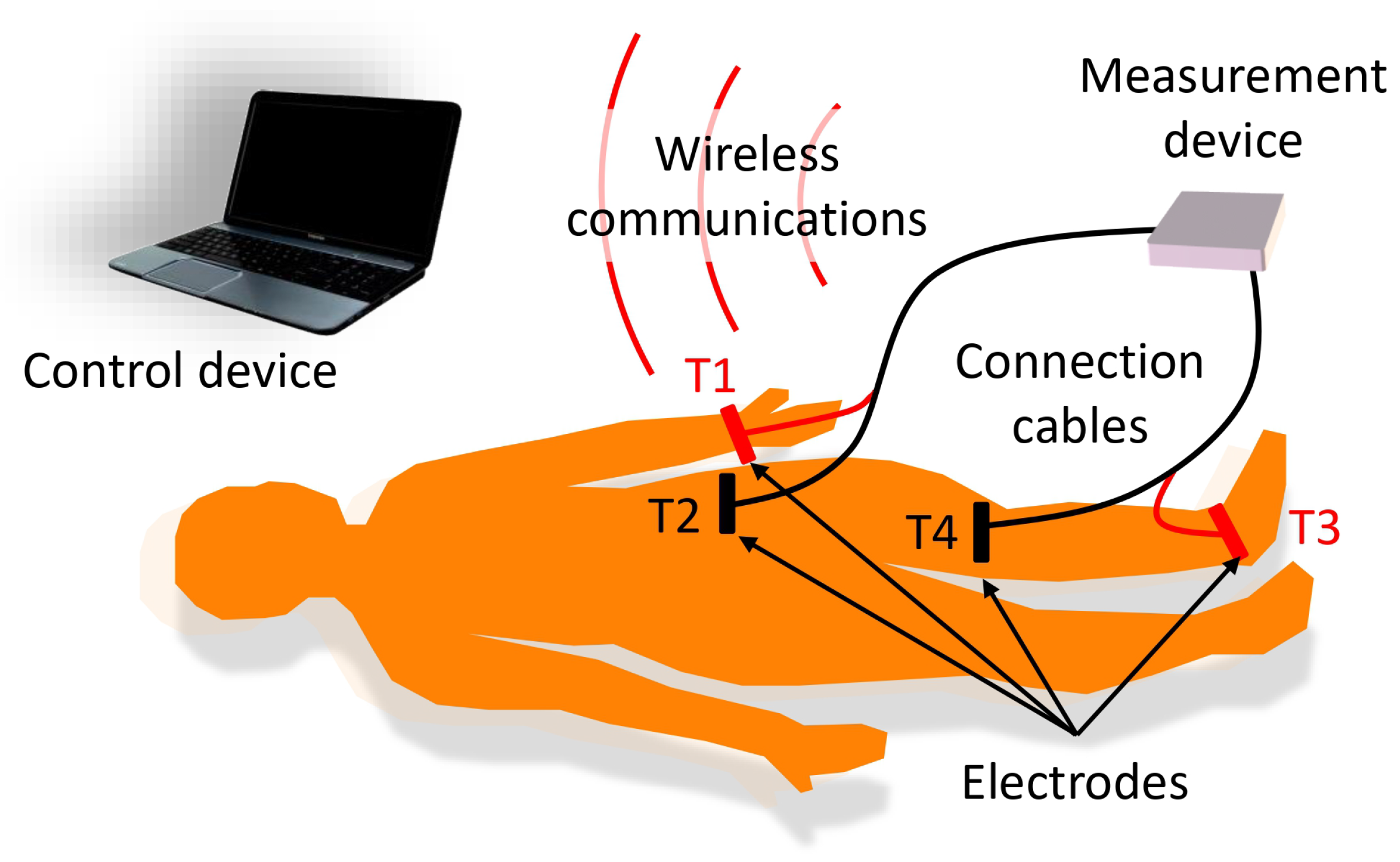
- For the bioimpedance measurements, four electrodes were used, following the measurement scheme described in [37], with two electrodes for current injection (T1 and T3 in Figure 2) and two electrodes for voltage measurement (T2 and T4 in Figure 2). The current injection electrodes were placed on the foot (dorsal surface at the level of the metatarsals) and the hand (dorsal surface above the knuckles) on the side of the body corresponding to the leg on which the measurements were made. The voltage measurement electrodes were located at the ends of the quadriceps region, one above the kneecap and the other at the level of the anterior superior iliac spine. Figure 2 shows a scheme of the measurement procedure and the location of the electrodes.
- In each measurement, the device estimated the real and imaginary part of the bioimpedance in 22 frequencies distributed logarithmically between 5 kHz and 1 MHz.
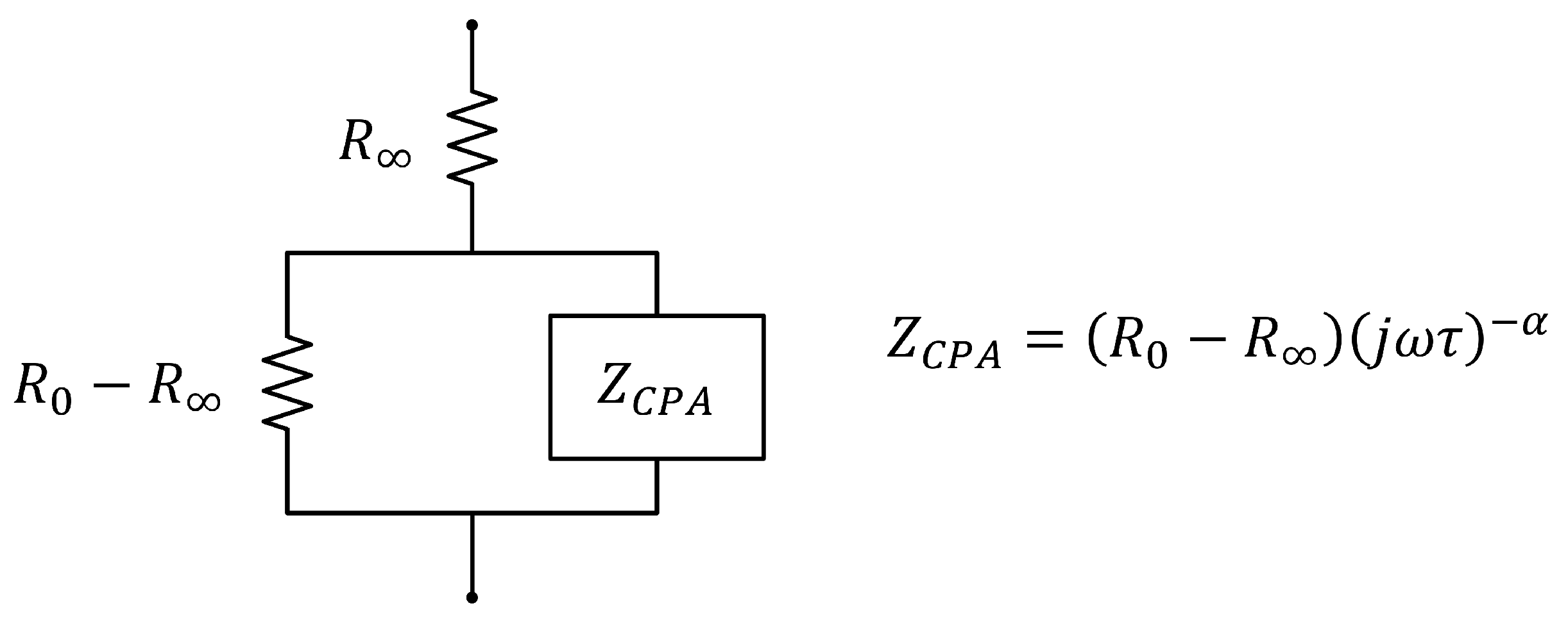
- The complex bioimpedance values were sent wirelessly to a laptop computer (control device, in the modular scheme described in Section 3.1), which estimated the parameters of the extended-Cole bioimpedance model that best fit with the bioimpedance values (see Table 1):The equations that define the extended-Cole model are the following, with being the angular frequency that corresponds to and f the frequency in Hz:
- The measurement process was repeated 3 times sequentially to allow the analysis of the repeatability of the measurements. Finally, the average value of the bioimpedance parameters of the three measurements was selected for the correlation and agreement analysis.
3. Results
3.1. Prototype Design and Development
- Electrodes: The electrodes validated in [37] were also used for measurements in the quadriceps region for the following reasons: being adhesive, movement artifacts are avoided; the conductive gel in contact with the skin and the Ag/AgCl chemical electrode reduces the impedance of the electrode-skin contact; the rectangular surface of 6 cm × 1.5 cm, with the shorter length in the current circulation line, reduces the impedance of the electrodes compared to standard solutions based on circular electrodes with a smaller contact surface.
- Measurement device: The device was battery-powered and bioimpedance measurements were based on the spectroscopy technique to allow differentiation between the extracellular and intracellular compartments. In each bioimpedance measurement, the module and phase of the bioimpedance were measured sequentially at the frequencies mentioned in Section 2. The measurement process lasted 25 s following the functional scheme described in [37]. The injected current was set at 0.4 mA to avoid any type of damage to the human body. The instrumentation amplifier was modified to obtain a gain of 26 dB in the measured voltage, a value chosen to provide the best signal-to-noise ratio considering that the impedance of the quadriceps is much lower than that pre-established for the whole body, and with sufficient robustness so that the signals do not saturate in any volunteer. To estimate the module and phase at the different frequencies, the quadrature signal demodulation method was used following the procedure described in [37].
- Connection cables: The electrodes were attached to the measurement device by two 140 cm-length cables, which provided sufficient ergonomics and flexibility to loosely arrange the measurement device in an area close to the measurement subject. Two isolated active lines were inside each cable, one for connection to a current injection electrode and another for connection to a voltage measurement electrode. The lines were twisted to minimize mutual interference and shielded on the outside to remove external interference. In the last 30 cm, the lines were separated to facilitate the connection to the electrodes, which in turn, was carried out by using self-adjusting crocodile-type clips.
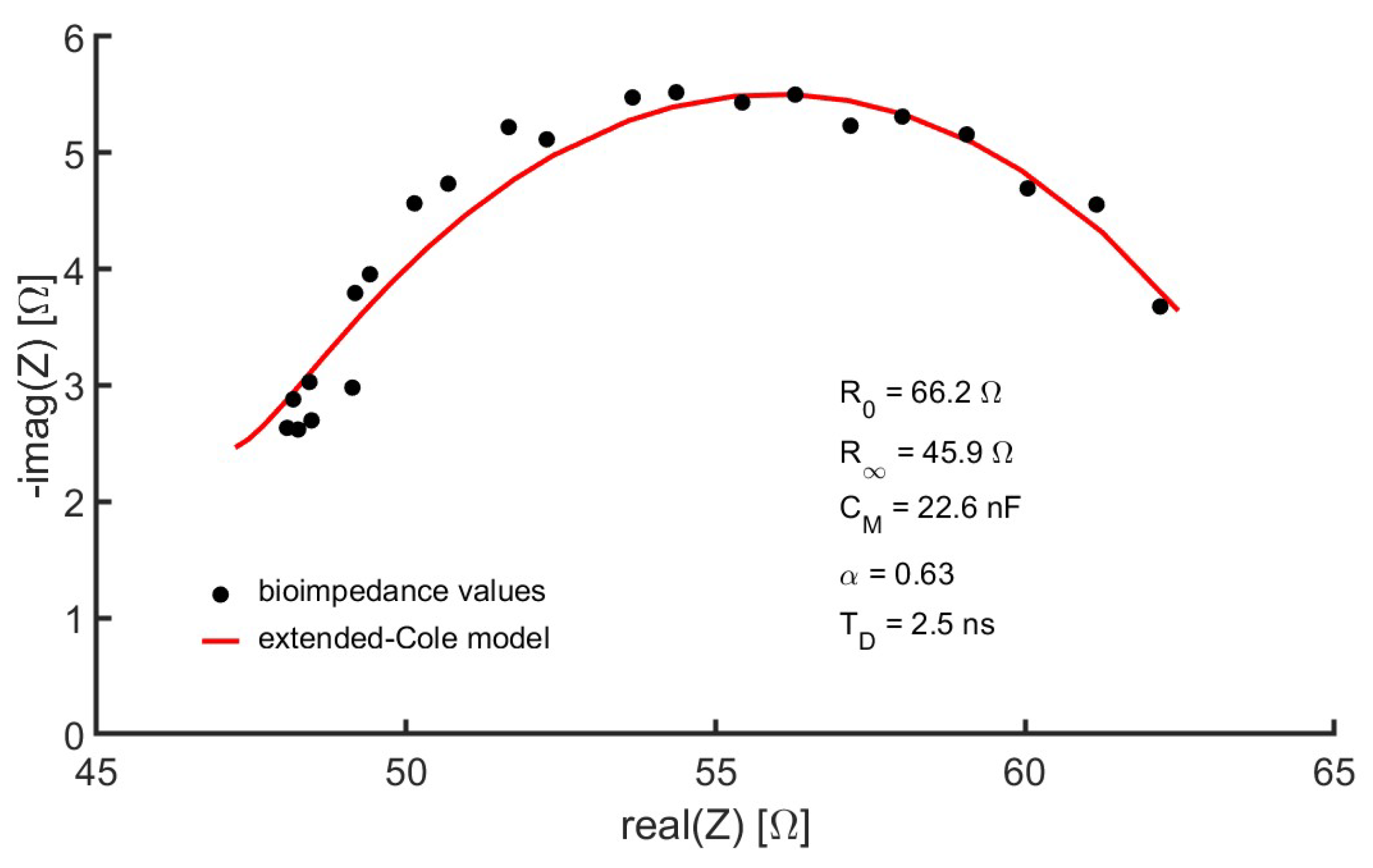
- Control device: A laptop computer served as the user interface for controlling the measuring device. This interface was programmed in the MATLAB environment (version R2022a) from GUI applications. Healthcare professionals helped establish GUI specifications based on requirements specified from interviews and questionnaires. The interface allowed the recording of the anthropometric parameters of the subject under study, such as weight, age, sex, height and external dimensions of the measurement area, taken with a flexible measuring tape: distance between the voltage measurement electrodes, perimeter of the leg at the point where the reference ultrasound-based measurements were performed (see Section 2). A button on the interface started the measurement process. The values of the module and phase of the bioimpedance at the different frequencies were converted into a representation in the complex plane. Using genetic algorithms, the parameters of the extended-Cole model that minimized the error with respect to the bioimpedance values were estimated and also displayed in the GUI. In this work, an implementation of the classical genetic algorithms [41,42] with a population of 10,000 and 12 generations has been used. The bioimpedance values and the processing results were stored in files with the date and time of measurement. Figure 4 shows an example of bioimpedance values in one of the measurements made on the quadriceps to one of the volunteers, along with the extended-Cole model that best fits the bioimpedance values.
- Wireless communications: A bidirectional wireless communications link was established between the measuring device and the control device. A protocol was developed for sending commands from the control device, including the measurement start command, and sending data from the measurement device in a sequence of information triplets (module, phase and frequency). To implement communications, the Microchip RN42 transceiver (Microchip Technology Inc., Chandler, Arizona, USA) was used, developing an SPP serial interface over the Bluetooth v2.1 standard.
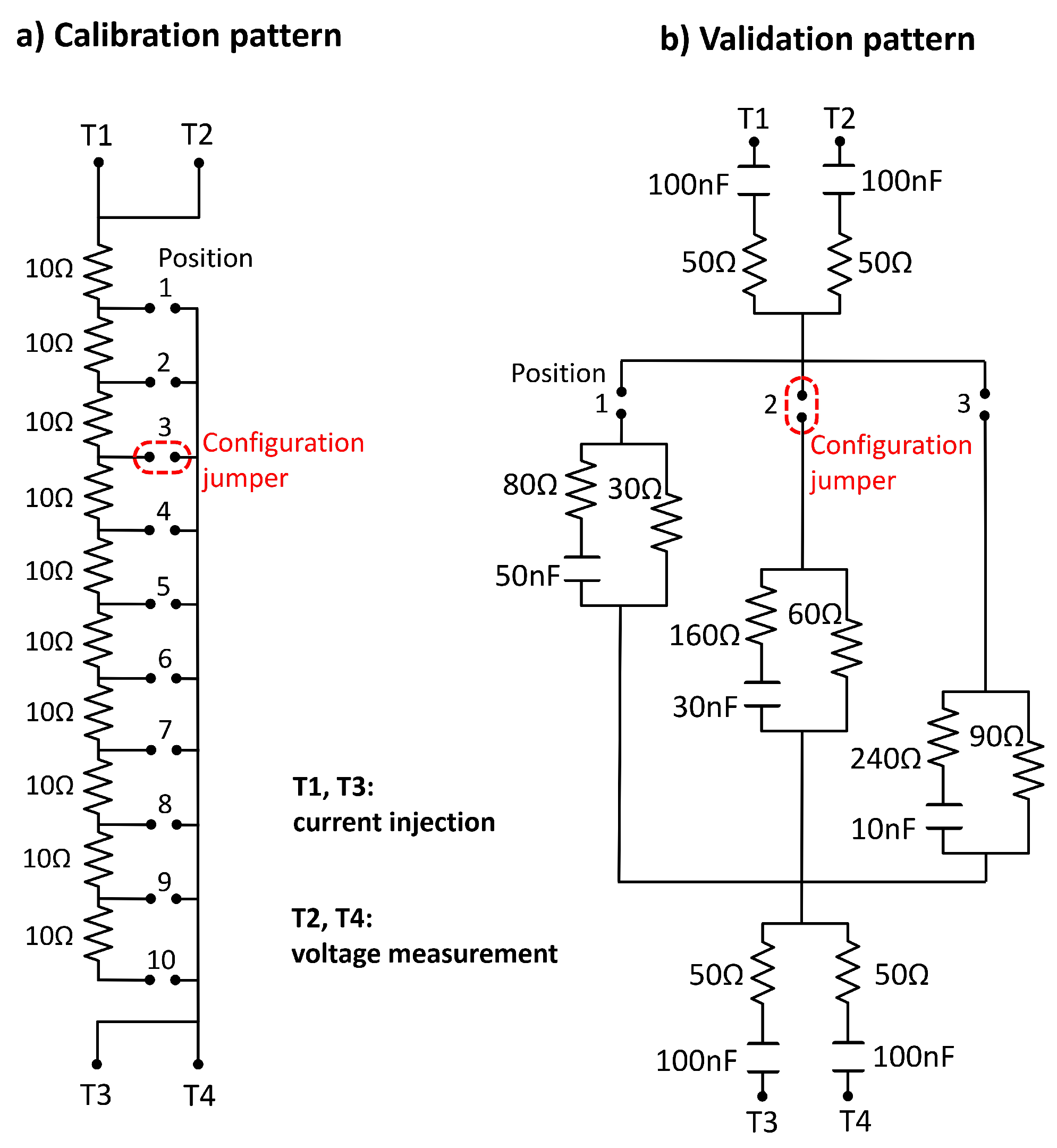
3.2. Technical Evaluation
3.3. Clinical Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mou, K.; Chan, S.M.; Vlahos, R. Musculoskeletal crosstalk in chronic obstructive pulmonary disease and comorbidities: Emerging roles and therapeutic potentials. Pharmacol. Ther. 2024, 257, 108635. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, C.; Savva, C.; Korakakis, V.; Ploutarchou, G.; Adamide, T.; Georgiou, A.; Xanthos, T. The effects of upper limb exercise training on upper limb muscle strength in people with chronic obstructive pulmonary disease: A systematic review and meta-analysis of randomized controlled trials. Ther. Adv. Respir. Dis. 2023, 17, 17534666231170813. [Google Scholar] [CrossRef] [PubMed]
- Nici, L. Pulmonary Rehabilitation: Mechanisms of Functional Loss and Benefits of Exercise. Respir. Care 2024, 69, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Tonga, K.O.; Oliver, B.G. Effectiveness of Pulmonary Rehabilitation for Chronic Obstructive Pulmonary Disease Therapy: Focusing on Traditional Medical Practices. J. Clin. Med. 2023, 12, 4815. [Google Scholar] [CrossRef]
- Bui, K.L.; Nyberg, A.; Rabinovich, R.; Saey, D.; Maltais, F. The Relevance of Limb Muscle Dysfunction in Chronic Obstructive Pulmonary Disease: A Review For Clinicians. Clin. Chest Med. 2019, 40, 367–383. [Google Scholar] [CrossRef]
- Souza, R.; Cardim, A.; Maia, T.; Rocha, L.; Bezerra, S.; Marinho, P. Inspiratory muscle strength, diaphragmatic mobility, and body composition in chronic obstructive pulmonary disease. Physiother. Res. Int. 2019, 24, e1766. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Sato, S.; Muro, S.; Yamada, M.; Hasegawa, K.; Kiyokawa, H.; Mishima, M.; Aoyama, T. Interdependence of physical inactivity, loss of muscle mass and low dietary intake: Extrapulmonary manifestations in older chronic obstructive pulmonary disease patients. Geriatr. Gerontol. Int. 2018, 18, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Jaitovich, A.; Barreiro, E. Skeletal muscle dysfunction in chronic obstructive pulmonary disease what we know and can do for our patients. Am. J. Respir. Crit. Care Med. 2018, 198, 175–186. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Lu, X.M.; Liu, R.; Han, Y.X.; Niu, M.; Qian, H.Y.; Zhao, Q. Impaired Skeletal Muscle in Patients with Stable Chronic Obstructive Pulmonary Disease (COPD) Compared with Non-COPD Patients. Int. J. COPD 2023, 18, 1525–1532. [Google Scholar] [CrossRef]
- Jiang, M.; Li, P.; Wang, Y.; Cao, Y.; Han, X.; Jiang, L.; Liu, X.; Wu, W. Role of Nrf2 and exercise in alleviating COPD-induced skeletal muscle dysfunction. Ther. Adv. Respir. Dis. 2023, 17, 17534666231208633. [Google Scholar] [CrossRef]
- Couillard, A.; Prefaut, C. From muscle disuse to myopathy in COPD: Potential contribution of oxidative stress. Eur. Respir. J. 2005, 26, 703–719. [Google Scholar] [CrossRef]
- Brunton, N.M.; Barbour, D.J.; Gelinas, J.C.; Yacyshyn, A.F.; Sasso, J.P.; Harper, M.I.; McNeil, C.J.; Melzer, B.; Agar, G.; Eves, N.D. Lower-limb resistance training reduces exertional dyspnea and intrinsic neuromuscular fatigability in individuals with chronic obstructive pulmonary disease. J. Appl. Physiol. 2023, 134, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Devaraj, U.; Patrick, B.; Saxena, D.; Venkatnarayan, K.; Louis, V.; Krishnaswamy, U.; D’Souza, G. Ultrasonographic assessment of skeletal muscle mass and diaphragm function in patients with chronic obstructive pulmonary disease: A case-control study. Lung India 2020, 37, 220–226. [Google Scholar]
- Ramírez-Fuentes, C.; Mínguez-Blasco, P.; Ostiz, F.; Sánchez-Rodríguez, D.; Messaggi-Sartor, M.; Macías, R.; Muniesa, J.; Rodríguez, D.; Vila, J.; Perkisas, S.; et al. Ultrasound assessment of rectus femoris muscle in rehabilitation patients with chronic obstructive pulmonary disease screened for sarcopenia: Correlation of muscle size with quadriceps strength and fat-free mass. Eur. Geriatr. Med. 2019, 10, 89–97. [Google Scholar] [CrossRef]
- Man, W.C.; Kemp, P.; Moxham, J.; Polkey, M. Exercise and muscle dysfunction in COPD: Implications for pulmonary rehabilitation. Clin. Sci. 2009, 117, 281–291. [Google Scholar] [CrossRef]
- Jing, Y.; Ma, Y.; Zhang, H.; Wu, Z.; Li, Y.; Li, H.; Huang, M.; Lin, L.; Xu, Y. Pulmonary rehabilitation integrated coached exercise training for patients with COPD: A study protocol for a randomized controlled trial. Trials 2023, 24, 69. [Google Scholar] [CrossRef] [PubMed]
- Lamberton, C.E.; Mosher, C.L. Review of the Evidence for Pulmonary Rehabilitation in COPD: Clinical Benefits and Cost-Effectiveness. Respir. Care 2024, 69, 686–696. [Google Scholar] [CrossRef]
- Alqahtani, J.; Oyelade, T.; Sreedharan, J.; Aldhahir, A.; Alghamdi, S.; Alrajeh, A.; Alqahtani, A.; Alsulayyim, A.; Aldabayan, Y.; Alobaidi, N.; et al. Diagnostic and clinical values of non-cardiac ultrasound in COPD: A systematic review. BMJ Open Respir. Res. 2020, 7, e000717. [Google Scholar] [CrossRef]
- Jenkins, A.; Gaynor-Sodeifi, K.; Lewthwaite, H.; Triandafilou, J.; Belo, L.; de Oliveira, M.; Jensen, D. Efficacy of interventions to alter measures of fat-free mass in people with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Eur. Respir. J. 2023; early access. [Google Scholar]
- Jenkins, T.; Patel, S.; Edwards, G.; Nolan, C.; Canavan, J.; Kon, S.; Jones, S.; Barker, R.; Littlemore, H.; Maddocks, M.; et al. Longitudinal change in ultrasound-derived rectus femoris cross-sectional area in COPD. Eur. Respir. J. 2024; early access. [Google Scholar]
- Menon, M.; Houchen, L.; Harrison, S.; Singh, S.; Morgan, M.; Steiner, M. Ultrasound assessment of lower limb muscle mass in response to resistance training in COPD. Respir. Res. 2012, 13, 119. [Google Scholar] [CrossRef]
- Mcauley, H.; Harvey-Dunstan, T.; Craner, M.; Richardson, M.; Singh, S.; Steiner, M.; Greening, N. Longitudinal changes to quadriceps thickness demonstrate acute sarcopenia following admission to hospital for an exacerbation of chronic respiratory disease. Thorax 2021, 76, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Liang, C.; Yin, Y.; Shu, J.; Zhou, X.; Wang, Q.; Hou, G.; Wang, C. Ultrasound assessment of the rectus femoris in patients with chronic obstructive pulmonary disease predicts poor exercise tolerance: An exploratory study. BMC Pulm. Med. 2021, 21, 304. [Google Scholar] [CrossRef] [PubMed]
- Zenta, M.; Hattori, T.; Ogawa, Y.; Yoshida, S.; Kido, S.; Maruoka, H. Relationship between quadriceps muscle thickness and activities of daily living of patients with chronic respiratory failure. Rigakuryoho Kagaku 2020, 35, 719–727. [Google Scholar] [CrossRef]
- Maynard-Paquette, A.C.; Poirier, C.; Chartrand-Lefebvre, C.; Dubé, B.P. Ultrasound evaluation of the quadriceps muscle contractile index in patients with stable chronic obstructive pulmonary disease: Relationships with clinical symptoms, disease severity and diaphragm contractility. Int. J. COPD 2020, 15, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Q.; Liu, J.M.; Zhang, X.; Xue, Y.T.; Shi, J.P.; Chen, W.; Zheng, X.Y. Estimation of skeletal muscle mass by bioimpedance and differences among skeletal muscle mass indices for assessing sarcopenia. Clin. Nutr. 2021, 40, 2308–2318. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.; Kim, S.Y.; Jiang, F.; Chung, S.; Ambegaonkar, J.; Park, J.H.; Kim, Y.J.; Kim, C.H. Prediction equations of the multifrequency standing and supine bioimpedance for appendicular skeletal muscle mass in korean older people. Int. J. Environ. Res. Public Health 2020, 17, 5847. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Wu, M.Y.; Chen, H.S.; Hung, S.C.; Lim, P.S. Development and validation of a multifrequency bioimpedance spectroscopy equation to predict appendicular skeletal muscle mass in hemodialysis patients. Clin. Nutr. 2021, 40, 3288–3295. [Google Scholar] [CrossRef] [PubMed]
- Cruz Rivera, P.N.; Goldstein, R.L.; Polak, M.; Lazzari, A.A.; Moy, M.L.; Wan, E.S. Performance of bioelectrical impedance analysis compared to dual X-ray absorptiometry (DXA) in Veterans with COPD. Sci. Rep. 2022, 12, 1946. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Hernández, D.; Reina-Tosina, J.; Min, M. Fundamentals, recent advances, and future challenges in bioimpedance devices for healthcare applications. J. Sens. 2019, 2019, 9210258. [Google Scholar] [CrossRef]
- Stupin, D.D.; Kuzina, E.A.; Abelit, A.A.; Emelyanov, A.K.; Nikolaev, D.M.; Ryazantsev, M.N.; Koniakhin, S.V.; Dubina, M.V. Bioimpedance Spectroscopy: Basics and Applications. ACS Biomater. Sci. Eng. 2021, 7, 1962–1986. [Google Scholar] [CrossRef]
- Arieta, L.R.; Giuliani-Dewig, H.K.; Gerstner, G.R.; Mota, J.A.; Ryan, E.D. Segmental bioelectrical impedance spectroscopy: A novel field assessment of muscle size and quality in normal weight and obese older men. Exp. Gerontol. 2022, 162, 111745. [Google Scholar] [CrossRef]
- Wohlgemuth, K.J.; Freeborn, T.J.; Hare, M.M.; Southall, K.E.; Mota, J.A. Bioimpedance Spectroscopy Compared to Ultrasound-derived Measures of Quadriceps Muscle Quality. Int. J. Exerc. Sci. 2023, 2, 23. [Google Scholar]
- Wohlgemuth, K.J.; Freeborn, T.J.; Southall, K.E.; Hare, M.M.; Mota, J.A. Can segmental bioelectrical impedance be used as a measure of muscle quality? Med Eng. Phys. 2024, 124, 104103. [Google Scholar] [CrossRef]
- Maltais, F.; Decramer, M.; Casaburi, R.; Barreiro, E.; Burelle, Y.; Debigare, R.; Dekhuijzen, P.R.; Franssen, F.; Gayan-Ramirez, G.; Gea, J.; et al. An official American thoracic society/european respiratory society statement: Update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014, 189, E15–E62. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, A.; Saey, D.; Maltais, F. Why and how limb muscle mass and function should be measured in patients with chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2015, 12, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Hernández, D.; Reina-Tosina, J.; Roa, L.; Barbarov-Rostán, G.; Aresté-Fosalba, N.; Lara-Ruiz, A.; Cejudo-Ramos, P.; Ortega-Ruiz, F. Smart bioimpedance spectroscopy device for body composition estimation. Sensors 2020, 20, 70. [Google Scholar] [CrossRef]
- Roa, L.; Reina-Tosina, J.; Naranjo-Hernández, D.; Estudillo-Valderrama, M. Intelligent Bioimpedance Sensor for Biomedical Applications. ES Patent 2537351, 7 May 2015. [Google Scholar]
- McAdams, E.; Jossinet, J. Tissue impedance: A historical overview. Physiol. Meas. 1995, 16, A1–A13. [Google Scholar] [CrossRef]
- Zhang, M.; Xing, X.; Wang, W. Smart Sensor-Based Monitoring Technology for Machinery Fault Detection. Sensors 2024, 24, 2470. [Google Scholar] [CrossRef]
- Gholami-Boroujeny, S.; Bolic, M. Extraction of Cole parameters from the electrical bioimpedance spectrum using stochastic optimization algorithms. Med Biol. Eng. Comput. 2016, 54, 643–651. [Google Scholar] [CrossRef]
- Halter, R.J.; Hartov, A.; Paulsen, K.D.; Schned, A.; Heaney, J. Genetic and least squares algorithms for estimating spectral EIS parameters of prostatic tissues. Physiol. Meas. 2008, 29, S111–S123. [Google Scholar] [CrossRef]
- Albulbul, A. Evaluating major electrode types for idle biological signal measurements for modern medical technology. Bioengineering 2016, 3, 20. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhu, C.; Han, R.; Steuer, A.; Kolb, J.F.; Shi, F. Uncertainty Quantification and Sensitivity Analysis for the Electrical Impedance Spectroscopy of Changes to Intercellular Junctions Induced by Cold Atmospheric Plasma. Molecules 2022, 27, 5861. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, Ø.G.; Heiskanen, A. Bioimpedance and Bioelectricity Basics, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–617. [Google Scholar]
- Yashiro, M.; Kotera, H. Association of bioimpedance-derived 50-kHz phase angle as marker of body composition with electrical parameters regarding the Cole-Cole model. Ther. Apher. Dial. 2021, 25, 166–178. [Google Scholar] [CrossRef]
- Moqadam, S.; Grewal, P.; Ramani, P.; Liu, J.; Thomas, S.; Golnaraghi, F.; Karvat, A.; Kohli, K. Use of Electrical Impedance Spectroscopy in vivo to Distinguish Active and Rest Stages of Salivary Glands. J. Med Biol. Eng. 2017, 37, 769–779. [Google Scholar] [CrossRef]
- Phan, K.N.; Tran, V.A.; Dang, T.T. Modified biological model of meat in the frequency range from 50 Hz to 1 MHz. Adv. Intell. Syst. Comput. 2020, 1125, 1055–1068. [Google Scholar]
- Seymour, J.; Ward, K.; Sidhu, P.; Puthucheary, Z.; Steier, J.; Jolley, C.; Rafferty, G.; Polkey, M.; Moxham, J. Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax 2009, 64, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Sipila, S.; Suominen, H. Ultrasound imaging of the quadriceps muscle in elderly athletes and untrained men. Muscle Nerve 1991, 14, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Matsumoto, T.; Harada, R.; Yoshikawa, R.; Ueda, Y.; Takamiya, D.; Sakai, Y. Reliability and Validity of Quadriceps Muscle Thickness Measurements in Ultrasonography: A Comparison with Muscle Mass and Strength. Prog. Rehabil. Med. 2023, 8, 20230008. [Google Scholar] [CrossRef] [PubMed]
- Abdulsalam, A.J.; Kara, M.; Özçakar, L. Ultrasound is mightier than bioimpedance spectrometry but not ad lib. J. Nephrol. 2024; in press. [Google Scholar]
- Nakanishi, N.; Tsutsumi, R.; Okayama, Y.; Takashima, T.; Ueno, Y.; Itagaki, T.; Tsutsumi, Y.; Sakaue, H.; Oto, J. Monitoring of muscle mass in critically ill patients: Comparison of ultrasound and two bioelectrical impedance analysis devices. J. Intensive Care 2019, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Garrido, P.; de Miguel-Díez, J.; Rejas-Gutierrez, J.; Martín-Centeno, A.; Gobartt-Vázquez, E.; Hernandez-Barrera, V.; Gil de Miguel, A.; Jimenez-Garcia, R. Characteristics of chronic obstructive pulmonary disease in Spain from a gender perspective. BMC Pulm. Med. 2009, 9, 2. [Google Scholar] [CrossRef]
- Engelen, M.P.; Kirschner, S.K.; Coyle, K.S.; Argyelan, D.; Neal, G.; Dasarathy, S.; Deutz, N.E. Sex related differences in muscle health and metabolism in chronic obstructive pulmonary disease. Clin. Nutr. 2023, 42, 1737–1746. [Google Scholar] [CrossRef]
- Fernández-García, S.; Represas-Represas, C.; Ruano-Raviña, A.; Mosteiro-Añón, M.; Mouronte-Roibas, C.; Fernández-Villar, A. Social Profile of Patients Admitted for COPD Exacerbations. A Gender Analysis. Arch. Bronconeumol. 2020, 56, 84–89. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Interpretation | Units |
|---|---|---|
| Resistance at zero frequency | ||
| Resistance at infinite frequency | ||
| Extracellular resistance | ||
| Intracellular resistance | ||
| Time constant that characterizes the frequency relaxation distribution | seconds (s) | |
| Effective membrane capacitance | Farads (F) | |
| (the physical meaning of this parameter is discussed in Section 4) | ||
| Characteristic order of frequency relaxation distribution | dimensionless | |
| Phase delay due to hardware and cable length | seconds (s) |
| Group I, Women | Group II, Men | Group III, Strength | Global | |
|---|---|---|---|---|
| Number of volunteers | 7 | 26 | 12 | 33 |
| Weight (kg) | 76.3 ± 20.5 | 84.7 ± 17.2 | 79.5 ± 14.5 | 82.9 ± 18.3 |
| Age (years) | 56.4 ± 8.5 | 64.6 ± 7.2 | 66.3 ± 5.7 | 62.8 ± 8.2 |
| Height (cm) | 157.1 ± 6.9 | 167.8 ± 7.1 | 166.8 ± 5.8 | 165.5 ± 8.3 |
| Body mass index () | 30.8 ± 7.1 | 30.0 ± 5.6 | 28.4 ± 4.5 | 30.2 ± 6 |
| (cm) | 2.5 ± 0.5 | 2.4 ± 0.6 | 2.3 ± 0.7 | 2.5 ± 0.6 |
| (cm) | 1.2 ± 0.3 | 1.2 ± 0.4 | 1.1 ± 0.3 | 1.2 ± 0.4 |
| (cm) | 3.8 ± 0.5 | 3.2 ± 0.8 | 2.9 ± 0.7 | 3.3 ± 0.8 |
| (cm) | 4.7 ± 0.5 | 4.8 ± 0.8 | 4.5 ± 0.6 | 4.8 ± 0.8 |
| () | 5.3 ± 0.6 | 5.3 ± 1.7 | 5.0 ± 2.0 | 5.3 ± 1.5 |
| (kg) | - | - | 27.8 ± 6.3 | - |
| (kg) | - | - | 55.1 ± 14.7 | - |
| () | 72.6 ± 17.4 | 60.9 ± 13.1 | 61.3 ± 11.6 | 63.3 ± 14.6 |
| () | 47.4 ± 12.1 | 41.6 ± 9.1 | 41.6 ± 8.0 | 42.9 ± 9.9 |
| (nF) | 25.4 ± 10.4 | 24.7 ± 10.9 | 23.9 ± 11.2 | 24.9 ± 10.7 |
| (dimensionless) | 0.62 ± 0.07 | 0.61 ± 0.09 | 0.59 ± 0.11 | 0.62 ± 0.08 |
| Mean Absolute Error | Mean Absolute Relative Error | |
|---|---|---|
| () | 0.3 ± 0.2 | 0.4% ± 0.3% |
| () | 0.3 ± 0.2 | 0.6% ± 0.5% |
| (nF) | 1.0 ± 0.8 | 3.9% ± 2.7% |
| (dimensionless) | 0.01 ± 0.01 | 2.1% ± 1.8% |
| Reference | Parameter | Units | Group I, Women | Group II, Men | Group III, Strength | Global |
|---|---|---|---|---|---|---|
| () | () | () | () | |||
| 0.11 (0.813) | 0.66 (<) | 0.74 (0.006) | 0.47 (0.006) | |||
| 0.11 (0.814) | 0.78 (<) | 0.95 (<) | 0.63 (<) | |||
| (cm) | 0.54 (0.214) | 0.68 (<) | 0.88 (<) | 0.65 (<) | ||
| F | 0.68 (0.090) | 0.69 (<) | 0.79 (0.002) | 0.69 (<) | ||
| s | 0.51 (0.241) | 0.41 (0.039) | 0.34 (0.280) | 0.42 (0.015) | ||
| - | 0.36 (0.429) | 0.09 (0.651) | 0.09 (0.781) | 0.03 (0.867) | ||
| 0.29 (0.533) | 0.64 (<) | 0.64 (0.024) | 0.42 (0.014) | |||
| 0.10 (0.817) | 0.67 (<) | 0.79 (0.002) | 0.51 (0.002) | |||
| (cm) | 0.31 (0.493) | 0.49 (0.011) | 0.71 (0.009) | 0.46 (0.007) | ||
| F | 0.47 (0.284) | 0.55 (0.004) | 0.67 (0.018) | 0.53 (0.001) | ||
| s | 0.42 (0.346) | 0.33 (0.103) | 0.29 (0.363) | 0.33 (0.059) | ||
| - | 0.29 (0.518) | 0.15 (0.455) | 0.13 (0.685) | 0.08 (0.639) | ||
| 0.14 (0.764) | 0.61 (0.001) | 0.75 (0.005) | 0.33 (0.061) | |||
| 0.02 (0.968) | 0.63 (0.001) | 0.73 (0.007) | 0.41 (0.017) | |||
| (cm) | 0.35 (0.442) | 0.45 (0.020) | 0.53 (0.077) | 0.39 (0.024) | ||
| F | 0.79 (0.033) | 0.52 (0.007) | 0.62 (0.032) | 0.53 (0.002) | ||
| s | 0.84 (0.017) | 0.32 (0.106) | 0.35 (0.264) | 0.46 (0.007) | ||
| - | 0.42 (0.341) | 0.20 (0.318) | 0.21 (0.521) | 0.13 (0.481) | ||
| 0.48 (0.283) | 0.08 (0.677) | 0.14 (0.657) | 0.02 (0.903) | |||
| 0.47 (0.295) | 0.13 (0.543) | 0.24 (0.446) | 0.06 (0.721) | |||
| (cm) | 0.28 (0.553) | 0.14 (0.493) | 0.26 (0.410) | 0.11 (0.553) | ||
| F | 0.10 (0.840) | 0.19 (0.342) | 0.53 (0.079) | 0.18 (0.320) | ||
| s | 0.40 (0.375) | 0.20 (0.325) | 0.63 (0.029) | 0.19 (0.281) | ||
| - | 0.02 (0.965) | 0.07 (0.725) | 0.01 (0.965) | 0.07 (0.713) | ||
| 0.11 (0.807) | 0.38 (0.053) | 0.57 (0.053) | 0.30 (0.086) | |||
| 0.11 (0.822) | 0.46 (0.017) | 0.62 (0.032) | 0.40 (0.020) | |||
| (cm) | 0.52 (0.228) | 0.41 (0.035) | 0.50 (0.097) | 0.41 (0.017) | ||
| F | 0.81 (0.028) | 0.43 (0.027) | 0.57 (0.055) | 0.45 (0.009) | ||
| s | 0.66 (0.104) | 0.29 (0.145) | 0.38 (0.222) | 0.29 (0.102) | ||
| - | 0.01 (0.990) | 0.27 (0.179) | 0.27 (0.392) | 0.25 (0.172) | ||
| 0.45 (0.143) | ||||||
| 0.68 (0.015) | ||||||
| (kg) | 0.70 (0.011) | |||||
| F | 0.62 (0.032) | |||||
| s | 0.24 (0.446) | |||||
| - | 0.16 (0.629) | |||||
| 0.47 (0.124) | ||||||
| 0.73 (0.007) | ||||||
| (kg) | 0.77 (0.004) | |||||
| F | 0.70 (0.011) | |||||
| s | 0.34 (0.276) | |||||
| - | 0.29 (0.354) |
| Prediction Equation | Unit | ME | MSE | r | p | N | MD ± CI | Group |
|---|---|---|---|---|---|---|---|---|
| cm | 0.1 | 0.2 | 0.68 | 0.092 | 7 | I | ||
| cm | 0.2 | 0.2 | 0.69 | < | 26 | II | ||
| cm | 0.2 | 0.2 | 0.79 | 0.002 | 12 | III | ||
| cm | 0.2 | 0.2 | 0.69 | < | 33 | Global | ||
| cm | 0.2 | 0.3 | 0.47 | 0.006 | 33 | Global | ||
| cm | 0.1 | 0.2 | 0.95 | < | 12 | III | ||
| cm | 0.2 | 0.3 | 0.63 | < | 33 | Global | ||
| cm | 0.2 | 0.2 | 0.65 | < | 33 | Global | ||
| cm | 0.2 | 0.3 | 0.42 | 0.016 | 33 | Global | ||
| cm | 0.1 | 0.1 | 0.47 | 0.283 | 7 | I | ||
| cm | 0.1 | 0.2 | 0.55 | 0.004 | 26 | II | ||
| cm | 0.1 | 0.1 | 0.67 | 0.018 | 12 | III | ||
| cm | 0.1 | 0.2 | 0.53 | 0.001 | 33 | Global | ||
| cm | 0.1 | 0.2 | 0.79 | 0.033 | 7 | I | ||
| cm | 0.3 | 0.4 | 0.52 | 0.007 | 26 | II | ||
| cm | 0.2 | 0.3 | 0.62 | 0.032 | 12 | III | ||
| cm | 0.3 | 0.4 | 0.53 | 0.002 | 33 | Global | ||
| cm2 | 0.2 | 0.3 | 0.81 | 0.028 | 7 | I | ||
| cm2 | 0.6 | 1.1 | 0.43 | 0.027 | 26 | II | ||
| cm2 | 0.7 | 1.3 | 0.57 | 0.054 | 12 | III | ||
| cm2 | 0.5 | 1.0 | 0.45 | 0.009 | 33 | Global | ||
| cm2 | 0.5 | 1.0 | 0.40 | 0.020 | 33 | Global | ||
| cm2 | 0.5 | 1.0 | 0.41 | 0.017 | 33 | Global | ||
| kg | 1.8 | 3.1 | 0.68 | 0.016 | 12 | III | ||
| kg | 2.0 | 2.9 | 0.70 | 0.011 | 12 | III | ||
| kg | 2.1 | 3.1 | 0.62 | 0.032 | 12 | III | ||
| kg | 4.2 | 9.5 | 0.73 | 0.007 | 12 | III | ||
| kg | 3.9 | 7.0 | 0.77 | 0.004 | 12 | III | ||
| kg | 4.5 | 8.6 | 0.70 | 0.011 | 12 | III |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naranjo-Hernández, D.; Reina-Tosina, J.; Roa, L.M.; Barbarov-Rostán, G.; Ortega-Ruiz, F.; Cejudo Ramos, P. Smart Bioimpedance Device for the Assessment of Peripheral Muscles in Patients with COPD. Sensors 2024, 24, 4648. https://doi.org/10.3390/s24144648
Naranjo-Hernández D, Reina-Tosina J, Roa LM, Barbarov-Rostán G, Ortega-Ruiz F, Cejudo Ramos P. Smart Bioimpedance Device for the Assessment of Peripheral Muscles in Patients with COPD. Sensors. 2024; 24(14):4648. https://doi.org/10.3390/s24144648
Chicago/Turabian StyleNaranjo-Hernández, David, Javier Reina-Tosina, Laura M. Roa, Gerardo Barbarov-Rostán, Francisco Ortega-Ruiz, and Pilar Cejudo Ramos. 2024. "Smart Bioimpedance Device for the Assessment of Peripheral Muscles in Patients with COPD" Sensors 24, no. 14: 4648. https://doi.org/10.3390/s24144648






