Evaluation of the Reliability of the CCM-300 Chlorophyll Content Meter in Measuring Chlorophyll Content for Various Plant Functional Types
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Paired, Two-Tailed T-Test
2.2.2. Linear Regression Analysis
3. Results
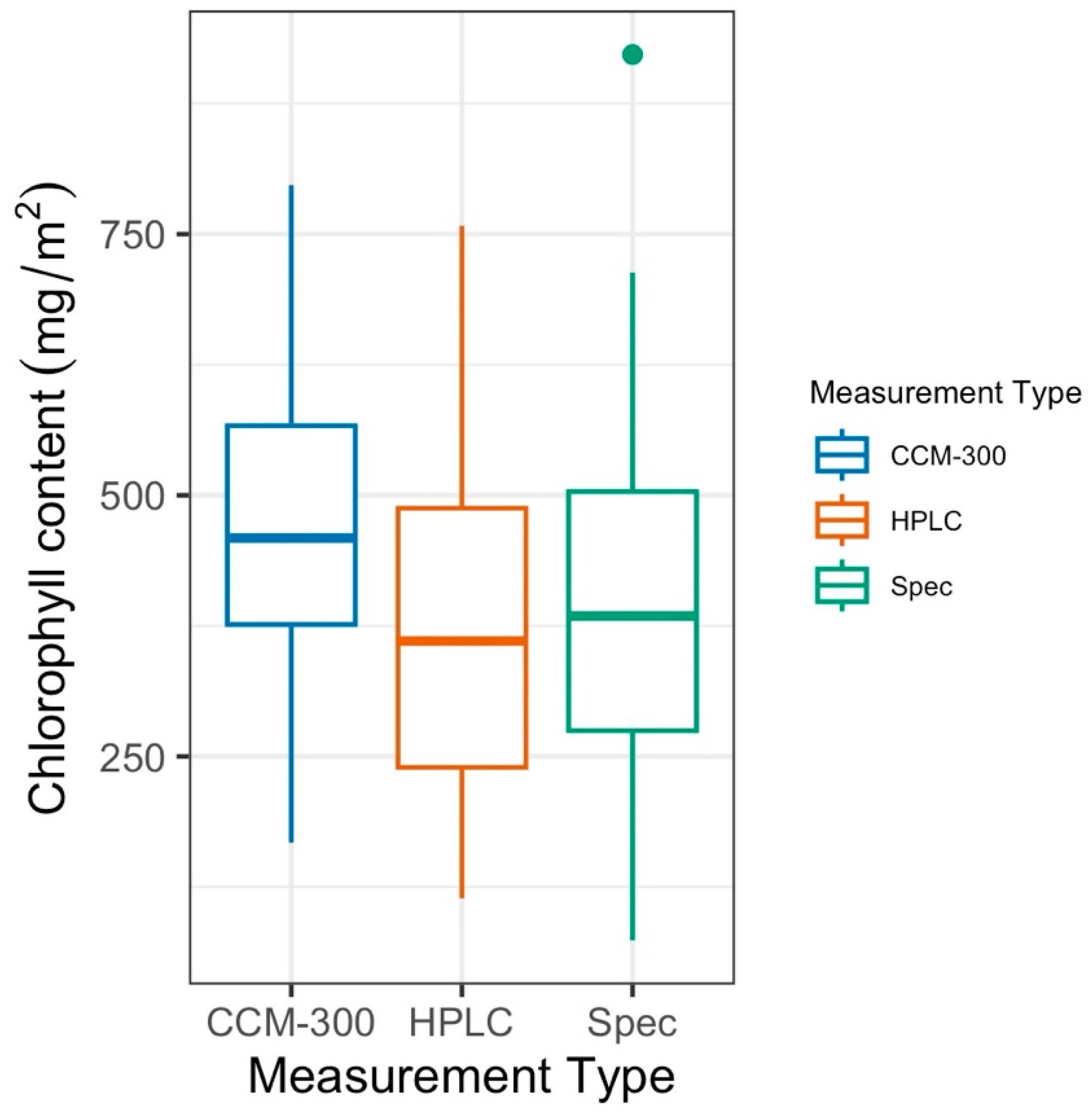
3.1. CCM-300 Measurements vs. Laboratory Chemistry Measurements
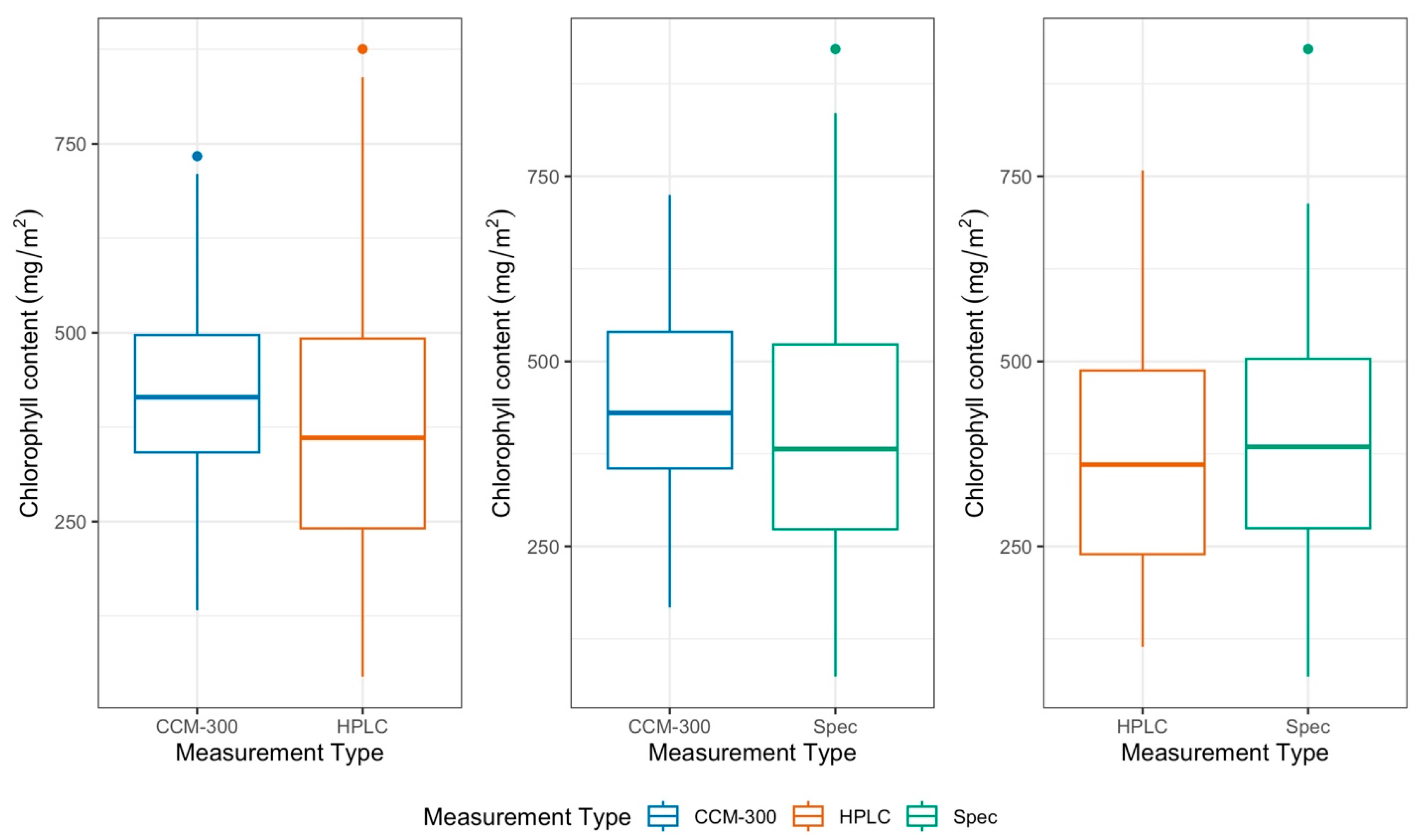
3.1.1. Paired T-Test for 41 Samples with All Three Measurements
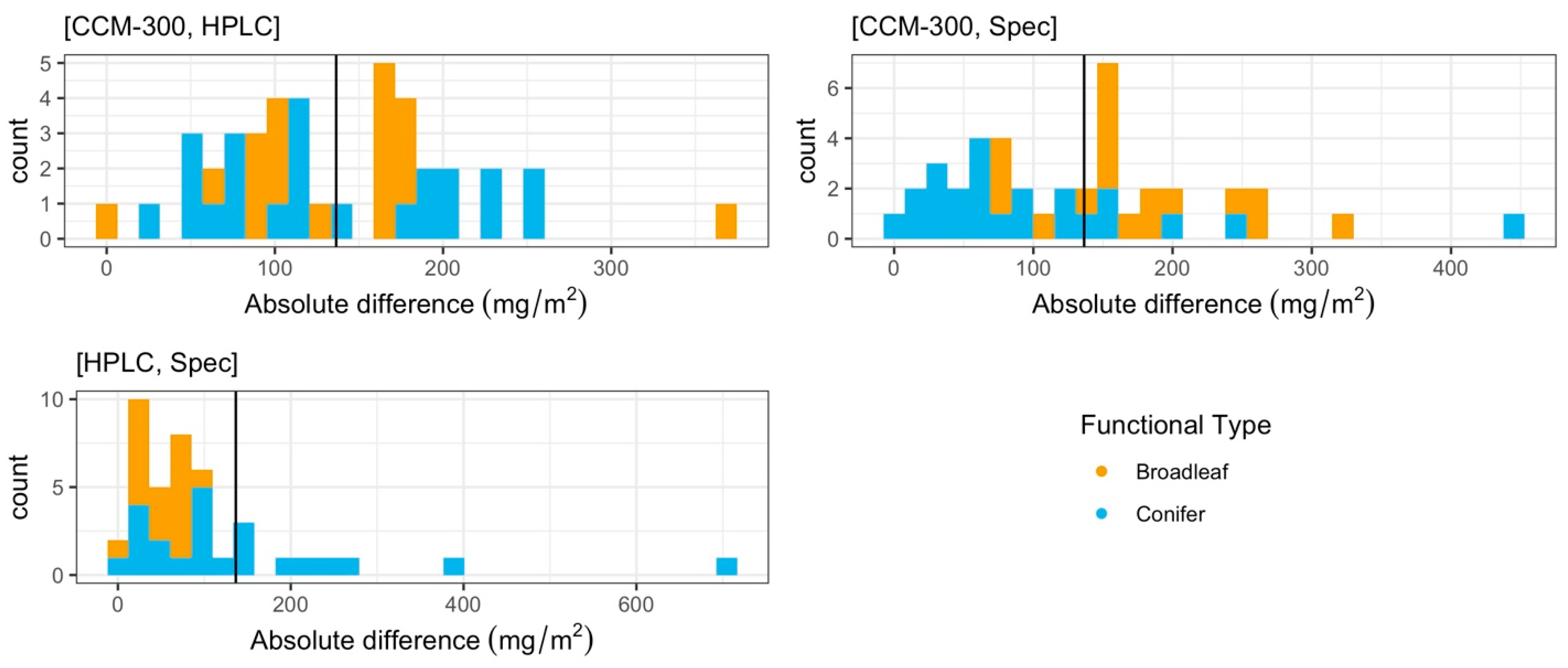
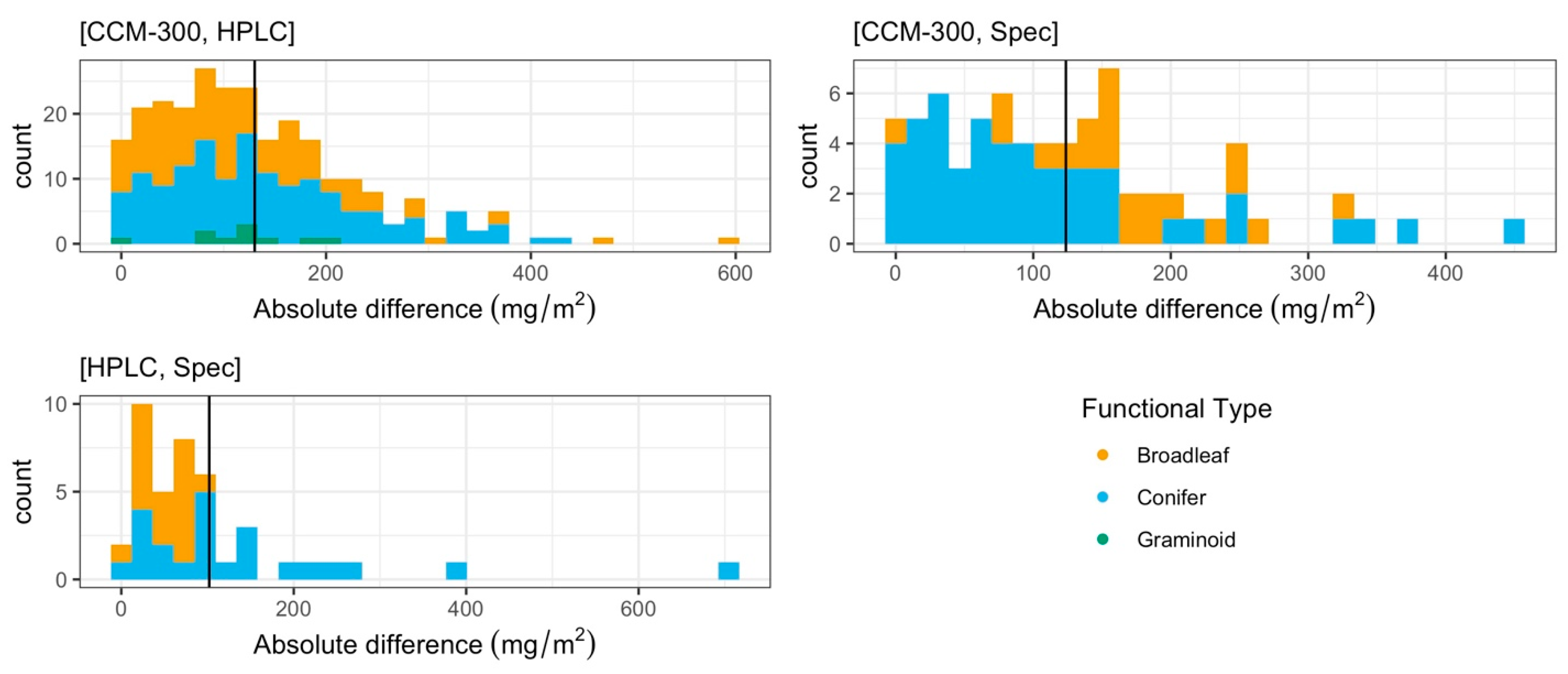
3.1.2. Paired T-Test of All Paired Samples
3.1.3. Iterative Subsampling for Randomized, Permutated Analysis
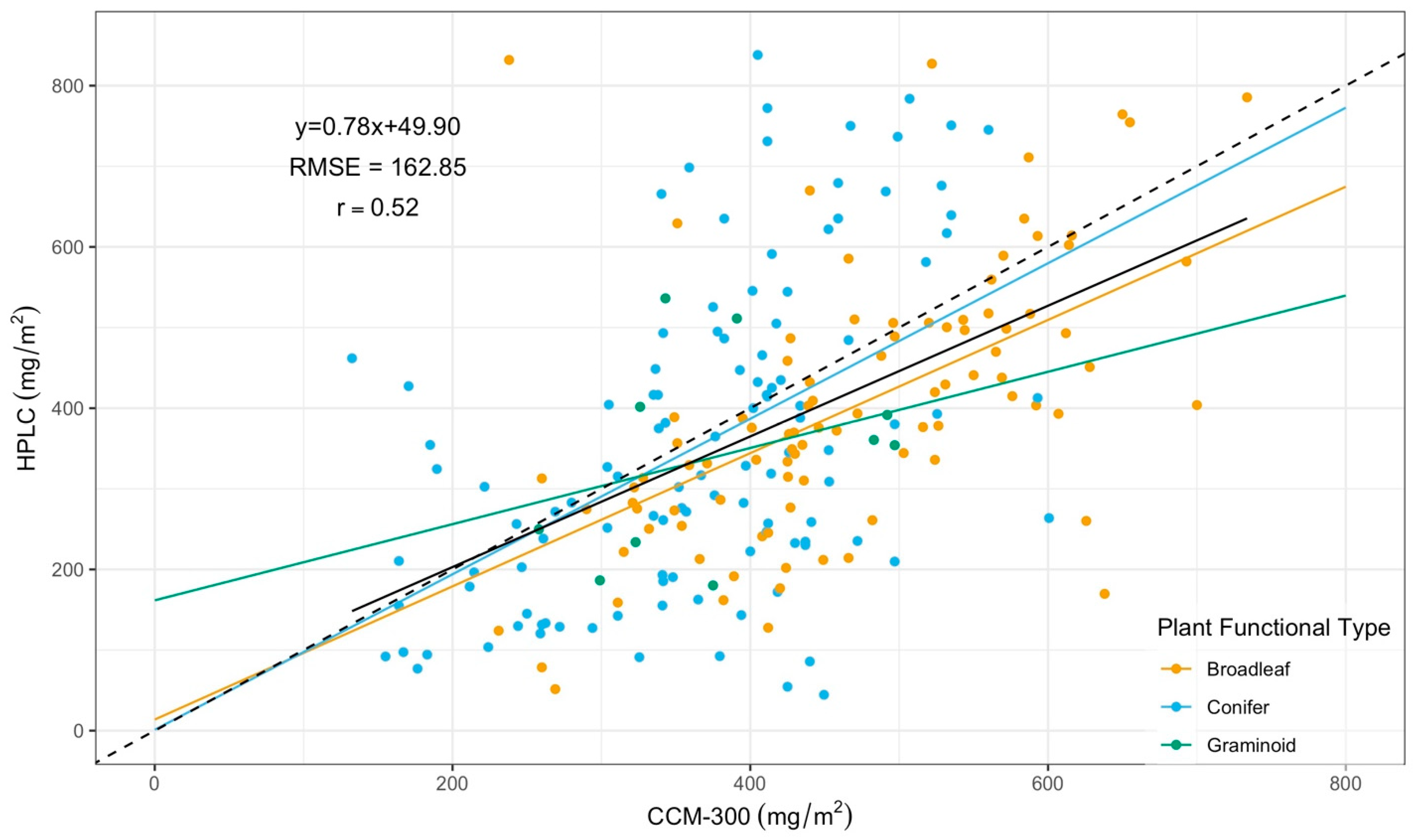
3.2. CCM-300 Measurements for Different Plant Functional Types
3.2.1. CCM-300 Measurements for Different Plant Functional Types
3.2.2. Needle Age Analysis
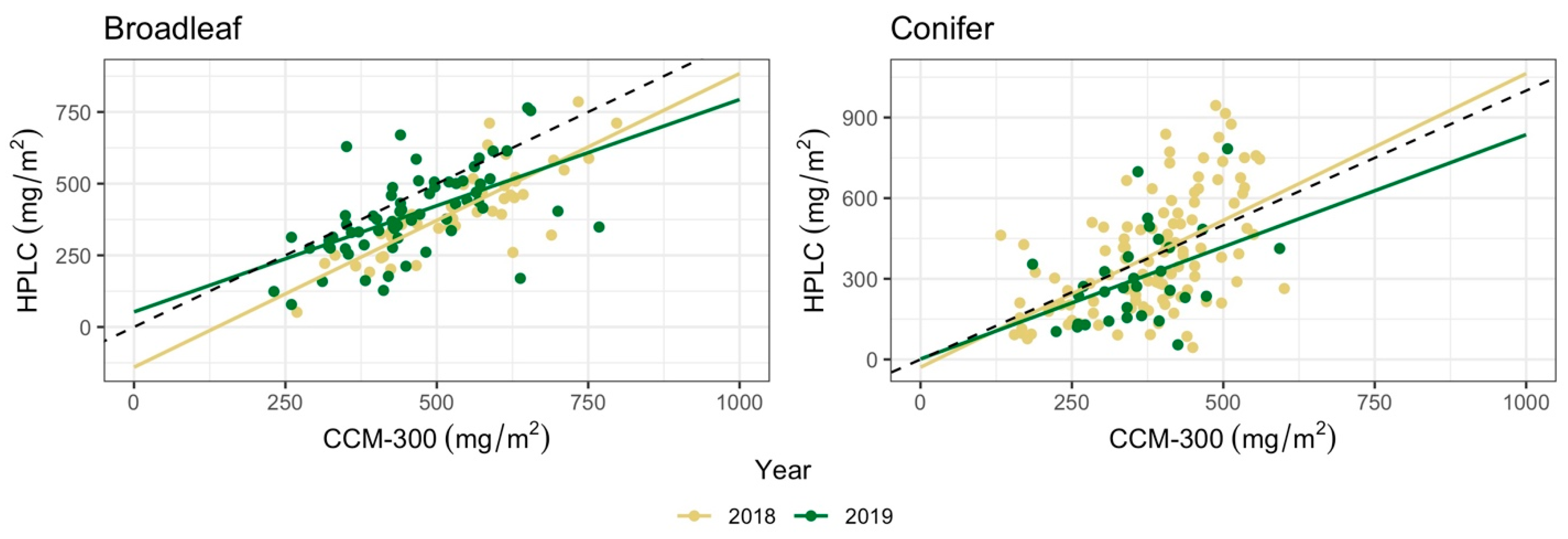
3.3. CCM-300 Performance across Years
4. Discussion
4.1. CCM-300 Measurements vs. Laboratory Chemistry Measurements
4.2. CCM-300 Measurements for Different Plant Functional Types
4.3. CCM-300 Performance across Years
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Croft, H.; Chen, J.M. 3.09—Leaf Pigment Content. In Comprehensive Remote Sensing; Liang, S., Ed.; Elsevier: Oxford, UK, 2018; pp. 117–142. [Google Scholar]
- Murchie, E.; Horton, P. Acclimation of photosynthesis to irradiance and spectral quality in British plant species: Chlorophyll content, photosynthetic capacity and habitat preference. Plant Cell Environ. 1997, 20, 438–448. [Google Scholar] [CrossRef]
- de Oliveira, J.G.; Alves, P.L.D.C.A.; Vitória, A.P. Alterations in chlorophyll a fluorescence, pigment concentrations and lipid peroxidation to chilling temperature in coffee seedlings. Environ. Exp. Bot. 2009, 67, 71–76. [Google Scholar] [CrossRef]
- Fleischer, W.E. The relation between chlorophyll content and rate of photosynthesis. J. Gen. Physiol. 1935, 18, 573. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, R.P.; Oxborough, K.; Pallett, K.E.; Baker, N.R. Rapid, noninvasive screening for perturbations of metabolism and plant growth using chlorophyll fluorescence imaging. Plant Physiol. 2003, 132, 485–493. [Google Scholar] [CrossRef] [PubMed]
- McAlister, E.D.; Myers, J. The time course of photosynthesis and fluorescence observed simultaneously. Smithson. Misc. Collect. 1940, 99, 1–37. [Google Scholar]
- Papageorgiou, G. 6—Chlorophyll Fluorescence: An Intrinsic Probe of Photosynthesis. In Energetics of Photosynthesis; Govindjee, Ed.; Academic Press: Cambridge, MA, USA, 1975; pp. 319–371. [Google Scholar]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Humphries, S.; Falkowski, P.G. Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Biol. 1994, 45, 633–662. [Google Scholar] [CrossRef]
- Ogaya, R.; Penuelas, J.; Asensio, D.; Llusià, J. Chlorophyll fluorescence responses to temperature and water availability in two co-dominant Mediterranean shrub and tree species in a long-term field experiment simulating climate change. Environ. Exp. Bot. 2011, 71, 123–127. [Google Scholar] [CrossRef]
- Peng, S.; Garcia, F.V.; Laza, R.C.; Sanico, A.L.; Visperas, R.M.; Cassman, K.G. Increased N-use efficiency using a chlorophyll meter on high-yielding irrigated rice. Field Crops Res. 1996, 47, 243–252. [Google Scholar] [CrossRef]
- Atta, B.M.; Saleem, M.; Ali, H.; Arshad, H.M.I.; Ahmed, M. Chlorophyll as a biomarker for early disease diagnosis. Laser Phys. 2018, 28, 065607. [Google Scholar] [CrossRef]
- Xu, M.; Liu, R.; Chen, J.M.; Shang, R.; Liu, Y.; Qi, L.; Croft, H.; Ju, W.; Zhang, Y.; He, Y.; et al. Retrieving global leaf chlorophyll content from MERIS data using a neural network method. ISPRS J. Photogramm. Remote Sens. 2022, 192, 66–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.M.; Miller, J.R.; Noland, T.L. Leaf chlorophyll content retrieval from airborne hyperspectral remote sensing imagery. Remote Sens. Environ. 2008, 112, 3234–3247. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J.; Tallis, M.J.; Llewellyn, G.M.; Taylor, G.; Snoeij, P. Validating the MERIS Terrestrial Chlorophyll Index (MTCI) with ground chlorophyll content data at MERIS spatial resolution. Int. J. Remote Sens. 2010, 31, 5513–5532. [Google Scholar] [CrossRef]
- Ling, Q.; Huang, W.; Jarvis, P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth. Res. 2011, 107, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Brown, L.A.; Williams, O.; Dash, J. Calibration and characterisation of four chlorophyll meters and transmittance spectroscopy for non-destructive estimation of forest leaf chlorophyll concentration. Agric. For. Meteorol. 2022, 323, 109059. [Google Scholar] [CrossRef]
- Dong, T.; Shang, J.; Chen, J.M.; Liu, J.; Qian, B.; Ma, B.; Morrison, M.J.; Zhang, C.; Liu, Y.; Shi, Y.; et al. Assessment of Portable Chlorophyll Meters for Measuring Crop Leaf Chlorophyll Concentration. Remote Sens. 2019, 11, 2706. [Google Scholar] [CrossRef]
- Ustin, S.L.; Jacquemoud, S. How the Optical Properties of Leaves Modify the Absorption and Scattering of Energy and Enhance Leaf Functionality. In Remote Sensing of Plant Biodiversity; Cavender-Bares, J., Gamon, J.A., Townsend, P.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 349–384. [Google Scholar]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Buschmann, C. Variability and application of the chlorophyll fluorescence emission ratio red/far-red of leaves. Photosynth. Res. 2007, 92, 261–271. [Google Scholar] [CrossRef]
- Roháček, K. Chlorophyll fluorescence parameters: The definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Li, R.H.; Guo, P.G.; Michael, B.; Stefania, G.; Salvatore, C. Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in barley. Agric. Sci. China 2006, 5, 751–757. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Buschmann, C.; Lichtenthaler, H.K. The chlorophyll fluorescence ratio F735/F700 as an accurate measure of the chlorophyll content in plants. Remote Sens. Environ. 1999, 69, 296–302. [Google Scholar] [CrossRef]
- Rascher, U.; Liebig, M.; Lüttge, U. Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ. 2000, 23, 1397–1405. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Chouinard, G.; Veilleux, J.; Pelletier, F.; Larose, M.; Philion, V.; Joubert, V.; Cormier, D. Impact of Exclusion Netting Row Covers on ‘Honeycrisp’ Apple Trees Grown under Northeastern North American Conditions: Effects on Photosynthesis and Fruit Quality. Insects 2019, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Colzi, I.; Renna, L.; Bianchi, E.; Castellani, M.B.; Coppi, A.; Pignattelli, S.; Loppi, S.; Gonnelli, C. Impact of microplastics on growth, photosynthesis and essential elements in Cucurbita pepo L. J. Hazard. Mater. 2022, 423, 127238. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.S.; Kim, K.-S.; Hamayun, M.; Kim, Y. Silicon Confers Soybean Resistance to Salinity Stress Through Regulation of Reactive Oxygen and Reactive Nitrogen Species. Front. Plant Sci. 2020, 10, 1725. [Google Scholar] [CrossRef] [PubMed]
- Opti-Sciences. CCM-300 Chlorophyll Content Meter Operation Manual. Available online: https://www.manualslib.com/manual/1390770/Opti-Sciences-Ccm-300.html (accessed on 3 February 2024).
- Zhang, Y.; Chen, J.M.; Thomas, S.C. Retrieving seasonal variation in chlorophyll content of overstory and understory sugar maple leaves from leaf-level hyperspectral data. Can. J. Remote Sens. 2007, 33, 406–415. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Schweiger, A.K.; Cavender-Bares, J.; Townsend, P.A.; Hobbie, S.E.; Madritch, M.D.; Wang, R.; Tilman, D.; Gamon, J.A. Plant spectral diversity integrates functional and phylogenetic components of biodiversity and predicts ecosystem function. Nat. Ecol. Evol. 2018, 2, 976–982. [Google Scholar] [CrossRef]
- Wang, Z.; Chlus, A.; Geygan, R.; Ye, Z.; Zheng, T.; Singh, A.; Couture, J.J.; Cavender-Bares, J.; Kruger, E.L.; Townsend, P.A. Foliar functional traits from imaging spectroscopy across biomes in eastern North America. New Phytol. 2020, 228, 494–511. [Google Scholar] [CrossRef] [PubMed]
- Thayer, S.S.; Björkman, O. Leaf Xanthophyll content and composition in sun and shade determined by HPLC. Photosynth. Res. 1990, 23, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Illowsky, B.; Dean, S. Introductory Statistics; OpenStax: Houston, TX, USA, 2018. [Google Scholar]
- Box, E.O. Plant Functional Types and Climate at the Global Scale. J. Veg. Sci. 1996, 7, 309–320. [Google Scholar] [CrossRef]
- Oren, R.; Werk, K.S.; Buchmann, N.; Zimmermann, R. Chlorophyll–nutrient relationships identify nutritionally caused decline in Piceaabies stands. Can. J. For. Res. 1993, 23, 1187–1195. [Google Scholar] [CrossRef]
- Gamon, J.A.; Surfus, J.S. Assessing leaf pigment content and activity with a reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- Lhotakova, Z.; (Charles University, Faculty of Science, Department of Plant Experimental Biology, Czech Republic); Neuwirthova, E.; (Charles University, Faculty of Science, Department of Plant Experimental Biology, Czech Republic); Potuckova, M.; (Charles University, Faculty of Science, Department of Applied Geoinformatics and Cartography, Czech Republic); Cervena, L.; (Charles University, Faculty of Science, Department of Applied Geoinformatics and Cartography, Czech Republic); Hunt, L.; (Charles University, Faculty of Science, Department of Plant Experimental Biology, Czech Republic); Kupkova, L.; (Charles University, Faculty of Science, Department of Applied Geoinformatics and Cartography, Czech Republic); Lukes, P.; (Global Change Research Institute of the Czech Academy of Sciences, Czech Republic); Campbell, P.; (Joint Center for Earth Systems Technology, NASA/Goddard Space Flight Center, Department of Geography And Environmental Sciences, USA); Albrechtova, J.; (Charles University, Faculty of Science, Department of Plant Experimental Biology, Czech Republic). Leaf structure matters for field evaluation of chlorophyll content with portable meters. 2024; (Unpublished work). [Google Scholar]
- Monje, O.A.; Bugbee, B. Inherent Limitations of Nondestructive Chlorophyll Meters: A Comparison of Two Types of Meters. HortScience 1992, 27, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Markwell, J.; Osterman, J.C.; Mitchell, J.L. Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Photosynth. Res. 1995, 46, 467–472. [Google Scholar] [CrossRef]
- Uddling, J.; Gelang-Alfredsson, J.; Piikki, K.; Pleijel, H. Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth. Res. 2007, 91, 37–46. [Google Scholar] [CrossRef]



| 2018 | 2019 | |||
|---|---|---|---|---|
| [CCM-300, HPLC] | [CCM-300, Spec] | [HPLC, Spec] | [CCM-300, HPLC] | |
| Broadleaf | 46 | 21 | 18 | 65 |
| Conifer | 109 | 51 | 23 | 31 |
| Graminoid | 10 | 0 | 0 | 0 |
| Total | 165 | 72 | 41 | 96 |
| [CCM-300, HPLC] | [CCM-300, Spec] | [HPLC, Spec] | |
|---|---|---|---|
| n | 261 | 72 | 41 |
| 420.0, 377.69 | 439.68, 403.1 | 375.57, 400.72 | |
| sx | 119.59, 181.23 | 132.07, 175.2 | 152.92, 161.71 |
| median | 414.5, 360.67 | 430.33, 381.48 | 360.46, 384.39 |
| Mean (mg/m2) | S.D. | ||
|---|---|---|---|
| Broadleaf | |||
| CCM-300 | 481.19 | 115.06 | |
| HPLC | 402.78 | 156.85 | |
| Conifer | |||
| CCM-300 | 374.44 | 102.93 | |
| HPLC | 360.45 | 200.09 | |
| Graminoid | |||
| CCM-300 | 378.70 | 85.60 | |
| HPLC | 340.59 | 126.25 |
| Functional Type | Regression | r | RMSE (mg/m2) |
|---|---|---|---|
| Broadleaf | Y = 0.75x + 41.53 | 0.55 | 154.76 |
| Conifer | Y = 1.01x − 16.29 | 0.52 | 171.16 |
| Graminoid | Y = 0.47x + 161.65 | 0.32 | 127.12 |
| Needle Age | Regression | r | RMSE (mg/m2) |
|---|---|---|---|
| New | Y = 1.09x − 64.61 | 0.70 | 91.50 |
| Old | Y = 1.24x − 0.32 | 0.49 | 222.48 |
| n | Regression | r | RMSE (mg/m2) | |
|---|---|---|---|---|
| Broadleaf | ||||
| 2018 | 47 | Y = 1.02x − 140.38 | 0.81 | 155.88 |
| 2019 | 65 | Y = 0.74x + 52.65 | 0.56 | 140.35 |
| Conifer | ||||
| 2018 | 111 | Y = 1.09x − 29.06 | 0.54 | 181.18 |
| 2019 | 31 | Y = 0.83x + 1.57 | 0.42 | 164.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Beek, J.M.; Zheng, T.; Wang, Z.; Kovach, K.R.; Townsend, P.A. Evaluation of the Reliability of the CCM-300 Chlorophyll Content Meter in Measuring Chlorophyll Content for Various Plant Functional Types. Sensors 2024, 24, 4784. https://doi.org/10.3390/s24154784
Van Beek JM, Zheng T, Wang Z, Kovach KR, Townsend PA. Evaluation of the Reliability of the CCM-300 Chlorophyll Content Meter in Measuring Chlorophyll Content for Various Plant Functional Types. Sensors. 2024; 24(15):4784. https://doi.org/10.3390/s24154784
Chicago/Turabian StyleVan Beek, Joelie M., Ting Zheng, Zhihui Wang, Kyle R. Kovach, and Philip A. Townsend. 2024. "Evaluation of the Reliability of the CCM-300 Chlorophyll Content Meter in Measuring Chlorophyll Content for Various Plant Functional Types" Sensors 24, no. 15: 4784. https://doi.org/10.3390/s24154784
APA StyleVan Beek, J. M., Zheng, T., Wang, Z., Kovach, K. R., & Townsend, P. A. (2024). Evaluation of the Reliability of the CCM-300 Chlorophyll Content Meter in Measuring Chlorophyll Content for Various Plant Functional Types. Sensors, 24(15), 4784. https://doi.org/10.3390/s24154784







