Monitoring Two Typical Marine Zooplankton Species Using Acoustic Methods in the South China Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Process of Acoustic Data
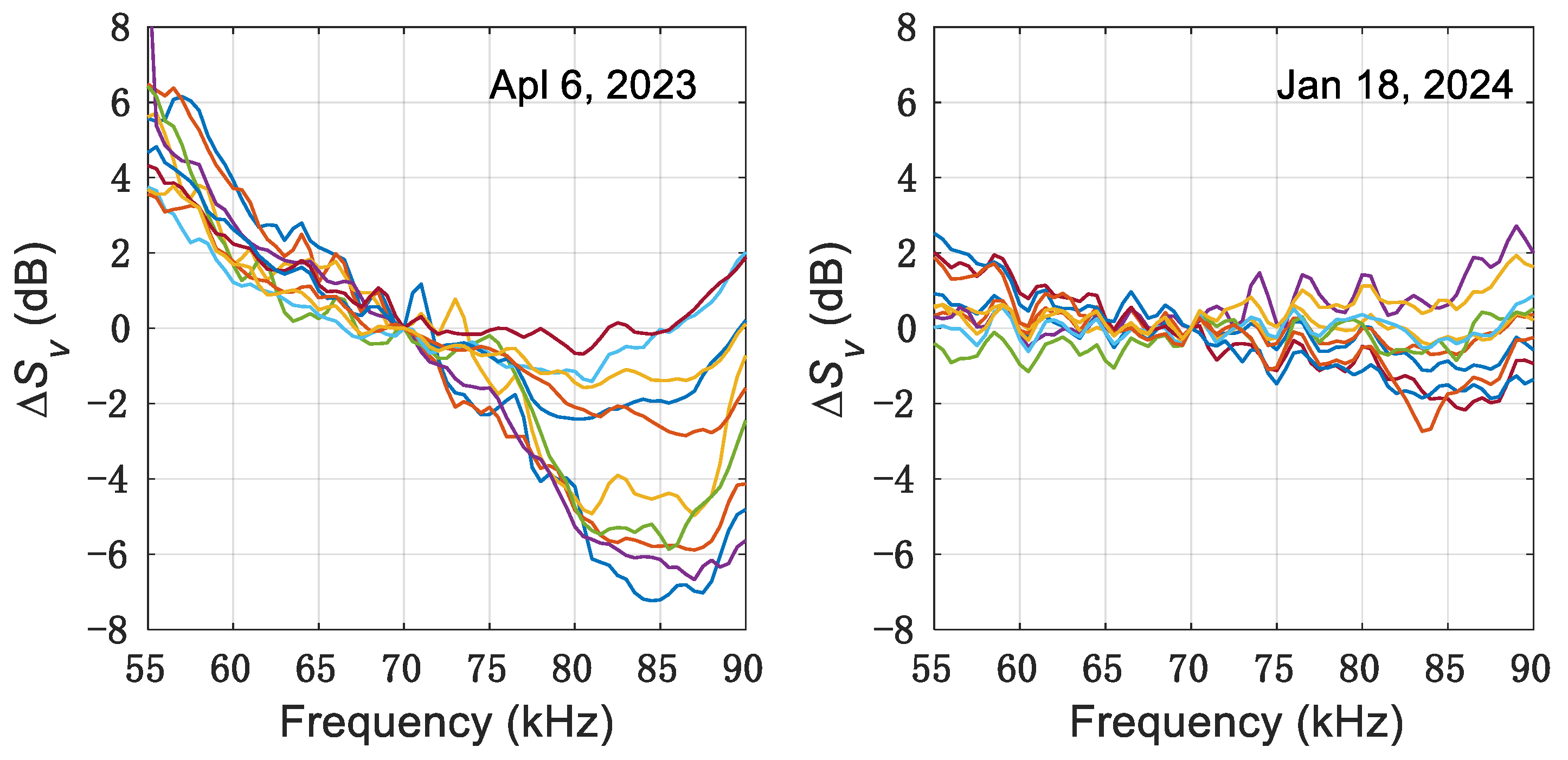
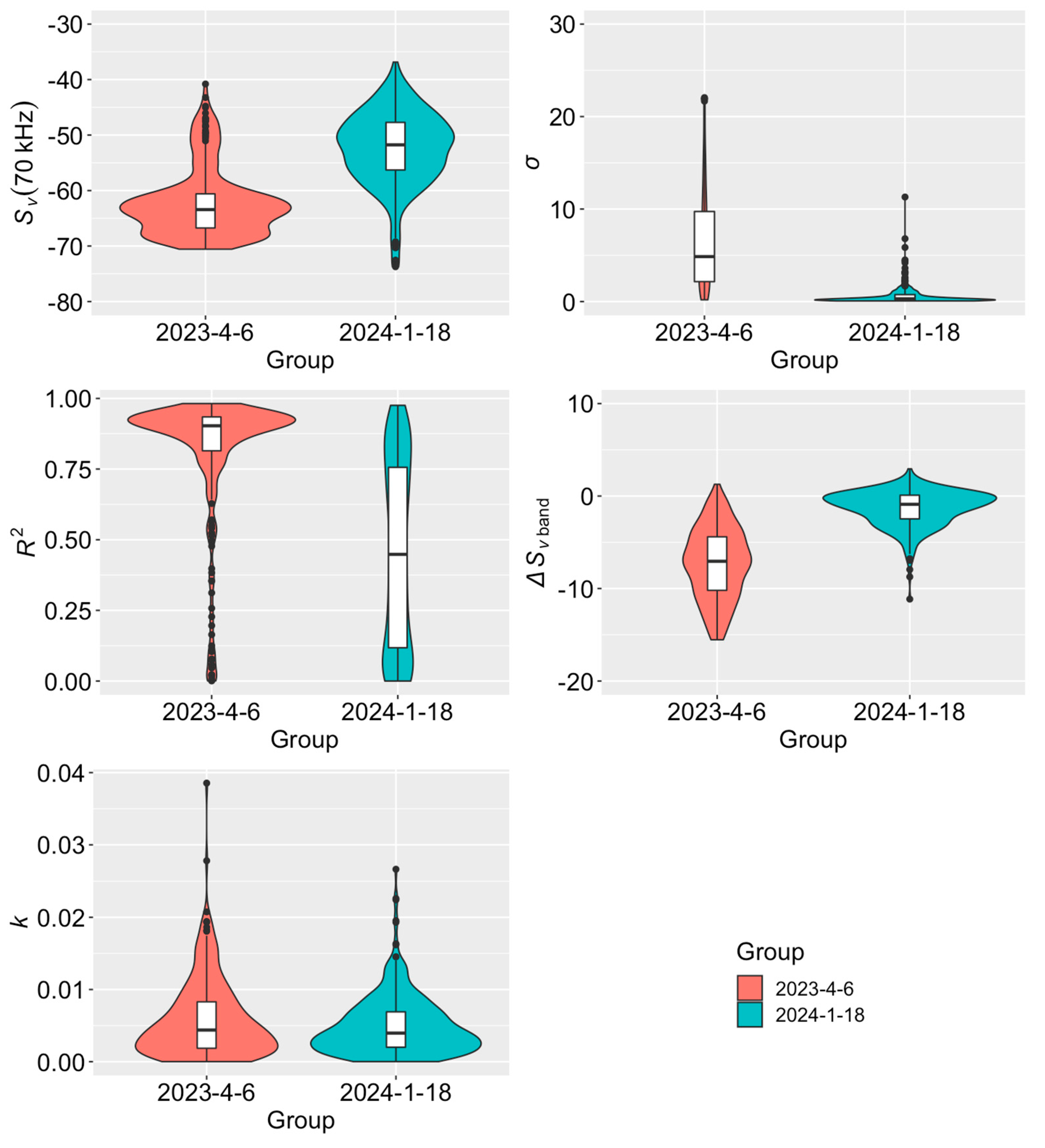
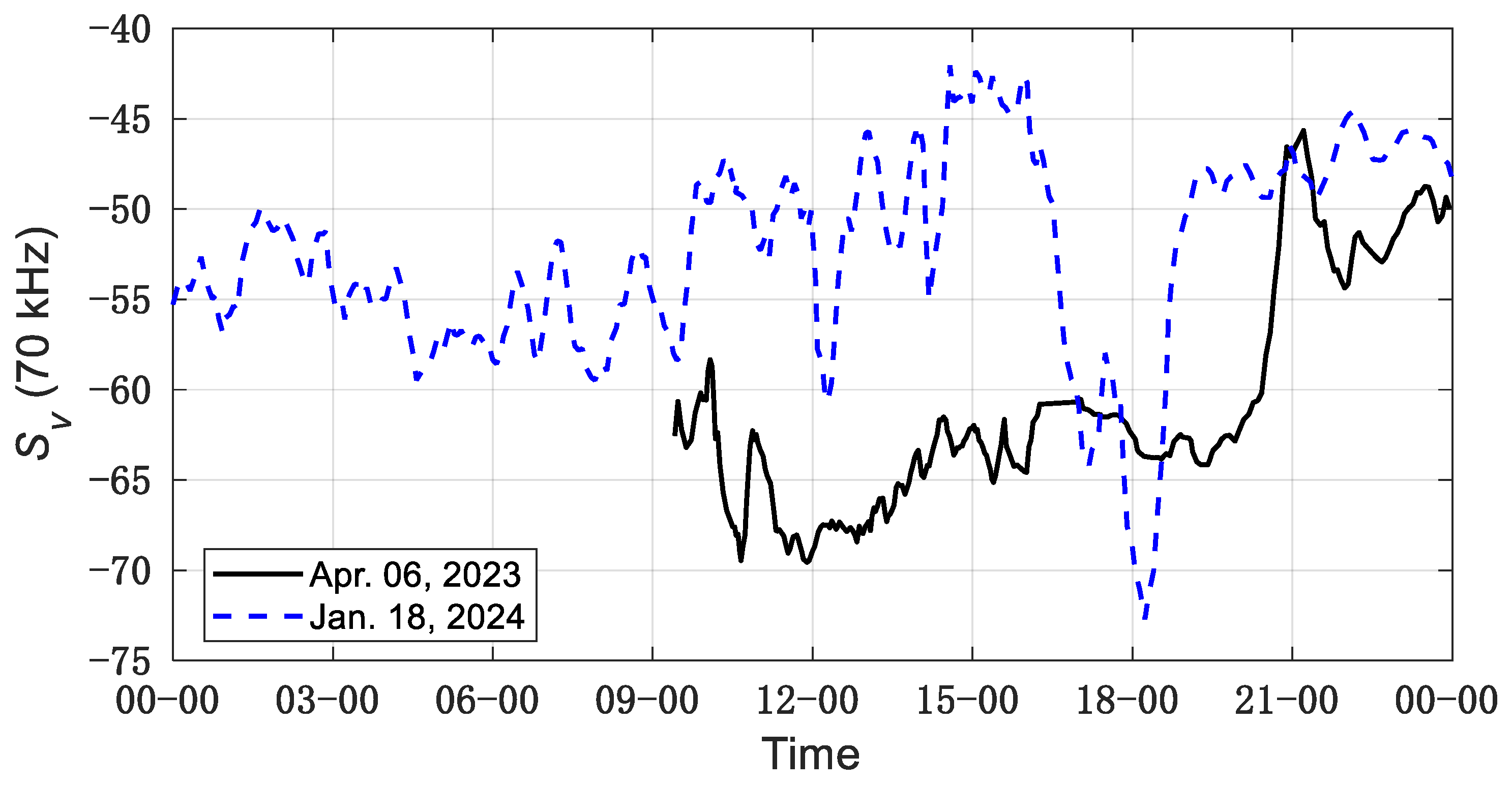
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omori, M. The Systematics, Biogeography, and Fishery of Epipelagic Shrimps of the Genus Acetes (Crustacea, Decapoda, Sergestidae); Ocean Research Institute, University of Tokyo: Tokyo, Japan, 1975. [Google Scholar]
- Oh, C.-W.; Jeong, I.-J. Reproduction and population dynamics of Acetes chinensis (Decapoda: Sergestidae) on the western coast of Korea, Yellow Sea. J. Crustac. Biol. 2003, 23, 827–835. [Google Scholar] [CrossRef]
- Liu, J.Y. Status of marine biodiversity of the China Seas. PLoS ONE 2013, 8, e50719. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Uye, S.I.; Burnett, J.; Mianzan, H. Stings of edible jellyfish (Rhopilema hispidum, Rhopilema esculentum and Nemopilema nomurai) in Japanese waters. Toxicon 2006, 48, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Suthers, I.; Bowling, L.; Kobayashi, T.; Rissik, D. Sampling methods for plankton. Plankton A Guide Their Ecol. Monit. Water Qual. 2009, 22, 73–114. [Google Scholar]
- Briseño-Avena, C.; Roberts, P.L.D.; Franks, P.J.S.; Jaffe, J.S. ZOOPS-O2: A broadband echosounder with coordinated stereo optical imaging for observing plankton in situ. Methods Oceanogr. 2015, 12, 36–54. [Google Scholar] [CrossRef]
- Cimino, M.A.; Patris, S.; Ucharm, G.; Bell, L.J.; Terrill, E. Jellyfish distribution and abundance in relation to the physical habitat of Jellyfish Lake, Palau. J. Trop. Ecol. 2018, 34, 17–31. [Google Scholar] [CrossRef]
- Thomas, G.L.; Kirsch, J. Nekton and plankton acoustics: An overview. Fish. Res. 2000, 47, 107–113. [Google Scholar] [CrossRef]
- Hariyanto, I.H.; Putranto, A.W.; Purwanto, B.; Sobarudin, D.P.; Saputro, P.D.; Khair, D.R.; Wibowo, M.A. Volume Backscattering Strength Estimation of Plankton from Water Column Multibeam Echosounder Data at Alor Strait, East Nusa Tenggara, Indonesia; IOP Publishing: Bristol, UK, 2023; p. 012065. [Google Scholar]
- Godlewska, M.; Balk, H.; Izydorczyk, K.; Kaczkowski, Z.; Mankiewicz-Boczek, J.; Ye, S. Rapid in situ assessment of high-resolution spatial and temporal distribution of cyanobacterial blooms using fishery echosounder. Sci. Total Environ. 2023, 857, 159492. [Google Scholar] [CrossRef]
- Simmonds, J.; MacLennan, D.N. Fisheries Acoustics: Theory and Practice; Blackwell Science: Oxford, UK, 2005. [Google Scholar]
- Hannachi, M.S.; Abdallah, L.B.; Marrakchi, O. Acoustic identification of small-pelagic fish species: Target strength analysis and school descriptor classification. MedSudMed Tech. Doc. 2004, 5, 90–99. [Google Scholar]
- Fernandes, P.G. Classification trees for species identification of fish-school echotraces. ICES J. Mar. Sci. 2009, 66, 1073–1080. [Google Scholar] [CrossRef]
- Korneliussen, R.J.; Heggelund, Y.; Eliassen, I.K.; Johansen, G.O. Acoustic species identification of schooling fish. ICES J. Mar. Sci. 2009, 66, 1111–1118. [Google Scholar] [CrossRef]
- Slonimer, A.L.; Dosso, S.E.; Albu, A.B.; Cote, M.; Marques, T.P.; Rezvanifar, A.; Ersahin, K.; Mudge, T.; Gauthier, S. Classification of Herring, Salmon, and Bubbles in Multifrequency Echograms Using U-Net Neural Networks. IEEE J. Ocean. Eng. 2023, 48, 1236–1254. [Google Scholar] [CrossRef]
- Stanton, T.K.; Chu, D.; Wiebe, P.H. Acoustic scattering characteristics of several zooplankton groups. ICES J. Mar. Sci. 1996, 53, 289–295. [Google Scholar] [CrossRef]
- Brierley, A.S.; Ward, P.; Watkins, J.L.; Goss, C. Acoustic discrimination of Southern Ocean zooplankton. Deep Sea Res. Part II: Top. Stud. Oceanogr. 1998, 45, 1155–1173. [Google Scholar] [CrossRef]
- Mutlu, E. Acoustical identification of the concentration layer of a copepod species, Calanus euxinus. Mar. Biol. 2003, 142, 517–523. [Google Scholar] [CrossRef]
- Mutlu, E. Acoustical (Echosounder and ADCP) observation of Calanus euxinus and Sagitta setosa in the Black. Hydroacoustics 2004, 7, 163–172. [Google Scholar]
- Lavery, A.C.; Chu, D.; Moum, J.N. Measurements of acoustic scattering from zooplankton and oceanic microstructure using a broadband echosounder. ICES J. Mar. Sci. 2010, 67, 379–394. [Google Scholar] [CrossRef]
- Ono, A.; Hashihama, F.; Amakasu, K.; Moteki, M. Estimated ammonium regeneration potentials of two common euphausiid species (Euphausia superba and E. crystallorophias) off Adélie Land, East Antarctica, in austral summer, 2008. Polar Biol. 2022, 45, 1523–1528. [Google Scholar] [CrossRef]
- Benoit-Bird, K.J.; Waluk, C.M. Exploring the promise of broadband fisheries echosounders for species discrimination with quantitative assessment of data processing effects. J. Acoust. Soc. Am. 2020, 147, 411–427. [Google Scholar] [CrossRef]
- Demer, D.A.; Berger, L.; Bernasconi, M.; Bethke, E.; Boswell, K.; Chu, D.; Domokos, R.; Dunford, A.; Fassler, S.; Gauthier, S. Calibration of Acoustic Instruments; ICES Cooperative Research Reports (CRR); International Council for the Exploration of the Sea (ICES): Copenhagen, Denmark, 2015. [Google Scholar]
- Demer, D.A.; Andersen, L.N.; Bassett, C.; Berger, L.; Chu, D.; Condiotty, J.; Hutton, B.; Korneliussen, R.; Bouffant, N.L.; Macaulay, G. 2016 USA–Norway EK80 Workshop Report: Evaluation of a Wideband Echosounder for Fisheries and Marine Ecosystem Science; ICES Cooperative Research Reports (CRR); International Council for the Exploration of the Sea (ICES): Copenhagen, Denmark, 2017. [Google Scholar]
- Ratliff, H. Curvature, Circumradius, and Circumcenter Formulas for any Three Points. Available online: https://hratliff.com/posts/2019/02/curvature-of-three-points/ (accessed on 4 February 2019).
- Bassett, C.; De Robertis, A.; Wilson, C.D. Broadband echosounder measurements of the frequency response of fishes and euphausiids in the Gulf of Alaska. ICES J. Mar. Sci. 2018, 75, 1131–1142. [Google Scholar] [CrossRef]
- Kahn, R.E.; Lavery, A.C.; Govindarajan, A.F. Broadband backscattering from scyphozoan jellyfish. J. Acoust. Soc. Am. 2023, 153, 3075. [Google Scholar] [CrossRef] [PubMed]
- Korneliussen, R.J. Acoustic Target Classification; ICES Cooperative Research Reports (CRR); International Council for the Exploration of the Sea (ICES): Copenhagen, Denmark, 2018. [Google Scholar]
- Reid, D.G. Report on Echo Trace Classification; ICES Cooperative Research Reports (CRR); International Council for the Exploration of the Sea (ICES): Copenhagen, Denmark, 2000. [Google Scholar]
- Haralabous, J.; Georgakarakos, S. Artificial neural networks as a tool for species identification of fish schools. ICES J. Mar. Sci. 1996, 53, 173–180. [Google Scholar] [CrossRef]
- McGehee, D.E.; O’Driscoll, R.L.; Traykovski, L.V.M. Effects of orientation on acoustic scattering from Antarctic krill at 120 kHz. Deep Sea Res. Part II Top. Stud. Oceanogr. 1998, 45, 1273–1294. [Google Scholar] [CrossRef]

| Settings | Values |
|---|---|
| Transducer type | ES70-70 |
| Nominal frequency (kHz) | 70 |
| Pulse duration (ms) | 1.024 |
| Transmit electric power (W) | 525 |
| Transmit signal type | LFM |
| Transmit frequency range (kHz) | 55–90 |
| Ramping | Fast |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Tang, Y. Monitoring Two Typical Marine Zooplankton Species Using Acoustic Methods in the South China Sea. Sensors 2024, 24, 4827. https://doi.org/10.3390/s24154827
Liu J, Tang Y. Monitoring Two Typical Marine Zooplankton Species Using Acoustic Methods in the South China Sea. Sensors. 2024; 24(15):4827. https://doi.org/10.3390/s24154827
Chicago/Turabian StyleLiu, Jing, and Yong Tang. 2024. "Monitoring Two Typical Marine Zooplankton Species Using Acoustic Methods in the South China Sea" Sensors 24, no. 15: 4827. https://doi.org/10.3390/s24154827
APA StyleLiu, J., & Tang, Y. (2024). Monitoring Two Typical Marine Zooplankton Species Using Acoustic Methods in the South China Sea. Sensors, 24(15), 4827. https://doi.org/10.3390/s24154827






