Electrochemical Detection of Ammonia in Water Using NiCu Carbonate Hydroxide-Modified Carbon Cloth Electrodes: A Simple Sensing Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Instruments and Reagents
2.2. Preparation of Nickel–Copper Hydroxide Electrode
2.3. Electrochemical Measurements
3. Results
3.1. Characterization
3.2. Electrochemical Behaviors
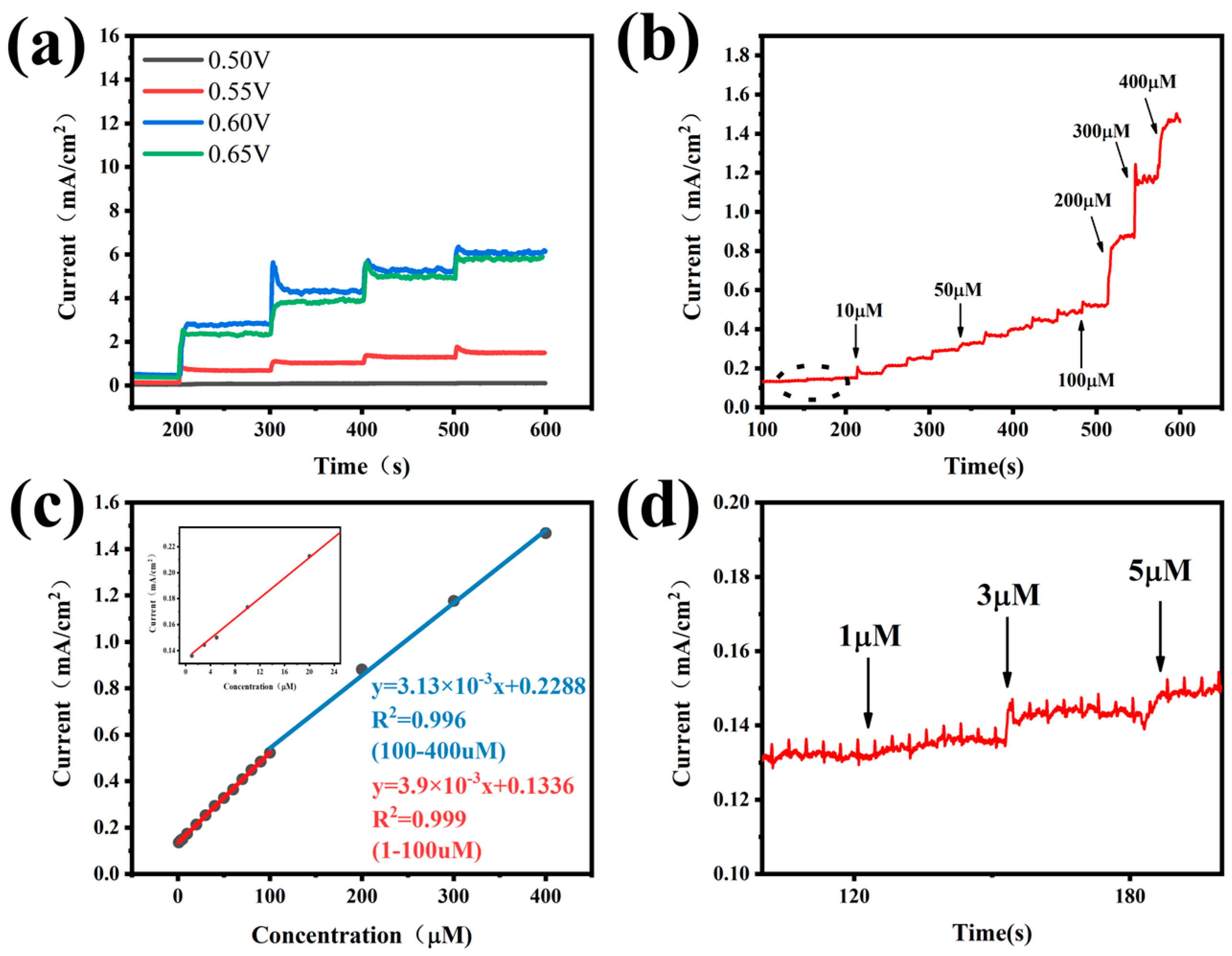
3.3. Sensitive Determination of Ammonia
3.4. Anti-Interference, Stability, Repeatability, and Real-Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, Z.F.; Yu, X.M.; Zheng, Y.Z.; Aghdam, E.; Sun, B.; Song, M.M.; Wang, A.J.; Han, J.L.; Zhang, J. Micropollutant abatement by the UV/chloramine process in potable water reuse: A review. J. Hazard. Mater. 2022, 424, 127341. [Google Scholar] [CrossRef]
- Devi, P.; Dalai, A.K. Implications of breakpoint chlorination on chloramines decay and disinfection by-products formation in brine solution. Desalination 2021, 504, 114961. [Google Scholar] [CrossRef]
- Wang, Z.; Liao, Y.F.; Li, X.W.; Shuang, C.D.; Pan, Y.; Li, Y.; Li, A.M. Effect of ammonia on acute toxicity and disinfection byproducts formation during chlorination of secondary wastewater effluents. Sci. Total Environ. 2022, 826, 153916. [Google Scholar] [CrossRef]
- Edwards, T.M.; Puglis, H.J.; Kent, D.B.; Durán, J.L.; Bradshaw, L.M.; Farag, A.M. Ammonia and aquatic ecosystems—A review of global sources, biogeochemical cycling, and effects on fish. Sci. Total Environ. 2024, 907, 167911. [Google Scholar] [CrossRef]
- Zhang, T.X.; Li, M.R.; Liu, C.; Wang, S.P.; Yan, Z.G. A review of the toxic effects of ammonia on invertebrates in aquatic environments. Environ. Pollut. 2023, 336, 122374. [Google Scholar] [CrossRef]
- Chamoli, A.; Bhambri, A.; Karn, S.K.; Raj, V. Ammonia, nitrite transformations and their fixation by different biological and chemical agents. Chem. Ecol. 2024, 40, 166–199. [Google Scholar] [CrossRef]
- Edition, F. Guidelines for drinking-water quality. WHO Chron. 2011, 38, 104–108. [Google Scholar]
- Wu, H.; Cao, A. Preparation and Adding Methods of Nessler’s Reagent Having Effects on Determination of Wa-ter Quality Ammonia Nitrogen. In Proceedings of the 2nd International Conference on Energy and Environmental Protection (ICEEP 2013), Guilin, China, 19–21 April 2013; pp. 1362–1366. [Google Scholar]
- Sahoo, P.R.; Sairam, K.; Kumar, R.; Rana, K.P.; Kumar, S. Synthesis and experimental investigations of a photoactive naphthopyran for sensing nanomolar concentration of ammonia. J. Photochem. Photobiol. A-Chem. 2024, 454, 115749. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, L.; Wu, F.; Bi, Z.; Hao, K.; Wen, Y.; Wang, D.; Fu, Z. Workplace Ammonia Determination by Ion Chromatography: Comparison of Different Sampling Tubes. J. Anal. Chem. 2022, 77, 1184–1193. [Google Scholar] [CrossRef]
- Calvo-López, A.; Rebollo-Calderon, B.; Ormazábal, A.; Artuch, R.; Rosell-Ferrer, J.; Alonso-Chamarro, J.; Puyol, M. Biomedical point-of-care microanalyzer for potentiometric determination of ammonium ion in plasma and whole blood. Anal. Chim. Acta 2022, 1205, 339782. [Google Scholar] [CrossRef]
- Darestani-Farahani, M.; Ma, F.Q.; Patel, V.; Selvaganapathy, P.R.; Kruse, P. An ion-selective chemiresistive platform as demonstrated for the detection of nitrogen species in water. Analyst 2023, 148, 5731–5744. [Google Scholar] [CrossRef]
- Nguyen, L.D.; Dang, C.M.; Doan, T.C.D. Highly stable ammonium ion-selective electrodes based on one-pot synthesized gold nanoparticle-reduced graphene oxide as ion-to-electron transducers. Microchem. J. 2023, 190, 108717. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Gu, J.L.; Zhao, X.Y.; Wang, X.Y. Facile one-step synthesis of Pt/Ni(OH)2 nanoflakes as sensitive electrode for detection of ammonia-nitrogen in drinking water. Mater. Lett. 2022, 328, 133090. [Google Scholar] [CrossRef]
- Wang, G.D.; Gao, J.; Sun, B.N.; He, D.; Zhao, C.; Suo, H. Enhanced ammonia sensitivity electrochemical sensors based on PtCu alloy nanoparticles in-situ synthesized on carbon cloth electrode. J. Electroanal. Chem. 2022, 922, 116721. [Google Scholar] [CrossRef]
- Wang, G.D.; Zhou, G.F.; Zhang, Q.Z.; He, D.; Zhao, C.; Suo, H. Sensitive Electrochemical Detection of Ammonia Nitrogen via a Platinum-Zinc Alloy Nanoflower-Modified Carbon Cloth Electrode. Sensors 2024, 24, 915. [Google Scholar] [CrossRef]
- Ahmed, M.; Zhao, R.H.; Xing, T.; Du, J.P. Constructing Netlike Nanosheets of ZnO/BiOCl with Heterojunction as Robust Material for Electrochemical Amine Detection. Chem.-A Eur. J. 2023, 29, e202202658. [Google Scholar] [CrossRef]
- Kosa, S.A.; Khan, A.N.; Al-Johani, B.; Taib, L.A.; Aslam, M.; Bawazir, W.A.; Hameed, A.; Soomro, M.T. Simple and Intelligent Electrochemical Detection of Ammonia over Cuprous Oxide Thin Film Electrode. Surfaces 2023, 6, 430–449. [Google Scholar] [CrossRef]
- Lu, H.; Hu, J.; Zhang, S.; Long, M.; Tang, A. Cuprous oxide and Ag modified titanium dioxide electrode for ultra-sensitive detection of ammonia nitrogen. J. Electroanal. Chem. 2023, 949, 117842. [Google Scholar] [CrossRef]
- Wang, C.; Wang, T.; Li, Z.; Xu, X.B.; Zhang, X.S.; Li, D.L. An Electrochemical Enzyme Biosensor for Ammonium Detection in Aquaculture Using Screen-Printed Electrode Modified by Gold Nanoparticle/Polymethylene Blue. Biosensors 2021, 11, 335. [Google Scholar] [CrossRef]
- Yang, S.F.; Zang, G.C.; Peng, Q.Y.; Fan, J.C.; Liu, Y.K.; Zhang, G.Y.; Zhao, Y.P.; Li, H.M.; Zhang, Y.C. In-situ growth of 3D rosette-like copper nanoparticles on carbon cloth for enhanced sensing of ammonia based on copper electrodissolution. Anal. Chim. Acta 2020, 1104, 60–68. [Google Scholar] [CrossRef]
- Mao, Z.; Tian, Y.; Guo, B.; Chen, R.; Zeng, Y.; Hou, F.; Yan, X.; Liang, J. Modulation of charge distribution enabling CuNi nano-alloys for efficient ammonia oxidation reaction to nitrite production. Chem. Eng. J. 2024, 484, 149570. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Diao, Y.; He, Q.; Lu, C.; Singh, A.; Kumar, A.; Liu, J.; Lan, Q. Recent advances in the electrochemical applications of Ni-based metal organic frameworks (Ni-MOFs) and their derivatives. Chemosphere 2022, 307, 135729. [Google Scholar] [CrossRef]
- Qi, H.D.; Huang, K.; Pan, F.H.K.; Ma, R.T.; Lian, C.; Liu, H.L.; Hu, J. Boosting Direct Seawater Electrolysis through Intercalation Engineering of Layered Double Hydroxides. Ind. Eng. Chem. Res. 2023, 62, 19674–19682. [Google Scholar] [CrossRef]
- Yadav, A.A.; Redekar, R.S.; Patil, K.V.; Kshirsagar, V.P.; Tarwal, N.L. Development of the nickel foam supported hydrothermally grown binder-free and highly porous magnesium-cobalt hydroxide films for supercapacitor application. J. Energy Storage 2024, 81, 110292. [Google Scholar] [CrossRef]
- Qin, X.; Teng, J.; Guo, W.; Wang, L.; Xiao, S.; Xu, Q.; Min, Y.; Fan, J. Magnetic Field Enhancing OER Electrocatalysis of NiFe Layered Double Hydroxide. Catal. Lett. 2023, 153, 673–681. [Google Scholar] [CrossRef]
- Li, B.; Dai, L.; Su, G.; Xia, Z.; Ye, Y.; Li, Z. Construction of defective MnCo-LDH nanoflowers with high activity for overall water splitting. Fuel 2024, 364, 130961. [Google Scholar] [CrossRef]
- Xu, W.; Du, D.; Lan, R.; Humphreys, J.; Miller, D.N.; Walker, M.; Wu, Z.; Irvin, J.T.S.; Tao, S. Electrodeposited NiCu bimetal on carbon paper as stable non-noble anode for efficient electrooxidation of ammonia. Appl. Catal. B-Environ. 2018, 237, 1101–1109. [Google Scholar] [CrossRef]
- Li, X.M.; Hao, X.G.; Wang, Z.D.; Abudula, A.; Guan, G.Q. In-situ intercalation of NiFe LDH materials: An efficient approach to improve electrocatalytic activity and stability for water splitting. J. Power Sources 2017, 347, 193–200. [Google Scholar] [CrossRef]
- Jiang, X.; Ying, D.W.; Liu, X.; Liu, M.C.; Zhou, S.; Guo, C.Y.; Zhao, G.H.; Wang, Y.L.; Jia, J.P. Identification of the role of Cu site in Ni-Cu hydroxide for robust and high selective electrochemical ammonia oxidation to nitrite. Electrochim. Acta 2020, 345, 136157. [Google Scholar] [CrossRef]
- Mohammadi, S.; Esmailpour, A.; Doustkhah, E.; Assadi, M.H.N. Stability Trends in Mono-Metallic 3d Layered Double Hydroxides. Nanomaterials 2022, 12, 1339. [Google Scholar] [CrossRef]
- Yang, A.Z.; Wang, J.C.; Su, K.Y.; Lei, W.; Qiu, X.Y.; Tang, Y.W. Modulating Hydroxyl-Rich Interfaces on Nickel-Copper Double Hydroxide Nanotyres to Pre-activate Alkaline Ammonia Oxidation Reactivity. Chem.-A Eur. J. 2021, 27, 4869–4875. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Wu, D.; Fu, J.; Hoque, N.A.; Ye, Y.; Xu, Z. A high-output triboelectric nanogenerator based on nickel-copper bimetallic hydroxide nanowrinkles for self-powered wearable electronics. J. Mater. Chem. A 2020, 8, 25995–26003. [Google Scholar] [CrossRef]
- Yuan, P.W.; Guo, S.H.; Gao, S.Q.; Wang, J.; Chen, W.Q.; Li, M.; Ma, K.Y.; Wang, N.; Liu, F.; Cheng, J.P. Influence of Ni/Cu ratio in nickel copper carbonate hydroxide on the phase and electrochemical properties. J. Alloys Compd. 2019, 780, 147–155. [Google Scholar] [CrossRef]
- Zheng, X.; Ye, Y.; Yang, Q.; Geng, B.; Zhang, X. Ultrafine nickel-copper carbonate hydroxide hierarchical nanowire networks for high-performance supercapacitor electrodes. Chem. Eng. J. 2016, 290, 353–360. [Google Scholar] [CrossRef]
- Pryce, M.; Just, J. Glaukosphaerite: A new nickel analogue of rosasite. Mineral. Mag. 1974, 39, 737–743. [Google Scholar] [CrossRef]
- Nickel, E.H.; Berry, L.G. The new mineral nullaginite and additional data on the related minerals rosasite and glaukosphaerite. Can. Miner. 1981, 19, 315–324. [Google Scholar]
- Hanawalt, J.; Rinn, H.; Frevel, L. Chemical analysis by X-ray diffraction. Ind. Eng. Chem. Anal. Ed. 1938, 10, 457–512. [Google Scholar] [CrossRef]
- Baby, J.N.; Stanley, M.M.; Sherlin, A.V.; George, M. Eutectic solvent mediated synthesis of carbonated CoFe-LDH nanorods: The effect of interlayer anion (Cl−, SO42−, CO32−) variants for comparing the bifunctional electrochemical sensing application. Chemosphere 2023, 315, 137716. [Google Scholar] [CrossRef]
- Deng, P.J.; Liu, Y.; Liu, H.L.; Li, X.A.; Lu, J.J.; Jing, S.Y.; Tsiakaras, P. Layered Double Hydroxides with Carbonate Intercalation as Ultra-Stable Anodes for Seawater Splitting at Ampere-Level Current Density. Adv. Energy Mater. 2024, 14, 2400053. [Google Scholar] [CrossRef]
- Mansour, A.; Melendres, C. Characterization of slightly hydrated Ni(OH)2 by XPS. Surf. Sci. Spectra 1994, 3, 247–254. [Google Scholar] [CrossRef]
- Moretti, G.; Fierro, G.; Lo Jacono, M.; Porta, P. Characterization of CuO–ZnO catalysts by X-ray photoelectron spectroscopy: Precursors, calcined and reduced samples. Surf. Interface Anal. 1989, 14, 325–336. [Google Scholar] [CrossRef]
- Talebi, P.; Greco, R.; Yamamoto, T.; Zeynali, M.; Asgharizadeh, S.; Cao, W. Hierarchical nickel carbonate hydroxide nanostructures for photocatalytic hydrogen evolution from water splitting. Mater. Adv. 2024, 5, 2968–2973. [Google Scholar] [CrossRef]
- Khan, S.I.; Motghare, R.V. Electrochemical determination of chlorophenaramine based on RTIL/CNT composite modified glassy carbon electrode in pharmaceutical samples. J. Electrochem. Soc. 2019, 166, B1202. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Butterworth-Heinemann: Oxford, UK, 1997. [Google Scholar]
- Novell-Leruth, G.; Valcarcel, A.; Pérez-Ramírez, J.; Ricart, J.M. Ammonia dehydrogenation over platinum-group metal surfaces. Structure, stability, and reactivity of adsorbed NH x species. J. Phys. Chem. C 2007, 111, 860–868. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, L.W.; Liu, J.X.; Gu, J.L.; Suo, H.; Zhao, C. One step and in situ synthesis of Ni foam-supported Pt-Ni(OH)2 nanosheets as electrochemical sensor for ammonia-nitrogen detection. Mater. Lett. 2022, 318, 132197. [Google Scholar] [CrossRef]
- Zhao, H.F.; Li, Y.; Cong, A.B.; Tong, J.H.; Bian, C. Ultramicro Interdigitated Array Electrode Chip with Optimized Construction for Detection of Ammonia Nitrogen in Water. Micromachines 2023, 14, 629. [Google Scholar] [CrossRef] [PubMed]
- Lahari, S.A.; Amreen, K.; Dubey, S.K.; Ponnalagu, R.N.; Goel, S. Modified Ultra Micro-Carbon Electrode for Efficient Ammonia Sensing for Water Quality Assessment. IEEE Trans. Nanobiosci. 2023, 22, 301–307. [Google Scholar] [CrossRef]
- Wang, J.Y.; Diao, P. Simultaneous detection of ammonia and nitrate using a modified electrode with two regions. Microchem. J. 2020, 154, 104649. [Google Scholar] [CrossRef]
- Wang, G.; Ma, G.; Gao, J.; He, D.; Zhao, C.; Suo, H. Enhanced Sensitivity of Electrochemical Sensors for Ammonia-Nitrogen via In-Situ Synthesis PtNi Nanoleaves on Carbon Cloth. Sensors 2024, 24, 387. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Peng, X.; Cui, Q.; He, D.; Zhao, C.; Suo, H. Fabrication of a Ni foam-supported platinum nanoparticles-silver/polypyrrole electrode for aqueous ammonia sensing. Synth. Met. 2020, 259, 116257. [Google Scholar] [CrossRef]







| Electrode Materials | Linear Range (μM) | Sensitivity (μA μM−1) | LOD | Method | Ref. |
|---|---|---|---|---|---|
| Cu NPs/CC | 5–9425 | 0.0062 | 1.25 μM | i-t | [21] |
| Pt-Ni(OH)2 | 0.05–600 | 0.191 | 39.2 nM | DPV | [47] |
| Pt-Ni(OH)2-NF | 5–1000 | 1.79 | 2.3 μM | DPV | [14] |
| MEMS | 8.82–117.65 | 0.4181 | / | DPV | [48] |
| UME/MWCNT | 10–60 | 0.049 | 8.69 μM | i-t | [49] |
| ZnO/BiOCl | 200–1000 | 11.8 | 0.25 μM | CV | [17] |
| Cu2O/GCE | 10–1000 | 0.05 | 6.23 μM | SWV | [18] |
| Pt/ITO | / | / | 3.9 μM | CV | [50] |
| SPEC/AuNPs/PMB | 0.65–300 | / | 0.65 μM | i-t | [20] |
| NiCu DHTs | / | 5.3 | 9 μM | i-t | [32] |
| PtCu/CC | 0.5–40 | 9.5 | 8.6 nM | DPV | [15] |
| 40–500 | 0.778 mA lg μM−1 cm−2 | ||||
| PtNi/CC | 0.5–500 | 7.83 | 24 nM | DPV | [51] |
| PtZn/CC | 1–1000 | 21.5 | 27.81 nM | DPV | [16] |
| Pt/Ag/ppy/Ni foam | 0.1–100 | 89 | 37 nM | LSV | [52] |
| (Ni,Cu)2(OH)2CO3/CC | 1–100 | 3.9 | 0.62 μM | i-t | this work |
| 100–400 | 3.1 |
| Sample | Initial (μM) | Added (μM) | Found (μM) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|---|
| Tap water | 0 | 20 | 20.50 | 102.5 | 2.0 |
| 50 | 52.94 | 105.9 | 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, G.; Wang, G.; Zhao, X.; He, D.; Zhao, C.; Suo, H. Electrochemical Detection of Ammonia in Water Using NiCu Carbonate Hydroxide-Modified Carbon Cloth Electrodes: A Simple Sensing Method. Sensors 2024, 24, 4824. https://doi.org/10.3390/s24154824
Zhou G, Wang G, Zhao X, He D, Zhao C, Suo H. Electrochemical Detection of Ammonia in Water Using NiCu Carbonate Hydroxide-Modified Carbon Cloth Electrodes: A Simple Sensing Method. Sensors. 2024; 24(15):4824. https://doi.org/10.3390/s24154824
Chicago/Turabian StyleZhou, Guangfeng, Guanda Wang, Xing Zhao, Dong He, Chun Zhao, and Hui Suo. 2024. "Electrochemical Detection of Ammonia in Water Using NiCu Carbonate Hydroxide-Modified Carbon Cloth Electrodes: A Simple Sensing Method" Sensors 24, no. 15: 4824. https://doi.org/10.3390/s24154824






