Mo-Doped LaFeO3 Gas Sensors with Enhanced Sensing Performance for Triethylamine Gas
Abstract
:1. Introduction
2. Experiment
2.1. Preparation of Mo-Doped LaFeO3 Samples
2.2. Characterizations of Gas-Sensitive Materials
2.3. Preparation of Gas-Sensitive Elements
2.4. Gas-Sensing Test
3. Results and Discussion
3.1. Characterizations of Sensing Materials
3.2. Gas-Sensing Performance
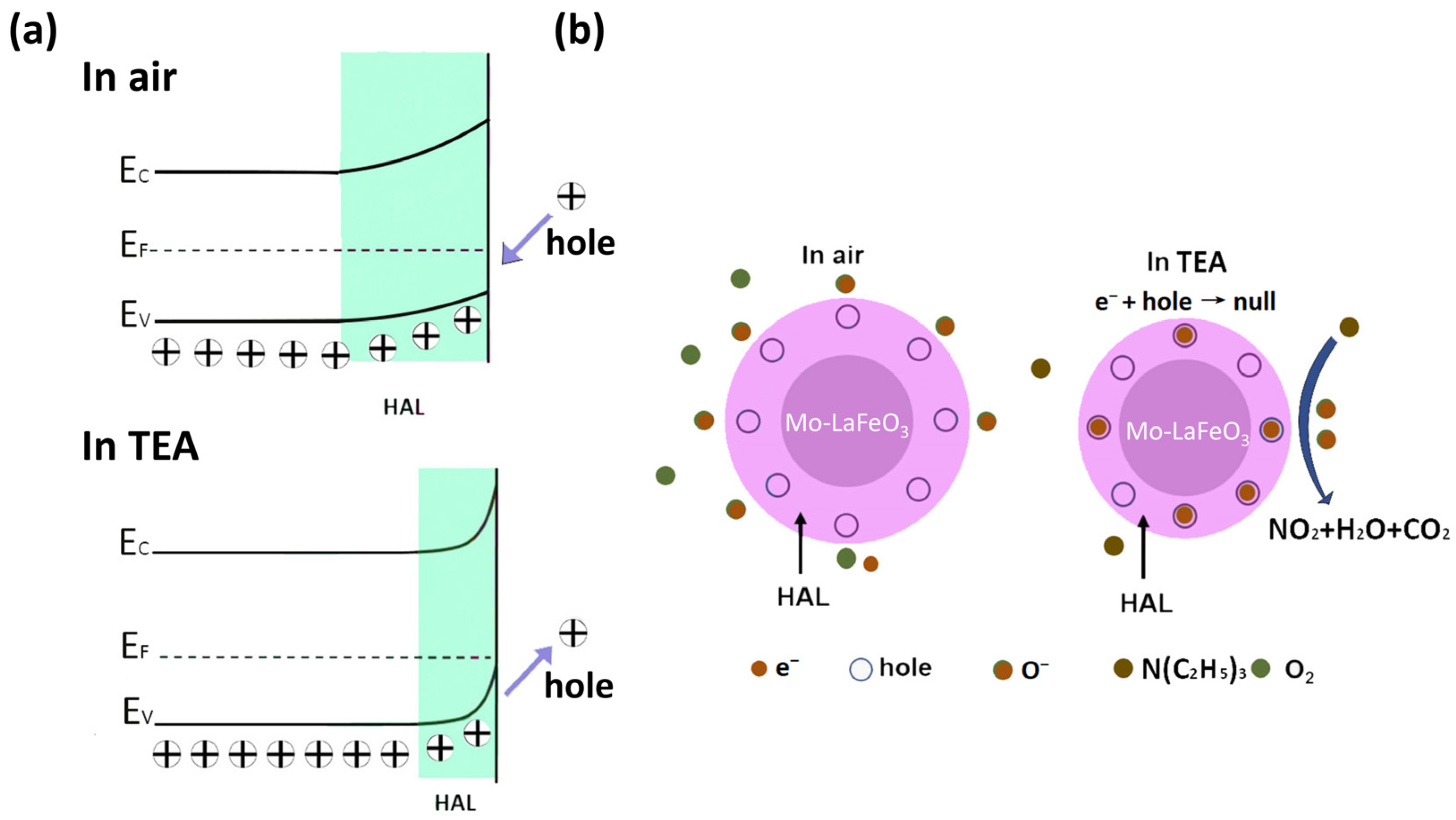
3.3. Gas-Sensing Mechanism
- (1)
- In the LaFeO3 lattice, the doping of Mo leads to distortion of the LaFeO3 lattice due to the difference in radii, which introduces defects. The presence of lattice defects provides more activation sites for the sensing reaction, which helps to improve the response value and selectivity [41];
- (2)
- The synergistic effect of morphology and surface composition is threefold. Firstly, the porous nanosheet structure, with its large specific surface area, exposes more reactive active sites, promoting the diffusion of TEA molecules and accelerating the adsorption–desorption rate of the target gas. Secondly, a higher content of adsorbed oxygen on the surface leads to the exposure of more active sites. Finally, the presence of oxygen vacancies on the material’s surface alters the electronic state of the metal cations and provides active sites for the gas-sensing process [32].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, S. Amorphous CoSnO3 for conductometric triethylamine gas sensing. Sens. Actuat. B-Chem. 2024, 401, 135086. [Google Scholar] [CrossRef]
- Li, X. Simple Thermocatalytic Oxidation Degradation of VOCs. Catal. Lett. 2022, 152, 1801–1818. [Google Scholar] [CrossRef]
- Duan, X. Enhancing the carbon dioxide sensing performance of LaFeO3 by Co doping. Sens. Actuat. B-Chem. 2024, 402, 135136. [Google Scholar] [CrossRef]
- Wu, M.; Chen, S.; Xiang, W. Oxygen vacancy induced performance enhancement of toluene catalytic oxidation using LaFeO3 perovskite oxides. Chem. Eng. J. 2020, 387, 124101. [Google Scholar] [CrossRef]
- Al-Hashem, M.; Sheikh, A.; Patricia, M. Role of Oxygen Vacancies in Nanostructured Metal-Oxide Gas Sensors: A Review. Sens. Actuat. B-Chem. 2019, 301, 126845. [Google Scholar] [CrossRef]
- Xiao, C. Formaldehyde gas sensor with 1 ppb detection limit based on In-doped LaFeO3 porous structure. Sens. Actuat. B-Chem. 2022, 371, 132558. [Google Scholar] [CrossRef]
- Gu, J. Synthesis of spindle-like Co-doped LaFeO3 porous microstructure for high performance n-butanol sensor. Sens. Actuat. B-Chem. 2021, 343, 130125. [Google Scholar] [CrossRef]
- Liu, J. Semiconductor Gas Sensor for Triethylamine Detection. Small 2022, 18, 2104984. [Google Scholar] [CrossRef]
- Zhang, J. LaMnO3/Co3O4 nanocomposite for enhanced triethylamine sensing properties. J. Rare Earths 2023, 42, 733–742. [Google Scholar] [CrossRef]
- Shuai, Y. NiO/BiVO4 p-n heterojunction microspheres for conductometric triethylamine gas sensors. Sens. Actuat. B-Chem. 2023, 384, 133625. [Google Scholar] [CrossRef]
- Zhao, C. Ultra-efficient trimethylamine gas sensor based on Au nanoparticles sensitized WO3 nanosheets for rapid assessment of seafood freshness. Food Chem. 2022, 392, 133318. [Google Scholar] [CrossRef] [PubMed]
- Wang, T. Construction of Zn2SnO4 decorated ZnO nanoparticles for sensing triethylamine with dramatically enhanced performance. Mater. Sci. Semicond. Process. 2022, 140, 106403. [Google Scholar] [CrossRef]
- Meng, D. In-situ growth of ordered Pd-doped ZnO nanorod arrays on ceramic tube with enhanced trimethylamine sensing performance. Appl. Surf. Sci. 2019, 463, 348–356. [Google Scholar] [CrossRef]
- Ma, C. Mixed-potential type triethylamine sensor based on NASICON utilizing SmMO3 (M = Al, Cr, Co) sensing electrodes. Sens. Actuat B-Chem. 2019, 284, 110–117. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, B.; Zhang, L. 0D/2D CdS/ZnO composite with n-n heterojunction for efficient detection of triethylamine. J. Colloid Interface Sci. 2021, 600, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Unraveling the high catalytic activity of single atom Mo-doped TiO2 toward NH3-SCR: Synergetic roles of Mo as acid sites and oxygen vacancies as oxidation sites. Chem. Eng. J. 2023, 465, 142759. [Google Scholar] [CrossRef]
- Ren, Z. Remarkable formaldehyde photo-oxidation efficiency of Zn2SnO4 co-modified by Mo doping and oxygen vacancies. Sep. Purif. Technol. 2023, 310, 123202. [Google Scholar] [CrossRef]
- Li, J. Significantly enhanced surface oxygen vacancies over W18O49 via Mo doping and plasma-induced surface reconstruction for oxidative desulfurization. Sep. Purif. Technol. 2023, 318, 123948. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, M.; Zhang, H. Room temperature gas sensor based on rGO/Bi2S3 heterostructures for ultrasensitive and rapid NO2 detection. Chem. Eng. J. 2024, 490, 151872. [Google Scholar] [CrossRef]
- Zhang, J. Facile strategy to synthesize porous GO/ZnO heterostructure for enhanced acetone gas sensing properties. Sens. Actuat. B-Chem. 2022, 359, 131601. [Google Scholar] [CrossRef]
- Qiao, X. Mo doped BiVO4 gas sensor with high sensitivity and selectivity towards H2S. Chem. Eng. J. 2020, 395, 125144. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L. Susceptible CoSnO3 nanoboxes with p-type response for triethylamine detection at low temperature. Crystengcomm 2020, 22, 2795–2805. [Google Scholar] [CrossRef]
- Liu, W. Engineering α-MoO3/TiO2 heterostructures derived from MOFs/MXene hybrids for high-performance triethylamine sensor. Chem. Eng. J. 2024, 483, 149340. [Google Scholar] [CrossRef]
- Souri, M.; Amoli, H.S. Gas sensing mechanisms in ABO3 perovskite materials at room temperature: A review. Mater. Sci. Semicond. Process. 2023, 156, 107271. [Google Scholar] [CrossRef]
- Lin, Z. The effect of Ni doping concentration on the gas sensing properties of Ni doped SnO2. Sens. Actuat. B-Chem. 2017, 239, 501–510. [Google Scholar] [CrossRef]
- Tian, R. Advanced triethylamine sensor utilizing 3D microspheres of La-doped MoO3: Performance enhancement and mechanism insights. Sens. Actuat. B-Chem. 2024, 412, 135817. [Google Scholar] [CrossRef]
- Liu, H. Innovative development on a p-type delafossite CuCrO2 nanoparticles based triethylamine sensor. Sens. Actuat. B-Chem. 2020, 324, 128743. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, Y.; Gu, J. Optimizing water electrolysis activity in Mo-doped Sr2FeCoO6−δ perovskites by balancing oxygen vacancies and structural stability. J. Power Sources 2024, 592, 233928. [Google Scholar] [CrossRef]
- Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Shen, Y. Enhanced peroxymonosulfate activation in the morphotropic phase boundary of molybdenum doped LaCoO3−δ perovskite. Chem. Eng. J. 2022, 446, 137352. [Google Scholar] [CrossRef]
- Zhang, G. LnFeO3 (Ln = La, Nd, Sm) derived from bimetallic organic frameworks for gas sensor. J. Alloys Compd. 2022, 902, 163803. [Google Scholar] [CrossRef]
- Wang, S. Oxygen-vacancy-mediated LaFe1−xMnxO3−δ perovskite nanocatalysts for degradation of organic pollutants through enhanced surface ozone adsorption and metal doping effects. Nanoscale 2021, 13, 12874–12884. [Google Scholar] [CrossRef] [PubMed]
- Bai, J. Redox effects on the structure and properties of Na-Mo-Fe-phosphate glasses. J. Non-Cryst. Solids 2021, 557, 120573. [Google Scholar] [CrossRef]
- Zhang, Y. Synergistic Effect of Electron Scattering and Space Charge Transfer Enabled Unprecedented Room Temperature NO2 Sensing Response of SnO2. Small 2023, 19, 2303631. [Google Scholar] [CrossRef] [PubMed]
- Yang, X. Enhanced gas sensing properties of monodisperse Zn2SnO4 octahedron functionalized by PdO nanoparticals. Sens. Actuat. B-Chem. 2018, 266, 302–310. [Google Scholar] [CrossRef]
- Liu, M. Highly sensitive and selective glycol gas sensor based on SmFeO3 microspheres. Ceram. Int. 2023, 49, 1108–1113. [Google Scholar] [CrossRef]
- Cheng, Z. Methane adsorption and dissociation on iron oxide oxygen carriers: The role of oxygen vacancies. Phys. Chem. Chem. Phys. 2016, 18, 16423–16435. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.S. Improved performance of triethylamine sensors through defect formation in La-doped MoO3 nanorods. Sens. Actuat. A-Phys. 2024, 366, 114975. [Google Scholar] [CrossRef]
- Liu, T. In-doped ZnO/NiO nanosheet as highly selective triethylamine sensor. J. Mater. Res. 2023, 38, 4747–4758. [Google Scholar] [CrossRef]
- Souri, M.; Amoli, H.S.; Yadolla, Y. Three-dimensionally ordered porous In-doped SmFeO3 perovskite gas sensor for highly sensitive and selective detection of formaldehyde. Sens. Actuat. B-Chem. 2024, 404, 135213. [Google Scholar] [CrossRef]
- Zhang, Y. Formaldehyde-sensing properties of LaFeO3 particles synthesized by citrate sol–gel method. J. Sol-Gel Sci. Technol. 2016, 79, 167–175. [Google Scholar] [CrossRef]
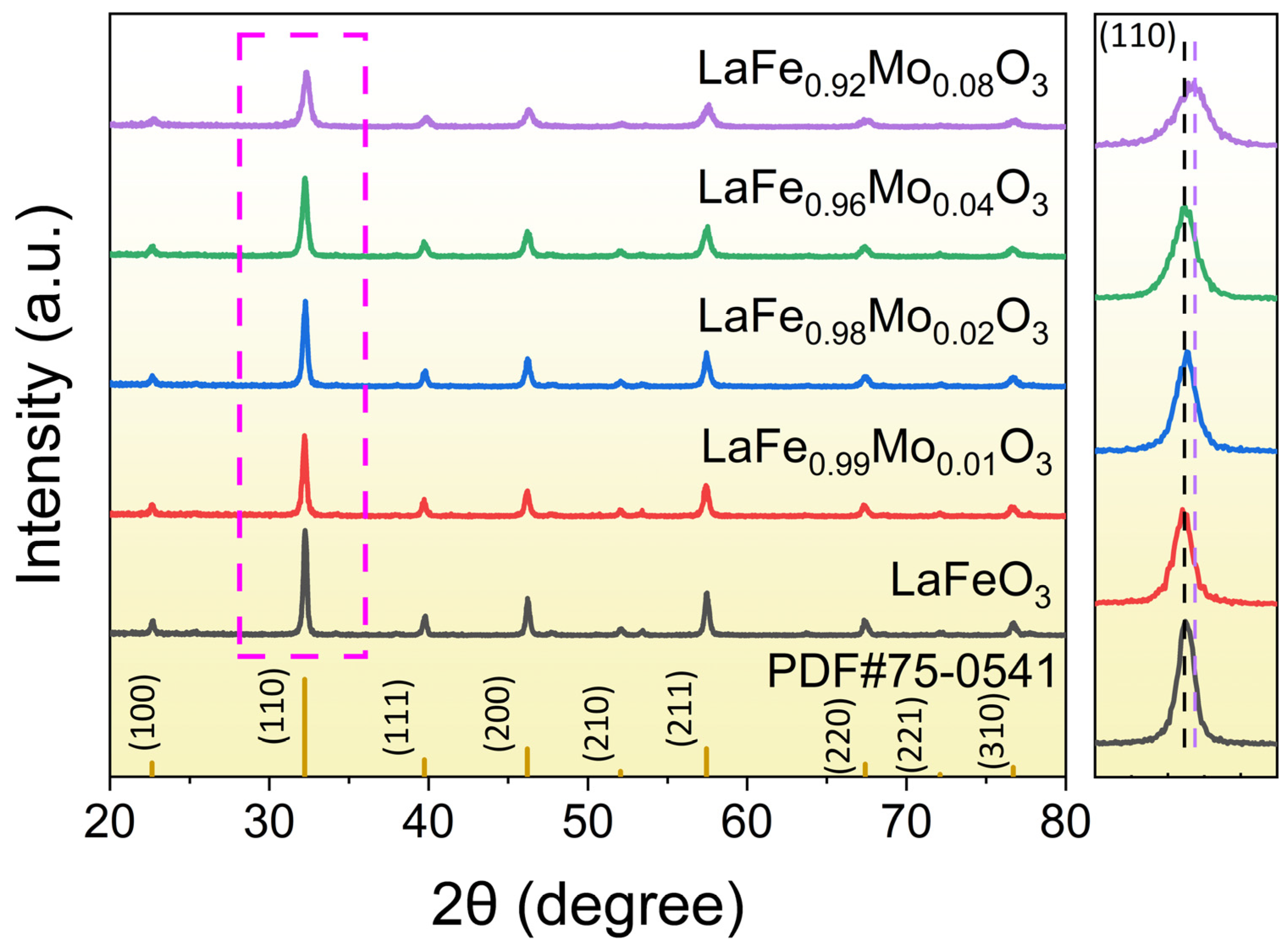



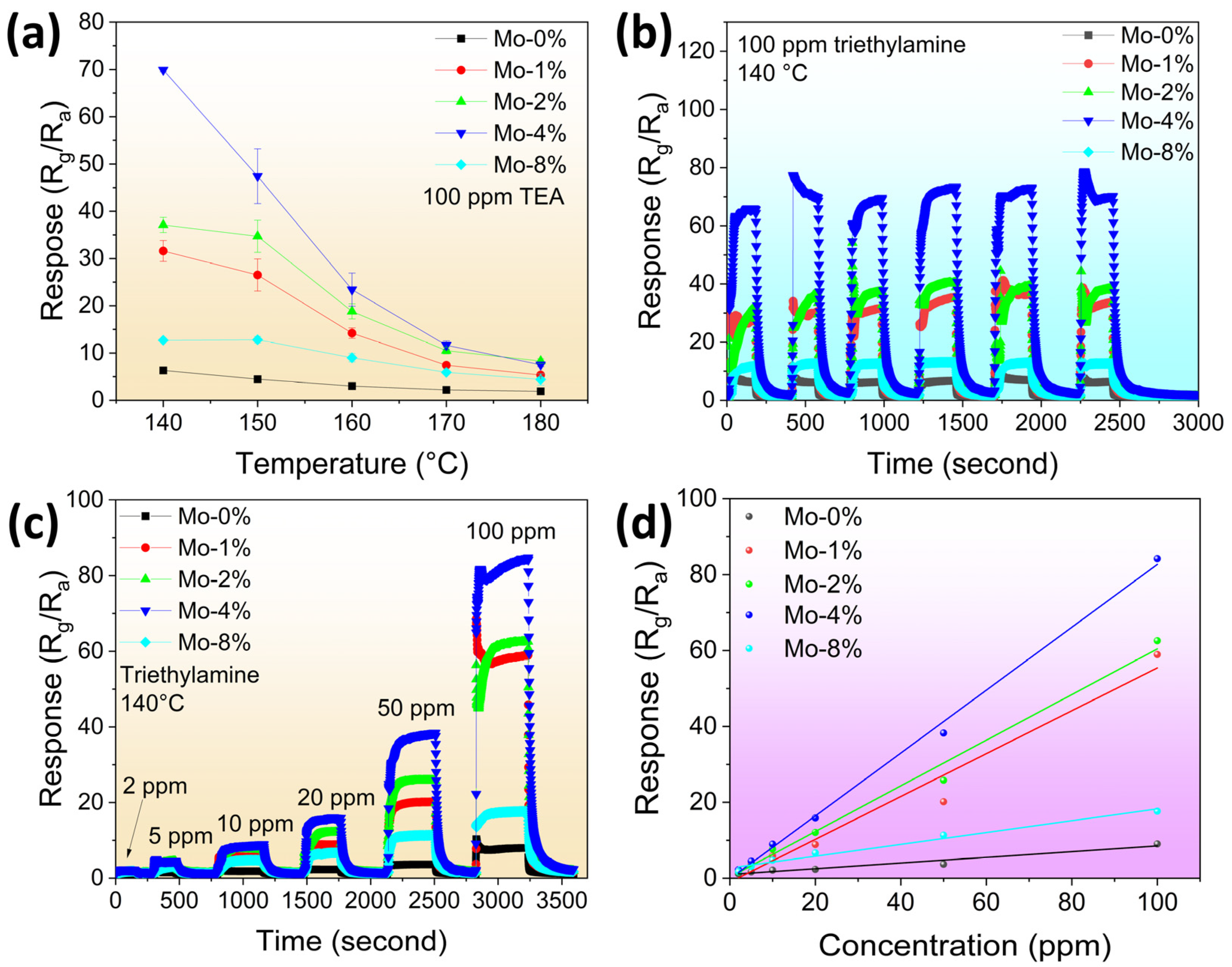
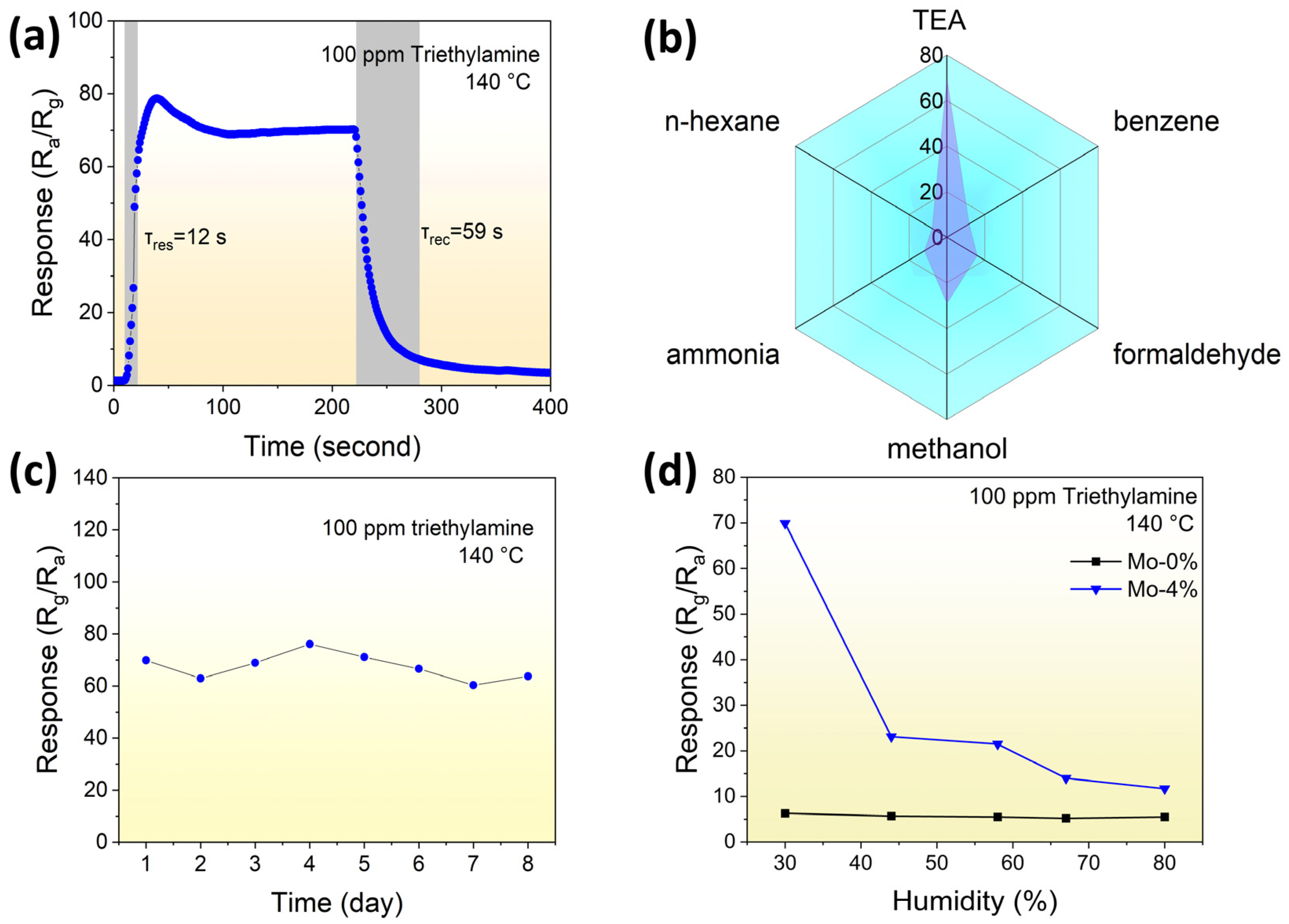
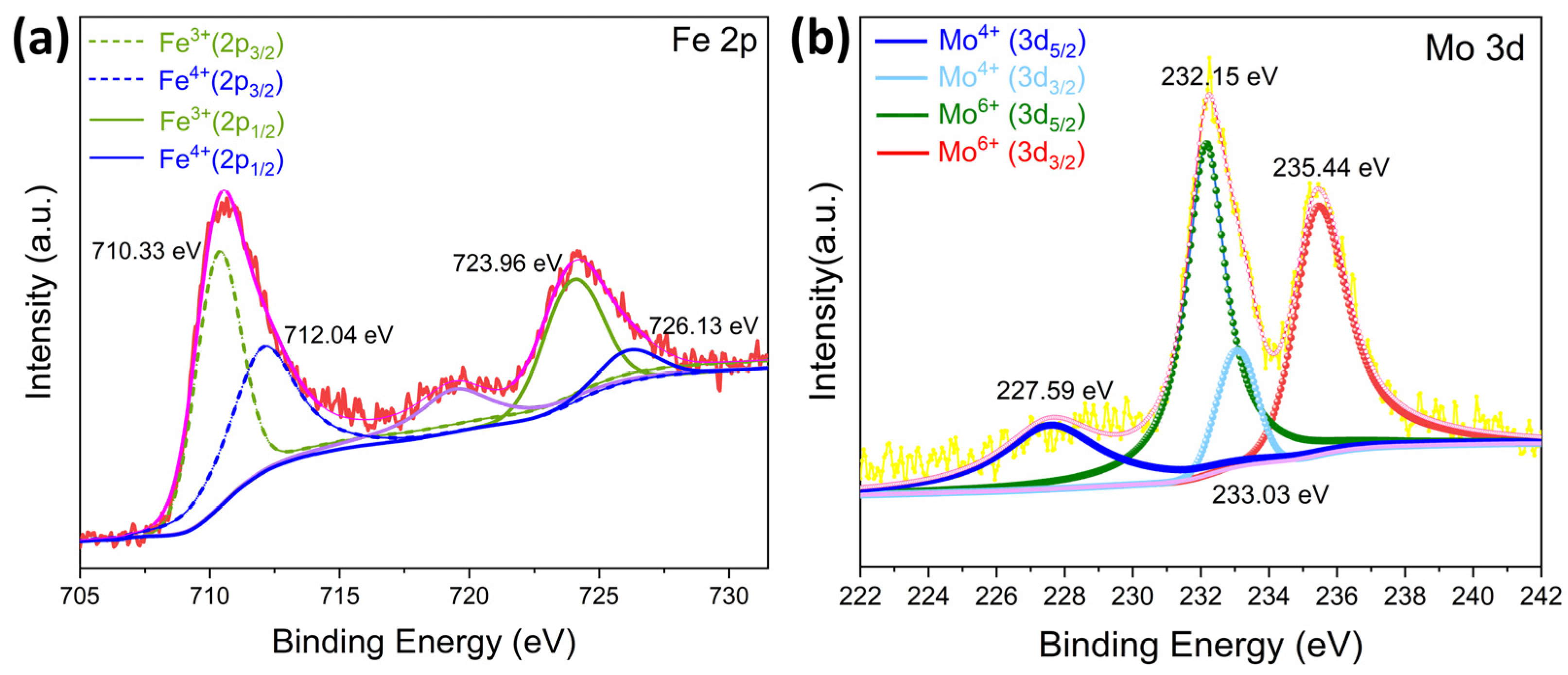

| Sample | a (Å) | b (Å) | c (Å) | Volume (Å3) | Crystallite Size (Å) |
|---|---|---|---|---|---|
| LaFeO3 | 5.55 | 7.84 | 5.55 | 241.4916 | 345 |
| LaFe0.99Mo0.01O3 | 5.55 | 7.87 | 5.54 | 241.9789 | 291 |
| LaFe0.98Mo0.02O3 | 5.55 | 7.85 | 5.54 | 241.3640 | 263 |
| LaFe0.96Mo0.04O3 | 5.54 | 7.86 | 5.54 | 241.2360 | 228 |
| LaFe0.92Mo0.02O3 | 3.91 | 3.91 | 3.95 | 60.3880 | 168 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.; Liang, H.; Zhao, Z.; Guo, S.; Chen, Y.; Tan, Z.; Song, X.-Z.; Wang, X. Mo-Doped LaFeO3 Gas Sensors with Enhanced Sensing Performance for Triethylamine Gas. Sensors 2024, 24, 4851. https://doi.org/10.3390/s24154851
Shen C, Liang H, Zhao Z, Guo S, Chen Y, Tan Z, Song X-Z, Wang X. Mo-Doped LaFeO3 Gas Sensors with Enhanced Sensing Performance for Triethylamine Gas. Sensors. 2024; 24(15):4851. https://doi.org/10.3390/s24154851
Chicago/Turabian StyleShen, Chenyu, Hongjian Liang, Ziyue Zhao, Suyi Guo, Yuxiang Chen, Zhenquan Tan, Xue-Zhi Song, and Xiaofeng Wang. 2024. "Mo-Doped LaFeO3 Gas Sensors with Enhanced Sensing Performance for Triethylamine Gas" Sensors 24, no. 15: 4851. https://doi.org/10.3390/s24154851
APA StyleShen, C., Liang, H., Zhao, Z., Guo, S., Chen, Y., Tan, Z., Song, X.-Z., & Wang, X. (2024). Mo-Doped LaFeO3 Gas Sensors with Enhanced Sensing Performance for Triethylamine Gas. Sensors, 24(15), 4851. https://doi.org/10.3390/s24154851








