Paper-Based Microfluidic Analytical Device Patterned by Label Printer for Point-of-Care Blood Glucose and Hematocrit Detection Using 3D-Printed Smartphone Cassette
Abstract
:1. Introduction
2. Materials and Methods
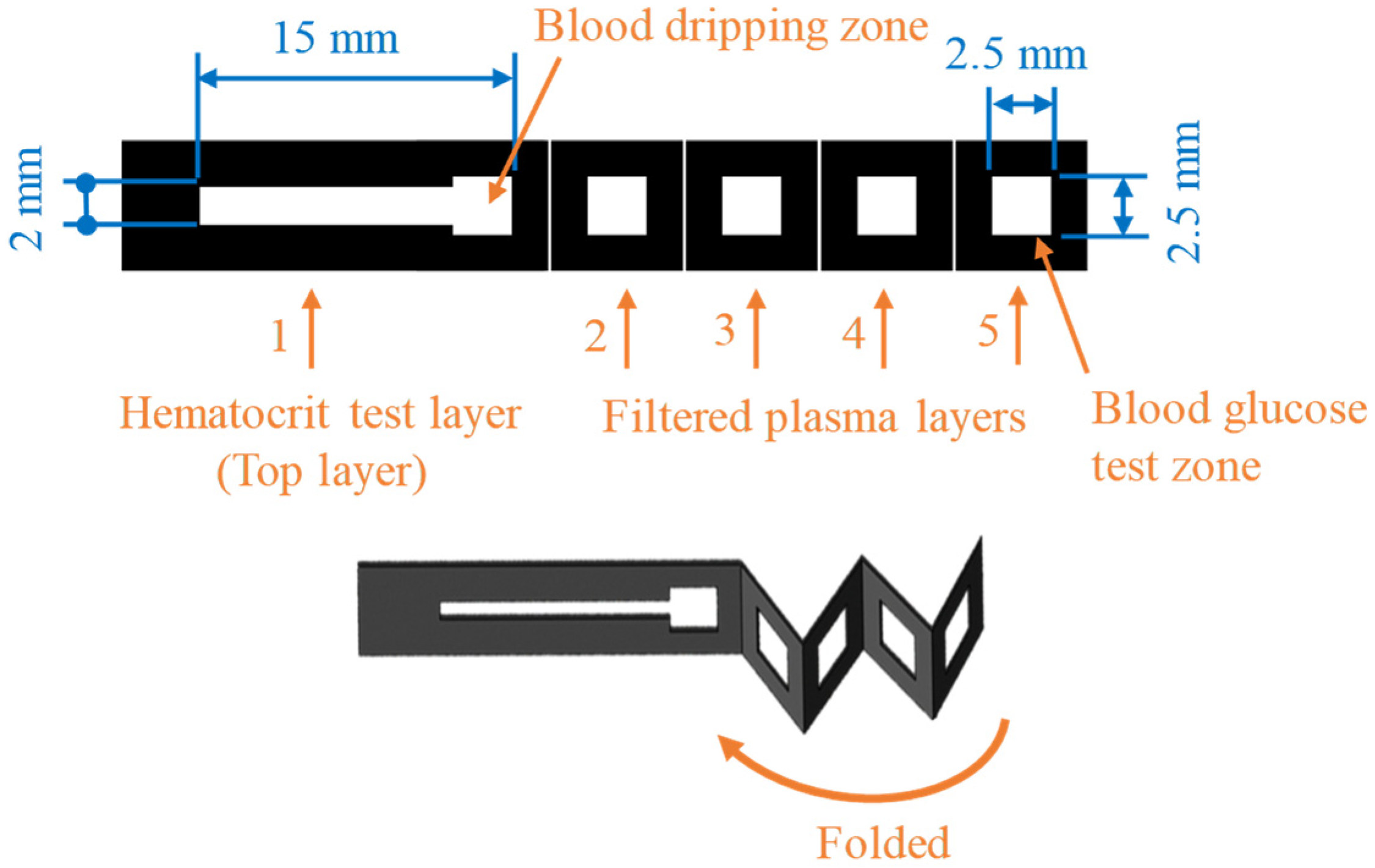
2.1. Design of Origami μPAD
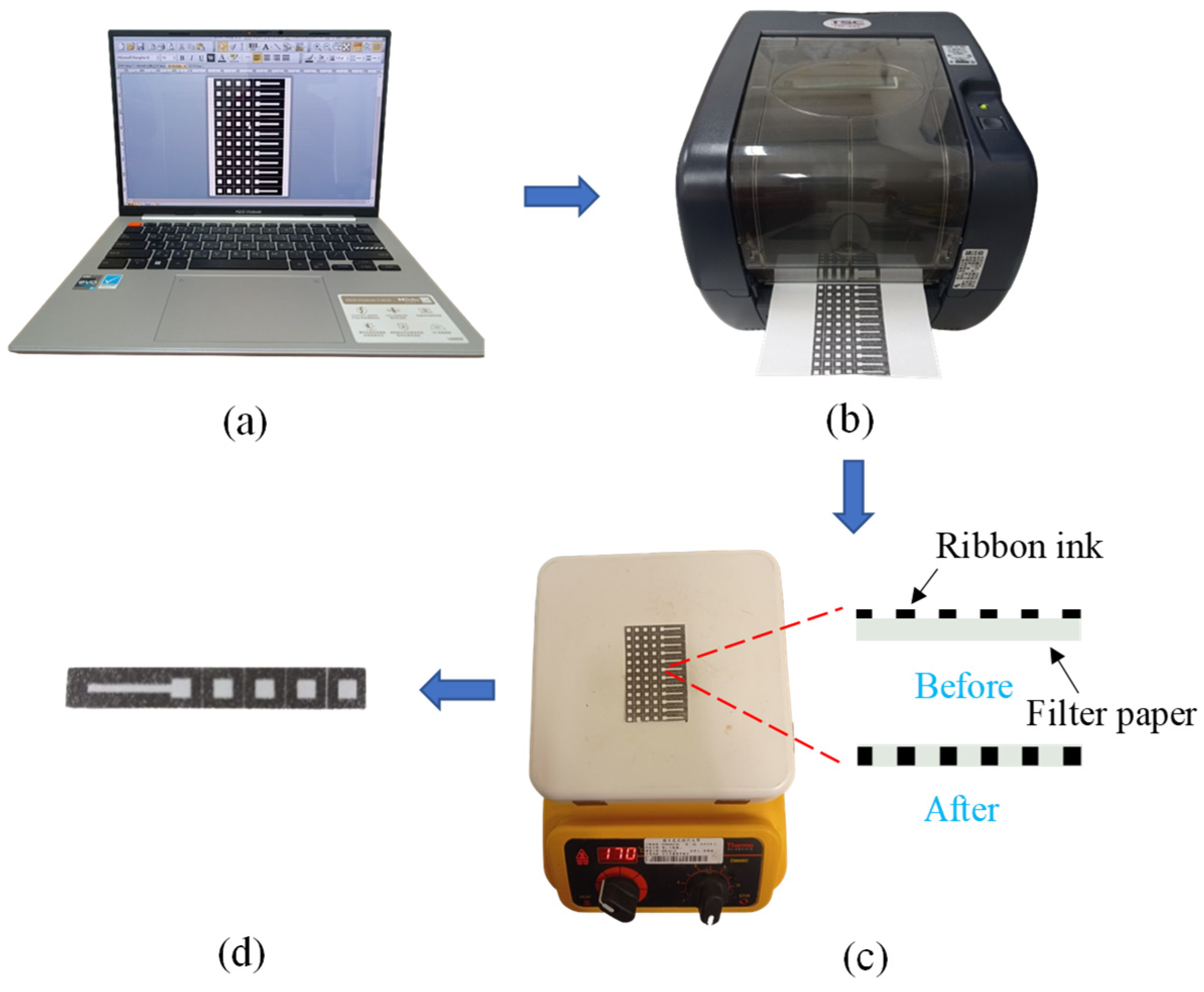
2.2. Fabrication of Origami μPAD
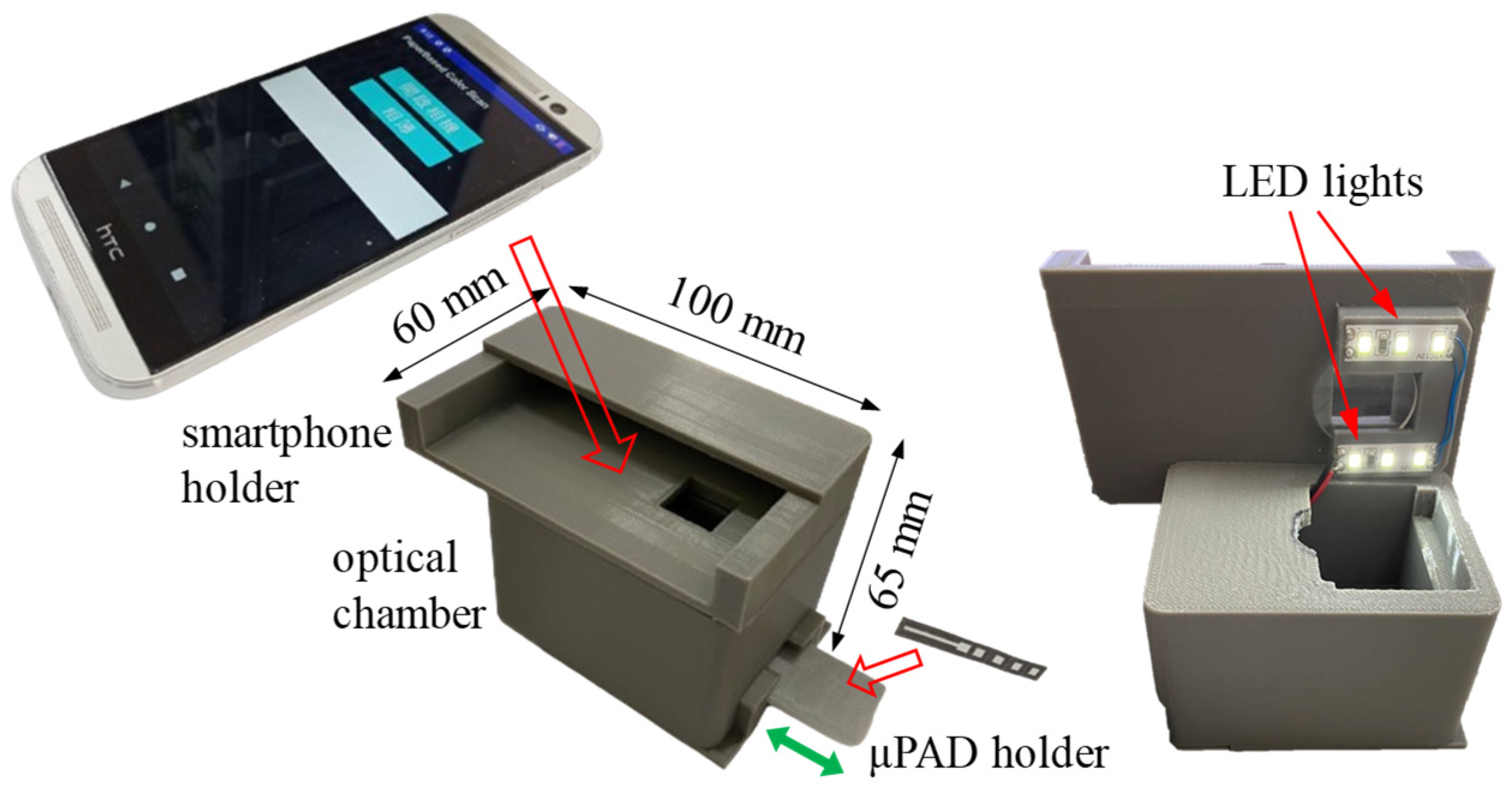
2.3. Smartphone-Based Optical Cassette and Self-Written Apps
2.4. Reagent Preparation and Glucose Colorimetric Assay
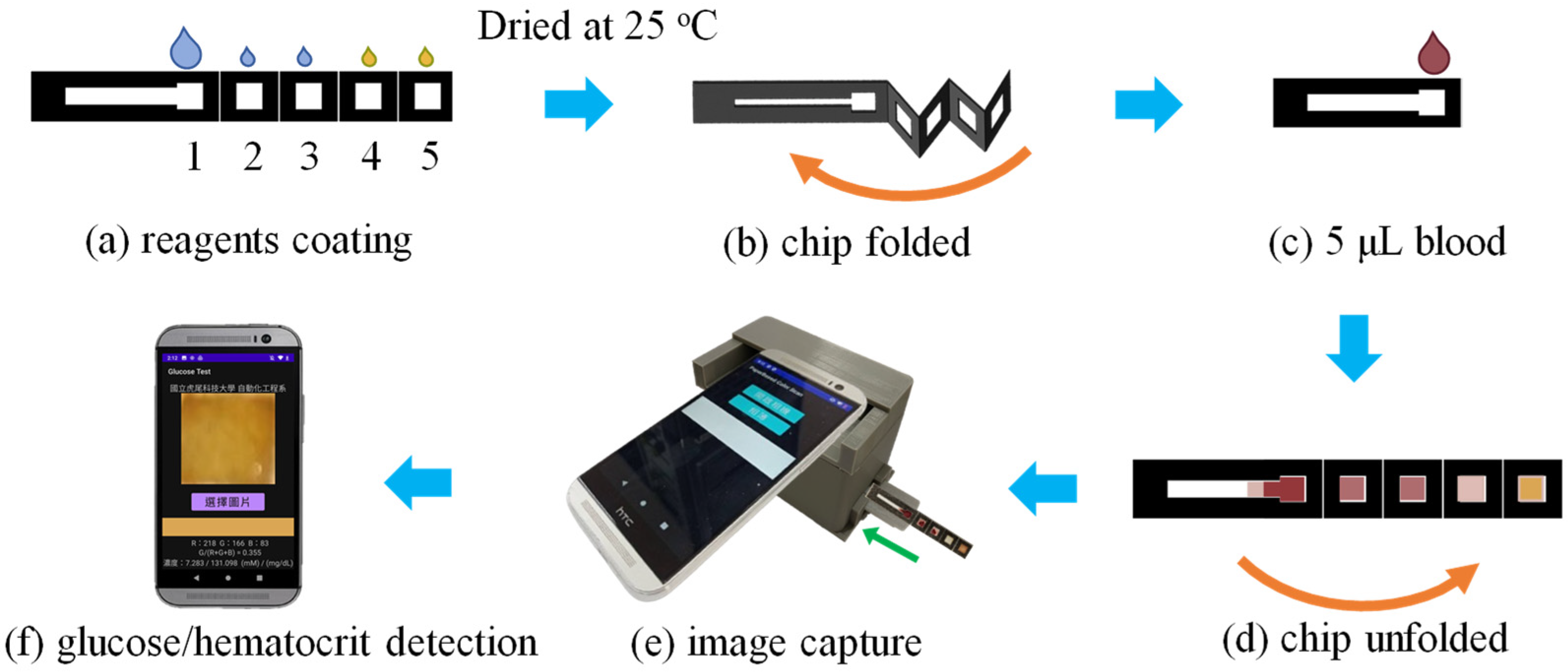
2.5. Experimental Process
3. Results and Discussion
3.1. Characterization of Label-Printed μPAD
3.2. Optimization of Plasma Separation Effect
3.3. Hematocrit Calibration Curve
3.4. Blood Glucose Calibration Curve
3.5. Practical Application of μPAD System to Hematocrit and Glucose Determination in Human Whole Blood Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2011, 34, S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mai, X.; Hong, X.; Chen, Y.; Li, X. Optical fiber SPR biosensor with a solid-phase enzymatic reaction device for glucose detection. Sens. Actuators B Chem. 2022, 366, 131984. [Google Scholar] [CrossRef]
- Bradna, P. Gout and diabetes. Vnitr. Lek. 2006, 52, 488–492. [Google Scholar] [PubMed]
- Maeda, E.; Kataoka, M.; Hino, M.; Kajimoto, K.; Kaji, N.; Tokeshi, M.; Kido, J.I.; Shinohara, Y.; Baba, Y. Determination of human blood glucose levels using microchip electrophoresis. Electrophoresis 2007, 28, 2927–2933. [Google Scholar] [CrossRef] [PubMed]
- Hu, J. The evolution of commercialized glucose sensors in China. Biosens. Bioelectron. 2009, 24, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, C.; Xue, Y.F.; McArthur, A. Self-monitoring of blood glucose in type 2 diabetes mellitus: A systematic review of economic evidence. J. Adv. Nurs. 2010, 66, 1931–1936. [Google Scholar]
- Shu, H.; Peng, S.; Lai, T.; Cui, X.; Ren, J.; Chen, T.; Xiao, X.; Wang, Y. Nickel foam electrode decorated with Fe-CdIn2O4 nanoparticles as an effective electrochemical sensor for non-enzymatic glucose detection. J. Electroanal. Chem. 2022, 919, 116524. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the developing world: Microfluidic paper-based analytical devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, K.; Xu, H.; Yan, W.; Jin, Q.; Cui, D. Detection platforms for point-of-care testing based on colorimetric, luminescent and magnetic assays: A review. Talanta 2019, 202, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Sun, X.; Dai, Z.; Gao, Y.; Wen, W. Point-of-care testing detection methods for COVID-19. Lab Chip 2021, 21, 1634–1660. [Google Scholar] [CrossRef] [PubMed]
- Parolo, C.; Merkoci, A. Paper-based nanobiosensors for diagnostics. Chem. Soc. Rev. 2013, 42, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Holmes, K.K.; Mabey, D.; Ronald, A. Rapid tests for sexually transmitted infections (STIs): The way forward. Sex. Transm. Infect. 2006, 82, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Yang, Y.; Liao, J.; He, B.; Liu, H. Bioinspired multistructured paper microfluidics for POCT. Lab Chip 2019, 19, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Laurenciano, C.J.D.; Tseng, C.C.; Chen, S.J.; Lu, S.Y.; Tayo, L.L.; Fu, L.M. Microfluidic colorimetric detection platform with sliding hybrid PMMA/paper microchip for human urine and blood sample analysis. Talanta 2021, 231, 122362. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yao, Y.; Liu, Q.; Chen, X. Photothermal hydrogel-integrated paper-based point-of-care platform for visible distance-readout of glucose. Anal. Chim. Acta 2024, 1285, 342035. [Google Scholar] [CrossRef] [PubMed]
- Nilghaz, A.; Mousavi, S.M.; Li, M.; Tian, J.; Cao, R.; Wang, X. Paper-based microfluidics for food safety and quality analysis. Trends Food Sci. Technol. 2021, 118, 273–284. [Google Scholar] [CrossRef]
- Hou, C.Y.; Fu, L.M.; Ju, W.J.; Wu, P.Y. Microfluidic colorimetric system for nitrite detection in foods. Chem. Eng. J. 2020, 398, 125573. [Google Scholar] [CrossRef]
- Cate, D.M.; Dungchai, W.; Cunningham, J.C.; Volckens, J.; Henry, C.S. Simple, distance-based measurement for paper analytical devices. Lab Chip 2013, 13, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Wei, H.X.; Qi, J.; Ma, H.; Liu, L.; Weng, J.; Zheng, X.; Li, Q.; Zhao, D.; Fang, H.; et al. Pulling-force spinning top for serum separation combined with paper-based microfluidic devices in COVID-19 ELISA diagnosis. ACS Sens. 2021, 6, 2709–2719. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.K.; Shaban, S.M.; Moon, B.S.; Pyun, D.G.; Kim, D.H. Smartphone-assisted point-of-care colorimetric biosensor for the detection of urea via pH-mediated AgNPs growth. Anal. Chim. Acta 2021, 1170, 338630. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhu, M.; Kong, J.; Li, Z.; Jiang, J.; Xi, Y.; Li, F. Microfluidic paper-based analytical device by using Pt nanoparticles as highly active peroxidase mimic for simultaneous detection of glucose and uric acid with use of a smartphone. Talanta 2022, 237, 122954. [Google Scholar] [CrossRef] [PubMed]
- Jalal, U.M.; Jin, G.J.; Shim, J.S. Paper-plastic hybrid microfluidic device for smartphone-based colorimetric analysis of urine. Anal. Chem. 2017, 89, 13160–13166. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Hemphill, M.A.; Gabrieli, D.; Wu, L.; Yelleswarapu, V.; Lawrence, G.; Pennycooke, W.; Singh, A.; Meaney, D.F.; Issadore, D. Smartphone-enabled optofluidic exosome diagnostic for concussion recovery. Sci. Rep. 2016, 6, 31215. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, I.; Speichert, M.; Granica, M.; Tymecki, L. Colorimetric point-of-care paper-based sensors for urinary creatinine with smartphone readout. Sens. Actuators B Chem. 2021, 340, 129915. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Wiley, B.J.; Gupta, M.; Whitesides, G.M. FLASH: A rapid method for prototyping paper-based microfluidic devices. Lab Chip 2008, 8, 2146–2150. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc. Natl. Acad. Sci. USA 2008, 105, 19606–19611. [Google Scholar] [CrossRef]
- Yu, L.; Shi, Z.Z. Microfluidic paper-based analytical devices fabricated by low-cost photolithography and embossing of Parafilm®. Lab Chip 2015, 15, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding wax printing: A simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef]
- Renault, C.; Koehne, J.; Ricco, A.J.; Crooks, R.M. Three-dimensional wax patterning of paper fluidic devices. Langmuir 2014, 30, 7030–7036. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, J.; Nguyen, T.; Shen, W. Paper-based microfluidic devices by plasma treatment. Anal. Chem. 2008, 80, 9131–9134. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, J.; Shen, W. Quantitative biomarker assay with microfluidic paper-based analytical devices. Anal. Bioanal. Chem. 2010, 396, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, G.; Ding, Z.W.; Chang, C.L.; Savran, C.A.; Ziaie, B. Laser-treated hydrophobic paper: An inexpensive microfluidic platform. Lab Chip 2011, 11, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.F.; Wang, Y.; Wu, Y.Y.; Xu, C.X.; Zhong, M.H.; Lai, H.Y.; Huang, J.S. Fabrication of a microfluidic paper-based analytical device by silanization of filter cellulose using a paper mask for glucose assay. Analyst 2014, 139, 4593–4598. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Forouzan, O.; Brown, T.P.; Shevkoplyas, S.S. Integrated separation of blood plasma from whole blood for microfluidic paper-based analytical devices. Lab Chip 2012, 12, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yang, J.; Xu, H.; Cao, B.; Qin, Q.; Liao, X.; Wo, Y.; Jin, Q.; Cui, D. Smartphone-imaged multilayered paper-based analytical device for colorimetric analysis of carcinoembryonic antigen. Anal. Bioanal. Chem. 2020, 412, 2517–2528. [Google Scholar] [CrossRef] [PubMed]
- Naveen Prasad, S.; Anderson, S.R.; Joglekar, M.V.; Hardikar, A.A.; Bansal, V.; Ramanathan, R. Bimetallic nanozyme mediated urine glucose monitoring through discriminant analysis of colorimetric signal. Biosens. Bioelectron. 2022, 212, 114386. [Google Scholar] [CrossRef] [PubMed]
- Noiphung, J.; Songjaroen, T.; Dungchai, W.; Henry, C.S.; Chailapakul, O.; Laiwattanapaisal, W. Electrochemical detection of glucose from whole blood using paper-based microfluidic devices. Anal. Chim. Acta 2013, 788, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, R.; Yang, Y.; Zhao, R.; Song, S.; Zhou, Y.; Shi, J.; Wang, L.; Song, H.; Hao, R. Multichannel immunosensor platform for the rapid detection of SARS-CoV-2 and influenza A(H1N1) virus. ACS Appl. Mater. Interfaces 2021, 13, 22262–22270. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Tan, M.K.; Go, D.B.; Yeo, L.Y.; Friend, J.R.; Chang, H.C. Paper-based microfluidic surface acoustic wave sample delivery and ionization source for rapid and sensitive ambient mass spectrometry. Anal. Chem. 2011, 83, 3260–3266. [Google Scholar] [CrossRef]
- Yu, J.H.; Wang, S.M.; Ge, L.; Ge, S.G. A novel chemiluminescence paper microfluidic biosensor based on enzymatic reaction for uric acid determination. Biosens. Bioelectron. 2011, 26, 3284–3289. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Luo, Y.; Shi, B.; Liu, X.M.; Gao, Z.G.; Du, Y.G.; Zhao, W.J.; Lin, B.C. Chemiluminescence diminishment on a paper-based analytical device: High throughput determination of beta-agonists in swine hair. Anal. Methods 2014, 6, 9684–9690. [Google Scholar] [CrossRef]
- Dou, M.W.; Dominguez, D.C.; Li, X.J.; Sanchez, J.; Scott, G. A versatile pdms/paper hybrid microfluidic platform for sensitive infectious disease diagnosis. Anal. Chem. 2014, 86, 7978–7986. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, D.; Qiu, Y.; Liu, X.; Huang, L.; Wen, H.; Hu, J. An europium functionalized carbon dot-based fluorescence test paper for visual and quantitative point-of-care testing of anthrax biomarker. Talanta 2020, 220, 121377. [Google Scholar] [CrossRef] [PubMed]
- Crowley, T.A.; Pizziconi, V. Isolation of plasma from whole blood using planar microfilters for lab-on-a-chip applications. Lab Chip 2005, 5, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Jalal, U.M.; Im, S.B.; Ko, S.; Shim, J.S. A smartphone-based optical platform for colorimetric analysis of microfluidic device. Sens. Actuators B Chem. 2017, 239, 52–59. [Google Scholar] [CrossRef]
- Solmaz, M.E.; Mutlu, A.Y.; Alankus, G.; Kılıç, V.; Bayram, A.; Horzum, N. Quantifying colorimetric tests using a smartphone app based on machine learning classifiers. Sens. Actuators B Chem. 2018, 255, 1967–1973. [Google Scholar] [CrossRef]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Use of multiple colorimetric indicators for paper-based microfluidic devices. Anal. Chim. Acta 2010, 674, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Choobbari, M.L.; Rad, M.B.; Jahanshahi, A.; Ghourchian, H. A sample volume independent paper microfluidic device for quantifying glucose in real human plasma. Microfluid. Nanofluid. 2020, 24, 74. [Google Scholar] [CrossRef]
- Lucio do Lago, C.; Torres da Silva, H.D.; Neves, C.A.; Alves Brito-Neto, J.G.; Fracassi da Silva, J.A. A dry process for production of microfluidic devices based on the lamination of laser-printed polyester films. Anal. Chem. 2003, 75, 3853–3858. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Gopalakrishnan, S.; Savitha, R.; Renganathan, T.; Pushpavanam, S. Fabrication of laser printed microfluidic paper-based analytical devices (LP-μPADs) for point-of-care applications. Sci. Rep. 2019, 9, 7896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Z.; Dai, J.; Zhang, W.; Jiang, Y.; Zhou, A. A low-cost mobile platform for whole blood glucose monitoring using colorimetric method. Microchem. J. 2021, 162, 105814. [Google Scholar] [CrossRef]
- Zhu, W.J.; Feng, D.Q.; Chen, M.; Chen, Z.D.; Zhu, R.; Fang, H.L.; Wang, W. Bienzyme colorimetric detection of glucose with self-calibration based on tree-shaped paper strip. Sens. Actuators B Chem. 2014, 190, 414–418. [Google Scholar] [CrossRef]




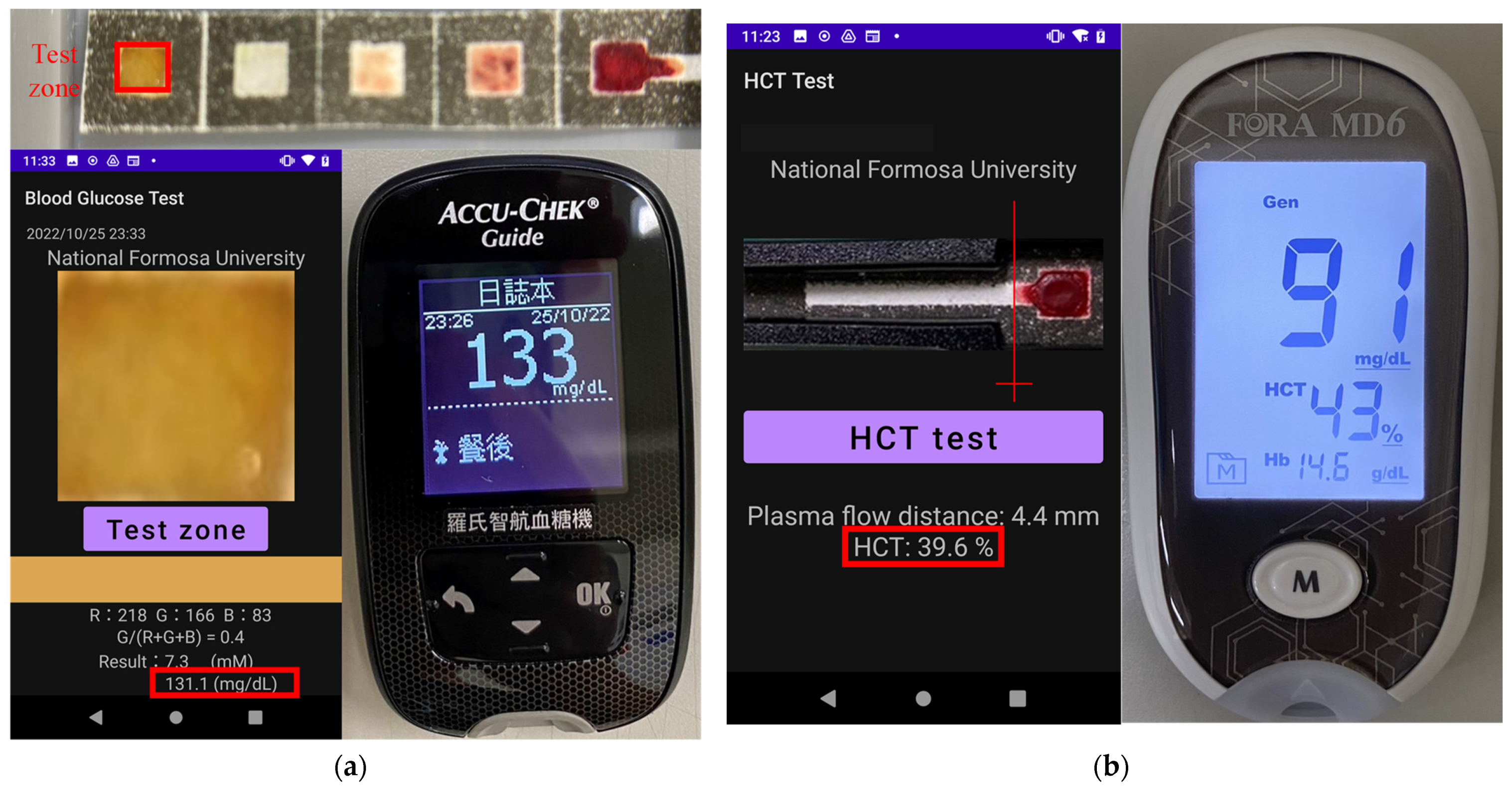
| Blood Glucose (mg/dL) | Hematocrit (%) | |||||
|---|---|---|---|---|---|---|
| Samples | μPAD | Glucose Meter | Relative Error (%) | μPAD | Hematocrit Meter | Relative Error (%) |
| 1 | 83.9 | 88 | 4.7 | 38.7 | 41 | 5.9 |
| 2 | 114.2 | 122 | 6.4 | 40.1 | 43 | 7.2 |
| 3 | 141.8 | 147 | 3.6 | 38.3 | 40 | 4.4 |
| 4 | 96.2 | 101 | 4.8 | 46.5 | 48 | 3.2 |
| 5 | 131.1 | 133 | 1.4 | 36.2 | 39 | 7.7 |
| 6 | 158.5 | 164 | 3.5 | 30.4 | 31 | 1.9 |
| 7 | 82.6 | 86 | 4.1 | 48.6 | 53 | 9.1 |
| 8 | 94.5 | 98 | 3.7 | 42.9 | 46 | 7.2 |
| 9 | 86.8 | 91 | 4.8 | 39.6 | 43 | 8.6 |
| 10 | 96.6 | 102 | 5.5 | 43.1 | 45 | 4.4 |
| 11 | 133.3 | 141 | 5.7 | 52.2 | 55 | 5.3 |
| 12 | 75.8 | 79 | 4.2 | 47.1 | 51 | 8.3 |
| 13 | 107.4 | 113 | 5.2 | 49.8 | 54 | 8.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.-X.; Jiang, M.-Z.; Chuang, Y.-J.; Kuo, J.-N. Paper-Based Microfluidic Analytical Device Patterned by Label Printer for Point-of-Care Blood Glucose and Hematocrit Detection Using 3D-Printed Smartphone Cassette. Sensors 2024, 24, 4792. https://doi.org/10.3390/s24154792
Cai Z-X, Jiang M-Z, Chuang Y-J, Kuo J-N. Paper-Based Microfluidic Analytical Device Patterned by Label Printer for Point-of-Care Blood Glucose and Hematocrit Detection Using 3D-Printed Smartphone Cassette. Sensors. 2024; 24(15):4792. https://doi.org/10.3390/s24154792
Chicago/Turabian StyleCai, Zong-Xiao, Ming-Zhang Jiang, Ya-Ju Chuang, and Ju-Nan Kuo. 2024. "Paper-Based Microfluidic Analytical Device Patterned by Label Printer for Point-of-Care Blood Glucose and Hematocrit Detection Using 3D-Printed Smartphone Cassette" Sensors 24, no. 15: 4792. https://doi.org/10.3390/s24154792




