1. Introduction
Chlorophyll is an essential compound within the chloroplast, the organelle responsible for housing the photosynthetic process that gives plants their green color. As a vital component of photosynthesis, chlorophyll captures light energy and converts it into chemical energy, which is used to yield glucose and oxygen [
1]. Photosynthetic efficiency is impacted by pigment concentrations [
2,
3,
4], thus quantification of chlorophyll content provides insights into plant growth [
5] and photosynthetic functioning [
6,
7], as well as plant response to environmental [
8,
9] and climatic [
10] interactions.
Increasingly, there is a demand for large volumes of rapidly collected chlorophyll measurements, particularly for agronomic applications requiring rapid assessments for nutrient or disease management [
11,
12]. However, laboratory methods for quantifying pigments, such as high-performance liquid chromatography, may be logistically infeasible for these applications due to both expense and the need for special handling of samples. Chlorophyll can also be estimated from remote sensing imagery [
13,
14], but calibration and validation of remote sensing maps require large sample sizes, often collected within a short time window to match remote sensing observations [
15].
To meet these demands, various types of portable chlorophyll meters have been developed to non-destructively estimate leaf chlorophyll content in situ. Most of these meters infer the chlorophyll content by measuring leaf spectral characteristics, especially in wavelengths between 400 nm and 1000 nm that have distinct pigment absorption, reflectance, or fluorescence features.
Absorptance meters, such as the SPAD-502 chlorophyll meter (Konica Minolta Sensing, Tokyo, Japan) and Opti-Sciences CCM-200plus Chlorophyll Content Meter (Opti-Sciences, Hudson, NH, USA), measure leaf absorptance at light wavelengths near 650 nm and 940 nm (red and near-infrared, respectively). Absorptance meters do not provide a direct output of chlorophyll content, but instead utilize the ratio of transmittance (which yields a ‘chlorophyll content index’ or ‘SPAD unit’, for example) to produce a relative chlorophyll content measurement, which is the sole output of absorptance meters. Regression analyses can be the basis to convert these measurements to units of chlorophyll content [
16]. Reflectance meters, like the UniSpec Spectral Analysis System (PP Systems, Amesbury, MA, USA), record leaf reflectance across many wavelengths (ultraviolet, visible, near-infrared). The output of reflectance meter measurements provides multiple data points, allowing for extensive analyses from a single leaf scan.
Chlorophyll meters based on leaf absorptance/reflectance features suffer from a few drawbacks. First, there is a documented decrease in the accuracy of chlorophyll estimations when leaf chlorophyll content increases due to saturation of absorption [
17]. Additionally, the indices generated by such meters require careful evaluation through time (e.g., assessment of calibration consistency), and across species and phenology [
18]. Finally, meter measurements are affected by factors other than chlorophyll content, such as differences in leaf structure [
19] and structural materials of cell walls [
20].
Leaf fluorescence measurements have emerged as an alternative to absorptance/reflectance for quantifying chlorophyll content in leaves. Light that is not absorbed by chlorophyll for photochemistry is re-emitted as heat or fluorescence. Fluorescence occurs during de-excitation after a fluorophore is transitioned into a state of excitement due to absorption of light [
21,
22,
23]. Previous studies have shown that the strength of the fluorescence signal closely tracks the leaf chlorophyll content [
24,
25,
26].
Unlike absorptance- and reflectance-based meters that measure light from an external source, fluorescence-based meters measure the light re-emitted from the leaf. Fluorescence meters, also referred to as fluorometers, measure peak wavelengths in the regions of 685–690 nm (red) and 730–740 nm (far-red, near-infrared). Fluorescence ratios of red and far-red regions [
23,
27,
28] enable direct estimates of chlorophyll content based on regression equations. For example, the widely used Opti-Sciences CCM-300 Chlorophyll Content Meter (hereafter referred to as CCM-300) measures emission ratios of red light at 700 nm to far-red emission at 735 nm (chlorophyll fluorescence ratio F735/F700) and estimates chlorophyll content (mg/m
2) based on equations from Gitelson et al. [
26]. To obtain consistent fluorescence measurements, the instrument is calibrated to a purple transparent fluorescent slide with a predetermined value (0.8 for our unit) before each measurement session.
The CCM-300, a portable, non-destructive device, has gained wide usage in field biology due to its ease of use and ability to provide rapid and repeatable measurements. Although the meter has been used to assess plant and ecosystem health in various studies [
29,
30,
31], the accuracy of the instrument has rarely been tested. Additionally, Gitelson’s [
26] equations used by the CCM-300 are based exclusively on broadleaf species, suggesting the need for testing on other leaf physiognomic types. Indeed, the Opti-Sciences CCM-300 Chlorophyll Content Meter Operation Manual [
32] encourages users to develop unique calibration parameters for vegetation types not represented by the Gitelson [
26] equations. However, despite its popularity, the validation of the CCM-300’s reliability across plant functional types is scarce, and we do not know of any published alternative equations for non-broadleaf species. As far as we are aware, our study is the first to evaluate the performance of the Opti-Sciences CCM-300 Chlorophyll Content Meter.
In this study, we assess the reliability of the Opti-Sciences CCM-300. We aim to (1) compare CCM-300 measurements against traditional laboratory chemistry analyses such as spectrophotometry and high-performance liquid chromatography (HPLC), (2) analyze meter consistency between plant functional types, and (3) determine meter reliability across years, assessing whether there is degradation in estimates as the instrument ages.
4. Discussion
4.1. CCM-300 Measurements vs. Laboratory Chemistry Measurements
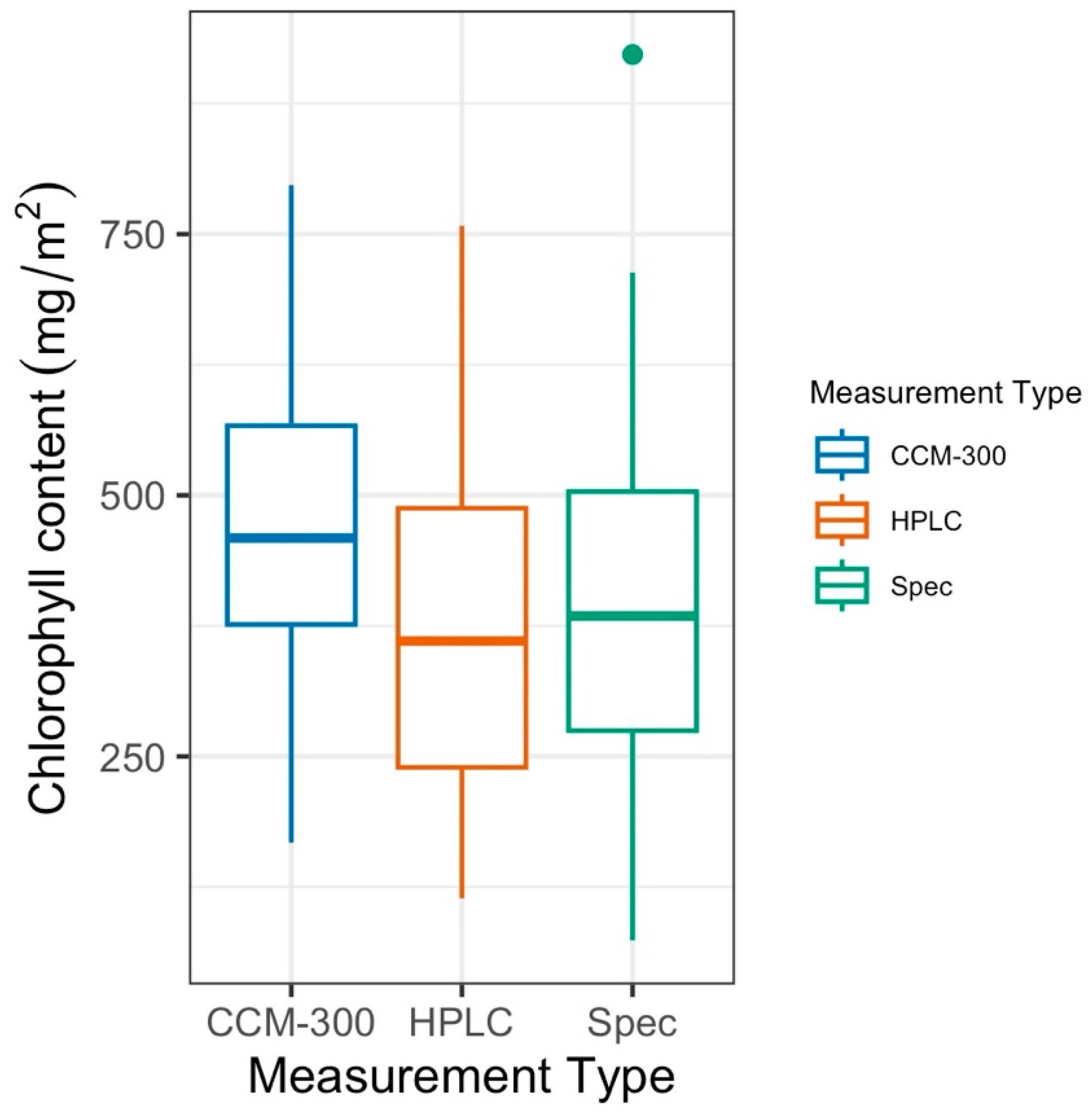
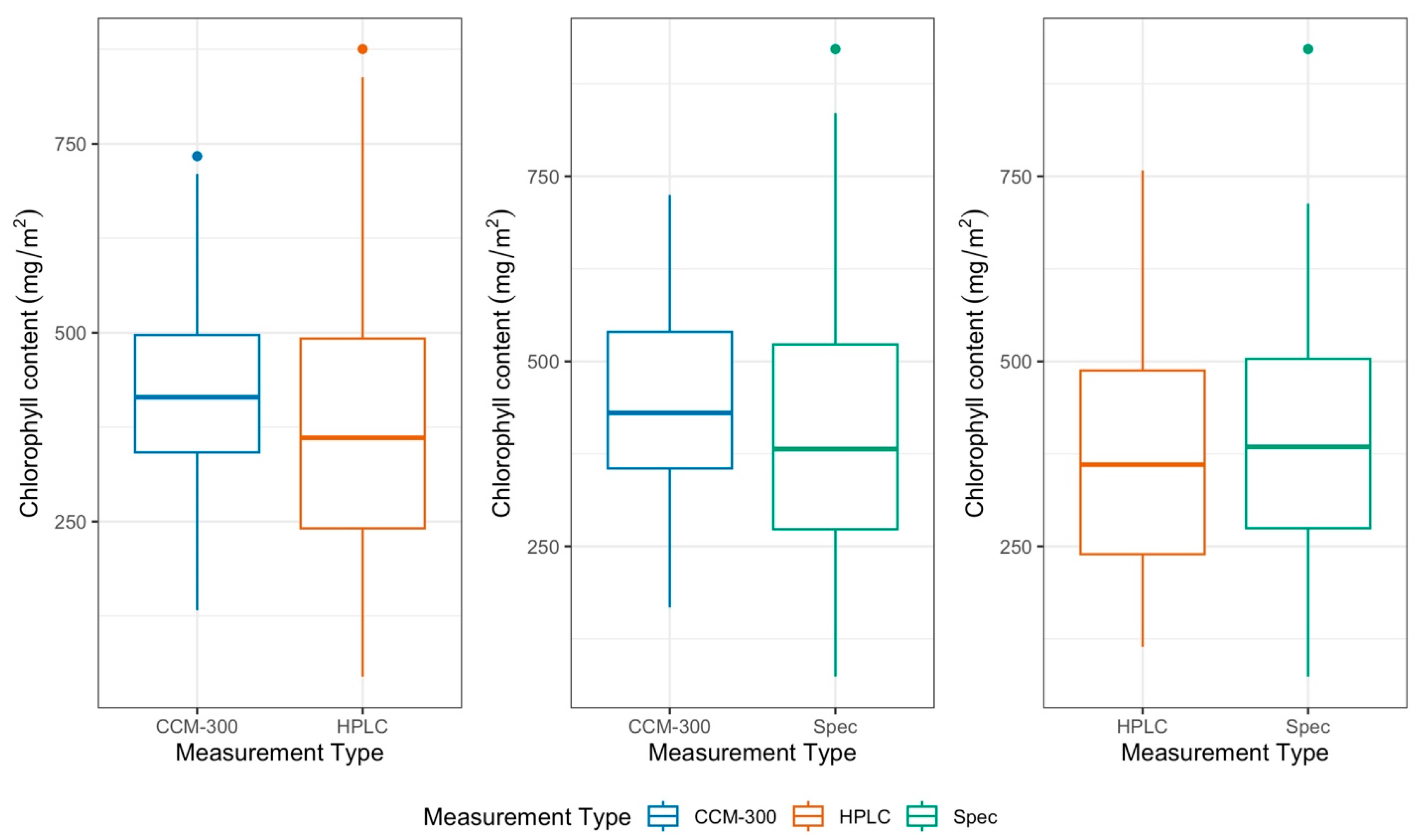
Based on the results of each of our paired T-tests, we found no statistically significant difference between HPLC and laboratory spectrophotometric (Spec) estimates of chlorophyll content (
p > 0.1). Both laboratory chemistry methods are reliable for obtaining chlorophyll content (
Figure 2 and
Figure 4). The biggest discrepancies between HPLC and Spec measurements are from old conifer samples (
Figure 3, pair [HPLC, Spec]). Old needles usually have higher chlorophyll content [
40] and it takes a longer time to fully extract the chlorophyll from the needle samples. During HPLC analyses, all samples were subjected to the same extraction procedure. While the extraction time is enough for most samples, it may not be for a few old needles with exceptionally high chlorophyll contents, which may cause discrepancies between HPLC and Spec.
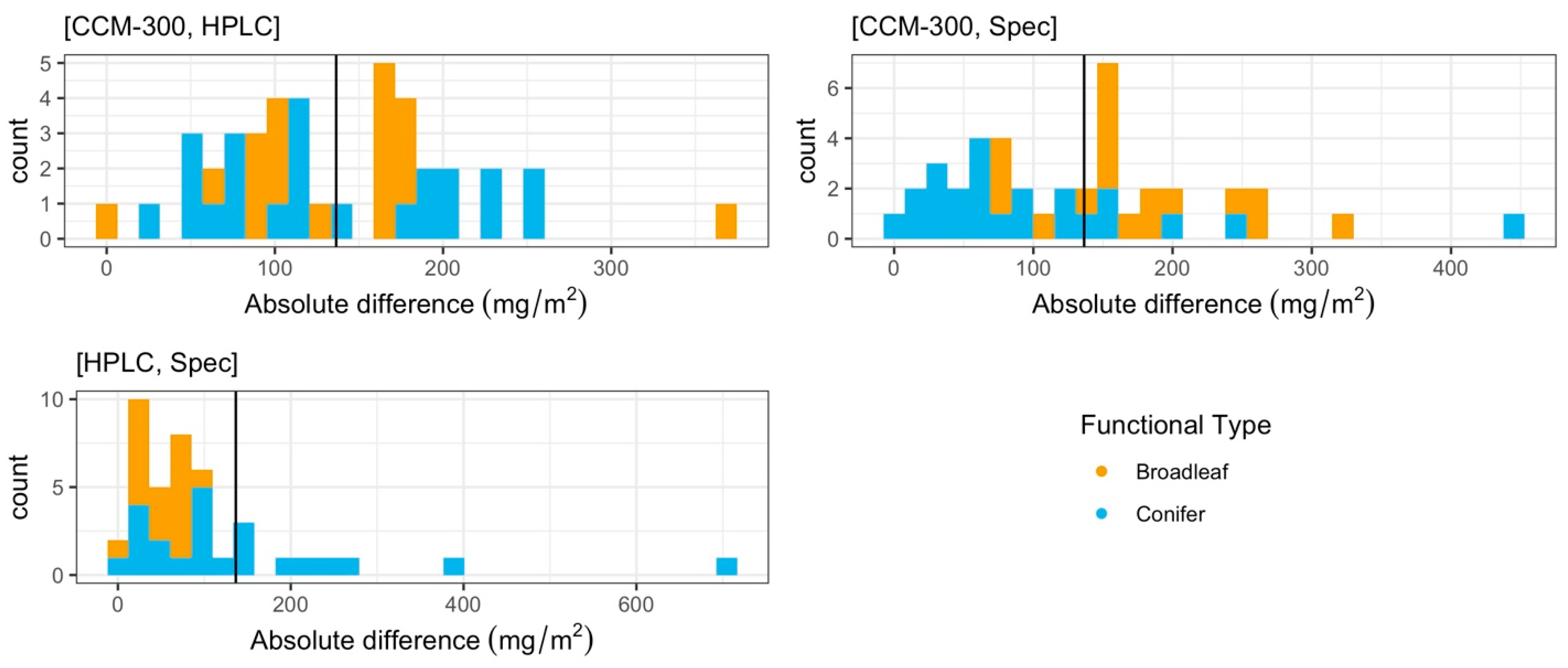
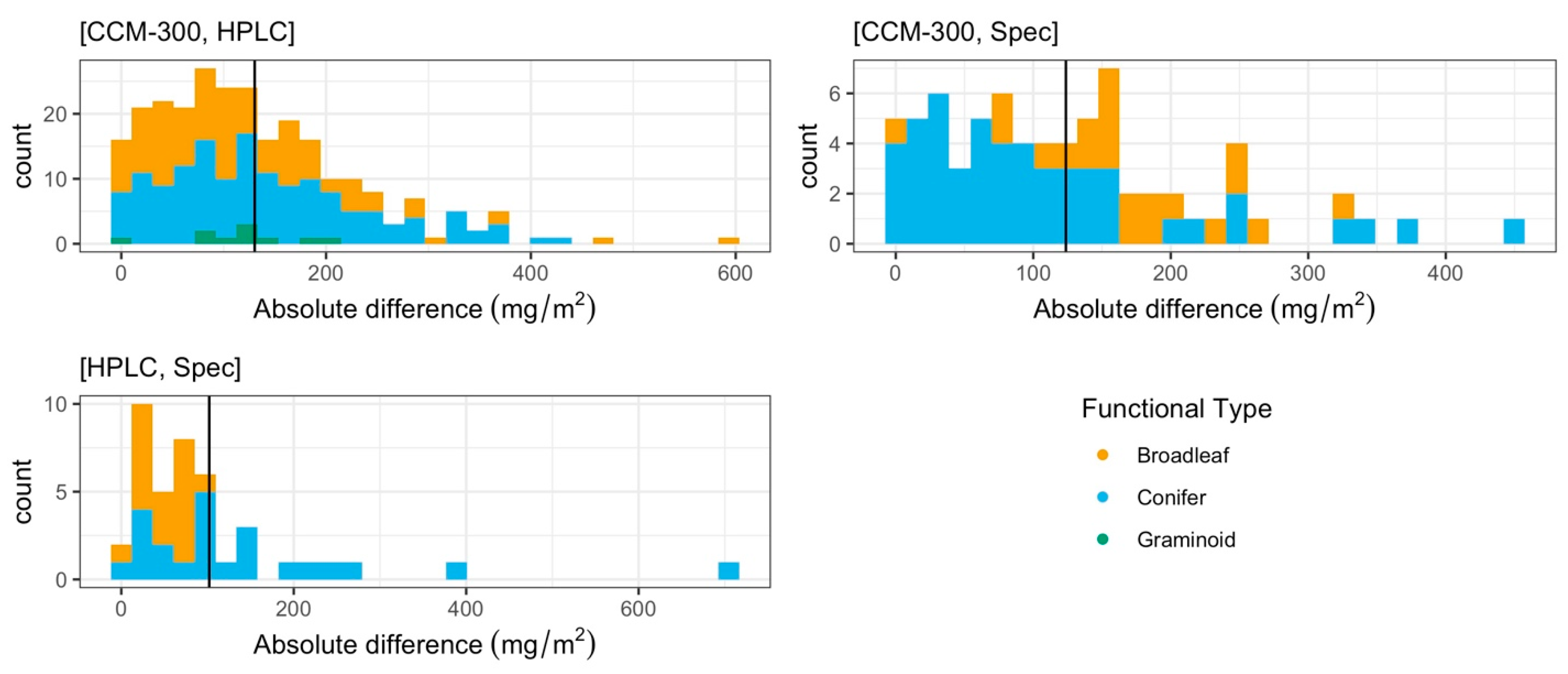
In contrast, we observed a significant difference between both CCM-300 and HPLC, and CCM-300 and laboratory spectrophotometry (Spec). Comparison of iteratively subsampled paired data (with 41 samples), all pairs, and peak values of permutation
p-value distribution did not influence the significance of paired T-test results, further supporting our inferences regarding the continued reliability of laboratory chemistry analyses and the potential limitations of the CCM-300. Our results are significantly different at α = 0.05, but the significance would change at α = 0.01 for some pairs. This change in alpha would result in no statistically significant difference for [CCM-300, Spec] pairs from all available paired data, as well as [CCM-300, HPLC] and [CCM-300, Spec] pairs from the permutation analysis (
Figure 6).
Additionally, for NEON data, CCM-300 measurements represented the average conditions of five samples while the laboratory chemistry only tested two to three samples. This mismatch could contribute to the observed difference between CCM-300 measurements and laboratory chemistry results.
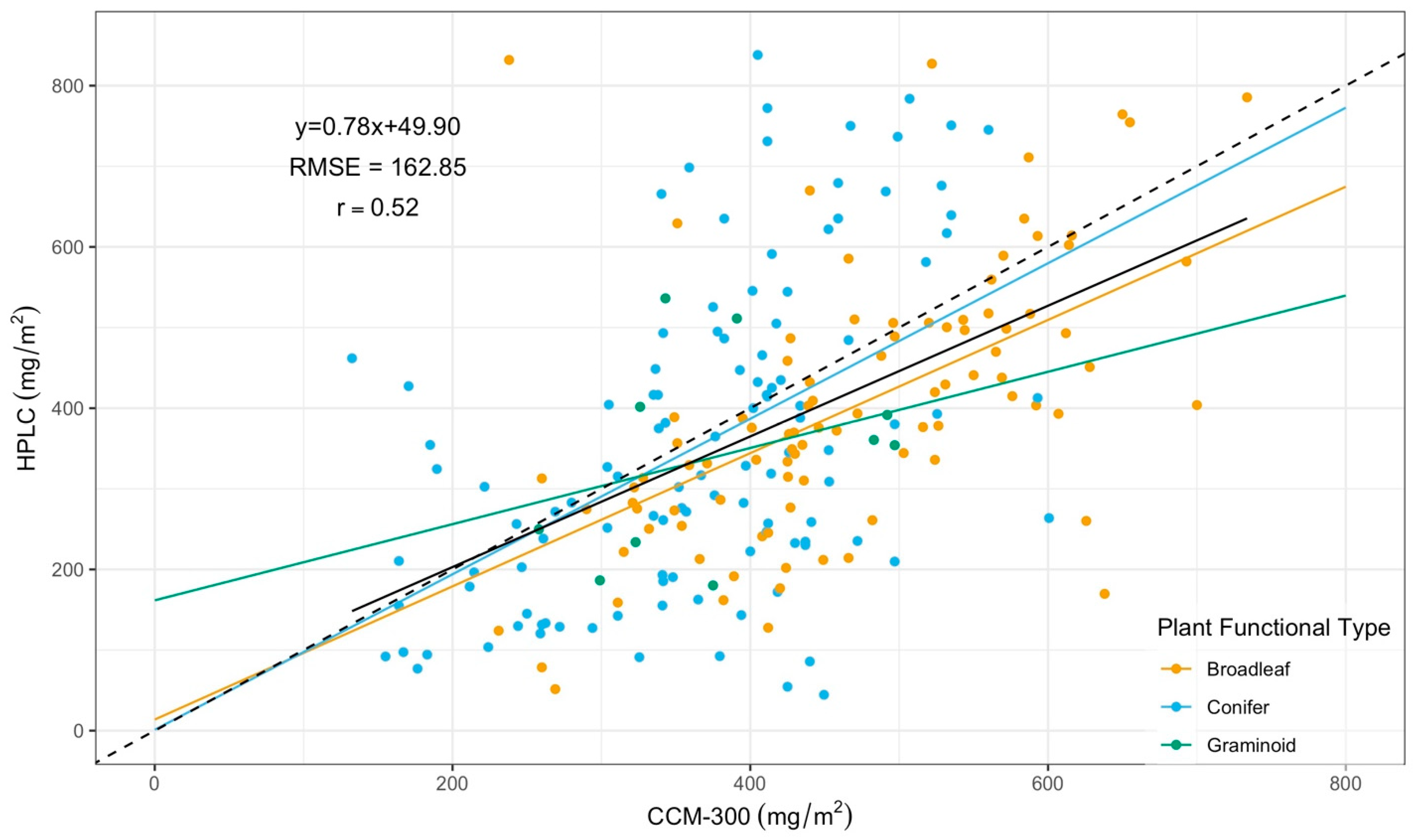
Despite the differences between CCM-300 and HPLC in the paired T-test, the regression model performed reasonably well when predicting HPLC using CCM-300 measurements (
Figure 7). The correlation coefficient (r) for [CCM-300, HPLC] was 0.52. Although the CCM-300 did not match HPLC exactly, it is able to track the variation in chlorophyll content. Nevertheless, laboratory chemistry analyses could produce more reliable estimates.
4.2. CCM-300 Measurements for Different Plant Functional Types
The grouping of data around the 1:1 line in
Figure 7 implies that the CCM-300 produces reasonable results for chlorophyll content across the plant functional types analyzed in this study. A regression relationship can be used to “correct” the trend in the CCM-300 data, but no single regression is suitable across plant functional types (
Table 4). This observation has been seen in other meters and has raised concern in the literature [
17,
41]. Richardson et al. [
17] encourage users of absorptance-based portable chlorophyll meters to consider the need for species-specific calibration when leaf structure varies among samples, while Gamon and Surfus [
41] concluded that the chlorophyll index used when analyzing leaf samples with a reflectance-based meter varies between species, specifically those with dissimilar leaf structures.
The meter performed the best for broadleaf species, followed by conifers, but poorly for graminoids. The poor performance for graminoids is likely due to the small variation in range (356 mg/m2 for graminoids, compared to 780.35 mg/m2 for broadleaf and 830.85 mg/m2 for conifer) represented by 10 samples for this functional type in our dataset.
We observed meter saturation around 625 mg/m
2 (
Figure 7) for conifers, indicating a deterioration in meter sensitivity and accuracy in measuring needles with chlorophyll content beyond this threshold. This saturation is the worst for old needles (
Figure 8) where our regression model has the highest RMSE (222.48 mg/m
2) (
Table 5). We suspect that differences in leaf anatomy, likely in the cuticle and epidermis, resulted in the saturation we observed. Needle leaves are known to have thicker and denser external layers compared to broadleaves; thus, the tough structure of old needles likely contributes to the saturation. Lhotáková et al. [
42] evaluated the effects of leaf structure on meter outputs and determined that the assessment of needle leaves has several constraints, and it is necessary to perform other biochemical assessments, such as spectrophotometry, for determination of chlorophyll content in needle leaves.
While meter measurements suffered from saturation for conifer samples, measurements are mostly reliable for broadleaves. This is not surprising, since the CCM-300 is based on the work of Gitelson et al. [
26], which used broadleaf species exclusively. To the best of our knowledge, Opti-Sciences has not updated the equations utilized by the meter. Our recommendation is to primarily use the CCM-300 for broadleaf species. When analyzing coniferous species with the meter, it is advisable to supplement the measurements with laboratory chemistry to account for any discrepancies [
42].
Other studies have reported potential factors contributing to the varied performance of absorptance-based portable chlorophyll meters. Richardson et al. [
17] suggest that the size of the measurement area on a given device has an impact on the output of hand-held chlorophyll meters. However, fluorescence-based meters, such as the CCM-300, do not suffer from this drawback, as fluorescence is an active technique measuring light actively emitted from the leaf itself. This does not require the entirety of the measurement window to be filled by a sample, allowing for the measurement of very small leaves. Various studies report that absorptance-based meters also produce less accurate estimations as chlorophyll content increases [
17,
19,
43,
44], which is consistent with the results of our study using the CCM-300. It is not surprising for the CCM-300 to be impacted by meter saturation, as other types of handheld devices experience the same phenomenon.
4.3. CCM-300 Performance across Years
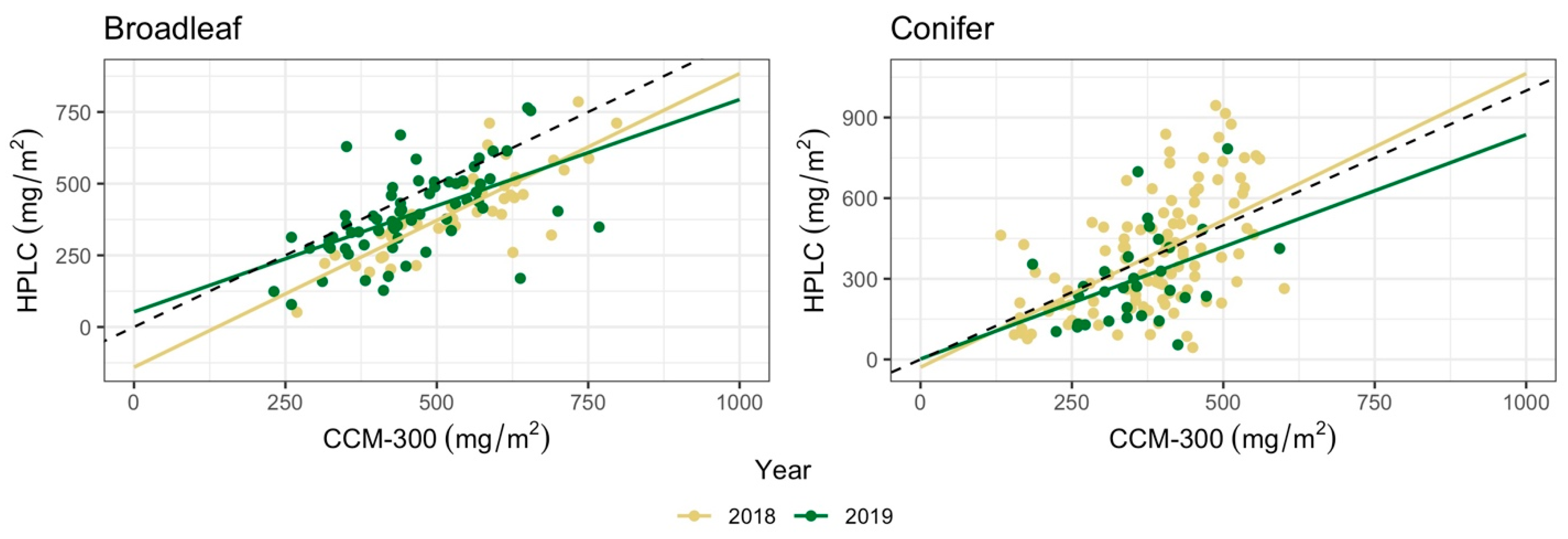
Based on the results of our analysis between years, the correlation coefficients (
r) suggest that the CCM-300 captured chlorophyll variation better in 2018 than in 2019 (
Table 6). This could be attributed to the lack of hardware calibration for the meter, and/or a deterioration in the quality of the calibration slide used before sample collection. After extensive usage in tough field conditions, the quality of the calibration slide may deteriorate. This could potentially cause drifts in the calibration and may have led to a worse performance of the meter in 2019.
As well, it is plausible that a larger overall sample size with greater uniformity among the sample size of all groups would yield results that are more consistent between both years and functional types. For broadleaf samples, the sample size from 2018 (n = 47) was slightly less than 2019 (n = 65). Within conifer samples, 2018 (n = 111) has significantly more samples than 2019 (n = 31).
4.4. Study Limitations
The Opti-Sciences CCM-300 provides reasonable results for chlorophyll content if users desire measurements of coarse-scale relative variations. However, broad applications of the CCM-300 for precise estimates may be problematic. Our study used opportunistic data and we cannot rule out that a larger sample size would better validate the precision of CCM-300 measurements, thus our findings may not fully capture the variability in CCM-300 performance. We also provide no absolute measure of accuracy for the HPLC and laboratory spectrophotometric analyses. Regardless, this study provides important evidence that users must carefully evaluate data from the CCM-300 for their applications.
Due to the expense of laboratory chemistry, our study was limited, to an extent, by cost. It is not realistic for many researchers to complete laboratory analyses for every sample in a study, thus portable chlorophyll content meters can be used in certain scenarios as a cost-effective, non-destructive method.
5. Conclusions
Our study utilizing opportunistic data suggests that the Opti-Sciences CCM-300 can produce reasonable results for estimating chlorophyll content, but is limited, especially if comparing measurements across functional physiognomic types. Laboratory chemistry analyses continue to be the most reliable method for measurement of chlorophyll content. The CCM-300 is most compatible with broadleaf species and is likely least reliable in measurements of old needles due to meter saturation. There is mild deterioration of the CCM-300 performance between years which could be due to calibration drift.
Moving forward, users of the CCM-300 for broad scale studies, such as in situ measurements to support remote sensing, should conduct a dedicated study with a large sample size composed of many samples from a wider variety of functional types. When measuring needle leaves, we recommend using equations, such as those represented in
Table 5, to correct for the large bias in the CCM-300 measurements. It is best practice, however, for users to develop their own equations tailored specifically to their studies.
It may be helpful for those planning future chlorophyll meter studies to consider that chlorophyll is not uniformly distributed within a leaf, impacting meter outputs depending on where the measurement is taken [
45]. It is recommended that multiple measurements be taken from each leaf then averaged to improve the accuracy of chlorophyll estimation [
32,
43]. When doing so, it is best practice to complete measurements of the same leaf within 3 min of the initial measurement to minimize the effects of chloroplast migration on meter outputs [
32]. The CCM-300 Chlorophyll Content Meter Operation Manual [
32] also recommends that ambient temperatures be considered when taking sample measurements, as this factor may have the potential to influence measurement results.
It is important to adjust measurement parameters to suit the study at hand, but we recommend completing studies with multiple replications of each sample. The CCM-300 includes a range of measuring options that allows users to average between 2 and 30 measurements. Samples with a lower fluorescence emission signal strength may require more measurements [
32].
Finally, one should take note of the conditions of the slide used to calibrate the CCM-300 meter. After repeated use, the calibration slide may undergo wear and tear that includes scratches, dents, and loss of adherence to the fluorescence coefficient it was once measured to be. Integrating an internal calibration slide into the meter would ensure consistent slide quality, and including a slide replacement schedule would enhance usability.
We hope that, ultimately, this study provides guidance on CCM-300 applications for large-scale chlorophyll content quantification in support of calibration and validation of a forthcoming generation of spaceborne imaging spectrometers, such as Surface Biology and Geology (SBG) and Copernicus Hyperspectral Imaging Mission for the Environment (CHIME) that will be used to map at high resolutions (30 m) seasonal chlorophyll worldwide at monthly or better time scales.