Taste Sensor Assessment of Bitterness in Medicines: Overview and Recent Topics
Abstract
1. Introduction
2. Bitterness Assessment in Medicines by Taste Sensor
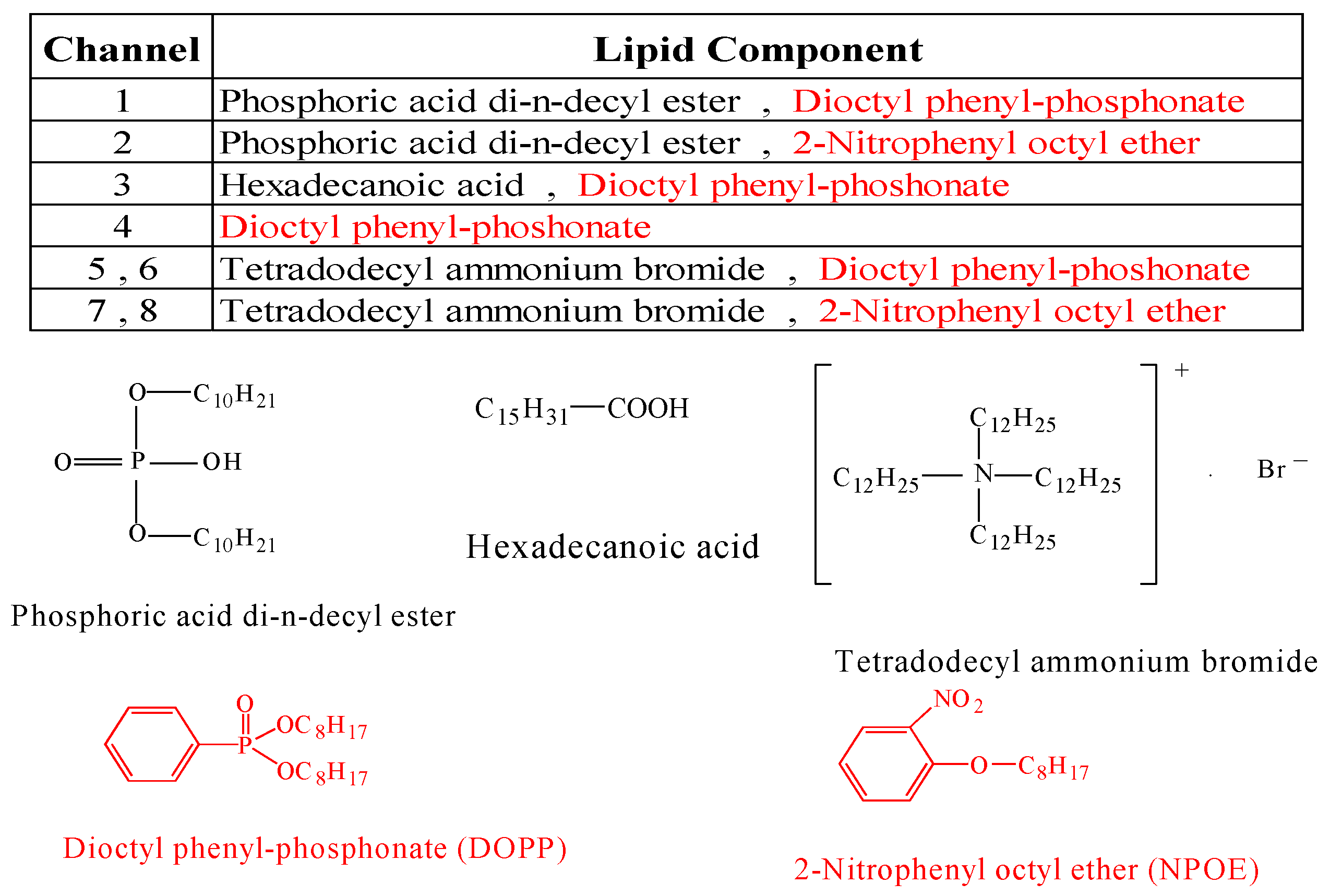
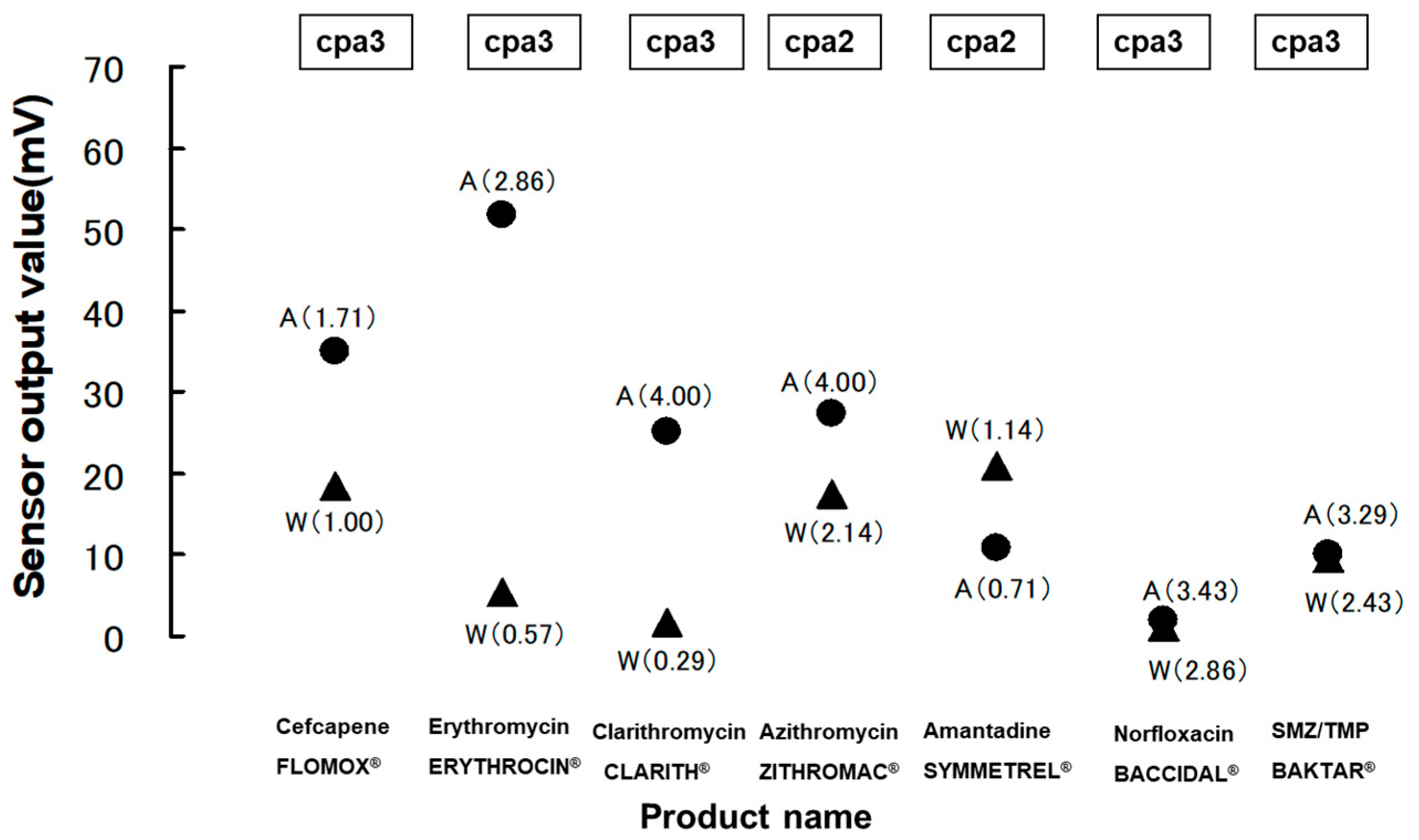
2.1. Bitterness Assessment in Medicines by Multichannel Taste Sensor
2.2. Prediction of Bitterness Enhancement in Bitter Medicines with Foods, Beverages, or Other Medications by Multichannel Taste Sensor
2.3. Assessment of Taste Masking in Medicines by Astree Sensor System
2.4. Characteristics of Insent Taste Sensor and Astree Sensor System in Pharmaceutical Applications
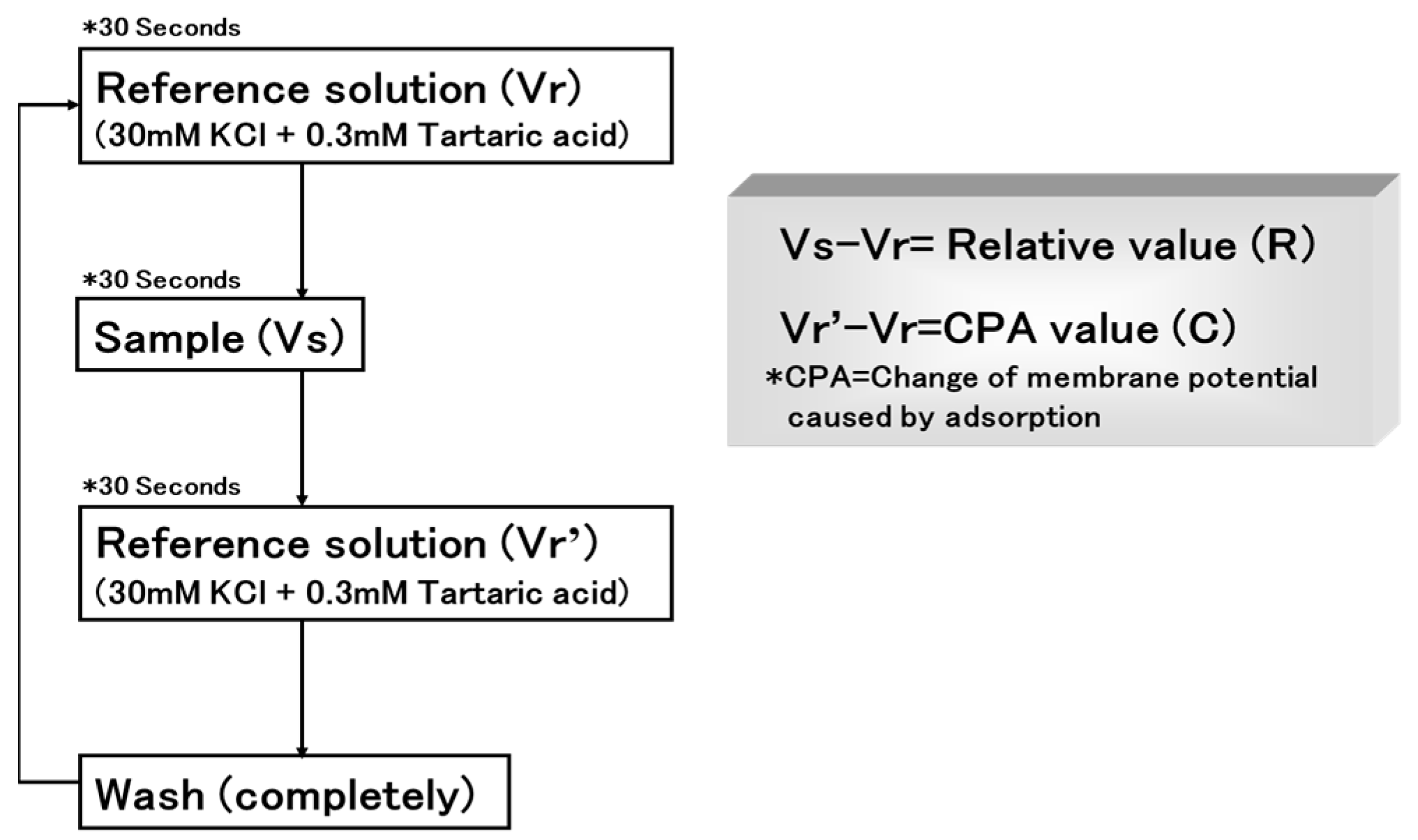
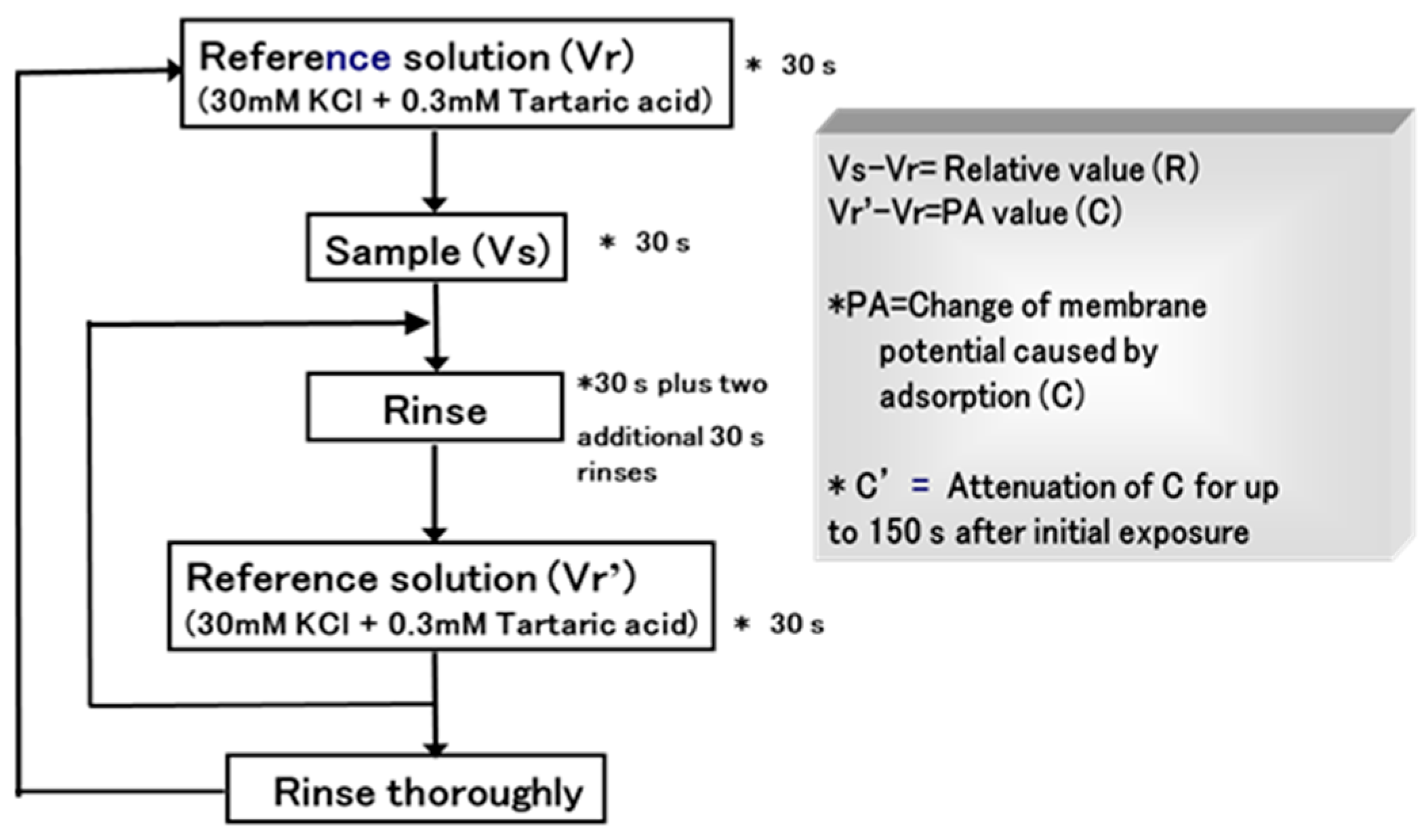
2.4.1. Insent Sensor
Overview
Advantages
- The Insent taste sensor can quantify the bitterness intensity of a wide range of pharmaceuticals, from oral medications to traditional Chinese medicines, as well as the degree of bitterness masking.
- The assessment is achieved using a lineup of bitterness-specific sensor membranes and astringency-specific membranes.
- It can predict an increase in bitterness when co-administered with certain foods/drinks or other pharmaceuticals.
- It can quantitatively assess bitterness at the moment pharmaceuticals are taken into the mouth and also assess the bitterness that lingers as an aftertaste, even after rinsing following ingestion.
- It can also quantify the bitterness intensity of pharmaceuticals and formulations in a suspended state.
Challenge
Duration
2.4.2. Astree Sensor
Overview
Advantages
- It is possible to objectively determine that formulations with smaller Euclidean distances between active drugs and placebos have weaker original drug flavors, meaning they exhibit stronger masking effects.
- It is also possible to compare between branded drugs and generic medicines.
- Utilizing sensors that respond to high-molecular-weight ingredients or sweeteners in formulation raw materials allows for the design of formulations that mask bitterness.
- Long durability.
Challenge
3. Advanced Taste Sensor Application to New Dosage Forms
3.1. Assessment of Taste Masking in ODTs by Multichannel Taste Sensors
3.2. Assessment of Taste Masking in ODTs by Astree Taste Sensor
3.3. Assessment of ODFs and Minitablets by Taste Sensor
3.4. Performance Qualifications of Taste Sensors
4. Taste Sensor Assessment in Medicinal Plants and Chinese (Herbal) Medicines
5. Taste Sensor Assessment for Nutritional Supplement Containing BCAA
6. Taste Sensor Assessment in Bitterness Suppression
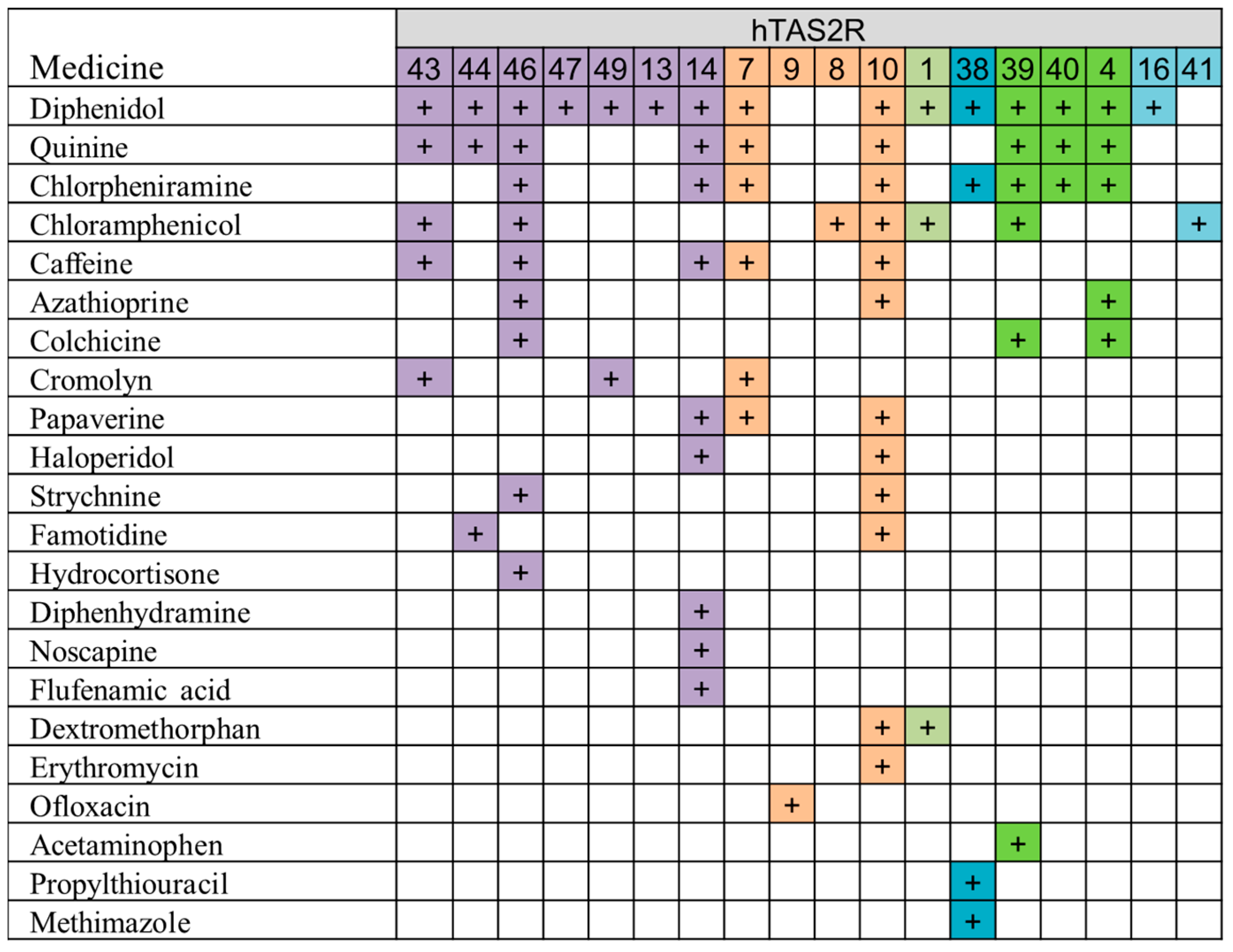
7. Correlation Evaluation between Taste Sensor Response and Human Bitter Receptor Response in BitterDB
8. Novel Taste Sensor Utilizing Allostery and Its Application to Non-Charged Substances
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nunn, T.; Williams, J. Formulation of medicines for children. Br. J. Clin. Pharmacol. 2005, 59, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Baguley, D.; Lim, E.; Bevan, A.; Pallet, A.; Faust, S.N. Prescribing for children—Taste and palatability affect adherence to antibiotics: A review. Arch. Dis. Child. 2012, 97, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Uchida, S.; Namiki, N. Taste-masking effect of physical and organoleptic methods on peppermint-scented orally disintegrating tablet of famotidine based on suspension spray-coating method. Chem. Pharm. Bull. 2012, 60, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Maeda, A.; Uchida, S.; Namiki, N. Preparation and evaluation of unpleasant taste-masked pioglitazone orally disintegrating tablets. Int. J. Pharm. 2013, 446, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Uchida, S.; Kashiwagura, Y.; Tanaka, S.; Hakamata, A.; Odagiri, K.; Inui, N.; Watanabe, H.; Namiki, N. Preparation of Cocoa Powder-Containing Orally Disintegrating Tablets of Rebamipide (Rebamipide Chocolet) and Evaluation of Their Clinical Palatability. Chem. Pharm. Bull. 2012, 60, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Iwamoto, K.; Okumura, H.; Hida, H.; Hiraoka, H.; Kamei, A.; Mori, D.; Yamada, K.; Ozaki, N. Sensory evaluation of the bitterness of asenapine using D-sorbitol pretreatment: Single-blind, placebo-controlled, crossover trial. BMC Psychiatry 2023, 23, 159. [Google Scholar] [CrossRef] [PubMed]
- Feeney, E.L.; Hayes, J.E. Exploring associations between taste perception, oral anatomy and polymorphisms in the carbonic anhydrase (gustin) gene CA6. Physiol. Behav. 2014, 128, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Sawai, A.; Motomura, T.; Oshima, T.; Sawai, S.; Fujikawa, T.; Fujii, H.; Bannai, Y.; Takeda, Y.; Ohno, M.; Tochikubo, O. Influence of Acute Mental Arithmetic Stress on Taste and Pungency. J. Nutr. Sci. Vitaminol. 2019, 65, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Angulo, R.; Bustamante, J.; Arévalo-Romero, C.A. Age, sex and pre-exposure effects on acquisition and generalization of conditioned taste aversion in rats. Behav. Brain Res. 2020, 394, 112813. [Google Scholar] [CrossRef] [PubMed]
- Altundag, A.; Cayonu, M. Chemical Senses in Cancer Patients. Curr. Pharm. Des. 2016, 22, 2264–2269. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Watanabe, K.; Kobayakawa, T.; Wada, M. Relationships between autistic traits, taste preference, taste perception, and eating behaviour. Eur. Eat. Disord. Rev. 2022, 30, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Latha, R.S.; Lakshmi, P.K. Electronic tongue: An analytical gustatory tool. J. Adv. Pharm. Technol. Res. 2012, 3, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Sato, T. The adaptation of the frog tongue to various taste solutions: The effect on gustatory neural responses to bitter stimuli. Comp. Biochem. Physiol. A Comp. Physiol. 1982, 73, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Okada, Y.; Miyamoto, T. Molecular mechanisms of gustatory transduction in frog taste cells. Prog. Neurobiol. 1995, 6, 239–287. [Google Scholar] [CrossRef] [PubMed]
- Meyerhof, W. Elucidation of mammalian bitter taste. Rev. Physiol. Biochem. Pharmacol. 2005, 154, 37–72. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Meyerhof, W. Mammalian bitter taste perception. In Chemosensory Systems in Mammals, Fishes, and Insects; Results and Problems in Cell Differentiation; Springer: Berlin/Heidelberg, Germany, 2009; Volume 47, pp. 77–96. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Son, H.J.; Kim, Y.; Misaka, T.; Rhyu, M.R. Umami-bitter interactions: The suppression of bitterness by umami peptides via human bitter taste receptor. Biochem. Biophys. Res. Commun. 2015, 456, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Rhyu, M.R.; Kim, Y.; Misaka, T. Suppression of hTAS2R16 Signaling by Umami Substances. Int. J. Mol. Sci. 2020, 21, 7045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sakurai, T.; Misaka, T.; Nagai, T.; Ishimaru, Y.; Matsuo, S.; Asakura, T.; Abe, K. pH-Dependent inhibition of the human bitter taste receptor hTAS2R16 by a variety of acidic substances. J. Agric. Food Chem. 2009, 57, 2508–2514. [Google Scholar] [CrossRef]
- Toko, K.; Tahara, Y.; Habara, M.; Kobayashi, K.; Ikezaki, H. Essentials of Machine Olfaction and Taste; Taste Sensor: Electronic Tongue with Global Selectivity; John Wiley & Sons Singapore Pte Ltd.: Singapore, 2016; pp. 87–174. [Google Scholar]
- Sharma, G.; Kumar, S.; Kumar, A.; Sharma, A.; Kumar, R.; Kaur, R.; Bhondekar, A.P. Development of lipid membrane based taste sensors for electronic tongue. Procedia Comput. Sci. 2015, 70, 146–152. [Google Scholar] [CrossRef]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. Taste sensing systems (electronic tongues) for pharmaceutical applications. Int. J. Pharm. 2011, 417, 256–271. [Google Scholar] [CrossRef]
- Ye, J.; Fan, M.; Zhang, X.; Liang, Q.; Zhang, Y.; Zhao, X.; Lin, C.T.; Zhang, D. A novel biomimetic electrochemicaltaste-biosensor based on conformational changes of the taste receptor. Biosens. Bioelectron. 2024, 249, 116001. [Google Scholar] [CrossRef] [PubMed]
- Khalilian, A.; Khan, M.R.R.; Kang, S.-W. Highly sensitive and wide-dynamic-range side-polished fiber-optic taste sensor. Sens. Actuators B Chem. 2017, 249, 700–707. [Google Scholar] [CrossRef]
- Chung, S.; Park, T.S.; Park, S.H.; Kim, J.Y.; Park, S.; Son, D.; Bae, Y.M.; Cho, S.I. Colorimetric sensor array for white wine tasting. Sensors 2015, 15, 18197–18208. [Google Scholar] [CrossRef]
- Kozitsina, A.; Svalova, T.; Malysheva, N.; Okhokhonin, A.; Vidrevich, M.; Brainina, K. Sensors based on bio and biomimetic receptors in medical diagnostic, environment, and food analysis. Biosensors 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Ciosek, P.; Wróblewski, W. Sensor arrays for liquid sensing—Electronic tongue systems. Analyst 2007, 132, 963–978. [Google Scholar] [CrossRef]
- Toko, K. Taste sensor with global selectivity. Mater. Sci. Eng. C 1996, 4, 69–82. [Google Scholar] [CrossRef]
- Wu, X.; Tahara, Y.; Yatabe, R.; Toko, K. Taste Sensor: Electronic tongue with lipid membranes. Anal. Sci. 2020, 36, 147–159. [Google Scholar] [CrossRef]
- Toko, K.; Tahara, Y.; Habara, M.; Kobayashi, Y.; Ikezaki, H. Taste Sensor: Electronic Tongue with Global Selectivity. In Essentials of Machine Olfaction and Taste; Wiley: Hoboken, NJ, USA, 2016; pp. 87–174. ISBN 9781118768495. [Google Scholar]
- Toko, K. Research and development of taste sensors as a novel analytical tool. Proc. Japan Acad. Ser. B 2023, 99, 173–189. [Google Scholar] [CrossRef]
- Takagi, S.; Toko, K.; Wada, K.; Yamada, H.; Toyoshima, K. Detection of suppression of bitterness by sweet substance using a multichannel taste sensor. J. Pharm. Sci. 1998, 87, 552–555. [Google Scholar] [CrossRef]
- Uchida, T.; Miyanaga, Y.; Tanaka, H.; Wada, K.; Kurosaki, S.; Ohki, T.; Yoshida, M.; Matsuyama, K. Quantitative evaluation of the bitterness of commercial medicines using a taste sensor. Chem. Pharm. Bull. 2000, 48, 1843–1845. [Google Scholar] [CrossRef]
- Uchida, T.; Kobayashi, Y.; Miyanaga, Y.; Toukubo, R.; Ikezaki, H.; Taniguchi, A.; Nishikata, M.; Matsuyama, K. A new method for evaluating the bitterness of medicines by semi-continuous measurement of adsorption using a taste sensor. Chem. Pharm. Bull. 2001, 49, 1336–1339. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Tanigake, A.; Miyanaga, Y.; Matsuyama, K.; Kunitomo, M.; Kobayashi, K.; Ikezaki, H.; Taniguchi, A. Evaluation of the bitterness of antibiotics using a taste sensor. J. Pharm. Pharmacol. 2003, 55, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Tanigake, Y.; Miyanaga, Y.; Nakamura, T.; Tsuji, E.; Matsuyama, K.; Kunitomo, M.; Uchida, T. The Bitterness Intensity of Clarithromycin Assessed by a Taste Sensor. Chem. Pharm. Bull. 2003, 51, 1241–1245. [Google Scholar] [CrossRef]
- Ishizaka, T.; Miyanaga, Y.; Mukai, J.; Asaka, K.; Nakai, Y.; Tsuji, E.; Uchida, T. Bitterness Evaluation of Medicines for Pediatric Use by a Taste Sensor. Chem. Pharm. Bull. 2004, 52, 943–948. [Google Scholar] [CrossRef]
- Ishizaka, T.; Okada, S.; Takemoto, E.; Tokuyama, E.; Tsuji, E.; Mukai, J.; Uchida, T. The Suppression of Enhanced Bitterness Intensity of Macrolide Dry Syrup Mixed with an Acidic Powder. Chem. Pharm. Bull. 2007, 55, 1452–1457. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Keeney, M.P. Taste Masking Analysis in Pharmaceutical Formulation Development Using an Electronic Tongue. Int. J. Pharm. 2006, 310, 118–124. [Google Scholar] [CrossRef]
- Wei, Y.; Nedley, M.P.; Bhaduri, S.B.; Bredzinski, X.; Boddu, S.H. Masking the bitter taste of injectable lidocaine HCl formulation for dental procedures. AAPS PharmSciTech. 2015, 16, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, J.; Hong, X.; Han, X.; Liu, B.; Li, X.; Zhang, H.; Gao, J.; Liu, N.; Gao, X.; et al. Taste MaskingStudy Based on an Electronic Tongue: The Formulation Design of 3D Printed Levetiracetam Instant-Dissolving Tablets. Pharm Res. 2021, 38, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Breitkreutz, J.; Boos, J. Paediatric and Geriatric Drug Delivery. Expert Opin. Drug Deliv. 2007, 4, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, T.; Tamura, T.; Kawai, H.; Kajiyama, A.; Itai, S. Formulation Design of an Oral, Fast-Disintegrating Dosage Form Containing Taste-Masked Particles of Famotidine. Chem. Pharm. Bull. 2008, 56, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Klingmann, V.; Pohly, C.E.; Meissner, T.; Mayatepek, E.; Möltner, A.; Flunkert, K.; Breitkreutz, J.; Bosse, H.M. Acceptability of an Orodispersible Film Compared to Syrup in Neonates and Infants: A Randomized Controlled Trial. Eur. J. Pharm. Biopharm. 2020, 151, 239–245. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Usui, R.; Ikezaki, H.; Tahara, K.; Takeuchi, H. An Advanced Technique Using an Electronic Taste-Sensing System to Evaluate the Bitterness of Orally Disintegrating Films and the Evaluation of Model Films. Int. J. Pharm. 2017, 531, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Poller, B.; Strachan, C.; Broadbent, R.; Walker, G.F. A Minitablet Formulation Made from Electrospun Nanofibers. Eur. J. Pharm. Biopharm. 2017, 114, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Meruva, S.; Singaraju, A.B.; Vinjamuri, B.P.; Ternik, R.; Stagner, W.C. Current State of Minitablet Product Design. J. Pharm. Sci. 2024, 113, 1123–1154. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.V.; Polk, A.N.; Patil, H.; Ye, X.; Pimparade, M.B.; Repka, M.A. Rat Palatability Study for Taste Assessment of Caffeine Citrate Formulation Prepared via Hot-Melt Extrusion Technology. AAPS PharmSciTech 2017, 18, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Boateng, J.S.; Bonnefille, M.; Aranyos, A.; Mitchell, J.C.; Douroumis, D. Taste Masking of Paracetamol by Hot-Melt Extrusion: An In Vitro and In Vivo Evaluation. Eur. J. Pharm. Biopharm. 2012, 80, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Al-Khattawi, A.; Mohammed, A.R. Challenges and Emerging Solutions in the Development of Compressed Orally Disintegrating Tablets. Expert. Opin. Drug Discov. 2014, 9, 1109–1120. [Google Scholar] [CrossRef]
- Dagan-Wiener, A.; Di Pizio, A.; Nissim, I.; Bahia, M.S.; Dubovski, N.; Margulis, E.; Niv, M.Y. BitterDB: Taste Ligands and Receptors Database in 2019. Nucleic Acids Res. 2019, 47, D1179–D1185. [Google Scholar] [CrossRef]
- Ni, K.; Che, B.; Gu, R.; Wang, C.; Xu, H.; Li, H.; Cen, S.; Luo, M.; Deng, L. BitterDB Database Analysis Plus Cell Stiffness Screening Identify Flufenamic Acid as the Most Potent TAS2R14-Based Relaxant of Airway Smooth Muscle Cells for Therapeutic Bronchodilation. Theranostics 2024, 14, 1744–1763. [Google Scholar] [CrossRef]
- Xue, A.Y.; Di Pizio, A.; Levit, A.; Yarnitzky, T.; Penn, O.; Pupko, T.; Niv, M.Y. Independent Evolution of Strychnine Recognition by Bitter Taste Receptor Subtypes. Mol. Biosci. 2018, 5, 9. [Google Scholar]
- Grau-Bové, C.; Grau-Bové, X.; Terra, X.; Garcia-Vallve, S.; Rodríguez-Gallego, E.; Beltran-Debón, R.; Blay, M.T.; Ardévol, A.; Pinent, M. Functional and Genomic Comparative Study of the Bitter Taste Receptor Family TAS2R: Insight into the Role of Human TAS2R5. FASEB J. 2022, 36, e22175. [Google Scholar] [CrossRef] [PubMed]
- Waterloo, L.A.W.; Löber, S.; Gmeiner, P. The Bitter Taste Receptor TAS2R14 as a Drug Target. Chimia 2022, 76, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Sainz, E.; Cavenagh, M.M.; Gutierrez, J.; Battey, J.F.; Northup, J.K.; Sullivan, S.L. Functional Characterization of Human Bitter Taste Receptors. Biochem. J. 2007, 403, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, W.; González-Nilo, F.; Agosin, E. Docking and Molecular Dynamics of Steviol Glycoside-Human Bitter Receptor Interactions. J. Agric. Food Chem. 2016, 64, 7585–7596. [Google Scholar] [CrossRef] [PubMed]
- BitterDB. Available online: https://bitterdb.agri.huji.ac.il (accessed on 1 June 2024).
- Haraguchi, T.; Uchida, T.; Yoshida, M.; Kojima, H.; Habara, M.; Ikezaki, H. The Utility of the Artificial Taste Sensor in Evaluating the Bitterness of Drugs: Correlation with Responses of Human TASTE2 Receptors (hTAS2Rs). Chem. Pharm. Bull. 2018, 66, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, S.; Haraguchi, T.; Nakamura, S.; Li, D.; Kojima, H.; Yoshida, M.; Uchida, T. Taste-masking effect of Chlorogenic acid (CGA) on bitter drugs assessed by taste sensor and surface plasmon resonance on the basis of CGA-drug interactions. Chem. Pharm. Bull. 2017, 65, 127–133. [Google Scholar] [CrossRef] [PubMed]
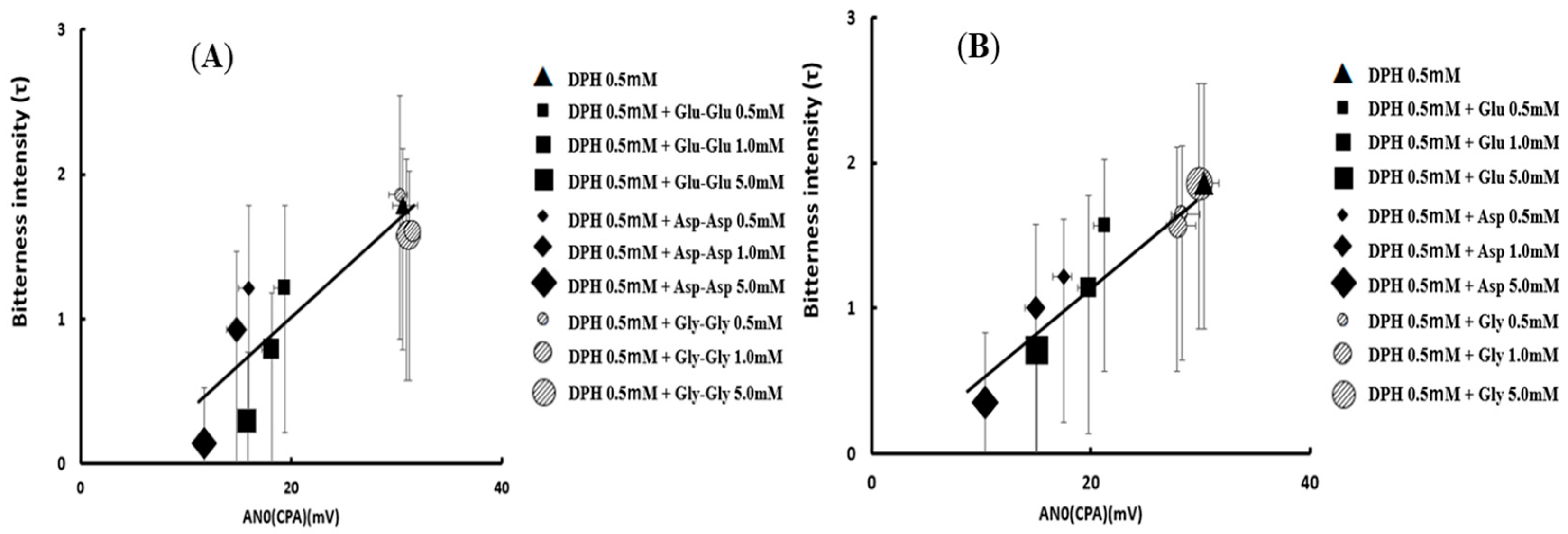
- Okuno, T.; Morimoto, S.; Nishikawa, H.; Haraguchi, T.; Kojima, H.; Tsujino, H.; Arisawa, M.; Yamashita, T.; Nishikawa, J.; Yoshida, M.; et al. Bitterness-Suppressing Effect of Umami Dipeptides and Their Constituent Amino Acids on Diphenhydramine: Evaluation by Gustatory Sensation and Taste Sensor Testing. Chem. Pharm. Bull. 2020, 68, 234–243. [Google Scholar] [CrossRef]
- Zhang, F.; Klebansky, B.; Fine, R.M.; Xu, H.; Pronin, A.; Liu, H.; Tachdjian, C.; Li, X. Molecular mechanism for the umami taste synergism. Proc. Natl. Acad. Sci. USA 2008, 105, 20930–20934. [Google Scholar] [CrossRef]
- Chen, K.Y.M.; Keri, D.; Barth, P. Computational design of G Protein-Coupled Receptor allosteric signal transductions. Nat. Chem. Biol. 2020, 16, 77–86. [Google Scholar] [CrossRef]
- Liu, J.; Nussinov, R. Allostery: An overview of its history, concepts, methods, and applications. PLoS Comput. Biol. 2016, 12, e1004966. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, J.; Toko, K.; Tahara, Y.; Ishida, M.; Habara, M.; Ikezaki, H.; Kojima, H.; Ikegami, S.; Yoshida, M.; Uchida, T. Development of taste sensor to detect non- charged bitter substances. Sensors 2020, 20, 3455. [Google Scholar] [CrossRef] [PubMed]
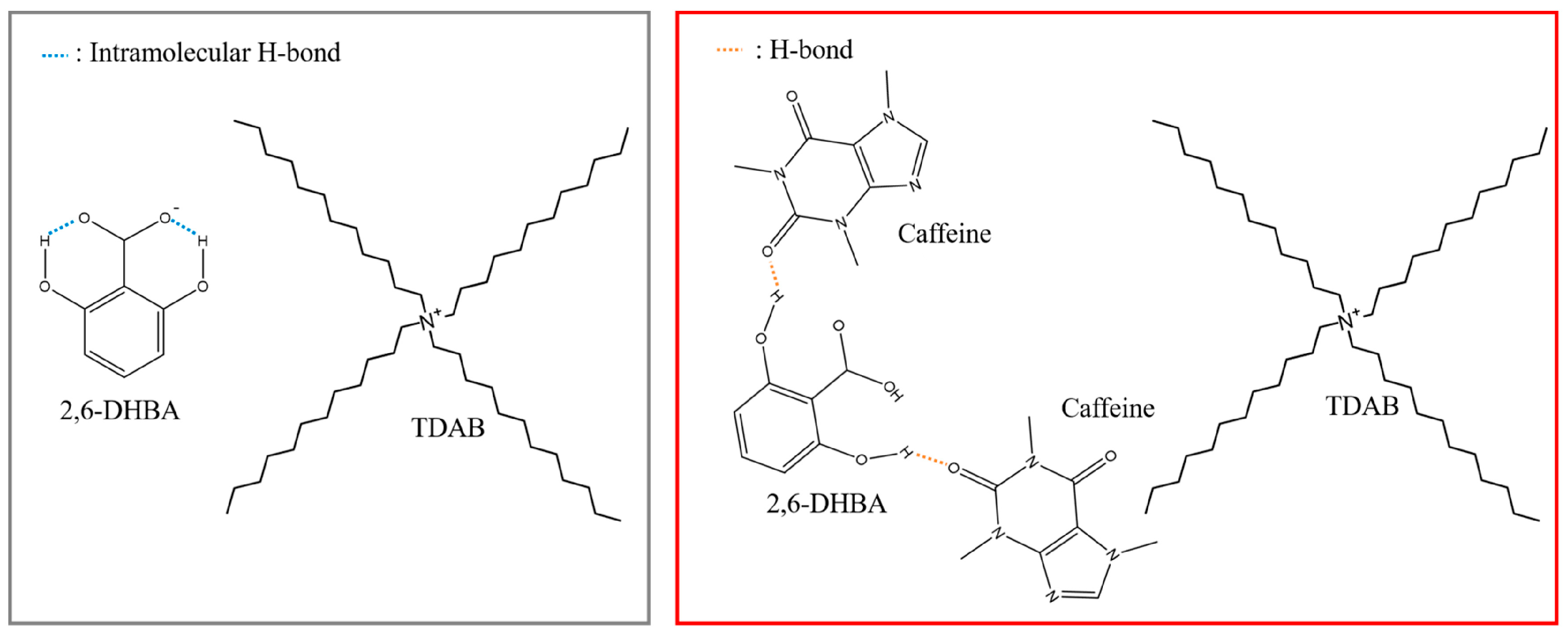
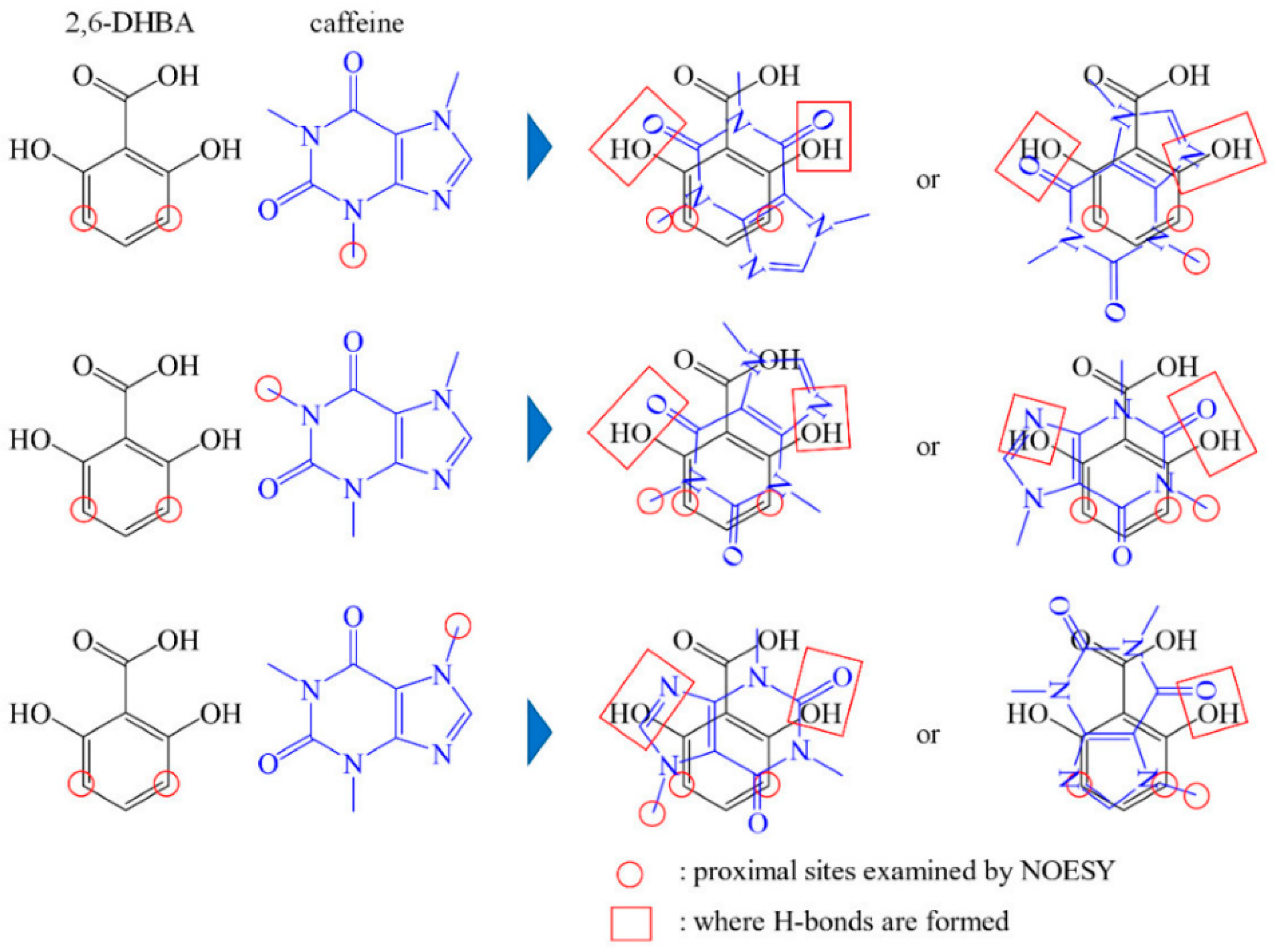
- Zhao, Z.; Ishida, M.; Onodera, T.; Toko, K. Effect of hydroxybenzoic acids on caffeine detection using taste sensor with lipid/polymer membranes. Sensors 2022, 22, 1607. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Ide, H.; Arima, K.; Zhao, Z.; Matsui, T.; Toko, K. Identification of the principle of taste sensors to detect non-charged bitter substances by 1H-NMR Measurement. Sensors 2022, 22, 2592. [Google Scholar] [CrossRef] [PubMed]
- Ikezaki, H.; Naito, Y.; Kobayashi, Y.; Toukubo, R.; Taniguchi, A.; Toko, K. Improvement of selectivity of taste sensor by control of charge density and hydrophobicity of lipid membrane. Tech. Rep. IEICE OME 2000, 100, 19–24. (In Japanese) [Google Scholar]
- Tahara, Y.; Toko, K. Electronic tongues-a review. IEEE Sens. J. 2013, 13, 3001–3011. [Google Scholar] [CrossRef]
- King, C.; Nightingale, R.; Phiri, T.; Zadutsa, B.; Kainja, E.; Makwenda, C.; Colbourn, T.; Stevenson, F. Non-adherence to oral antibiotics for community paediatric pneumonia treatment in Malawi—A qualitative investigation. PLoS ONE 2018, 13, e0206404. [Google Scholar] [CrossRef] [PubMed]
- Eells, S.J.; Nguyen, M.; Jung, J.; Macias-Gil, R.; May, L.; Miller, L.G. Relationship between Adherence to Oral Antibiotics and Postdischarge Clinical Outcomes among Patients Hospitalized with Staphylococcus aureus Skin Infections. Antimicrob. Agents Chemother. 2016, 60, 2941–2948. [Google Scholar] [CrossRef]
- Amarilyo, G.; Chodick, G.; Zalcman, J.; Koren, G.; Levinsky, Y.; Somekh, I.; Harel, L. Poor long-term adherence to secondary penicillin prophylaxis in children with history of rheumatic fever. Semin. Arthritis Rheum. 2019, 48, 1019–1024. [Google Scholar] [CrossRef]
- Coupland, J.N.; Hayes, J.E. Physical approaches to masking bitter taste: Lessons from food and pharmaceuticals. Pharm. Res. 2014, 31, 2921–2939. [Google Scholar] [CrossRef] [PubMed]
- Felton, L.A. Use of polymers for taste-masking pediatric drug products. Drug Dev. Ind. Pharm. 2018, 44, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Felton, L.A.; Binzet, B.; Wiley, C.; McChesney, D.; McConville, J.; Ҫelik, M.; Muttil, P. Spray drying Eudragit® E-PO with acetaminophen using 2- and 3-fluid nozzles for taste masking. Int. J. Pharm. 2024, 658, 124191. [Google Scholar] [CrossRef]
- Funasaki, N.; Uratsuji, I.; Okuno, T.; Hirota, S.; Neya, S. Masking mechanisms of bitter taste of drugs studied with ion selective electrodes. Chem. Pharm. Bull. 2006, 54, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Higashi, T.; Motoyama, K. Improvement of the bitter taste of drugs by complexation with cyclodextrins: Applications, evaluations and mechanisms. Ther. Deliv. 2012, 3, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Kaur, B.; Kumar, P.; Arora, S. Masking the unpleasant taste of etoricoxib by crosslinked acrylic polymer based ion-exchange resin complexation. Polim. Med. 2010, 40, 19–26. [Google Scholar] [PubMed]
- Zhao, X.; Li, Q.; Wang, C.; Hu, S.; He, X.; Sun, C.C. Simultaneous taste-masking and oral bioavailability enhancement of Ligustrazine by forming sweet salts. Int. J. Pharm. 2020, 577, 119089. [Google Scholar] [CrossRef] [PubMed]
- An, J.P.; Wang, Y.; Munger, S.D.; Tang, X. A review on natural sweeteners, sweet taste modulators and bitter masking compounds: Structure-activity strategies for the discovery of novel taste molecules. Crit. Rev. Food Sci. Nutr. 2024; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Faisal, W.; Farag, F.; Abdellatif, A.A.H.; Abbas, A.J. Taste Masking Approaches for Medicines. Curr Drug Deliv. 2018, 15, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Tafere, C.; Yilma, Z.; Abrha, S.; Yehualaw, A. Formulation, in vitro characterization and optimization of taste-masked orally disintegrating co-trimoxazole tablet by direct compression. PLoS ONE. 2021, 16, e0246648. [Google Scholar] [CrossRef] [PubMed]
- Matsui, R.; Uchida, S.; Namiki, N. Combination effect of physical and gustatory taste masking for propiverine hydrochloride orally disintegrating tablets on palatability. Biol. Pharm. Bull. 2015, 38, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Yajima, T.; Fukushima, Y.; Itai, S.; Kawashima, Y. Method of evaluation of the bitterness of clarithromycin dry syrup. Chem. Pharm. Bull. 2002, 50, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Yajima, T.; Umeki, N.; Itai, S. Optimum spray congealing conditions for masking the bitter taste of clarithromycin in wax matrix. Chem. Pharm. Bull. 1999, 47, 220–225. [Google Scholar] [CrossRef]
- Yajima, T.; Itai, S.; Takeuchi, H.; Kawashima, Y. Optimum heat treatment conditions for masking the bitterness of the clarithromycin wax matrix. Chem. Pharm. Bull. 2003, 5, 1223–1226. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Habara, M.; Ikezaki, H.; Chen, R.; Naito, Y.; Toko, K. Advanced Taste Sensors Based on Artificial Lipids with Global Selectivity to Basic Taste Qualities and High Correlation to Sensory Scores. Sensors 2010, 10, 3411–3433. [Google Scholar] [CrossRef]
- Katsuragi, Y.; Mitsui, Y.; Umeda, T.; Sugiura, Y.; Otsuji, K.; Kurihara, K. Basic studies for the practical use of bitterness inhibitors: Selective inhibition of bitterness by phospholipid. Pharm. Res. 1997, 14, 720–724. [Google Scholar] [CrossRef]
- Kondo, H.; Sako, K. Trade-offs in oral drug product development. Yakugaku Zasshi 2015, 135, 229–235. (In Japanese) [Google Scholar] [CrossRef] [PubMed][Green Version]
- Package Insert for Clarithromycin Dry Syrup. Available online: https://medical.taisho.co.jp/di/brand/cl-tds/product.php?bdname=cl50&brand=cl-tds (accessed on 3 June 2024). (In Japanese).
- Package Insert for L-Carbocysteine Dry Syrup. Available online: https://www.kegg.jp/medicus-bin/japic_med?japic_code=00070591 (accessed on 3 June 2024). (In Japanese).
- Abu-Khalaf, N.; Zaid, A.N.; Jaradat, N.; AlKilany, A.; Abu Rumaila, B.; Al Ramahi, R.; Shweiki, S.; Nidal, S.; Surakhi, N. Clarithromycin pharmaceutical suspensions (the brand Klacid® and two generic K1 and K2). Sensors 2018, 18, 454. [Google Scholar] [CrossRef] [PubMed]
- Kayumba, P.C.; Huyghebaert, N.; Cordella, C.; Ntawukuliryayo, J.D.; Vervaet, C.; Remon, J.D. Quinine sulphate pellets for flexible pediatric drug dosing: Formulation development and evaluation of taste-masking efficiency using the electronic tongue. Eur. J. Pharm. Biopharm. 2007, 66, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Naini, V.; Ahmed, S.U. Utilization of a modified special-cubic design and an electronic tongue for bitterness masking formula-tion optimization. J. Pharm. Sci. 2007, 96, 2723–2734. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, X.; Zhang, L.; Gao, X.; Li, H.; Shi, J.; Li, X. Bitterness intensity prediction of berberine hydrochloride using an electronic tongue and a GA-BP neual network. Exp. Ther. Med. 2014, 7, 1696–1702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kovacs, Z.; Szöllősi, D.; Zaukuu, J.-L.Z.; Bodor, Z.; Vitális, F.; Aouadi, B.; Zsom-Muha, V.; Gillay, Z. Factors Influencing the Long-Term Stability of Electronic Tongue and Application of Improved Drift Correction. Biosensors 2020, 10, 74. [Google Scholar] [CrossRef]
- Liu, F.; Ghaffur, A.; Bains, J.; Hamdy, S. Acceptability of oral solid medicines in older adults with and without dysphagia: A nested pilot validation questionnaire based observational study. Int. J. Pharm. 2016, 512, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Khan, A. Optimization of the process variables of roller compaction, on the basis of granules characteristics (flow, mechanical strength, and disintegration behavior): An application of SeDeM-ODT expert system. Drug Dev. Ind. Pharm. 2019, 45, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Kadota, K.; Terada, H.; Fujimoto, A.; Nogami, S.; Uchiyama, H.; Tozuka, Y. Formulation and evaluation of bitter taste-masked orally disintegrating tablets of high memantine hydrochloride loaded granules coated with polymer via layering technique. Int. J. Pharm. 2021, 604, 120725. [Google Scholar] [CrossRef] [PubMed]
- Shiino, K.; Oshima, T.; Sonoda, R.; Kimura, S.; Itai, S.; Iwao, Y. Controlled-Release Fine Particles Prepared by Melt Adsorption for Orally Disintegrating Tablets. Chem. Pharm. Bull. 2019, 67, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Tahara, Y.; Toko, K. Study of the Relationship between Taste Sensor Response and the Amount of Epigallocatechin Gallate Adsorbed onto a Lipid-Polymer Membrane. Sensors 2015, 15, 6241–6249. [Google Scholar] [CrossRef] [PubMed]
- Altan, S.; Francois, M.; Inghelbrecht, S.; Manola, A.; Shen, Y. An Application of Serially Balanced Designs for the Study of Known Taste Samples with the Alpha-ASTREE Electronic Tongue. AAPS PharmSciTech 2014, 15, 1439–1446. [Google Scholar] [CrossRef]
- Li, Y.; Langley, N.; Zhang, J. Recent Advances in Bitterness-Sensing Systems. Biosensors 2023, 13, 414. [Google Scholar] [CrossRef]
- Kinnamon, S.C.; Finger, T.E. Recent Advances in Taste Transduction and Signaling. F1000Research 2019, 8, 2117. [Google Scholar] [CrossRef]
- Pydi, S.P.; Upadhyaya, J.; Singh, N.; Bhullar, R.P.; Chelikani, P. Recent Advances in Structure and Function Studies on Human Bitter Taste Receptors. Curr. Protein Pept. Sci. 2012, 13, 501–508. [Google Scholar] [CrossRef]
- Shigemura, N.; Ninomiya, Y. Recent Advances in Molecular Mechanisms of Taste Signaling and Modifying. Int. Rev. Cell Mol. Biol. 2016, 323, 71–106. [Google Scholar]
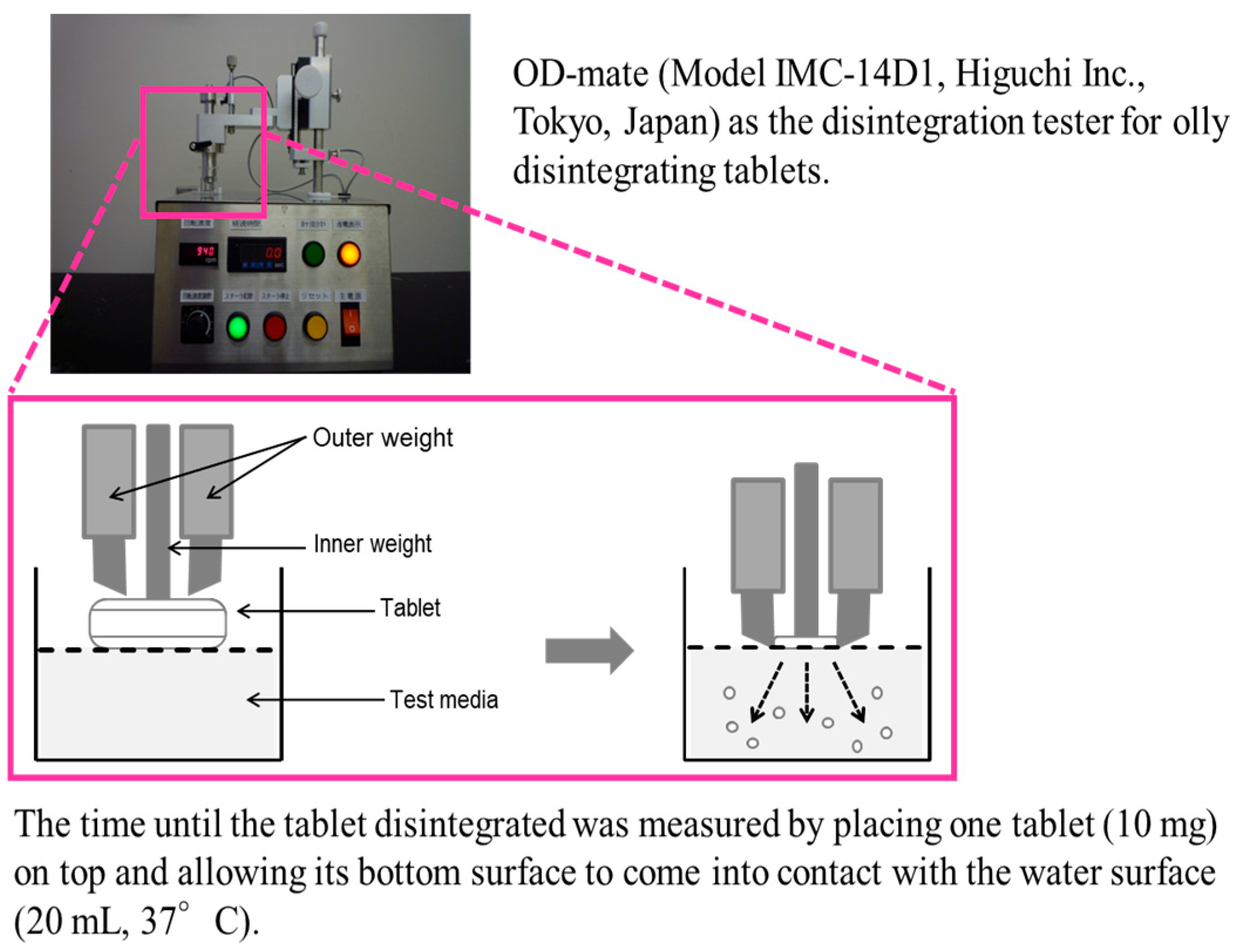
- Harada, T.; Uchida, T.; Yoshida, M.; Kobayashi, Y.; Narazaki, R.; Ohwaki, T. A new method for evaluating the bitterness of medicines in development using a taste sensor and a disintegration testing apparatus. Chem. Pharm. Bull. 2010, 58, 1009–1014. [Google Scholar] [CrossRef]
- Harada, T.; Sakurai, M.; Hondo, S.; Yasui, M.; Owaki, T. Application of taste sensor to medicines in research, development and market. Yakugaku Zasshi 2014, 134, 325–331. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uchida, T.; Yoshida, M.; Hazekawa, M.; Haraguchi, T.; Furuno, H.; Teraoka, M.; Ikezaki, H. Evaluation of palatability of 10 commercial amlodipine orally disintegrating tablets by gustatory sensation testing, OD-mate as a new disintegration apparatus and the artificial taste sensor. J. Pharm. Pharmacol. 2013, 65, 1312–1320. [Google Scholar] [CrossRef]
- Suzuki, T.; Kurano, T.; Kanazawa, T.; Suzuki, N. Establishment of a Novel Method to Evaluate Water Permeation Rates by Combining the Dynamic Contact Angle and Thermographic Approach: Significance of Disintegration Properties of Orally Disintegrating Tablets. Yakugaku Zasshi 2020, 140, 1071–1080. [Google Scholar] [CrossRef]
- Haraguchi, T.; Miyazaki, A.; Yoshida, M.; Uchida, T. Bitterness evaluation of intact and crushed Vesicare orally disintegrating tablets using taste sensors. J. Pharm. Pharmacol. 2013, 65, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Uchida, S.; Sugiura, T.; Namiki, N. The prediction of the palatability of orally disintegrating tablets by an electronic gustatory system. Int. J. Pharm. 2015, 493, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Cho, S.M.; Cui, J.H.; Cao, Q.R.; Oh, E.; Lee, B.J. In vitro and in vivo correlation of disintegration and bitter taste masking using orally disintegrating tablet containing ion exchange resin-drug complex. Int. J. Pharm. 2013, 455, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Amelian, A.; Wasilewska, K.; Wesoły, M.; Ciosek-Skibińska, P.; Winnicka, K. Taste-masking assessment of orally disintegrating tablets and lyophilisates with cetirizine dihydrochloride microparticles. Saudi Pharm. J. 2017, 25, 1144–1150. [Google Scholar] [CrossRef]
- European Pharmacopoeia 10th Edition (Ph. Eur. 10) stipulation (Chapter 2.9.1) page 939. Available online: https://www.edqm.eu/en/european-pharmacopoeia (accessed on 1 July 2024).
- Birer, M.; Acartürk, F. Electrospun orally disintegrating film formulation of telmisartan. Pharm. Dev. Technol. 2021, 26, 661–672. [Google Scholar] [CrossRef]
- Hu, J.; Fitaihi, R.; Abukhamees, S.; Abdelhakim, H.E. Formulation and Characterisation of Carbamazepine Orodispersible 3D-Printed Mini-Tablets for Paediatric Use. Pharmaceutics 2023, 15, 250. [Google Scholar] [CrossRef]
- Wesoły, M.; Kluk, A.; Sznitowska, M.; Ciosek, P.; Wróblewski, W. Influence of Experimental Conditions on Electronic Tongue Results-Case of Valsartan Minitablets Dissolution. Sensors 2016, 16, 1353. [Google Scholar] [CrossRef]
- Ekweremadu, C.S.; Abdelhakim, H.E.; Craig, D.Q.M.; Barker, S.A. Development and Evaluation of Feline Tailored Amlodipine Besylate Mini-Tablets Using L-lysine as a Candidate Flavouring Agent. Pharmaceutics 2020, 12, 917. [Google Scholar] [CrossRef]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. Performance qualification of an electronic tongue based on ICH guideline Q2. J. Pharm. Biomed. Anal. 2010, 51, 497–506. [Google Scholar] [CrossRef]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. A comparative study on two electronic tongues for pharmaceutical formulation development. J. Pharm. Biomed. Anal. 2011, 55, 272–281. [Google Scholar] [CrossRef]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. Rational development of taste masked oral liquids guided by an electronic tongue. Int. J. Pharm. 2010, 400, 114–123. [Google Scholar] [CrossRef]
- Richter, E.; Geetha, T.; Burnett, D.; Broderick, T.L.; Babu, J.R. The Effects of Momordica charantia on Type 2 Diabetes Mellitus and Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 4643. [Google Scholar] [CrossRef]
- Kamyab, R.; Namdar, H.; Torbati, M.; Ghojazadeh, M.; Araj-Khodaei, M.; Fazljou, S.M.B. Medicinal Plants in the Treatment of Hypertension: A Review. Adv. Pharm. Bull. 2021, 11, 601–617. [Google Scholar] [CrossRef]
- Kataoka, M.; Miyanaga, Y.; Tsuji, E.; Uchida, T. Evaluation of Bottled Nutritive Drinks Using a Taste Sensor. Int. J. Pharm. 2004, 279, 107–114. [Google Scholar] [CrossRef]
- Kataoka, M.; Tokuyama, E.; Miyanaga, Y.; Uchida, T. The Taste Sensory Evaluation of Medicinal Plants and Chinese Medicines. Int. J. Pharm. 2008, 351, 36–44. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Liu, R.; Wang, J.; Wu, Z.; Zhang, L.; Li, H.; Gui, X.; Kang, B.; Shi, J. Optimization and validation of the protocol used to analyze the taste of traditional Chinese medicines using an electronic tongue. Exp. Ther. Med. 2016, 12, 2949–2957. [Google Scholar] [CrossRef][Green Version]
- Jayasundar, R.; Singh, A.; Kumar, D. Challenges in using electronic tongue to study rasa of plants: I. Finding the right tool for the right job. J. Ayurveda Integr. Med. 2021, 12, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Jayasundar, R.; Ghatak, S.; Kumar, D.; Singh, A.; Bhosle, P. No ambiguity: Chemosensory-based ayurvedic classification of medicinal plants can be fingerprinted using E-tongue coupled with multivariate statistical analysis. Front. Pharmacol. 2022, 13, 1025591. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Singh, A.; Jayasundar, R.J. Challenges in using Electronic tongue to study rasa of plants: II. Impact of solvent and concentration on sensor response and taste ranking. Ayurveda Integr. Med. 2021, 12, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Baiu, I.; Spain, D.A. Enteral Nutrition. JAMA 2019, 321, 2040. [Google Scholar] [CrossRef] [PubMed]
- Warners, M.J.; Vlieg-Boerstra, B.J.; Bredenoord, A.J. Elimination and elemental diet therapy in eosinophilic oesophagitis. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 793–803. [Google Scholar] [CrossRef]
- Ohara, N.; Mizushima, T.; Iijima, H.; Takahashi, H.; Hiyama, S.; Haraguchi, N.; Inoue, T.; Nishimura, J.; Shinzaki, S.; Hata, T.; et al. Adherence to an elemental diet for preventing postoperative recurrence of Crohn’s disease. Surg. Today 2017, 47, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Anthony, T.G.; Watford, M.; Hou, Y.; Wu, G.; et al. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids 2016, 48, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Miyanaga, Y.; Tanigake, A.; Nakamura, T.; Kobayashi, Y.; Ikezaki, H.; Taniguchi, A.; Matsuyama, K.; Uchida, T. Prediction of the Bitterness of Single, Binary- and Multiple-Component Amino Acid Solutions Using a Taste Sensor. Int. J. Pharm. 2002, 248, 207–218. [Google Scholar] [CrossRef]
- Mukai, J.; Miyanaga, Y.; Ishizaka, T.; Asaka, K.; Nakai, Y.; Tsuji, E.; Uchida, T. Quantitative Taste Evaluation of Total Enteral Nutritions. Chem. Pharm. Bull. 2004, 52, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. Branched-chain amino acids and muscle protein synthesis in humans: Myth or reality? J. Int. Soc. Sports Nutr. 2017, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Adamkiewicz, L.; Szeleszczuk, Ł. Review of Applications of Cyclodextrins as Taste-Masking Excipients for Pharmaceutical Purposes. Molecules 2023, 28, 6964. [Google Scholar] [CrossRef] [PubMed]
- Preis, M.; Grother, L.; Alex, P.; Breitkreutz, J. In-vitro and in-vivo evaluation of taste-masked cetirizine hydrochloride formulated in oral lyophilisates. Int. J. Pharm. 2015, 491, 8–16. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, T. Taste Sensor Assessment of Bitterness in Medicines: Overview and Recent Topics. Sensors 2024, 24, 4799. https://doi.org/10.3390/s24154799
Uchida T. Taste Sensor Assessment of Bitterness in Medicines: Overview and Recent Topics. Sensors. 2024; 24(15):4799. https://doi.org/10.3390/s24154799
Chicago/Turabian StyleUchida, Takahiro. 2024. "Taste Sensor Assessment of Bitterness in Medicines: Overview and Recent Topics" Sensors 24, no. 15: 4799. https://doi.org/10.3390/s24154799
APA StyleUchida, T. (2024). Taste Sensor Assessment of Bitterness in Medicines: Overview and Recent Topics. Sensors, 24(15), 4799. https://doi.org/10.3390/s24154799





