Unveiling Colorectal Cancer Biomarkers: Harnessing Biosensor Technology for Volatile Organic Compound Detection
Abstract
:1. Introduction
2. Overview of Colorectal Cancer and Current Diagnostic Challenges
2.1. Current Diagnostic Challenges
2.2. The Imperative for Non-Invasive and Accessible Diagnostics
3. Volatile Organic Compounds as Biomarkers in Colorectal Cancer
3.1. Specific VOC Signatures Associated with CRC
| Volatile Organic Compound | References |
|---|---|
| cyclohexanone dimethyldecane dodecane 4-ethyl-1-octyn-3-ol Ethylaniline cyclooctylmethanol trans-2-dodecen-1-ol 3-hydroxy-2,4,4-trimethylpentyl 2-methylpropanoate | Wang et al. [105] |
| tetradecane, ethyl-benzene, methylbenzene, acetic acid, 5,9-undecadien-2-one, 6,10-dimethyl(E), decane, benzaldehyde, benzoic acid, 1,3 bis(1-metiletenil) benzene, decanal, unidentified compound T22_75, dodecane, 2-ethyl-1-hexanol and ethanone, 1[4-(1-methylethenyl)phenyl] | Altomare et al. [106] |
| acetone ethyl acetate 4-methyl-octane ethanol | Amal et al. [107] |
| 1,1′-(1-butenylidene)bis benzene 1,3-dimethyl benzene 1-iodo nonane [(1,1-dimethylethyl)thio] acetic acid 4-(4-propylcyclohexyl)-40-cyano[1,10-biphenyl]-4-yl ester benzoic acid 2-amino-5-isopropyl-8-methyl-1-azulenecarbonitrile | Peng et al. [108] |
3.2. Techniques Used for Breath VOCs
3.3. Breath Sampling and Analysis Technologies
4. CRC Diagnosis and Nanotechnology
4.1. Nanoparticles
4.2. Microfluidics
5. Discussion and Conclusions
5.1. Future Perspectives
5.2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Colorectal Cancer. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer (accessed on 11 July 2023).
- Moen, L.; Liu, B.; Bukirwa, P.; Chingonzoh, T.; Chokunonga, E.; Finesse, A.; Korir, A.; Lamin, B.; Lorenzoni, C.F.; Manraj, S.S.; et al. Trends in the incidence of colorectal cancer in sub-Saharan Africa: A population-based registry study. Int. J. Cancer 2024, 155, 675–682. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Schreuders, E.H.; Ruco, A.; Rabeneck, L.; E Schoen, R.; Sung, J.J.Y.; Young, G.P.; Kuipers, E.J. Colorectal cancer screening: A global overview of existing programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Helsingen, L.M.; Vandvik, P.O.; Jodal, H.C.; Agoritsas, T.; Lytvyn, L.; Anderson, J.C.; Auer, R.; Murphy, S.B.; Almadi, M.A.; Corley, D.A.; et al. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: A clinical practice guideline. BMJ 2019, 367, l5515. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Carethers, J.M. Stool-Based Screening Tests for Colorectal Cancer. JAMA 2023, 329, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.; Abosheaishaa, H.; Salem, A.; Hassan, N.; Jaber, F.; Mahmoud, M.; Abuelazm, M.; Zulqarnin, M.; Ghoz, H. Mo1149 evolving trends in colorectal cancer incidence among young patients under 45: A 22-year analysis of CDC wonder database. Gastroenterology 2024, 166, S-953. [Google Scholar] [CrossRef]
- Patel, S.G.; Karlitz, J.J.; Yen, T.; Lieu, C.H.; Boland, C.R. The rising tide of early-onset colorectal cancer: A comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol. Hepatol. 2022, 7, 262–274. [Google Scholar] [CrossRef]
- Vuik, F.E.; Nieuwenburg, S.A.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J.; Zadnik, V.; Pellisé, M.; Esteban, L.; Kaminski, M.F.; et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019, 68, 1820–1826. [Google Scholar] [CrossRef]
- Ilyas, F.; Ahmed, E.; Ali, H.; Ilyas, M.; Sarfraz, S.; Khalid, M.; Khalaf, M.; Mudireddy, P.R. Temporal trends in colorectal cancer mortality rates (1999–2022) in the United States. Cancer Rep. 2024, 7, e2012. [Google Scholar] [CrossRef] [PubMed]
- Force, U.P.S.T. Screening for Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2008, 149, 627–637. [Google Scholar] [CrossRef]
- Demb, J.; Kolb, J.M.; Dounel, J.; Fritz, C.D.; Advani, S.M.; Cao, Y.; Coppernoll-Blach, P.; Dwyer, A.J.; Perea, J.; Heskett, K.M.; et al. Red Flag Signs and Symptoms for Patients With Early-Onset Colorectal Cancer: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2024, 7, e2413157. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. JNCI J. Natl. Cancer Inst. 2017, 109, djw322. [Google Scholar] [CrossRef] [PubMed]
- Spaander, M.C.; Zauber, A.G.; Syngal, S.; Blaser, M.J.; Sung, J.J.; You, Y.N.; Kuipers, E.J. Young-onset colorectal cancer. Nat. Rev. Dis. Primers 2023, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.R.; Galvani, A.P. Impact of the COVID-19 pandemic on cancer incidence and mortality. Lancet Public Health 2022, 7, e490–e491. [Google Scholar] [CrossRef] [PubMed]
- Bommi, J.R.; Kummari, S.; Lakavath, K.; Sukumaran, R.A.; Panicker, L.R.; Marty, J.L.; Goud, K.Y. Recent Trends in Biosensing and Diagnostic Methods for Novel Cancer Biomarkers. Biosensors 2023, 13, 398. [Google Scholar] [CrossRef] [PubMed]
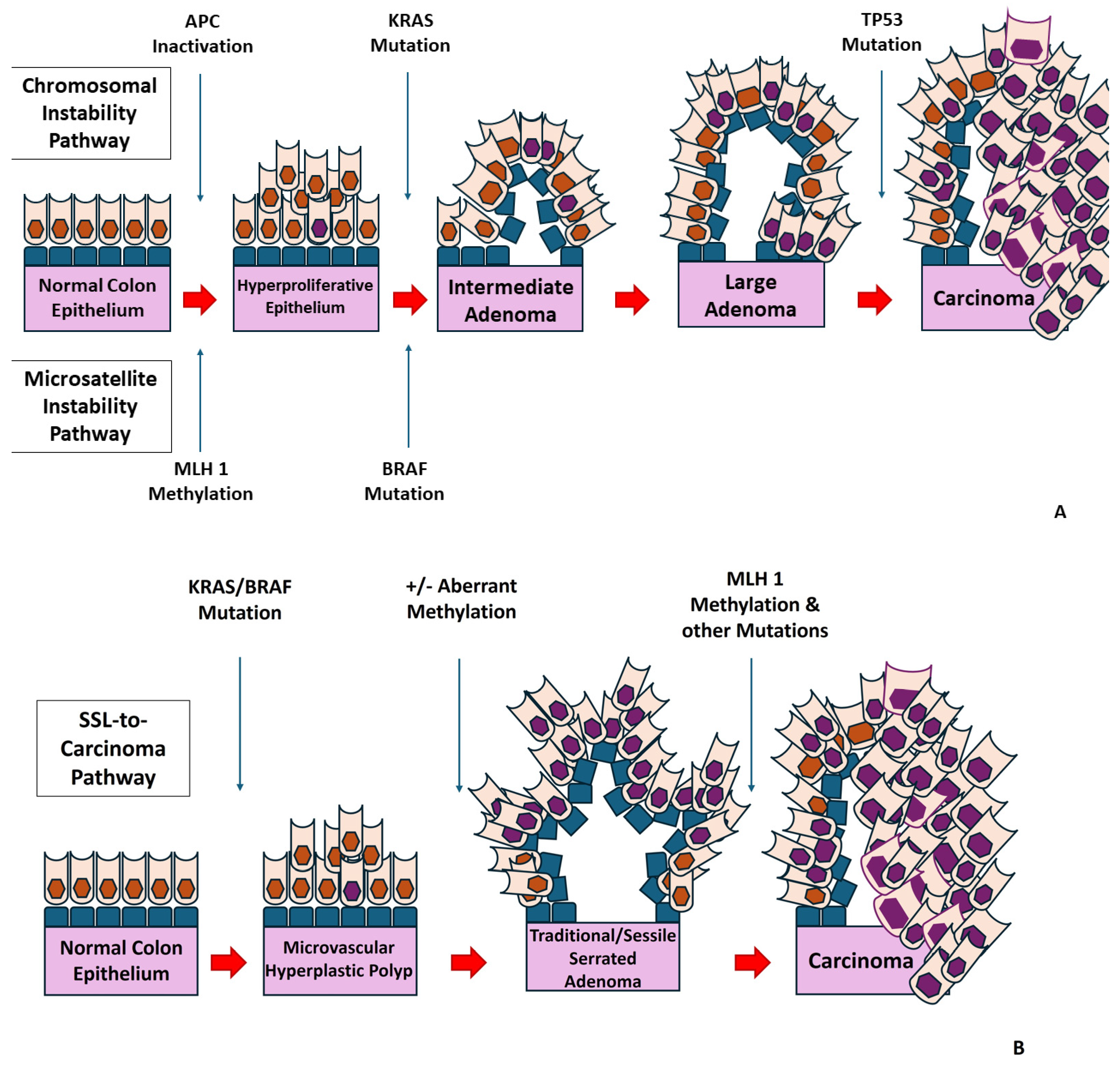
- Conteduca, V.; Sansonno, D.; Russi, S.; Dammacco, F. Precancerous colorectal lesions (Review). Int. J. Oncol. 2013, 43, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Waldum, H.; Fossmark, R. Gastritis, Gastric Polyps and Gastric Cancer. Int. J. Mol. Sci. 2021, 22, 6548. [Google Scholar] [CrossRef]
- Marley, A.R.; Nan, H. Epidemiology of colorectal cancer. Int. J. Mol. Epidemiol. Genet. 2016, 7, 105–114. [Google Scholar]
- Sameer, A.S. Colorectal Cancer: Molecular Mutations and Polymorphisms. Front. Oncol. 2013, 3, 114. [Google Scholar] [CrossRef]
- Nassar, D.; Blanpain, C. Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 47–76. [Google Scholar] [CrossRef]
- Medema, J.P. Cancer stem cells: The challenges ahead. Nature 2013, 15, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Paschke, S.; Jafarov, S.; Staib, L.; Kreuser, E.-D.; Maulbecker-Armstrong, C.; Roitman, M.; Holm, T.; Harris, C.C.; Link, K.-H.; Kornmann, M. Are Colon and Rectal Cancer Two Different Tumor Entities? A Proposal to Abandon the Term Colorectal Cancer. Int. J. Mol. Sci. 2018, 19, 2577. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Yamane, L.; Scapulatempo-Neto, C.; Reis, R.M.; Guimarães, D.P. Serrated pathway in colorectal carcinogenesis. World J. Gastroenterol. 2014, 20, 2634–2640. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.D.; East, J.; Rees, C.J.; Cripps, N.; Docherty, J.; Dolwani, S.; Kaye, P.V.; Monahan, K.J.; Novelli, M.R.; Plumb, A.; et al. British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England post-polypectomy and post-colorectal cancer resection surveillance guidelines. Gut 2019, 69, 201–223. [Google Scholar] [CrossRef] [PubMed]
- Perea García, J.; Arribas, J.; Cañete, Á.; García, J.L.; Álvaro, E.; Tapial, S.; Narváez, C.; Vivas, A.; Brandáriz, L.; Hernández-Villafranca, S.; et al. Association of Polyps with Early-Onset Colorectal Cancer and Throughout Surveillance: Novel Clinical and Molecular Implications. Cancers 2019, 11, 1900. [Google Scholar] [CrossRef]
- Kim, M.; Vogtmann, E.; Ahlquist, D.A.; Devens, M.E.; Kisiel, J.B.; Taylor, W.R.; White, B.A.; Hale, V.L.; Sung, J.; Chia, N.; et al. Fecal Metabolomic Signatures in Colorectal Adenoma Patients Are Associated with Gut Microbiota and Early Events of Colorectal Cancer Pathogenesis. mBio 2020, 11, 110–128. [Google Scholar] [CrossRef]
- Noffsinger, A.E. Serrated Polyps and Colorectal Cancer: New Pathway to Malignancy. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 343–364. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K. Serrated Polyps in the Colon. Gastroenterol. Hepatol. 2014, 10, 671–674. [Google Scholar]
- Snover, D.C.; Batts, K.P. Serrated Colorectal Neoplasia. Surg. Pathol. Clin. 2010, 3, 207–240. [Google Scholar] [CrossRef]
- Snover, D.C.; Jass, J.R.; Fenoglio-Preiser, C.; Batts, K.P. Serrated polyps of the large intestine: A morphologic and molecular review of an evolving concept. Am. J. Clin. Pathol. 2005, 124, 380–391. [Google Scholar] [CrossRef]
- E East, J.; Atkin, W.S.; Bateman, A.C.; Clark, S.K.; Dolwani, S.; Ket, S.N.; Leedham, S.J.; Phull, P.S.; Rutter, M.D.; A Shepherd, N.; et al. British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut 2017, 66, 1181–1196. [Google Scholar] [CrossRef]
- Kahi, C.J.; Hewett, D.G.; Norton, D.L.; Eckert, G.J.; Rex, D.K. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin. Gastroenterol. Hepatol. 2010, 9, 42–46. [Google Scholar] [CrossRef]
- Sweetser, S.; Smyrk, T.C.; Sugumar, A. Serrated polyps: Critical precursors to colorectal cancer. Expert. Rev. Gastroenterol. Hepatol. 2011, 5, 627–635. [Google Scholar] [CrossRef]
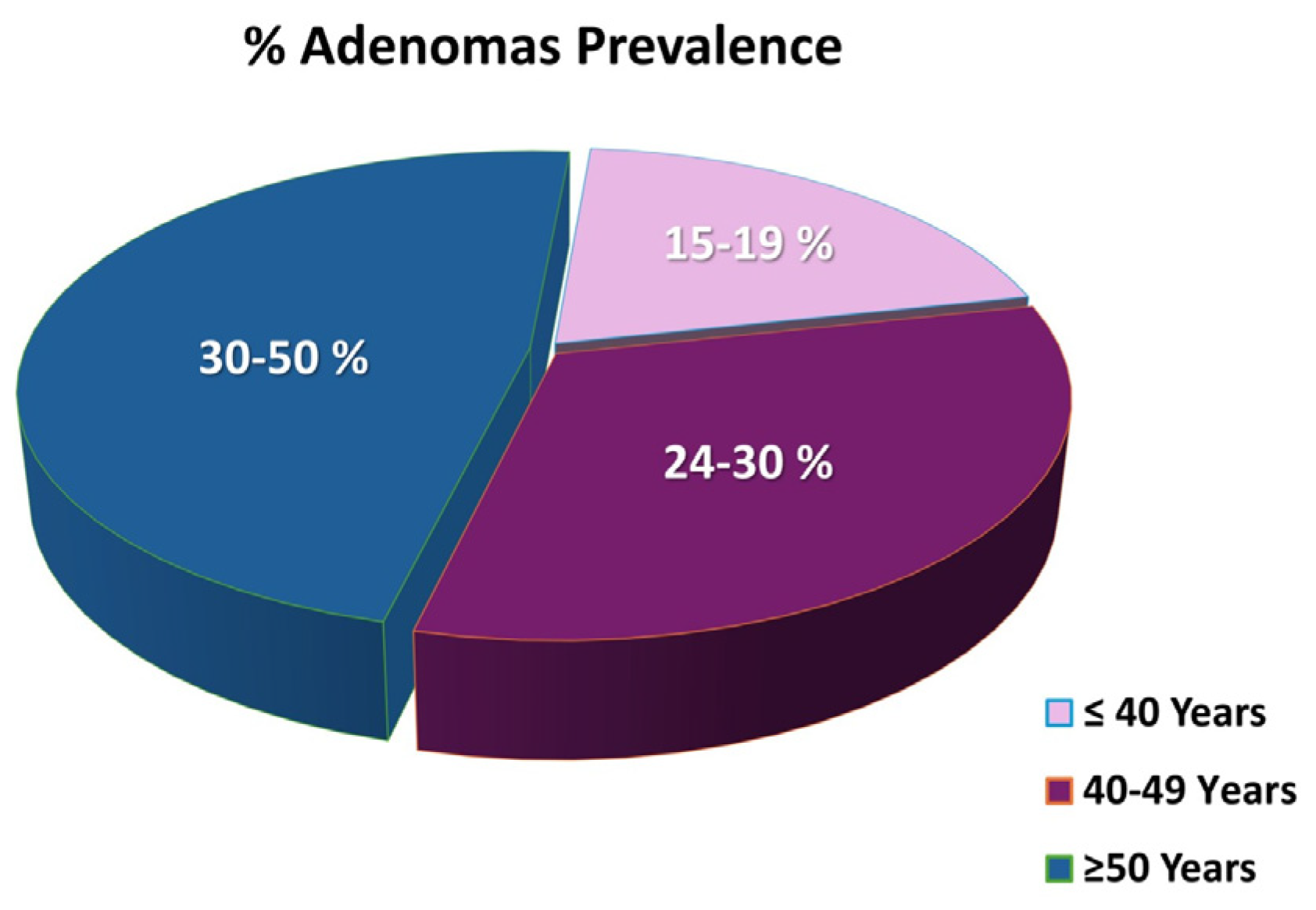
- Fairley, K.J.; Li, J.; Komar, M.; Steigerwalt, N.; Erlich, P. Predicting the risk of recurrent adenoma and incident colorectal cancer based on findings of the baseline colonoscopy. Clin. Transl. Gastroenterol. 2014, 5, e64. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gong, X.; Xu, K.; Luo, S.; Gao, W.; Li, B.; Jing, D. Risk factor analysis of malignant adenomas detected during colonoscopy. Front. Med. 2023, 10, 1106272. [Google Scholar] [CrossRef] [PubMed]
- Simon, K.; Balchen, V. Colorectal cancer development and advances in screening. Clin. Interv. Aging 2016, 11, 967–976. [Google Scholar] [CrossRef]
- Huang, Y.; Gong, W.; Su, B.; Zhi, F.; Liu, S.; Jiang, B. Risk and cause of interval colorectal cancer after colonoscopic polypectomy. Digestion 2012, 86, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kolb, J.M.; Molmenti, C.L.; Patel, S.G.; Lieberman, D.A.; Ahnen, D.J. Increased Risk of Colorectal Cancer Tied to Advanced Colorectal Polyps: An Untapped Opportunity to Screen First-Degree Relatives and Decrease Cancer Burden. Am. J. Gastroenterol. 2020, 115, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.E.; Cross, A.J.; Schoen, R.E.; Senore, C.; Pinsky, P.F.; Miller, E.A.; Segnan, N.; Wooldrage, K.; Wieszczy-Szczepanik, P.; Armaroli, P.; et al. Effectiveness of Colonoscopy Screening vs Sigmoidoscopy Screening in Colorectal Cancer. JAMA Netw. Open 2024, 7, e240007. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.E.; Cross, A.J.; Schoen, R.E.; Senore, C.; Pinsky, P.; Miller, E.; Segnan, N.; Wooldrage, K.; Wieszczy-Szczepanik, P.; Armaroli, P.; et al. 15-Year Benefits of Sigmoidoscopy Screening on Colorectal Cancer Incidence and Mortality: A Pooled Analysis of Randomized Trials. Ann. Intern. Med. 2022, 175, 1525–1533. [Google Scholar] [CrossRef]
- Jain, S.; Maque, J.; Galoosian, A.; Osuna-Garcia, A.; May, F.P. Optimal Strategies for Colorectal Cancer Screening. Curr. Treat. Options Oncol. 2022, 23, 474–493. [Google Scholar] [CrossRef]
- Bhagatwala, J.; Singhal, A.; Aldrugh, S.; Sherid, M.; Sifuentes, H.; Sridhar, S. Colonoscopy—Indications and Contraindications. In Screening for Colorectal Cancer with Colonoscopy; Rajunor, E., Ed.; IntechOpen: Rijeka, Croatia, 2015; Chapter 3. [Google Scholar]
- Kim, S.Y.; Kim, H.-S.; Park, H.J. Adverse events related to colonoscopy: Global trends and future challenges. World J. Gastroenterol. 2019, 25, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Kahi, C.J.; Burke, C.A.; Rabeneck, L.; Sauer, B.G.; Rex, D.K. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am. J. Gastroenterol. 2021, 116, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, E.; Kelly, O.B.; Hall, B. Advances in colon capsule endoscopy: A review of current applications and challenges. Front. Gastroenterol. 2023, 2, 1316334. [Google Scholar] [CrossRef]
- Kroijer, R.; Kobaek-Larsen, M.; Qvist, N.; Knudsen, T.; Baatrup, G. Colon capsule endoscopy for colonic surveillance. Color. Dis. 2019, 21, 532–537. [Google Scholar] [CrossRef]
- Zorzi, M.; Fedeli, U.; Schievano, E.; Bovo, E.; Guzzinati, S.; Baracco, S.; Fedato, C.; Saugo, M.; Tos, A.P.D. Impact on colorectal cancer mortality of screening programmes based on the faecal immunochemical test. Gut 2014, 64, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.F.; Lieberman, D.A.; Durbin, T.E.; Weiss, D.G. Accuracy of screening for fecal occult blood on a single stool sam-ple obtained by digital rectal examination: A comparison with recommended sampling practice. Ann. Intern. Med. 2005, 142, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, M.; Hassan, C.; Capodaglio, G.; Narne, E.; Turrin, A.; Baracco, M.; Dal Cin, A.; Fiore, A.; Martin, G.; Repici, A.; et al. Divergent Long-Term Detection Rates of Proximal and Distal Advanced Neoplasia in Fecal Immunochemical Test Screening Programs: A Retrospective Cohort Study. Ann. Intern. Med. 2018, 169, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Barnell, E.K.; Wurtzler, E.M.; La Rocca, J.; Fitzgerald, T.; Petrone, J.; Hao, Y.; Kang, Y.; Holmes, F.L.; Lieberman, D.A. Multitarget Stool RNA Test for Colorectal Cancer Screening. JAMA 2023, 330, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, T.F.; Ransohoff, D.F.; Itzkowitz, S.H.; Levin, T.R.; Lavin, P.; Lidgard, G.P.; Ahlquist, D.A.; Berger, B.M. Multitarget Stool DNA Testing for Colorectal-Cancer Screening. N. Engl. J. Med. 2014, 370, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Carethers, J.M. Fecal DNA Testing for Colorectal Cancer Screening. Annu. Rev. Med. 2020, 71, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; Kubik, M.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965–1977. [Google Scholar]
- Anand, S.; Liang, P.S. A Practical Overview of the Stool DNA Test for Colorectal Cancer Screening. Clin. Transl. Gastroenterol. 2022, 13, e00464. [Google Scholar] [CrossRef]
- Daniel, C.L.; Kohler, C.L.; Stratton, K.L.; Oeffinger, K.C.; Leisenring, W.M.; Waterbor, J.W.; Whelan, K.F.; Armstrong, G.T.; Henderson, T.O.; Krull, K.R.; et al. Predictors of colorectal cancer surveillance among survivors of childhood cancer treated with radiation: A report from the Childhood Cancer Survivor Study. Cancer 2015, 121, 1856–1863. [Google Scholar] [CrossRef]
- Lee, J.K.; Jensen, C.D.; Levin, T.R.; Doubeni, C.A.; Zauber, A.G.; Chubak, J.; Kamineni, A.S.; Schottinger, J.E.; Ghai, N.R.; Udaltsova, N.; et al. Long-term Risk of Colorectal Cancer and Related Death After Adenoma Removal in a Large, Community-based Population. Gastroenterology 2020, 158, 884–894.e5. [Google Scholar] [CrossRef]
- Hassan, C.; Antonelli, G.; Dumonceau, J.-M.; Regula, J.; Bretthauer, M.; Chaussade, S.; Dekker, E.; Ferlitsch, M.; Gimeno-Garcia, A.; Jover, R.; et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guide-line—Update 2020. Endoscopy 2020, 52, 687–700. [Google Scholar] [CrossRef]
- Tran, A.H.; Ngor, E.W.M.; Wu, B.U. Surveillance colonoscopy in elderly patients: A retrospective cohort study. JAMA Intern. Med. 2014, 174, 1675–1682. [Google Scholar] [CrossRef]
- Cross, A.J.; Wooldrage, K.; Robbins, E.C.; Kralj-Hans, I.; MacRae, E.; Piggott, C.; Stenson, I.; Prendergast, A.; Patel, B.; Pack, K.; et al. Faecal immunochemical tests (FIT) versus colonoscopy for surveillance after screening and polypectomy: A diagnostic accuracy and cost-effectiveness study. Gut 2018, 68, 1642–1652. [Google Scholar] [CrossRef]
- Mulder, S.A.; Kranse, R.; Damhuis, R.A.; Rob, J.; Kuipers, E.J.; van Leerdam, M.E. The incidence and risk factors of metachronous colorectal cancer: An indication for follow-up. Dis. Colon. Rectum 2012, 55, 522–531. [Google Scholar] [CrossRef]
- Fuccio, L.; Rex, D.; Ponchon, T.; Frazzoni, L.; Dinis-Ribeiro, M.; Bhandari, P.; Dekker, E.; Pellisè, M.; Correale, L.; van Hooft, J.; et al. New and Recurrent Colorectal Cancers After Resection: A Systematic Review and Meta-analysis of Endoscopic Surveillance Studies. Gastroenterology 2019, 156, 1309–1323.e3. [Google Scholar] [CrossRef]
- Hassan, C.; Wysocki, P.T.; Fuccio, L.; Seufferlein, T.; Dinis-Ribeiro, M.; Brandão, C.; Regula, J.; Frazzoni, L.; Pellise, M.; Alfieri, S.; et al. Endoscopic surveillance after surgical or endoscopic resection for colorectal cancer: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Digestive Oncology (ESDO) Guideline. Endoscopy 2019, 51, 266–277. [Google Scholar] [CrossRef]
- Aziz, Z.; Wagner, S.; Agyekum, A.; Pumpalova, Y.S.; Prest, M.; Lim, F.; Rustgi, S.; Kastrinos, F.; Grady, W.M.; Hur, C. Cost-Effectiveness of Liquid Biopsy for Colorectal Cancer Screening in Patients Who Are Unscreened. JAMA Netw. Open 2023, 6, e2343392. [Google Scholar] [CrossRef]
- Gavin, D.R.; Valori, R.M.; Anderson, J.T.; Donnelly, M.T.; Williams, J.G.; Swarbrick, E.T. The national colonoscopy audit: A nationwide assessment of the quality and safety of colonoscopy in the UK. Gut 2012, 62, 242–249. [Google Scholar] [CrossRef]
- Shinji, S.; Yamada, T.; Matsuda, A.; Sonoda, H.; Ohta, R.; Iwai, T.; Takeda, K.; Yonaga, K.; Masuda, Y.; Yoshida, H. Recent Advances in the Treatment of Colorectal Cancer: A Review. J. Nippon. Med. Sch. 2022, 89, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.A.; Rees, C.J.; Sharp, L.; Koo, S. A risk-stratified approach to colorectal cancer prevention and diagnosis. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Landegren, U.; Hammond, M. Cancer diagnostics based on plasma protein biomarkers: Hard times but great expectations. Mol. Oncol. 2020, 15, 1715–1726. [Google Scholar] [CrossRef] [PubMed]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Kalinich, M.; Haber, D.A. Cancer detection: Seeking signals in blood. Science 2018, 359, 866–867. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, K.; Nguyen, L.H.; Joshi, A.; Cao, Y.; Nishihara, R.; Wu, K.; Ogino, S.; Giovannucci, E.L.; Song, M.; et al. Association of Screening Lower Endoscopy With Colorectal Cancer Incidence and Mortality in Adults Older Than 75 Years. JAMA Oncol. 2021, 7, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Shaheed, S.-U.; Tait, C.; Kyriacou, K.; Linforth, R.; Salhab, M.; Sutton, C. Evaluation of nipple aspirate fluid as a diagnostic tool for early detection of breast cancer. Clin. Proteom. 2018, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, C.V.; Pereira, F.; Pereira, J.A.M.; Camara, J.S. Volatilomics: An Emerging and Promising Avenue for the Detection of Potential Prostate Cancer Biomarkers. Cancers 2022, 14, 3982. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Stenzl, A.; Sharma, A.; Vasdev, N. Urinary biomarkers in bladder cancer: A review of the current landscape and future directions. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Xiang, L.; Wu, S.; Hua, Q.; Bao, C.; Liu, H. Volatile Organic Compounds in Human Exhaled Breath to Diagnose Gastrointestinal Cancer: A Meta-Analysis. Front. Oncol. 2021, 11, 606915. [Google Scholar] [CrossRef] [PubMed]
- Brasier, N.; Eckstein, J. Sweat as a Source of Next-Generation Digital Biomarkers. Digit. Biomark. 2019, 3, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L.; Robinson, A.B.; Teranishi, R.; Cary, P. Quantitative Analysis of Urine Vapor and Breath by Gas-Liquid Partition Chromatography. Proc. Natl. Acad. Sci. USA 1971, 68, 2374–2376. [Google Scholar] [CrossRef]
- Oakley-Girvan, I.; Davis, S.W. Breath based volatile organic compounds in the detection of breast, lung, and colorectal cancers: A systematic review. Cancer Biomark. 2017, 21, 29–39. [Google Scholar] [CrossRef]
- Hussain, M.S.; Gupta, G.; Mishra, R.; Patel, N.; Gupta, S.; Alzarea, S.I.; Kazmi, I.; Kumbhar, P.; Disouza, J.; Dureja, H.; et al. Unlocking the secrets: Volatile Organic Compounds (VOCs) and their devastating effects on lung cancer. Pathol. Res. Pract. 2024, 255, 155157. [Google Scholar] [CrossRef] [PubMed]
- Sutaria, S.R.; Gori, S.S.; Morris, J.D.; Xie, Z.; Fu, X.-A.; Nantz, M.H. Lipid Peroxidation Produces a Diverse Mixture of Saturated and Unsaturated Aldehydes in Exhaled Breath That Can Serve as Biomarkers of Lung Cancer—A Review. Metabolites 2022, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Mezmale, L.; Leja, M.; Lescinska, A.M.; Pčolkins, A.; Kononova, E.; Bogdanova, I.; Polaka, I.; Stonans, I.; Kirsners, A.; Ager, C.; et al. Identification of Volatile Markers of Colorectal Cancer from Tumor Tissues Using Volatilomic Approach. Molecules 2023, 28, 5990. [Google Scholar] [CrossRef] [PubMed]
- Janfaza, S.; Nojavani, M.B.; Khorsand, B.; Nikkhah, M.; Zahiri, J. Cancer Odor Database (COD): A critical databank for cancer diagnosis research. Database 2017, 2017, bax055. [Google Scholar] [CrossRef]
- Janfaza, S.; Khorsand, B.; Nikkhah, M.; Zahiri, J. Digging deeper into volatile organic compounds associated with cancer. Biol. Methods Protoc. 2019, 4, bpz014. [Google Scholar] [CrossRef]
- Mika, A.; Pakiet, A.; Czumaj, A.; Kaczynski, Z.; Liakh, I.; Kobiela, J.; Perdyan, A.; Adrych, K.; Makarewicz, W.; Sledzinski, T. Decreased Triacylglycerol Content and Elevated Contents of Cell Membrane Lipids in Colorectal Cancer Tis-sue: A Lipidomic Study. J. Clin. Med. 2020, 9, 1095. [Google Scholar] [CrossRef]
- Guillen, M.D.; Goicoechea, E. Toxic oxygenated alpha,beta-unsaturated aldehydes and their study in foods: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 119–136. [Google Scholar] [CrossRef]
- Lee, N.; Spears, M.E.; Carlisle, A.E.; Kim, D. Endogenous toxic metabolites and implications in cancer therapy. Oncogene 2020, 39, 5709–5720. [Google Scholar] [CrossRef]
- Carley, A.N.; Maurya, S.K.; Fasano, M.; Wang, Y.; Selzman, C.H.; Drakos, S.G.; Lewandowski, E.D. Short-Chain Fatty Acids Outpace Ketone Oxidation in the Failing Heart. Circulation 2021, 143, 1797–1808. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Carneiro-Freire, N.; Seco-Filgueira, M.; Fernández-Fernández, C.; Mouriño-Bayolo, D. Mitochondrial beta-oxidation of saturated fatty acids in humans. Mitochondrion 2019, 46, 73–90. [Google Scholar] [CrossRef]
- van der Sluis, R.; Erasmus, E. Xenobiotic/medium chain fatty acid: CoA ligase—A critical review on its role in fatty acid metabolism and the detoxification of benzoic acid and aspirin. Expert. Opin. Drug Metab. Toxicol. 2016, 12, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [PubMed]
- Cotter, D.G.; Schugar, R.C.; Crawford, P.A. Ketone body metabolism and cardiovascular disease. Am. J. Physiol.-Heart Circ. Physiol. 2013, 304, H1060–H1076. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P. The monocarboxylate transporter family—Structure and functional characterization. IUBMB Life 2011, 64, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.J. Analysis of Volatile Organic Compounds in the Exhaled Breath for the Diagnosis of Lung Cancer. J. Thorac. Oncol. 2008, 3, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Mochalski, P.; Sponring, A.; King, J.; Unterkofler, K.; Troppmair, J.; Amann, A. Release and uptake of volatile organic compounds by human hepatocellular carcinoma cells (HepG2) in vitro. Cancer Cell Int. 2013, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Ratiu, I.A.; Ligor, T.; Bocos-Bintintan, V.; A Mayhew, C.; Buszewski, B. Volatile Organic Compounds in Exhaled Breath as Fingerprints of Lung Cancer, Asthma and COPD. J. Clin. Med. 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- De Vietro, N.; Aresta, A.; Rotelli, M.T.; Zambonin, C.; Lippolis, C.; Picciariello, A.; Altomare, D.F. Relationship between cancer tissue derived and exhaled volatile organic compound from colorectal can-cer patients. Preliminary results. J. Pharm. Biomed. Anal. 2020, 180, 113055. [Google Scholar] [CrossRef] [PubMed]
- Dima, A.C.; Balaban, D.V.; Dima, A. Diagnostic Application of Volatile Organic Compounds as Potential Biomarkers for Detecting Digestive Neoplasia: A Systematic Review. Diagnostics 2021, 11, 2317. [Google Scholar] [CrossRef]
- Vernia, F.; Valvano, M.; Fabiani, S.; Stefanelli, G.; Longo, S.; Viscido, A.; Latella, G. Are Volatile Organic Compounds Accurate Markers in the Assessment of Colorectal Cancer and Inflammatory Bowel Diseases? A Review. Cancers 2021, 13, 2361. [Google Scholar] [CrossRef]
- Ferrari, A.; Neefs, I.; Hoeck, S.; Peeters, M.; Van Hal, G. Towards Novel Non-Invasive Colorectal Cancer Screening Methods: A Comprehensive Review. Cancers 2021, 13, 1820. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ke, C.; Wang, X.; Chi, C.; Guo, L.; Luo, S.; Guo, Z.; Xu, G.; Zhang, F.; Li, E. Noninvasive detection of colorectal cancer by analysis of exhaled breath. Anal. Bioanal. Chem. 2014, 406, 4757–4763. [Google Scholar] [CrossRef] [PubMed]
- Altomare, D.F.; Picciariello, A.; Rotelli, M.T.; De Fazio, M.; Aresta, A.; Zambonin, C.G.; Vincenti, L.; Trerotoli, P.; De Vietro, N. Chemical signature of colorectal cancer: Case–control study for profiling the breath print. BJS Open 2020, 4, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Amal, H.; Leja, M.; Funka, K.; Lasina, I.; Skapars, R.; Sivins, A.; Ancans, G.; Kikuste, I.; Vanags, A.; Tolmanis, I.; et al. Breath testing as potential colorectal cancer screening tool. Int. J. Cancer 2015, 138, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of na-nosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef] [PubMed]
- van Keulen, K.E.; Jansen, M.E.; Schrauwen, R.W.; Kolkman, J.J.; Siersema, P.D. Volatile organic compounds in breath can serve as a non-invasive diagnostic biomarker for the detec-tion of advanced adenomas and colorectal cancer. Aliment. Pharmacol. Ther. 2020, 51, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, T.; Rezaloo, F. Toluene chemiresistor sensor based on nano-porous toluene-imprinted polymer. Int. J. Environ. Anal. Chem. 2012, 93, 919–934. [Google Scholar] [CrossRef]
- Janfaza, S.; Banan Nojavani, M.; Nikkhah, M.; Alizadeh, T.; Esfandiar, A.; Ganjali, M.R. A selective chemiresistive sensor for the cancer-related volatile organic compound hexanal by using molecularly imprinted polymers and multiwalled carbon nanotubes. Mikrochim. Acta 2019, 186, 137. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, M.; Janfaza, S.; Tahmooressi, H.; Tasnim, N.; Hoorfar, M. Selective detection of VOCs using microfluidic gas sensor with embedded cylindrical microfeatures coated with graphene oxide. J. Hazard. Mater. 2021, 424, 127566. [Google Scholar] [CrossRef]
- Paknahad, M.; Mcintosh, C.; Hoorfar, M. Selective detection of volatile organic compounds in microfluidic gas detectors based on “like dissolves like”. Sci. Rep. 2019, 9, 161. [Google Scholar] [CrossRef]
- Soyer, O.U.; Dizdar, E.A.; Keskin, O.; Lilly, C.; Kalayci, O. Comparison of two methods for exhaled breath condensate collection. Allergy 2006, 61, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Marie-Desvergne, C.; Dubosson, M.; Mossuz, V.C. Evaluation of a new method for the collection and measurement of 8-isoprostane in exhaled breath for future application in nanoparticle exposure biomonitoring. J. Breath Res. 2018, 12, 031001. [Google Scholar] [CrossRef] [PubMed]
- Zamuruyev, K.; A Aksenov, A.; Pasamontes, A.; Brown, J.F.; Pettit, D.R.; Foutouhi, S.; Weimer, B.C.; Schivo, M.; Kenyon, N.J.; Delplanque, J.-P.; et al. Human breath metabolomics using an optimized non-invasive exhaled breath condensate sampler. J. Breath. Res. 2016, 11, 016001. [Google Scholar] [CrossRef] [PubMed]
- Li, D. Recent advances in colorectal cancer screening. Chronic Dis. Transl. Med. 2018, 4, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Trevethan, R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public Health 2017, 5, 307. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, P.; Kaur, G.; Singh, N.K. Nanotechnology for colorectal cancer detection and treatment. World J. Gastroenterol. 2022, 28, 6497–6511. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, X.; Fu, K.; Zhang, X.; Shang, L.; Su, Z. Highly fluorescent carbon dots as novel theranostic agents for biomedical applications. Nanoscale 2021, 13, 17236–17253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, M.; Wu, M.; Zhu, J.; Zhang, X. Multifunctional Carbon-Based Nanomaterials: Applications in Biomolecular Imaging and Therapy. ACS Omega 2018, 3, 9126–9145. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Xue, F.; Chen, H.; Wu, X.; Zhang, H.; Chen, G.; Lu, J.; Cai, L.; Xiang, G.; Deng, Z.; et al. A multi-center study of using carbon nanoparticles to track lymph node metastasis in T1–2 colorectal cancer. Surg. Endosc. 2014, 28, 3315–3321. [Google Scholar] [CrossRef]
- Mirahadi, M.; Ghanbarzadeh, S.; Ghorbani, M.; Gholizadeh, A.; Hamishehkar, H. A review on the role of lipid-based nanoparticles in medical diagnosis and imaging. Ther. Deliv. 2018, 9, 557–569. [Google Scholar] [CrossRef]
- Alizadeh, T. Chemiresistor sensors array optimization by using the method of coupled statistical techniques and its applica-tion as an electronic nose for some organic vapors recognition. Sens. Actuators B Chem. 2010, 143, 740–749. [Google Scholar] [CrossRef]
- Alizadeh, T.; Hamed, L. Graphene/graphite/molecularly imprinted polymer nanocomposite as the highly selective gas sensor for nitrobenzene vapor recognition. J. Environ. Chem. Eng. 2014, 2, 1514–1526. [Google Scholar] [CrossRef]
- Regmi, S.; Poudel, C.; Adhikari, R.; Luo, K.Q. Applications of Microfluidics and Organ-on-a-Chip in Cancer Research. Biosensors 2022, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Erkocyigit, B.A.; Ozufuklar, O.; Yardim, A.; Celik, E.G.; Timur, S. Biomarker Detection in Early Diagnosis of Cancer: Recent Achievements in Point-of-Care Devices Based on Paper Microfluidics. Biosensors 2023, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Ogunwobi, O.O.; Mahmood, F.; Akingboye, A. Biomarkers in Colorectal Cancer: Current Research and Future Prospects. Int. J. Mol. Sci. 2020, 21, 5311. [Google Scholar] [CrossRef] [PubMed]
- Haick, H.; Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A. Assessment, origin, and implementation of breath volatile cancer markers. Chem. Soc. Rev. 2013, 43, 1423–1449. [Google Scholar] [CrossRef] [PubMed]
- Mochalski, P.; Leja, M.; Gasenko, E.; Skapars, R.; Santare, D.; Sivins, A.; Aronsson, D.E.; Ager, C.; Jaeschke, C.; Shani, G.; et al. Ex vivo emission of volatile organic compounds from gastric cancer and non-cancerous tissue. J. Breath Res. 2018, 12, 046005. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.L.; Passos, M.; Câmara, J.S. Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. Br. J. Cancer 2011, 105, 1894–1904. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; McFarlane, M.J.; Ryan-Fisher, C.; Westenbrink, E.; Hodges, P.; Thomas, M.G.; Chambers, S.; O’Connell, N.; Bailey, C.; Harmston, C.; et al. Detection of Colorectal Cancer (CRC) by Urinary Volatile Organic Compound Analysis. PLoS ONE 2014, 9, e108750. [Google Scholar] [CrossRef]
- Gosho, M.; Nagashima, K.; Sato, Y. Study Designs and Statistical Analyses for Biomarker Research. Sensors 2012, 12, 8966–8986. [Google Scholar] [CrossRef]
- Xiang, W.; Wang, R.; Bai, D.; Yu, T.-H.; Chen, X.-Z.; on behalf of the SIGES Research Groups. Helicobacter Pylori Related Gastric Cancer Screening and Cost-Effectiveness Analysis: A Hospital-Based Cross-Sectional Study (SIGES). Nutr. Cancer 2022, 74, 2769–2778. [Google Scholar] [CrossRef]
- Selleck, M.J.; Senthil, M.; Wall, N.R. Making Meaningful Clinical Use of Biomarkers. Biomark. Insights 2017, 12, 1177271917715236. [Google Scholar] [CrossRef]
- Pitkänen, A.; Löscher, W.; Vezzani, A.; Becker, A.J.; Simonato, M.; Lukasiuk, K.; Gröhn, O.; Bankstahl, J.P.; Friedman, A.; Aronica, E.; et al. Advances in the development of biomarkers for epilepsy. Lancet Neurol. 2016, 15, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H. Recent developments in biomarkers in Parkinson disease. Curr. Opin. Neurol. 2013, 26, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Parikh, C.R. Current concepts and advances in biomarkers of acute kidney injury. Crit. Rev. Clin. Lab. Sci. 2021, 58, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Tsou, P.-H.; Lin, Z.-L.; Pan, Y.-C.; Yang, H.-C.; Chang, C.-J.; Liang, S.-K.; Wen, Y.-F.; Chang, C.-H.; Chang, L.-Y.; Yu, K.-L.; et al. Exploring Volatile Organic Compounds in Breath for High-Accuracy Prediction of Lung Cancer. Cancers 2021, 13, 1431. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, V.A. What do dogs, ancient Romans, Linus Pauling, and mass spectrometry have in common? Early lung cancer and exhaled breath. J. Thorac. Cardiovasc. Surg. 2015, 151, 313–314. [Google Scholar] [CrossRef]
- Heaney, L.M.; Kang, S.; Turner, M.A.; Lindley, M.R.; Thomas, C.L.P. The Impact of a Graded Maximal Exercise Protocol on Exhaled Volatile Organic Compounds: A Pilot Study. Molecules 2022, 27, 370. [Google Scholar] [CrossRef]
- Alsaadi, D.; Clements, N.; Gabuniya, N.; Francis, N.; Chand, M. Exhaled volatile organic compounds in the detection of colorectal cancer: A systematic review and meta-analysis. EXCLI J. 2024, 23, 795–810. [Google Scholar] [CrossRef]
- Gower, H.; Danielson, K.; Dennett, A.P.E.; Deere, J. Potential role of volatile organic compound breath testing in the Australasian colorectal cancer pathway. ANZ J. Surg. 2023, 93, 1159–1161. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, Y.; Tan, S.; Li, Z.; Zheng, R.; Ren, Y.; Jiang, Y.; Huang, X. Diagnostic performance of volatile organic compounds analysis and electronic noses for detecting colorectal cancer: A systematic review and meta-analysis. Front. Oncol. 2024, 14, 1397259. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, K.; Mineta, D.; Ishida, K.; Katayama, K.; Kondo, T. Mixed effects of moderate exercise and subsequent various food ingestion on breath acetone. J. Breath Res. 2022, 17, 016004. [Google Scholar] [CrossRef] [PubMed]
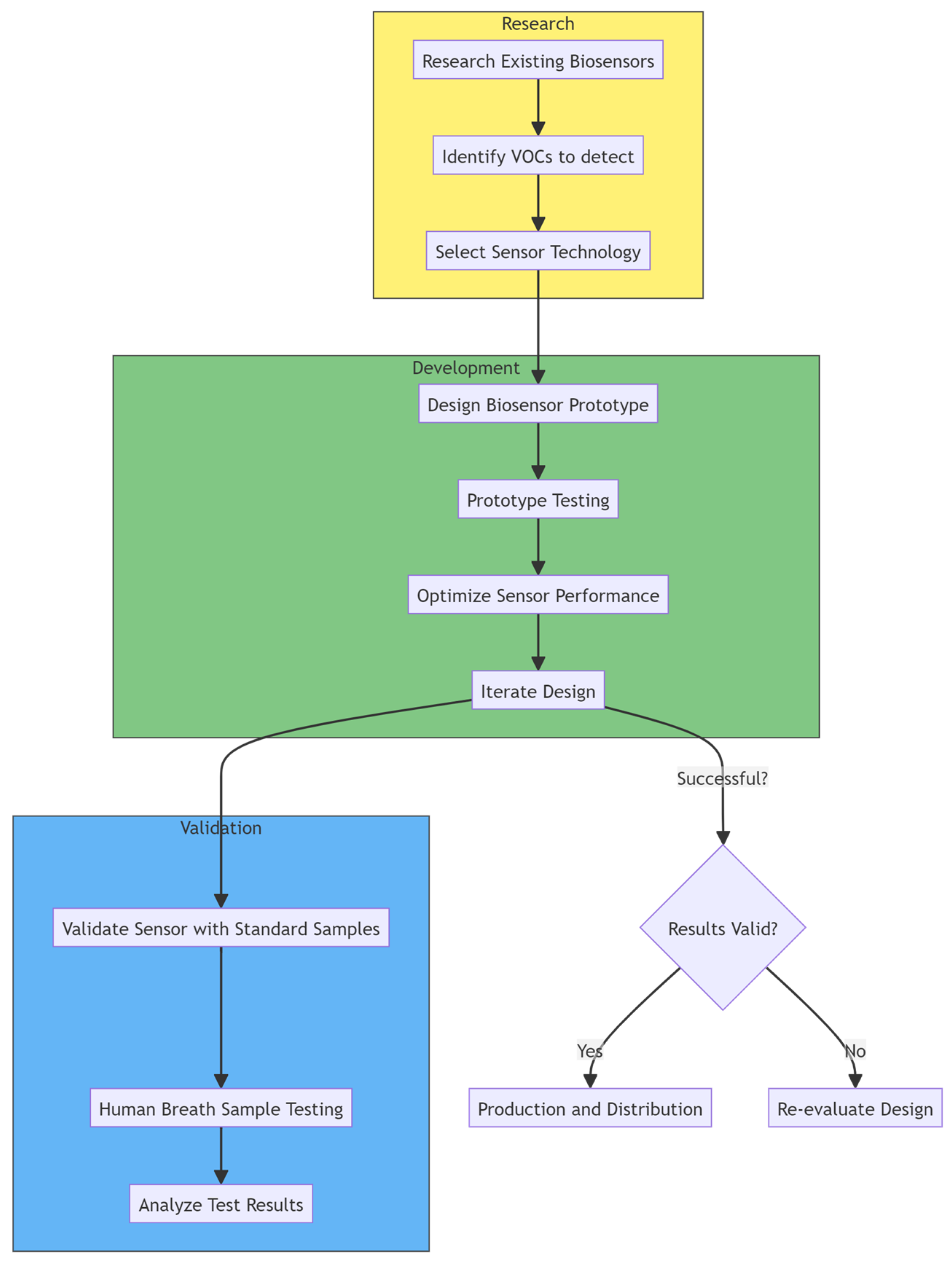

| Technique | Sample Used | CRC-Related VOCs Identified: | Reference | |
|---|---|---|---|---|
| 1 | Solid-phase microextraction gas chromatography/mass spectrometry (SPME-GC/MS) | Exhaled breath from patients and healthy subjects | 3-hydroxy-2,4,4-trimethylpentyl 2-methylpropanoate, cyclohexanone, 2,2-dimethyldecane,cyclooctylmethanol, dodecane, 4-ethyl-1-octyn-3-ol,ethylaniline, trans-2-dodecen-1-ol, and 6-t-butyl-2,2,9,9-tetramethyl-3,5-decadien-7-yne | Wang et al. [105] |
| 2 | Gas chromatography–mass spectrometry | Exhaled breath from patients and healthy subjects | tetradecane, ethyl-benzene, methylbenzene, acetic acid, 5,9-undecadien-2-one, 6,10-dimethyl (E), decane, benzaldehyde, benzoicacid, 1,3 bis(1-metiletenil) benzene, decanal, unidenti-fied compound T22_75, dodecane, 2-ethyl-1-hexanoland ethanone, 1[4-(1-methylethenyl)phenyl] | Altomare et al. [106] |
| 3 | E-nose technology | Exhaled breath from patients and healthy subjects | Van Keulen et al. [109] | |
| 4 | GC-MS and cross-reactive nanoarrays in combination with pattern recognition methods | Exhaled breath from patients and healthy subjects | Ethanol, acetone, ethyl acetate, -methyl octane | Amal et al. [107] |
| 5 | Functionalized gold nanoparticles (GNPs) | Exhaled breath from patients and healthy subjects | ¼1,10-(1-butenylidene)bis benzene; ¼1,3-dimethyl benzene; ¼1-iodo nonane; ¼[(1,1-dimethylethyl)thio] aceticacid; ¼4-(4-propylcyclohexyl)-40-cyano[1,10-biphenyl]-4-yl ester benzoic acid; ¼2-amino-5-isopropyl-8-methyl-1-azulenecarbonitrile | Peng et al. [108] |
| 6 | Molecular imprinted polymers (MIPs) chemiresistor sensor | - | toluene | Alizadeh and Rezaloo [110] |
| 7 | Molecular imprinted polymer nanoparticles (MIPs) chemiresistive sensor | - | hexanal | Janfaza et al. [111] |
| 8 | Microfluidic gas sensor— 3D printing and coating with Graphine Oxide (GO) | - | methanol, ethanol, pentanol, hexanal, toluene | Ghazi et al. [112] |
| 9 | Microfluidic-based gas detector—3D-printed with a metal oxide semiconductor (MOS) gas sensor | - | acetone, methanol | Paknahad et al. [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golfinopoulou, R.; Hatziagapiou, K.; Mavrikou, S.; Kintzios, S. Unveiling Colorectal Cancer Biomarkers: Harnessing Biosensor Technology for Volatile Organic Compound Detection. Sensors 2024, 24, 4712. https://doi.org/10.3390/s24144712
Golfinopoulou R, Hatziagapiou K, Mavrikou S, Kintzios S. Unveiling Colorectal Cancer Biomarkers: Harnessing Biosensor Technology for Volatile Organic Compound Detection. Sensors. 2024; 24(14):4712. https://doi.org/10.3390/s24144712
Chicago/Turabian StyleGolfinopoulou, Rebecca, Kyriaki Hatziagapiou, Sophie Mavrikou, and Spyridon Kintzios. 2024. "Unveiling Colorectal Cancer Biomarkers: Harnessing Biosensor Technology for Volatile Organic Compound Detection" Sensors 24, no. 14: 4712. https://doi.org/10.3390/s24144712






