Biomimetic Neuromorphic Sensory System via Electrolyte Gated Transistors
Abstract
:1. Introduction
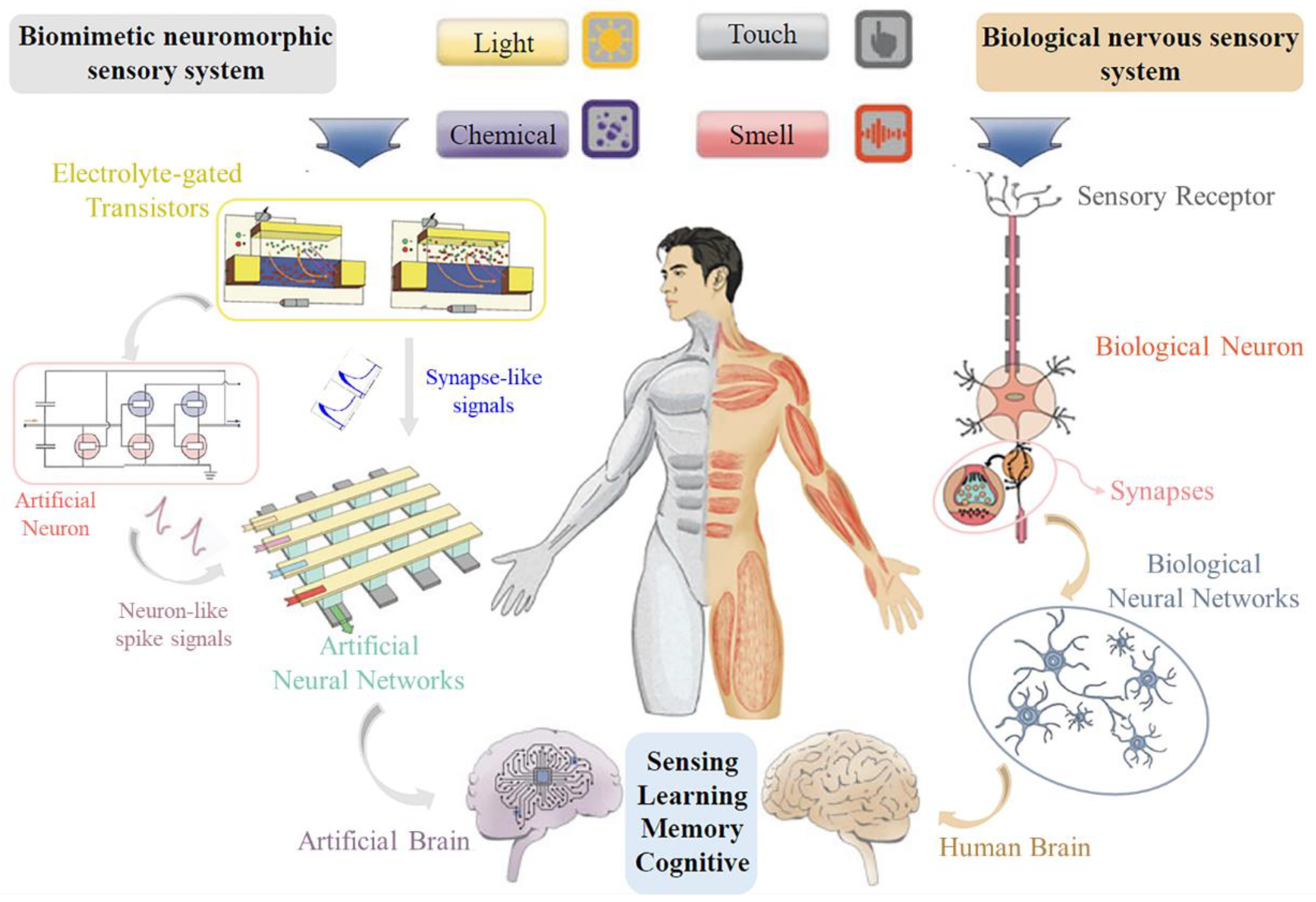
2. Fundamentals of Nervous Sensory System
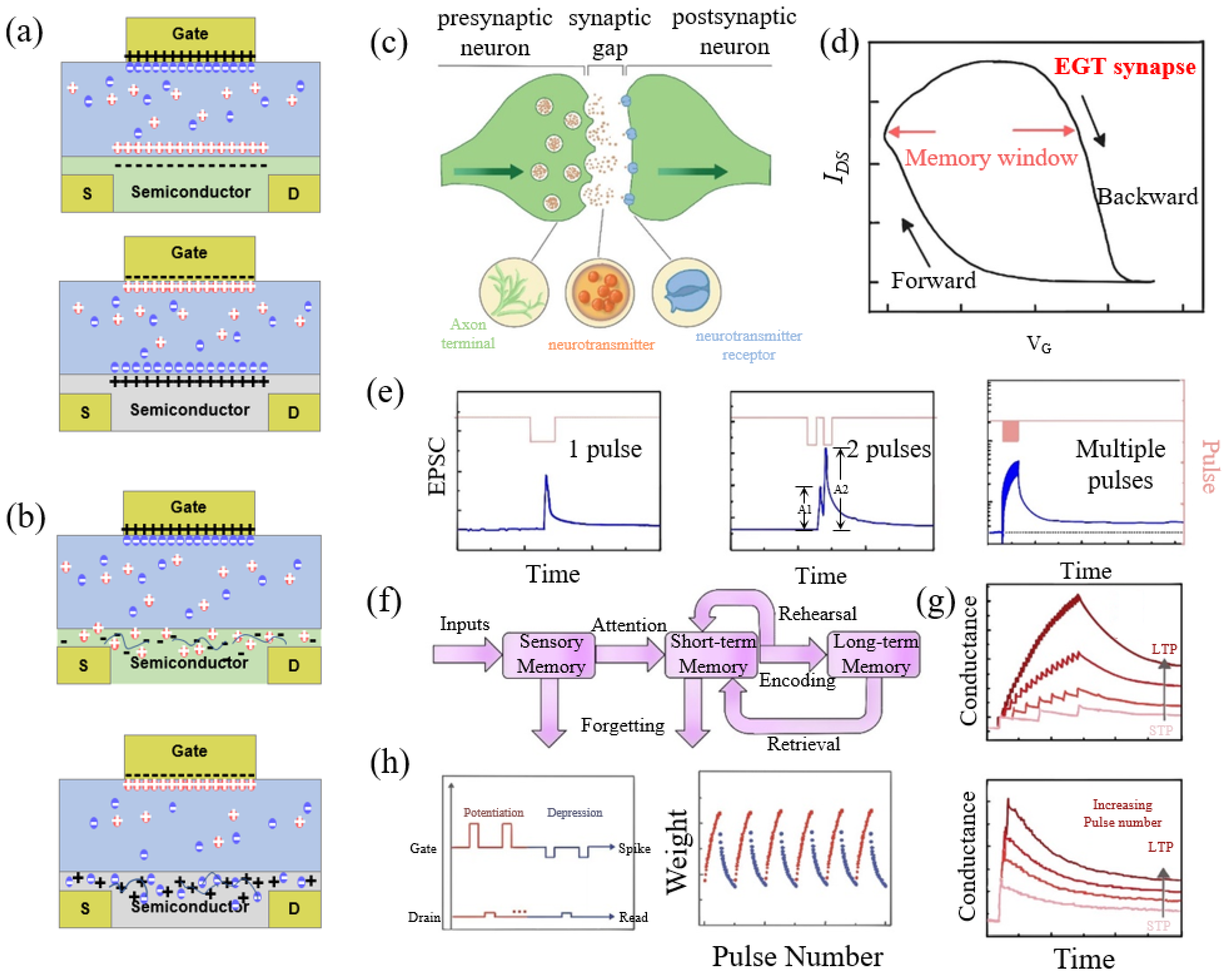
2.1. Electrolyte-Gated Neuromorphic Transistors
2.2. Electrolyte-Gated Transistor Synapse
2.3. Electrolyte-Gated Transistor Neurons

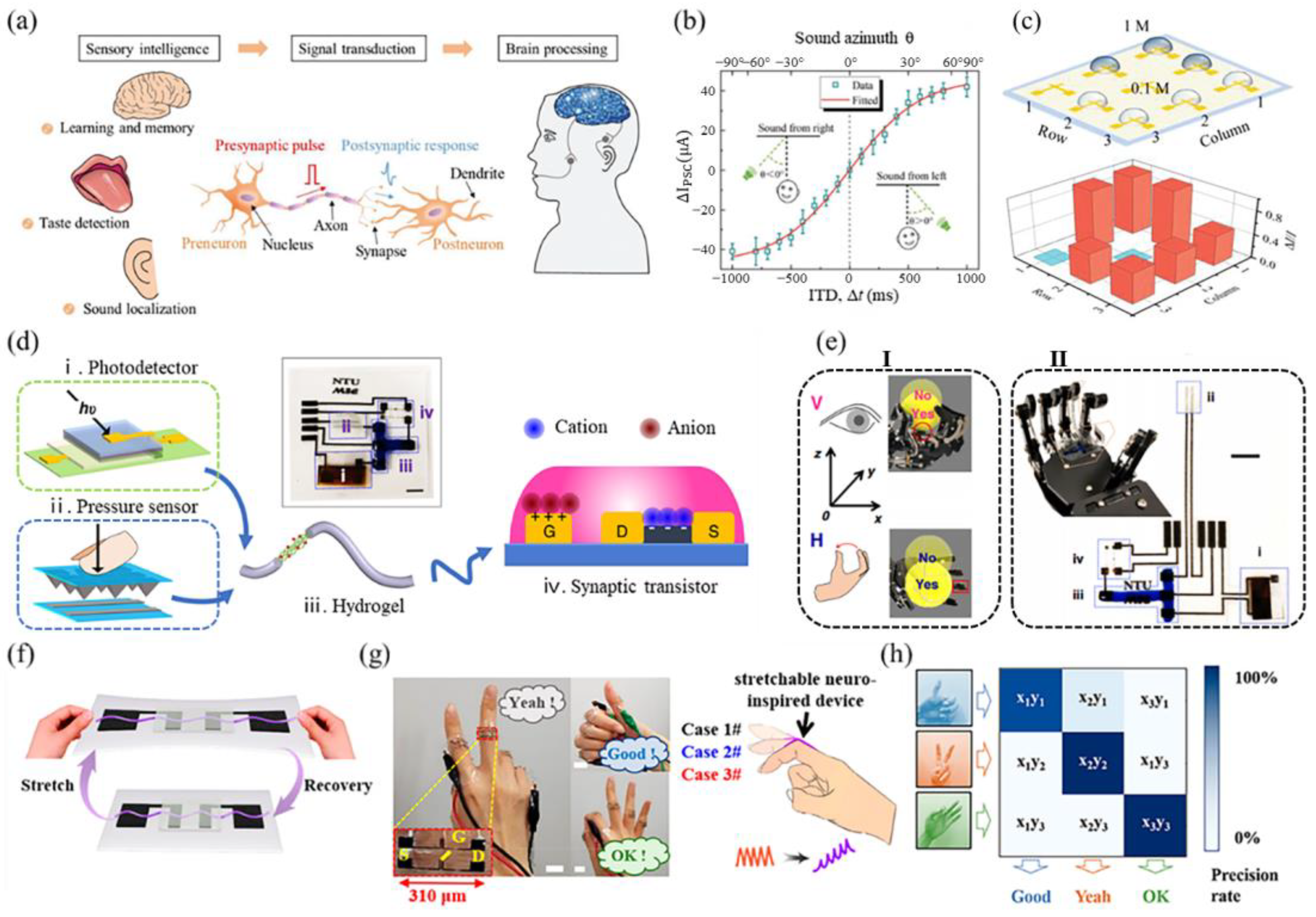
3. Application of Biomimetic Neuromorphic Sensory Systems
3.1. Tactile System
3.2. Visual System
3.3. Chemical System
3.4. Multimodal System
4. Summary and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Oteiza, P.; Baldwin, M.W. Evolution of sensory systems. Curr. Opin. Neurobiol. 2021, 71, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, R.; Aier, I.; Semwal, R.; Tyagi, P.; Varadwaj, P. Sense of Smell: Structural, Functional, Mechanistic Advancements and Challenges in Human Olfactory Research. Curr. Neuropharmacol. 2019, 17, 891–911. [Google Scholar] [CrossRef] [PubMed]
- Handler, A.; Ginty, D.D. The mechanosensory neurons of touch and their mechanisms of activation. Nat. Rev. Neurosci. 2021, 22, 521–537. [Google Scholar] [CrossRef] [PubMed]
- Barlow, L.A. The sense of taste: Development, regeneration, and dysfunction. WIREs Mech. Dis. 2022, 14, e1547. [Google Scholar] [CrossRef] [PubMed]
- Kohn, A. Visual Adaptation: Physiology, Mechanisms, and Functional Benefits. J. Neurophysiol. 2007, 97, 3155–3164. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, T.W. Organic Synapses for Neuromorphic Electronics: From Brain-Inspired Computing to Sensorimotor Nervetronics. Acc. Chem. Res. 2019, 52, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Sun, J.; Kong, L.A.; Gou, G.; Yang, J.; He, J.; Gao, Y.; Wan, Q. Artificial Synapses Based on in-Plane Gate Organic Electrochemical Transistors. ACS Appl. Mater. Interfaces 2016, 8, 26169–26175. [Google Scholar] [CrossRef]
- Mu, B.; Guo, L.; Liao, J.; Xie, P.; Ding, G.; Lv, Z.; Zhou, Y.; Han, S.-T.; Yan, Y. Near-Infrared Artificial Synapses for Artificial Sensory Neuron System. Small 2021, 17, 2103837. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, L.; Huang, W.; Li, Y.; Huang, S.; Zhu, Y.; Yang, D.; Pi, X. Optoelectronic Synaptic Devices for Neuromorphic Computing. Adv. Intell. Syst. 2021, 3, 2000099. [Google Scholar] [CrossRef]
- Meng, Y.; Cheng, G. Human somatosensory systems based on sensor-memory-integrated technology. Nanoscale 2024, 16, 11928–11958. [Google Scholar] [CrossRef]
- Sun, F.; Lu, Q.; Feng, S.; Zhang, T. Flexible Artificial Sensory Systems Based on Neuromorphic Devices. ACS Nano 2021, 15, 3875–3899. [Google Scholar] [CrossRef] [PubMed]
- Park, H.L.; Lee, Y.; Kim, N.; Seo, D.G.; Go, G.T.; Lee, T.W. Flexible Neuromorphic Electronics for Computing, Soft Robotics, and Neuroprosthetics. Adv. Mater. 2020, 32, e1903558. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhu, L.; Zhu, Y.; Chen, C.; Jiang, S.; Liu, R.; Shi, Y.; Wan, Q. Recent Progress on Emerging Transistor-Based Neuromorphic Devices. Adv. Intell. Syst. 2021, 3, 2000210. [Google Scholar] [CrossRef]
- van de Burgt, Y.; Melianas, A.; Keene, S.T.; Malliaras, G.; Salleo, A. Organic electronics for neuromorphic computing. Nat. Electron. 2018, 1, 386–397. [Google Scholar] [CrossRef]
- Mehonic, A.; Kenyon, A.J. Brain-inspired computing needs a master plan. Nature 2022, 604, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Turing, A.M. I.—Computing Machinery and Intelligence. Mind 1950, LIX, 433–460. [Google Scholar] [CrossRef]
- Liu, S.C.; Delbruck, T. Neuromorphic sensory systems. Curr. Opin. Neurobiol. 2010, 20, 288–295. [Google Scholar] [CrossRef]
- Zhu, Y.; Mao, H.; Zhu, Y.; Wang, X.; Fu, C.; Ke, S.; Wan, C.; Wan, Q. CMOS-compatible neuromorphic devices for neuromorphic perception and computing: A review. Int. J. Extrem. Manuf. 2023, 5, 042010. [Google Scholar] [CrossRef]
- Tang, J.; Yuan, F.; Shen, X.; Wang, Z.; Rao, M.; He, Y.; Sun, Y.; Li, X.; Zhang, W.; Li, Y.; et al. Bridging Biological and Artificial Neural Networks with Emerging Neuromorphic Devices: Fundamentals, Progress, and Challenges. Adv. Mater. 2019, 31, 1902761. [Google Scholar] [CrossRef]
- Zidan, M.A.; Strachan, J.P.; Lu, W.D. The future of electronics based on memristive systems. Nat. Electron. 2018, 1, 22–29. [Google Scholar] [CrossRef]
- Burr, G.W.; Shelby, R.M.; Sebastian, A.; Kim, S.; Kim, S.; Sidler, S.; Virwani, K.; Ishii, M.; Narayanan, P.; Fumarola, A.; et al. Neuromorphic computing using non-volatile memory. Adv. Phys. X 2017, 2, 124–189. [Google Scholar] [CrossRef]
- Hu, M.; Graves, C.E.; Li, C.; Li, Y.; Ge, N.; Montgomery, E.; Davila, N.; Jiang, H.; Williams, R.S.; Yang, J.J.; et al. Memristor-Based Analog Computation and Neural Network Classification with a Dot Product Engine. Adv. Mater. 2018, 30, 1705914. [Google Scholar] [CrossRef] [PubMed]
- Prezioso, M.; Merrikh-Bayat, F.; Hoskins, B.D.; Adam, G.C.; Likharev, K.K.; Strukov, D.B. Training and operation of an integrated neuromorphic network based on metal-oxide memristors. Nature 2015, 521, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Rokade, K.A.; Kumbhar, D.D.; Patil, S.L.; Sutar, S.S.; More, K.V.; Dandge, P.B.; Kamat, R.K.; Dongale, T.D. CogniFiber: Harnessing Biocompatible and Biodegradable 1D Collagen Nanofibers for Sustainable Nonvolatile Memory and Synaptic Learning Applications. Adv. Mater. 2024, 36, 2312484. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.K.; Hersam, M.C. Neuromorphic nanoelectronic materials. Nat. Nanotechnol. 2020, 15, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, S.R.; Le Gallo, M.; Boybat, I.; Rajendran, B.; Sebastian, A.; Eleftheriou, E. A phase-change memory model for neuromorphic computing. J. Appl. Phys. 2018, 124, 152135. [Google Scholar] [CrossRef]
- Tuma, T.; Pantazi, A.; Le Gallo, M.; Sebastian, A.; Eleftheriou, E. Stochastic phase-change neurons. Nat. Nanotechnol. 2016, 11, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Suri, M.; Sousa, V.; Perniola, L.; Vuillaume, D.; DeSalvo, B. Phase change memory for synaptic plasticity application in neuromorphic systems. In Proceedings of the 2011 International Joint Conference on Neural Networks, San Jose, CA, USA, 31 July–5 August 2011; pp. 619–624. [Google Scholar]
- Liu, L.; Wang, D.; Wang, D.; Sun, Y.; Lin, H.; Gong, X.; Zhang, Y.; Tang, R.; Mai, Z.; Hou, Z.; et al. Domain wall magnetic tunnel junction-based artificial synapses and neurons for all-spin neuromorphic hardware. Nat. Commun. 2024, 15, 4534. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Li, X.; Xu, N.; Chen, H.; Cabrini, S.; Khizroev, S.; Bokor, J.; You, L. A Dual Magnetic Tunnel Junction-Based Neuromorphic Device. Adv. Intell. Syst. 2020, 2, 2000143. [Google Scholar] [CrossRef]
- Sengupta, A.; Roy, K. Spin-Transfer Torque Magnetic neuron for low power neuromorphic computing. In Proceedings of the 2015 International Joint Conference on Neural Networks (IJCNN), Killarney, Ireland, 12–17 July 2015; pp. 1–7. [Google Scholar]
- Chang, L.; Ma, X.; Wang, Z.; Zhang, Y.; Xie, Y.; Zhao, W. PXNOR-BNN: In/with Spin-Orbit Torque MRAM Preset-XNOR Operation-Based Binary Neural Networks. IEEE Trans. Very Large Scale Integr. Syst. 2019, 27, 2668–2679. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, W.; Wang, M.; Pan, B.; Cao, K.; Guo, M.; Zhang, T.; Cheng, H.; Li, S.; Zhu, D.; et al. Spin-Torque Memristors Based on Perpendicular Magnetic Tunnel Junctions for Neuromorphic Computing. Adv. Sci. 2021, 8, 2004645. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, D.; Liang, X.; Lu, W.D. Ionic modulation and ionic coupling effects in MoS2 devices for neuromorphic computing. Nat. Mater. 2019, 18, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Aziz, J.; Khan, M.F.; Neumaier, D.; Miao, Z.; Elahi, E.; Kim, H.; Chavan, V.D.; Ghafoor, F.; Ghfar, A.A.; Kadam, K.D.; et al. Controlled charge transport in ZrO2 and its bilayer structures for low-power memory. J. Alloys Compd. 2024, 1001, 175103. [Google Scholar] [CrossRef]
- Grollier, J.; Querlioz, D.; Camsari, K.Y.; Everschor-Sitte, K.; Fukami, S.; Stiles, M.D. Neuromorphic spintronics. Nat. Electron. 2020, 3, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cheng, H.; Zhang, B.; Yin, J.; Zhu, D.; Cai, W.; Li, S.; Zhao, W. Tunneling Magnetoresistance Materials and Devices for Neuromorphic Computing. Mater. Futures 2023, 2, 032302. [Google Scholar] [CrossRef]
- Bu, X.; Xu, H.; Shang, D.; Li, Y.; Lv, H.; Liu, Q. Ion-Gated Transistor: An Enabler for Sensing and Computing Integration. Adv. Intell. Syst. 2020, 2, 2000156. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, Y.; Wang, Y.; Zhang, J.; Fang, L.; Jin, S.; Shao, Y.; Huang, J. Recent Advances in Transistor-Based Artificial Synapses. Adv. Funct. Mater. 2019, 29, 1903700. [Google Scholar] [CrossRef]
- Ling, H.; Koutsouras, D.A.; Kazemzadeh, S.; van de Burgt, Y.; Yan, F.; Gkoupidenis, P. Electrolyte-gated transistors for synaptic electronics, neuromorphic computing, and adaptable biointerfacing. Appl. Phys. Rev. 2020, 7, 011307. [Google Scholar] [CrossRef]
- Cho, S.W.; Kwon, S.M.; Kim, Y.-H.; Park, S.K. Recent Progress in Transistor-Based Optoelectronic Synapses: From Neuromorphic Computing to Artificial Sensory System. Adv. Intell. Syst. 2021, 3, 2000162. [Google Scholar] [CrossRef]
- Friedlein, J.T.; McLeod, R.R.; Rivnay, J. Device physics of organic electrochemical transistors. Org. Electron. 2018, 63, 398–414. [Google Scholar] [CrossRef]
- Khan, M.A.; Yim, S.; Rehman, S.; Ghafoor, F.; Kim, H.; Patil, H.; Khan, M.F.; Eom, J. Two-dimensional materials memory devices with floating metal gate for neuromorphic applications. Mater. Today Adv. 2023, 20, 100438. [Google Scholar] [CrossRef]
- Strakosas, X.; Wei, B.; Martin, D.C.; Owens, R.M. Biofunctionalization of polydioxythiophene derivatives for biomedical applications. J. Mater. Chem. B 2016, 4, 4952–4968. [Google Scholar] [CrossRef]
- Bisri, S.Z.; Shimizu, S.; Nakano, M.; Iwasa, Y. Endeavor of Iontronics: From Fundamentals to Applications of Ion-Controlled Electronics. Adv. Mater. 2017, 29, 1607054. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hong, K.; Xie, W.; Lee, K.H.; Zhang, S.; Lodge, T.P.; Frisbie, C.D. Electrolyte-gated transistors for organic and printed electronics. Adv. Mater. 2013, 25, 1822–1846. [Google Scholar] [CrossRef] [PubMed]
- Khodagholy, D.; Rivnay, J.; Sessolo, M.; Gurfinkel, M.; Leleux, P.; Jimison, L.H.; Stavrinidou, E.; Herve, T.; Sanaur, S.; Owens, R.M.; et al. High transconductance organic electrochemical transistors. Nat. Commun. 2013, 4, 2133. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, D.; Rivnay, J.; Matsuhisa, N.; Lonjaret, T.; Yokota, T.; Yawo, H.; Sekino, M.; Malliaras, G.G.; Someya, T. Integration of Organic Electrochemical and Field-Effect Transistors for Ultraflexible, High Temporal Resolution Electrophysiology Arrays. Adv. Mater. 2016, 28, 9722–9728. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, F.; Adrahtas, D.Z.; Bao, Z.; Berggren, M.; Biscarini, F.; Bonfiglio, A.; Bortolotti, C.A.; Frisbie, C.D.; Macchia, E.; Malliaras, G.G.; et al. Electrolyte-gated transistors for enhanced performance bioelectronics. Nat. Rev. Methods Primers 2021, 1, 66. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chen, J.; Wang, G.; Yao, Y.; Zhuang, X.; Pankow, R.M.; Cheng, Y.; Marks, T.J.; Facchetti, A. Dielectric materials for electrolyte gated transistor applications. J. Mater. Chem. C 2021, 9, 9348–9376. [Google Scholar] [CrossRef]
- Wang, G.Y.; Lian, K.; Chu, T.Y. Electrolyte-Gated Field Effect Transistors in Biological Sensing: A Survey of Electrolytes. IEEE J. Electron. Devices Soc. 2021, 9, 939–950. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Kim, J.E.; Kim, J.S.; Chun, S.Y.; Soh, K.; Yoon, J.H. Artificial sensory system based on memristive devices. Exploration 2024, 4, 20220162. [Google Scholar] [CrossRef]
- Jacobson, S.; Marcus, E.M. Introduction to the Nervous System. In Neuroanatomy for the Neuroscientist; Jacobson, S., Marcus, E.M., Eds.; Springer: Boston, MA, USA, 2011; pp. 3–15. [Google Scholar]
- Joos, K.; Gilles, A.; Van de Heyning, P.; De Ridder, D.; Vanneste, S. From sensation to percept: The neural signature of auditory event-related potentials. Neurosci. Biobehav. Rev. 2014, 42, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.J.; Jolivet, R.; Attwell, D. Synaptic energy use and supply. Neuron 2012, 75, 762–777. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zou, H.; Wang, M.; Wang, J.; Wang, T.; Wang, L.; Chen, X. Conformal Neuromorphic Bioelectronics for Sense Digitalization. Adv. Mater. 2024, 2403444. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Qin, S.; Gao, G.; Xu, C.; Lin Wang, Z.; Sun, Q. Bioinspired interactive neuromorphic devices. Mater. Today 2022, 60, 158–182. [Google Scholar] [CrossRef]
- Fletcher, A. Nerve cell function and synaptic mechanisms. Anaesth. Intensive Care Med. 2022, 23, 177–182. [Google Scholar] [CrossRef]
- Delmas, P.; Hao, J.; Rodat-Despoix, L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat. Rev. Neurosci. 2011, 12, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Roper, S.D.; Chaudhari, N. Taste buds: Cells, signals and synapses. Nat. Rev. Neurosci. 2017, 18, 485–497. [Google Scholar] [CrossRef]
- Südhof, T.C. The cell biology of synapse formation. J. Cell Biol. 2021, 220, e202103052. [Google Scholar] [CrossRef] [PubMed]
- Nadim, F.; Bucher, D. Neuromodulation of neurons and synapses. Curr. Opin. Neurobiol. 2014, 29, 48–56. [Google Scholar] [CrossRef]
- Severson, K.S.; Xu, D.; Van de Loo, M.; Bai, L.; Ginty, D.D.; O’Connor, D.H. Active Touch and Self-Motion Encoding by Merkel Cell-Associated Afferents. Neuron 2017, 94, 666–676.e9. [Google Scholar] [CrossRef]
- Balasubramanian, V. Brain power. Proc. Natl. Acad. Sci. USA 2021, 118, e2107022118. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yu, H.; Chai, Y. Low-Power Computing with Neuromorphic Engineering. Adv. Intell. Syst. 2021, 3, 2000150. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, B.; Zhou, J.; Chen, Y.; Chu, Y.; Huang, J. Distinguishable Detection of Ultraviolet, Visible, and Infrared Spectrum with High-Responsivity Perovskite-Based Flexible Photosensors. Small 2018, 14, 1800527. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, M.; Lee, A.; Park, H.-L.; Jang, J.; Bae, J.-H.; Kang, I.M.; Kim, E.-S.; Lee, S.-H. Organic Memristor-Based Flexible Neural Networks with Bio-Realistic Synaptic Plasticity for Complex Combinatorial Optimization. Adv. Sci. 2023, 10, 2300659. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Gao, L.; Liu, C.; Deng, J.; Xie, M.; Bai, L.; Wang, G.; Cheng, Y.; Huang, W.; Yu, J. Stretchable organic electrochemical transistors via three-dimensional porous elastic semiconducting films for artificial synaptic applications. Nano Res. 2023, 16, 10206–10214. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Li, E.; Cao, C.; Zheng, W.; Chen, H.; Li, W. Stretchable Transistor-Structured Artificial Synapses for Neuromorphic Electronics. Small 2023, 19, 2205395. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Zhang, X.; Liu, S.; Lu, J.; Wang, Y.; Jiang, H.; Yang, Y.; Sun, Y.; Wei, W.; Wang, J.; et al. Compact artificial neuron based on anti-ferroelectric transistor. Nat. Commun. 2022, 13, 7018. [Google Scholar] [CrossRef]
- Wang, T.; Wang, M.; Wang, J.; Yang, L.; Ren, X.; Song, G.; Chen, S.; Yuan, Y.; Liu, R.; Pan, L.; et al. A chemically mediated artificial neuron. Nat. Electron. 2022, 5, 586–595. [Google Scholar] [CrossRef]
- Sarkar, T.; Lieberth, K.; Pavlou, A.; Frank, T.; Mailaender, V.; McCulloch, I.; Blom, P.W.M.; Torricelli, F.; Gkoupidenis, P. An organic artificial spiking neuron for in situ neuromorphic sensing and biointerfacing. Nat. Electron. 2022, 5, 774–783. [Google Scholar] [CrossRef]
- Yang, G.R.; Wang, X.-J. Artificial Neural Networks for Neuroscientists: A Primer. Neuron 2020, 107, 1048–1070. [Google Scholar] [CrossRef]
- Kiani, F.; Yin, J.; Wang, Z.; Yang, J.J.; Xia, Q. A fully hardware-based memristive multilayer neural network. Sci. Adv. 2021, 7, eabj4801. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ge, C.; Liu, Z.; Zhong, H.; Guo, E.; He, M.; Wang, C.; Yang, G.; Jin, K. Electrolyte-gated transistors for neuromorphic applications. J. Semicond. 2021, 42, 013103. [Google Scholar] [CrossRef]
- Mao, B.; Zhou, K.; Xiang, Y.; Zhang, Y.; Yuan, Q.; Hao, H.; Chen, Y.; Liu, H.; Wang, X.; Wang, X.; et al. A Bioinspired Robotic Finger for Multimodal Tactile Sensing Powered by Fiber Optic Sensors. Adv. Intell. Syst. 2024, 2400175. [Google Scholar] [CrossRef]
- Fang, L.; Mao, C.; Wang, H.; Ding, Q.; Jiao, W.; Li, B.; Zhang, Y.; Gong, D. Recent progress of organic artificial synapses in biomimetic sensory neural systems. J. Mater. Chem. C 2024, 12, 8586–8610. [Google Scholar] [CrossRef]
- Lee, J.; Kaake, L.G.; Cho, J.H.; Zhu, X.Y.; Lodge, T.P.; Frisbie, C.D. Ion Gel-Gated Polymer Thin-Film Transistors: Operating Mechanism and Characterization of Gate Dielectric Capacitance, Switching Speed, and Stability. J. Phys. Chem. C 2009, 113, 8972–8981. [Google Scholar] [CrossRef]
- Liu, C.; Deng, J.; Gao, L.; Cheng, J.; Peng, Y.; Zeng, H.; Huang, W.; Feng, L.-W.; Yu, J. Multilayer Porous Polymer Films for High-Performance Stretchable Organic Electrochemical Transistors. Adv. Electron. Mater. 2023, 9, 2300119. [Google Scholar] [CrossRef]
- Gao, L.; Wu, M.; Yu, X.; Yu, J. Device design principles and bioelectronic applications for flexible organic electrochemical transistors. Int. J. Extrem. Manuf. 2024, 6, 012005. [Google Scholar] [CrossRef]
- Owens, R.M.; Malliaras, G.G. Organic Electronics at the Interface with Biology. MRS Bull. 2010, 35, 449–456. [Google Scholar] [CrossRef]
- Cea, C.; Zhao, Z.; Wisniewski, D.J.; Spyropoulos, G.D.; Polyravas, A.; Gelinas, J.N.; Khodagholy, D. Integrated internal ion-gated organic electrochemical transistors for stand-alone conformable bioelectronics. Nat. Mater. 2023, 22, 1227–1235. [Google Scholar] [CrossRef]
- Jo, Y.J.; Kim, H.; Ok, J.; Shin, Y.-J.; Shin, J.H.; Kim, T.H.; Jung, Y.; Kim, T.-I. Biocompatible and Biodegradable Organic Transistors Using a Solid-State Electrolyte Incorporated with Choline-Based Ionic Liquid and Polysaccharide. Adv. Funct. Mater. 2020, 30, 1909707. [Google Scholar] [CrossRef]
- Fang, R.; Wang, S.; Zhang, W.; Ren, K.; Sun, W.; Wang, F.; Lai, J.; Zhang, P.; Xu, X.; Luo, Q.; et al. Oxide-Based Electrolyte-Gated Transistors with Stable and Tunable Relaxation Responses for Deep Time-Delayed Reservoir Computing. Adv. Electron. Mater. 2024, 10, 2300652. [Google Scholar] [CrossRef]
- Sporea, R.A. Tuning electrolyte-gated transistors to order. Nat. Electron. 2022, 5, 836–837. [Google Scholar] [CrossRef]
- Gao, X.; Yin, J.; Zhu, J.; Chang, J.; Zhang, J.; Hao, Y. Electrolyte-Gated Flexible MoS2 Synaptic Transistors with Short-Term Plasticity. IEEE Electron. Device Lett. 2024, 45, 605–608. [Google Scholar] [CrossRef]
- Harikesh, P.C.; Tu, D.; Fabiano, S. Organic electrochemical neurons for neuromorphic perception. Nat. Electron. 2024. [Google Scholar] [CrossRef]
- Du, H.; Lin, X.; Xu, Z.; Chu, D. Electric double-layer transistors: A review of recent progress. J. Mater. Sci. 2015, 50, 5641–5673. [Google Scholar] [CrossRef]
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Donahue, M.J.; Williamson, A.; Strakosas, X.; Friedlein, J.T.; McLeod, R.R.; Gleskova, H.; Malliaras, G.G. High-Performance Vertical Organic Electrochemical Transistors. Adv. Mater. 2018, 30, 1705031. [Google Scholar] [CrossRef]
- Hormuzdi, S.G.; Filippov, M.A.; Mitropoulou, G.; Monyer, H.; Bruzzone, R. Electrical synapses: A dynamic signaling system that shapes the activity of neuronal networks. Biochim. Biophys. Acta (BBA) Biomembr. 2004, 1662, 113–137. [Google Scholar] [CrossRef]
- Park, J.Y.; Choe, D.-H.; Lee, D.H.; Yu, G.T.; Yang, K.; Kim, S.H.; Park, G.H.; Nam, S.-G.; Lee, H.J.; Jo, S.; et al. Revival of Ferroelectric Memories Based on Emerging Fluorite-Structured Ferroelectrics. Adv. Mater. 2023, 35, 2204904. [Google Scholar] [CrossRef]
- Amin, H.U.; Malik, A. Memory Retention and Recall Process. In EEG/ERP Analysis; CRC Press: Boca Raton, FL, USA, 2014; pp. 219–237. [Google Scholar]
- Bliss, T.V.P.; Collingridge, G.L.; Morris, R.G.M.; Reymann, K.G. Long-term potentiation in the hippocampus: Discovery, mechanisms and function. Neuroforum 2018, 24, A103–A120. [Google Scholar] [CrossRef]
- Zhang, F.; Li, C.; Li, Z.; Dong, L.; Zhao, J. Recent progress in three-terminal artificial synapses based on 2D materials: From mechanisms to applications. Microsyst. Nanoeng. 2023, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Pereda, A.E. Electrical synapses and their functional interactions with chemical synapses. Nat. Rev. Neurosci. 2014, 15, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Inal, S.; Malliaras, G.G.; Rivnay, J. Benchmarking organic mixed conductors for transistors. Nat. Commun. 2017, 8, 1767. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shang, D.; Luo, Q.; An, J.; Li, Y.; Wu, S.; Yao, Z.; Zhang, W.; Xu, X.; Dou, C.; et al. A low-power vertical dual-gate neurotransistor with short-term memory for high energy-efficient neuromorphic computing. Nat. Commun. 2023, 14, 6385. [Google Scholar] [CrossRef] [PubMed]
- Chouhdry, H.H.; Lee, D.H.; Bag, A.; Lee, N.-E. A flexible artificial chemosensory neuronal synapse based on chemoreceptive ionogel-gated electrochemical transistor. Nat. Commun. 2023, 14, 821. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic electrochemical transistors. Nat. Rev. Mater. 2018, 3, 17086. [Google Scholar] [CrossRef]
- Bernards, D.A.; Malliaras, G.G. Steady-State and Transient Behavior of Organic Electrochemical Transistors. Adv. Funct. Mater. 2007, 17, 3538–3544. [Google Scholar] [CrossRef]
- Sun, H.; Gerasimov, J.; Berggren, M.; Fabiano, S. n-Type organic electrochemical transistors: Materials and challenges. J. Mater. Chem. C 2018, 6, 11778–11784. [Google Scholar] [CrossRef]
- Jackman, S.L.; Regehr, W.G. The Mechanisms and Functions of Synaptic Facilitation. Neuron 2017, 94, 447–464. [Google Scholar] [CrossRef]
- Heller, E.; Zhang, W.; Selimi, F.; Earnheart, J.; Slimak-Mastrobuoni, M.; Santos-Torres, J.; Ibañez-Tallon, I.; Aoki, C.; Chait, B.; Heintz, N. The Biochemical Anatomy of Cortical Inhibitory Synapses. PLoS ONE 2012, 7, e39572. [Google Scholar] [CrossRef]
- Isaacson, J.S.; Scanziani, M. How inhibition shapes cortical activity. Neuron 2011, 72, 231–243. [Google Scholar] [CrossRef]
- Fernandes, D.; Carvalho, A.L. Mechanisms of homeostatic plasticity in the excitatory synapse. J. Neurochem. 2016, 139, 973–996. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Y.; Lin, Q.; Liu, T.; Wang, S.; Chen, H.; Li, C.; Gu, X.; Zhang, X.; Huang, H. Organic Optoelectronic Synapses for Sound Perception. Nanomicro Lett. 2023, 15, 133. [Google Scholar] [CrossRef]
- Zucker, R.S.; Regehr, W.G. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002, 64, 355–405. [Google Scholar] [CrossRef]
- Fioravante, D.; Regehr, W.G. Short-term forms of presynaptic plasticity. Curr. Opin. Neurobiol. 2011, 21, 269–274. [Google Scholar] [CrossRef] [PubMed]
- López, J.C. A fresh look at paired-pulse facilitation. Nat. Rev. Neurosci. 2001, 2, 307. [Google Scholar] [CrossRef]
- Yang, Y.-M.; Wang, W.; Fedchyshyn, M.J.; Zhou, Z.; Ding, J.; Wang, L.-Y. Enhancing the fidelity of neurotransmission by activity-dependent facilitation of presynaptic potassium currents. Nat. Commun. 2014, 5, 4564. [Google Scholar] [CrossRef] [PubMed]
- Gkoupidenis, P.; Koutsouras, D.A.; Malliaras, G.G. Neuromorphic device architectures with global connectivity through electrolyte gating. Nat. Commun. 2017, 8, 15448. [Google Scholar] [CrossRef]
- Xie, D.; Jiang, J.; Hu, W.; He, Y.; Yang, J.; He, J.; Gao, Y.; Wan, Q. Coplanar Multigate MoS2 Electric-Double-Layer Transistors for Neuromorphic Visual Recognition. ACS Appl. Mater. Interfaces 2018, 10, 25943–25948. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lei, T.; Wong, M. Parallel Dual-Gate Thin-Film Transistors for Sensing and Neuromorphic Computing. In Proceedings of the 2022 IEEE 16th International Conference on Solid-State & Integrated Circuit Technology (ICSICT), Nanjing, China, 25–28 October 2022; pp. 1–4. [Google Scholar]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Shiffrin, R.M.; Atkinson, R.C. Storage and retrieval processes in long-term memory. Psychol. Rev. 1969, 76, 179–193. [Google Scholar] [CrossRef]
- Xu, W.; Min, S.-Y.; Hwang, H.; Lee, T.-W. Organic core-sheath nanowire artificial synapses with femtojoule energy consumption. Sci. Adv. 2016, 2, e1501326. [Google Scholar] [CrossRef] [PubMed]
- Keene, S.T.; Melianas, A.; Fuller, E.J.; van de Burgt, Y.; Talin, A.A.; Salleo, A. Optimized pulsed write schemes improve linearity and write speed for low-power organic neuromorphic devices. J. Phys. D Appl. Phys. 2018, 51, 224002. [Google Scholar] [CrossRef]
- Xu, N. On the Concept of Resting Potential—Pumping Ratio of the Na+/K+ Pump and Concentration Ratios of Potassium Ions Outside and Inside the Cell to Sodium Ions Inside and Outside the Cell. J. Membr. Biol. 2013, 246, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Stojilkovic, S.S. Ion Channels, Transporters, and Electrical Signaling. In Neuroscience in Medicine; Conn, P.M., Ed.; Humana Press: Totowa, NJ, USA, 2008; pp. 53–89. [Google Scholar]
- Isacoff, E.Y.; Jan, L.Y.; Minor, D.L. Conduits of Life’s Spark: A Perspective on Ion Channel Research since the Birth of Neuron. Neuron 2013, 80, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A. Action potential: Generation and propagation. Anaesth. Intensive Care Med. 2011, 12, 258–262. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. Bull. Math. Biol. 1990, 52, 25–71. [Google Scholar] [CrossRef] [PubMed]
- Häusser, M. The Hodgkin-Huxley theory of the action potential. Nat. Neurosci. 2000, 3, 1165. [Google Scholar] [CrossRef]
- Chow, C.C.; White, J.A. Spontaneous action potentials due to channel fluctuations. Biophys. J. 1996, 71, 3013–3021. [Google Scholar] [CrossRef]
- Eliasmith, C.; Anderson, C.H. Neural Engineering: Computation, Representation, and Dynamics in Neurobiological Systems. IEEE Trans. Neural Netw. 2004, 15, 528–529. [Google Scholar] [CrossRef]
- Gerstner, W.; Kistler, W.M. Spiking Neuron Models: Single Neurons, Populations, Plasticity; Cambridge University Press: New York, NY, USA, 2002. [Google Scholar]
- Segee, B. Methods in Neuronal Modeling: From Ions to Networks, 2nd Edition. Comput. Sci. Eng. 1999, 1, 81. [Google Scholar] [CrossRef]
- Harikesh, P.C.; Yang, C.-Y.; Tu, D.; Gerasimov, J.Y.; Dar, A.M.; Armada-Moreira, A.; Massetti, M.; Kroon, R.; Bliman, D.; Olsson, R.; et al. Organic electrochemical neurons and synapses with ion mediated spiking. Nat. Commun. 2022, 13, 901. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-Y.; Huang, J.-D.; Jeong, S.Y.; Liu, T.; Wu, Z.; van der Pol, T.; Wang, Q.; Stoeckel, M.-A.; Li, Q.; Fahlman, M.; et al. Stable organic electrochemical neurons based on p-type and n-type ladder polymers. Mater. Horiz. 2023, 10, 4213–4223. [Google Scholar] [CrossRef]
- Belleri, P.; Pons i Tarrés, J.; McCulloch, I.; Blom, P.W.M.; Kovács-Vajna, Z.M.; Gkoupidenis, P.; Torricelli, F. Unravelling the operation of organic artificial neurons for neuromorphic bioelectronics. Nat. Commun. 2024, 15, 5350. [Google Scholar] [CrossRef]
- Harikesh, P.C.; Yang, C.-Y.; Wu, H.-Y.; Zhang, S.; Donahue, M.J.; Caravaca, A.S.; Huang, J.-D.; Olofsson, P.S.; Berggren, M.; Tu, D.; et al. Ion-tunable antiambipolarity in mixed ion–electron conducting polymers enables biorealistic organic electrochemical neurons. Nat. Mater. 2023, 22, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Y.; Sun, Q.; Abdurehim, A.; Xu, J.; Xie, J.; Zhang, Y. Taste and its receptors in human physiology: A comprehensive look. Food Front. 2024, 5, 1512–1533. [Google Scholar] [CrossRef]
- Wu, M.; Zhuang, Q.; Yao, K.; Li, J.; Zhao, G.; Zhou, J.; Li, D.; Shi, R.; Xu, G.; Li, Y.; et al. Stretchable, skin-conformable neuromorphic system for tactile sensory recognizing and encoding. InfoMat 2023, 5, e12472. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, Y.; Zhong, D.; Zhang, Z.; Choudhury, S.; Lai, J.-C.; Gong, H.; Niu, S.; Yan, X.; Zheng, Y.; et al. Neuromorphic sensorimotor loop embodied by monolithically integrated, low-voltage, soft e-skin. Science 2023, 380, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, H.; Liu, T.; Wei, Y.; Yao, G.; Lin, Q.; Han, X.; Zhang, C.; Huang, H. Retina-Inspired Organic Photonic Synapses for Selective Detection of SWIR Light. Angew. Chem. Int. Ed. 2023, 62, e202213733. [Google Scholar] [CrossRef]
- Zhuge, X.; Wang, J.; Zhuge, F. Photonic Synapses for Ultrahigh-Speed Neuromorphic Computing. Phys. Status Solidi (RRL) Rapid Res. Lett. 2019, 13, 1900082. [Google Scholar] [CrossRef]
- Dan, S.; Paramanik, S.; Pal, A.J. Introducing Chiro-optical Activities in Photonic Synapses for Neuromorphic Computing and In-Memory Logic Operations. ACS Nano 2024, 18, 14457–14468. [Google Scholar] [CrossRef]
- Zhang, Q.; Jin, T.; Ye, X.; Geng, D.; Chen, W.; Hu, W. Organic Field Effect Transistor-Based Photonic Synapses: Materials, Devices, and Applications. Adv. Funct. Mater. 2021, 31, 2106151. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, S.; Zhao, Y.; Zhang, J.; Huang, J. Recent Progress in Photonic Synapses for Neuromorphic Systems. Adv. Intell. Syst. 2020, 2, 1900136. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Guo, Y. Intrinsically flexible organic phototransistors for bioinspired neuromorphic sensory system. Wearable Electron. 2024, 1, 41–52. [Google Scholar] [CrossRef]
- Chen, K.; Hu, H.; Song, I.; Gobeze, H.B.; Lee, W.-J.; Abtahi, A.; Schanze, K.S.; Mei, J. Organic optoelectronic synapse based on photon-modulated electrochemical doping. Nat. Photonics 2023, 17, 629–637. [Google Scholar] [CrossRef]
- Hao, D.; Chen, T.; Guo, P.; Liu, D.; Wang, X.; Huang, H.; Huang, J.; Shan, F.; Yang, Z. Artificial optoelectronic synaptic devices based on vertical organic field-effect transistors with low energy consumption. Adv. Compos. Hybrid Mater. 2023, 6, 129. [Google Scholar] [CrossRef]
- Li, Y.; Shen, G. Advances in optoelectronic artificial synapses. Cell Rep. Phys. Sci. 2022, 3, 101037. [Google Scholar] [CrossRef]
- Lee, H.; Jiang, Z.; Yokota, T.; Fukuda, K.; Park, S.; Someya, T. Stretchable organic optoelectronic devices: Design of materials, structures, and applications. Mater. Sci. Eng. R Rep. 2021, 146, 100631. [Google Scholar] [CrossRef]
- van Doremaele, E.R.W.; Ji, X.; Rivnay, J.; van de Burgt, Y. A retrainable neuromorphic biosensor for on-chip learning and classification. Nat. Electron. 2023, 6, 765–770. [Google Scholar] [CrossRef]
- Keene, S.T.; Lubrano, C.; Kazemzadeh, S.; Melianas, A.; Tuchman, Y.; Polino, G.; Scognamiglio, P.; Cinà, L.; Salleo, A.; van de Burgt, Y.; et al. A biohybrid synapse with neurotransmitter-mediated plasticity. Nat. Mater. 2020, 19, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, Q.; Shi, W.; Liu, Y.; Liu, K.; Yang, X.; Shao, M.; Guo, A.; Huang, X.; Zhang, F.; et al. Ultralow-Power and Multisensory Artificial Synapse Based on Electrolyte-Gated Vertical Organic Transistors. Adv. Funct. Mater. 2022, 32, 2200959. [Google Scholar] [CrossRef]
- Wan, C.; Cai, P.; Guo, X.; Wang, M.; Matsuhisa, N.; Yang, L.; Lv, Z.; Luo, Y.; Loh, X.J.; Chen, X. An artificial sensory neuron with visual-haptic fusion. Nat. Commun. 2020, 11, 4602. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, W.; Ni, Y.; Xu, Z.; Cui, B.; Liu, J.; Wei, H.; Xu, W. Stretchable Neuromorphic Transistor That Combines Multisensing and Information Processing for Epidermal Gesture Recognition. ACS Nano 2022, 16, 2282–2291. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Gao, L.; Liu, C.; Guo, H.; Yu, J. Biomimetic Neuromorphic Sensory System via Electrolyte Gated Transistors. Sensors 2024, 24, 4915. https://doi.org/10.3390/s24154915
Li S, Gao L, Liu C, Guo H, Yu J. Biomimetic Neuromorphic Sensory System via Electrolyte Gated Transistors. Sensors. 2024; 24(15):4915. https://doi.org/10.3390/s24154915
Chicago/Turabian StyleLi, Sheng, Lin Gao, Changjian Liu, Haihong Guo, and Junsheng Yu. 2024. "Biomimetic Neuromorphic Sensory System via Electrolyte Gated Transistors" Sensors 24, no. 15: 4915. https://doi.org/10.3390/s24154915





