A Rapid Nanofocusing Method for a Deep-Sea Gene Sequencing Microscope Based on Critical Illumination
Abstract
:1. Introduction
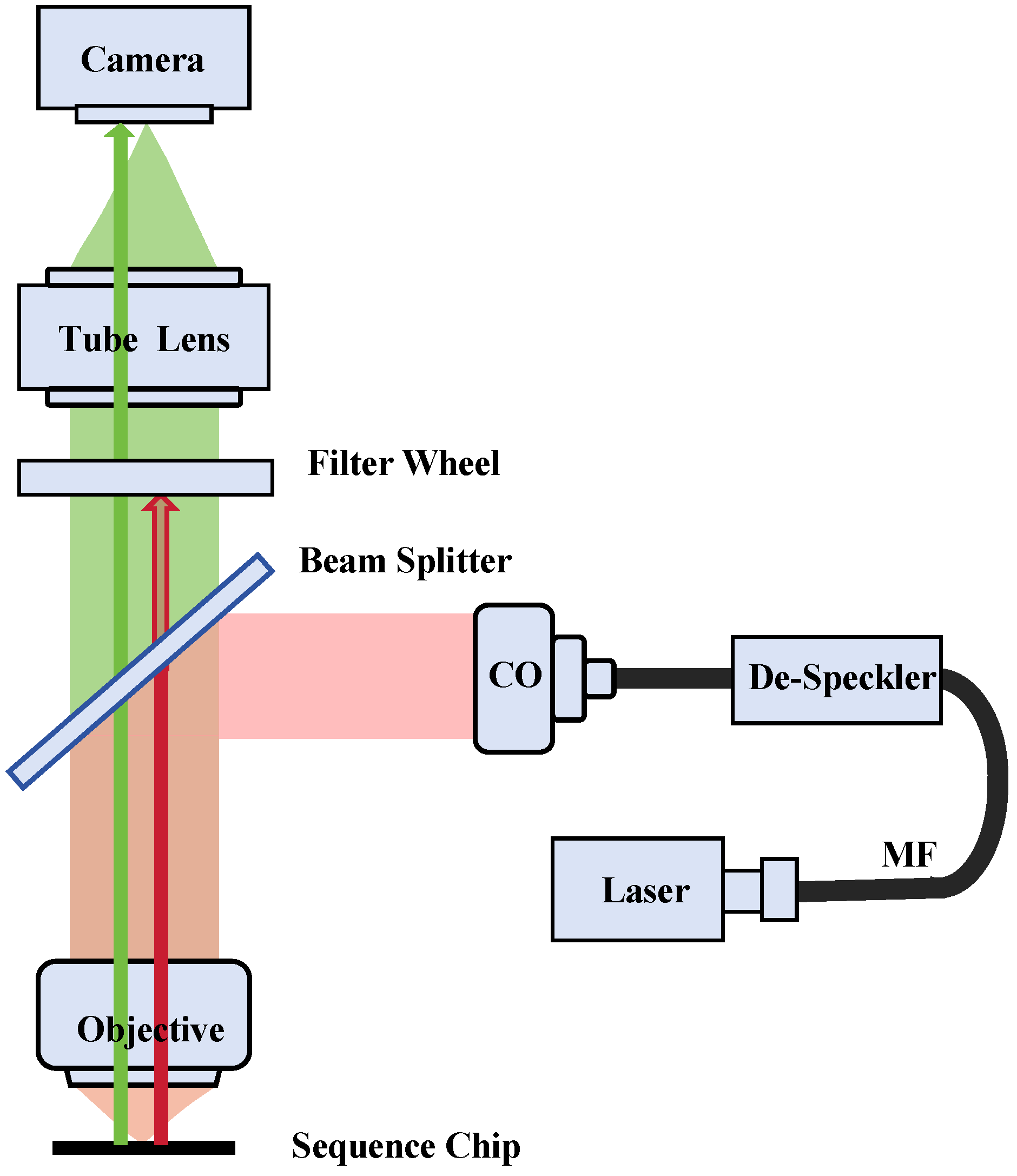
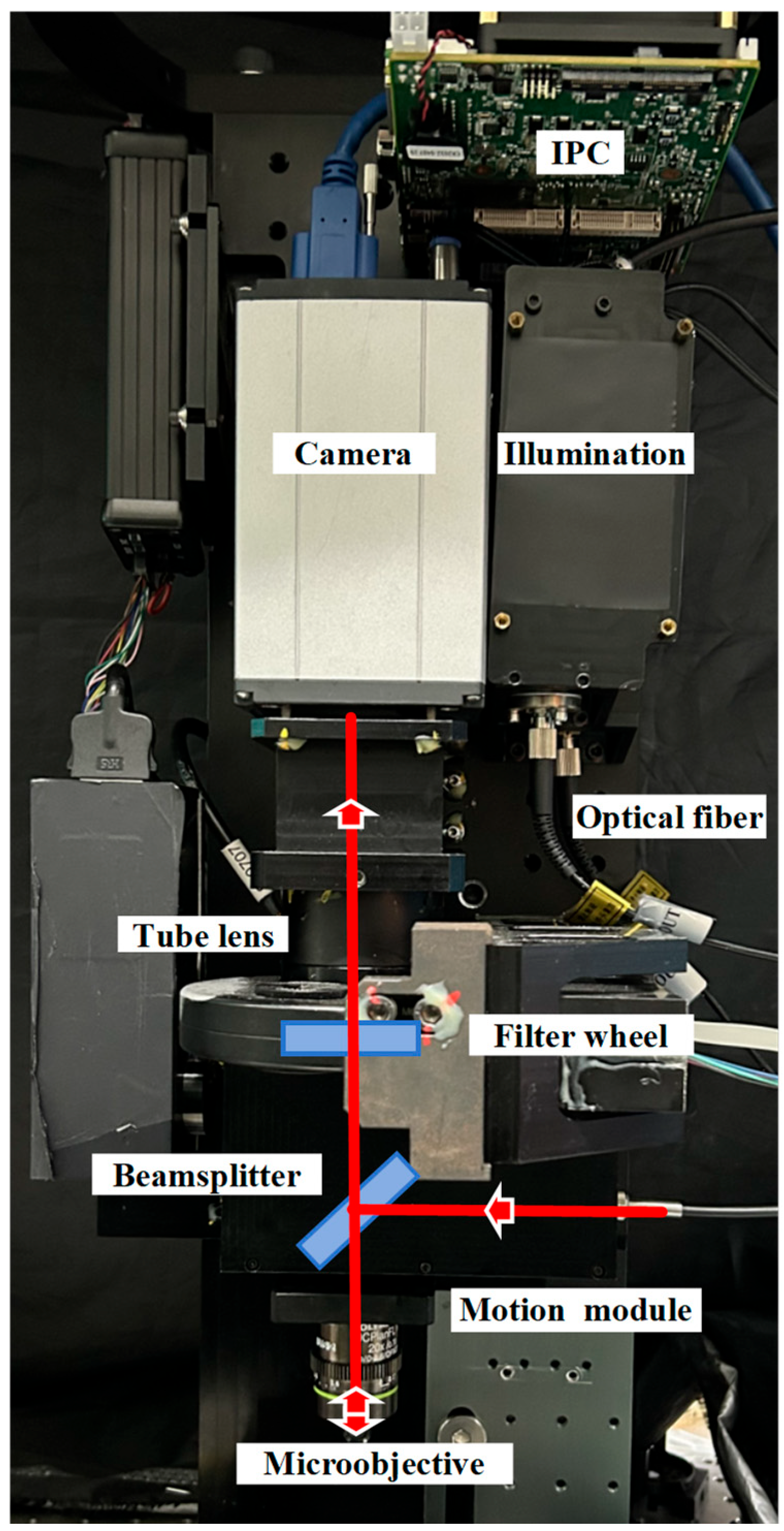
2. Methods
3. Results
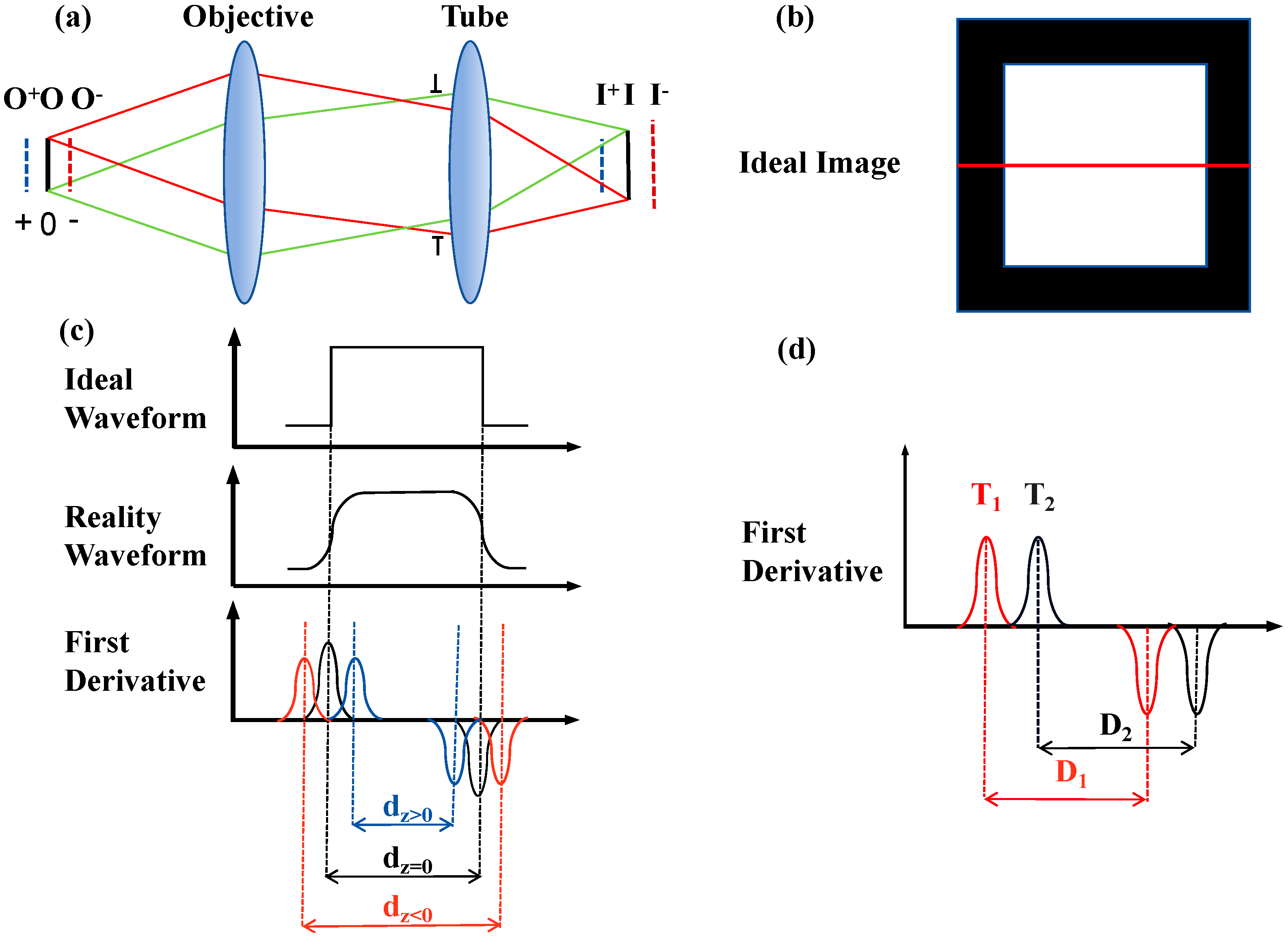
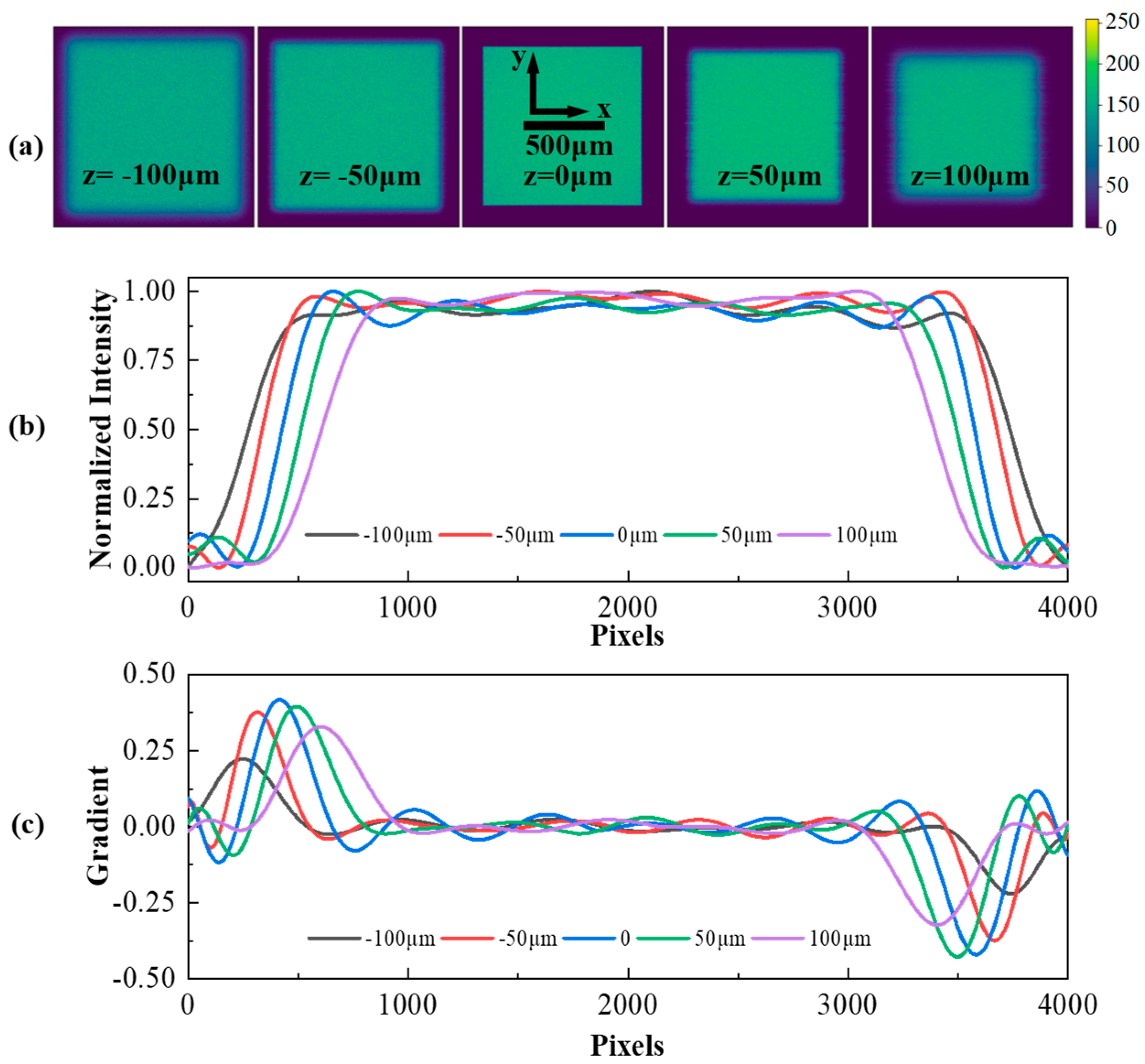
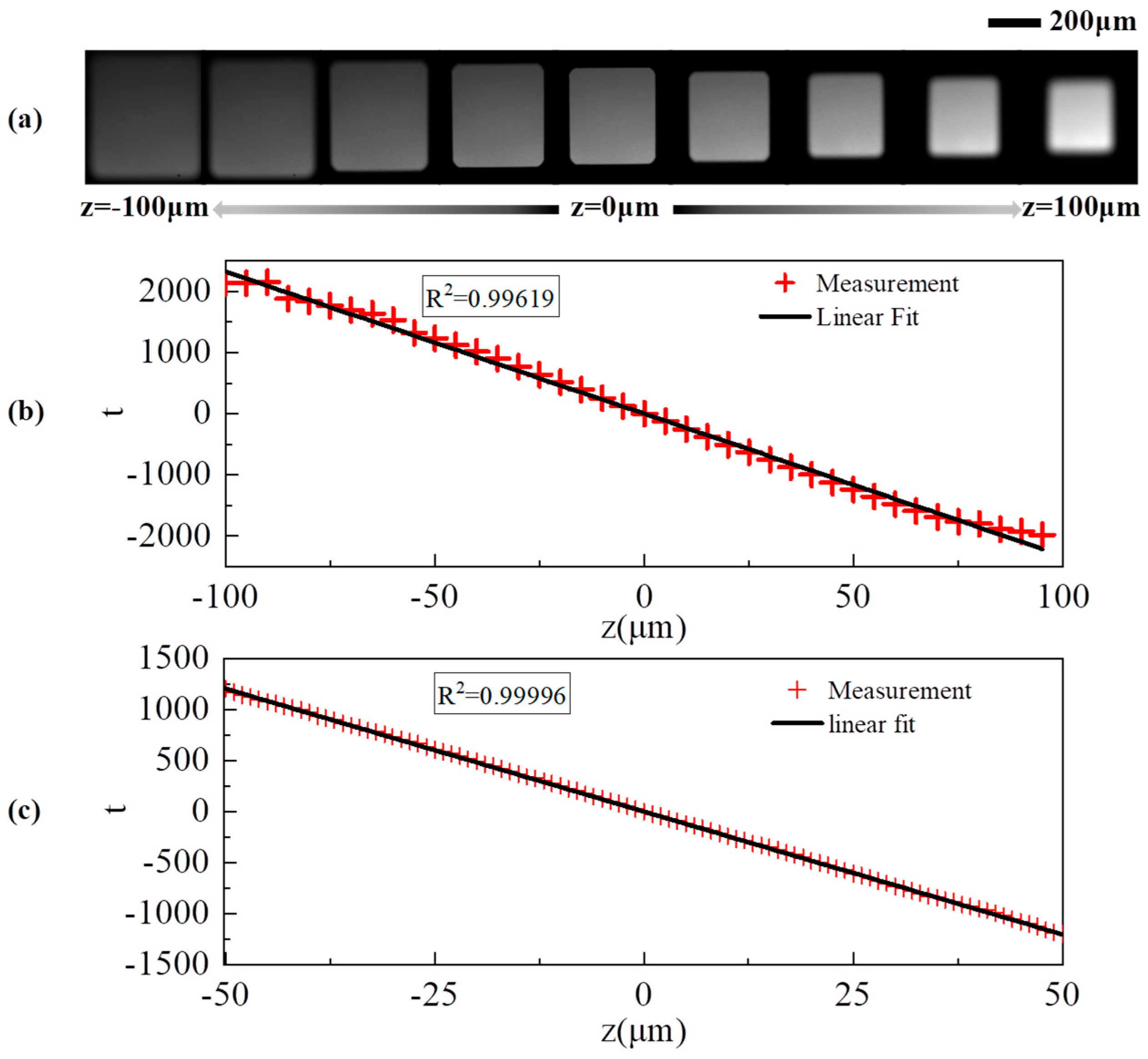
3.1. Simulation Analysis
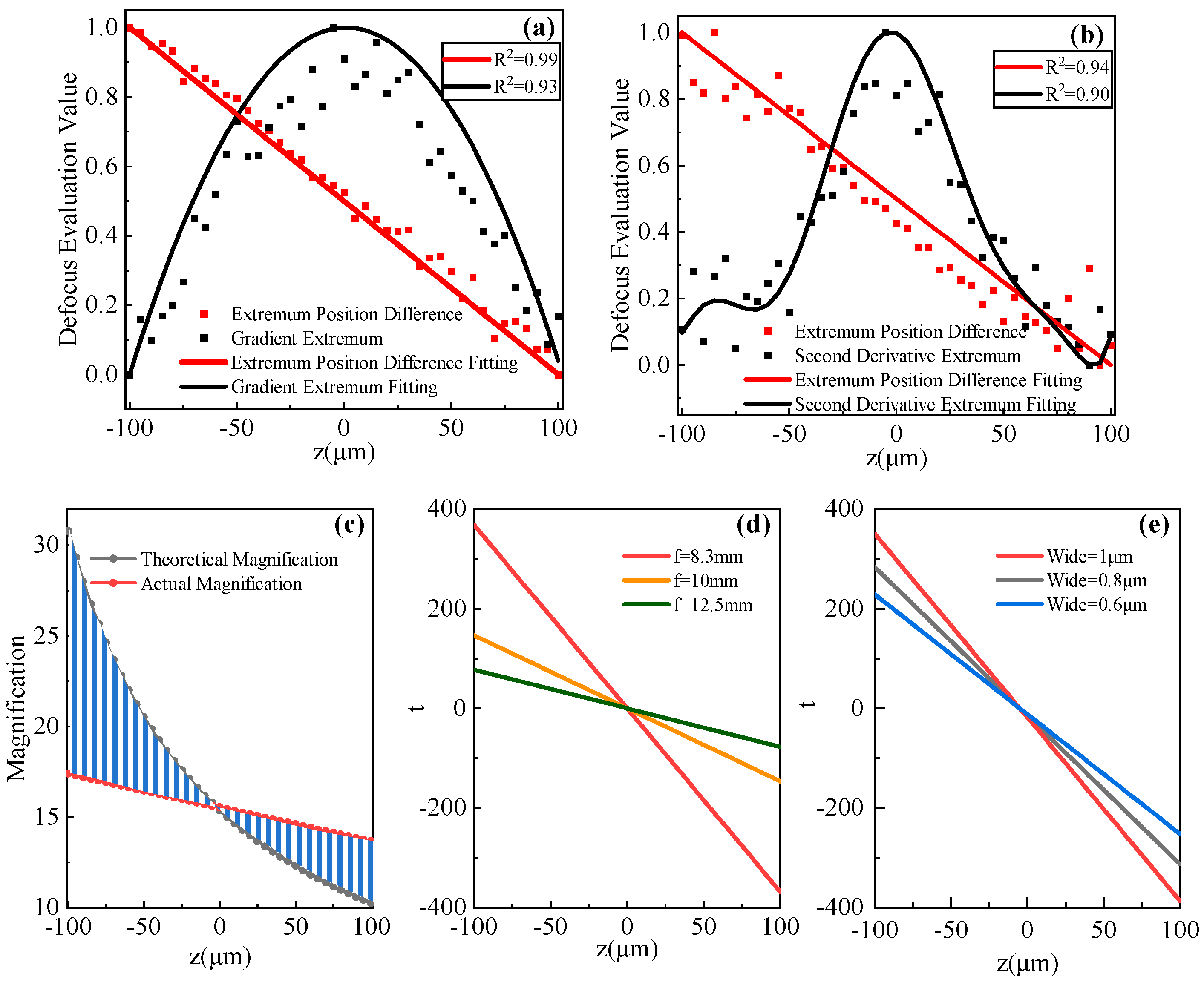
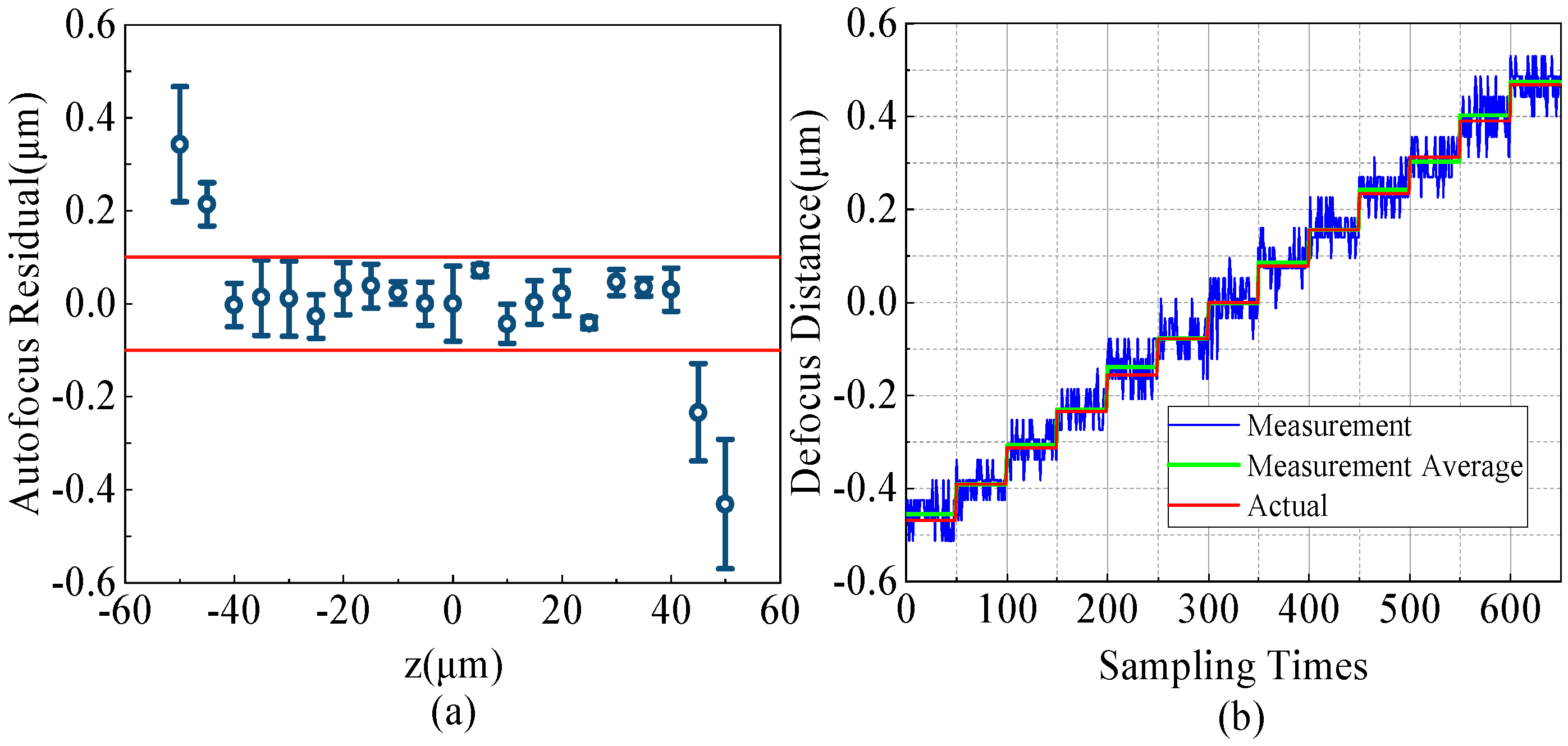
3.2. Accuracy Test
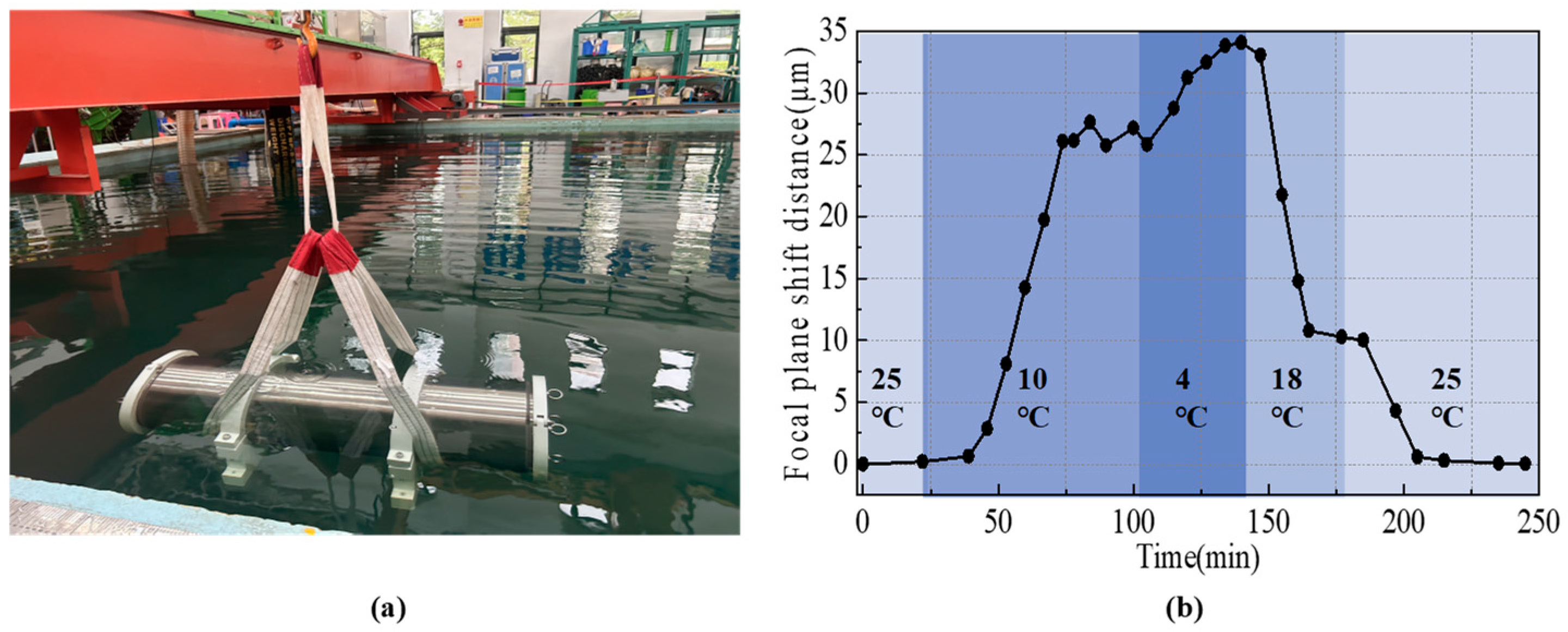
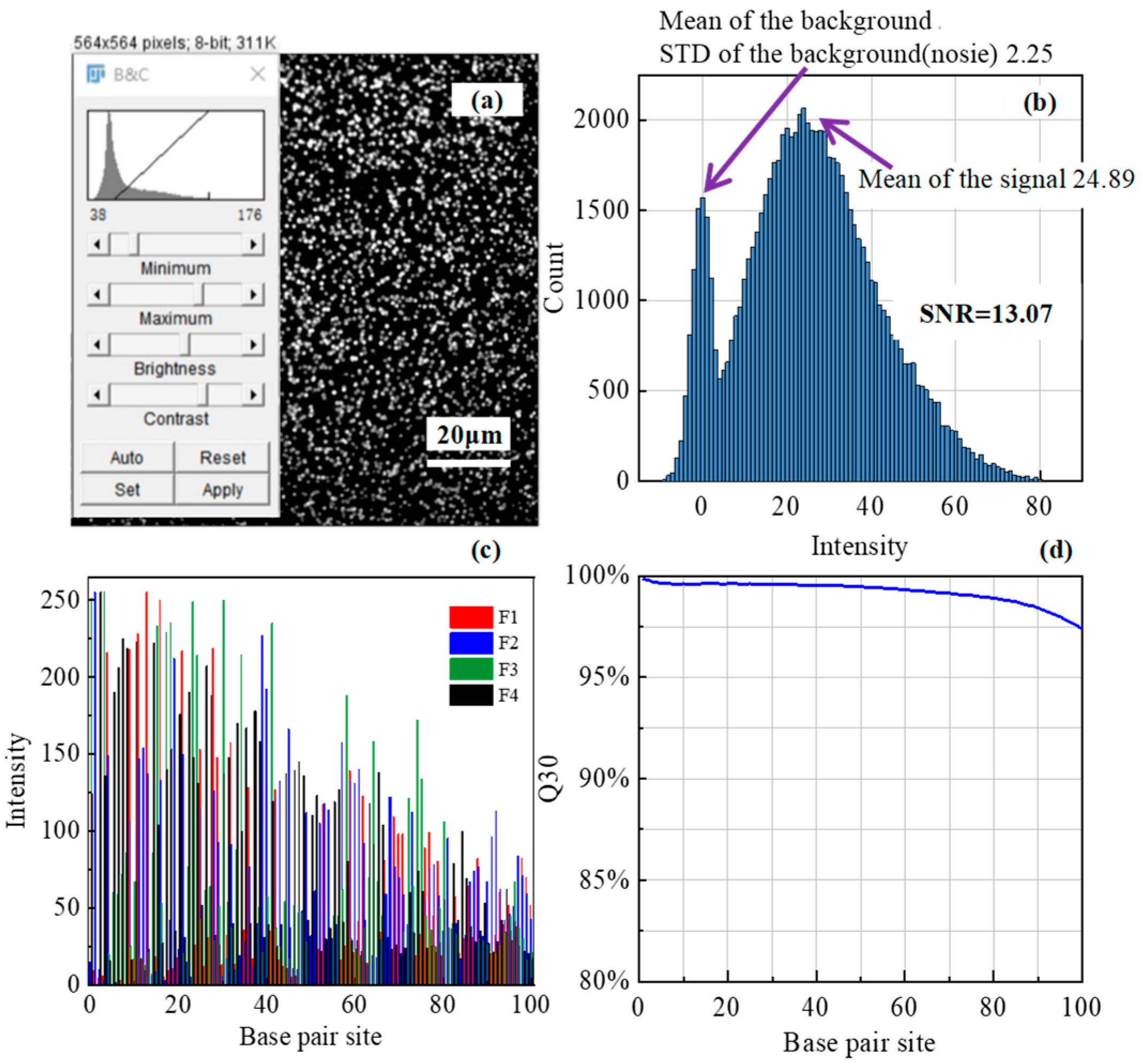
3.3. Sequencing Experiment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, M.; Gai, Y.; Guo, X.; Hou, Y.; Zeng, R. Properties and applications of extremozymes from deep-sea extremophilic microorganisms: A mini review. Mar. Drugs 2019, 17, 656. [Google Scholar] [CrossRef]
- Ge, M.; Li, L.; Zhang, X.; Luan, Z.; Du, Z.; Xi, S.; Yan, J. A Piecewise Model for In Situ Raman Measurement of the Chlorinity of Deep-Sea High-Temperature Hydrothermal Fluids. Appl. Spectrosc. 2021, 75, 1178–1188. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Xiao, X.; Xu, J. An integrative genomic island affects the adaptations of the piezophilic hyperthermophilic archaeon Pyrococcus yayanosii to high temperature and high hydrostatic pressure. Front. Microbiol. 2016, 7, 228167. [Google Scholar] [CrossRef]
- Li, L.; Du, Z.; Zhang, X.; Xi, S.; Wang, B.; Luan, Z.; Lian, C.; Yan, J. In situ Raman spectral characteristics of carbon dioxide in a deep-sea simulator of extreme environments reaching 300 °C and 30 MPa. Appl. Spectrosc. 2018, 72, 48–59. [Google Scholar] [CrossRef]
- He, T.; Li, H.; Zhang, X. Deep-sea hydrothermal vent viruses compensate for microbial metabolism in virus-host interactions. mBio 2017, 8, e00893-17. [Google Scholar] [CrossRef]
- Chen, X.; Tang, K.; Zhang, M.; Liu, S.; Chen, M.; Zhan, P.; Fan, W.; Chen, C.-T.A.; Zhang, Y. Genome-centric insight into metabolically active microbial population in shallow-sea hydrothermal vents. Microbiome 2022, 10, 170. [Google Scholar] [CrossRef]
- Raddadi, N.; Cherif, A.; Daffonchio, D.; Neifar, M.; Fava, F. Biotechnological applications of extremophiles, extremozymes and extremolytes. Appl. Microbiol. Biotechnol. 2015, 99, 7907–7913. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Gao, X.; Wang, K.; Wang, W.; Wang, Q.; Yan, P. Isolation of a novel deep-sea Bacillus circulus strain and uniform design for optimization of its anti-aflatoxigenic bioactive metabolites production. Bioengineered 2019, 10, 13–22. [Google Scholar] [CrossRef]
- Guardiola, M.; Uriz, M.J.; Taberlet, P.; Coissac, E.; Wangensteen, O.S.; Turon, X. Deep-sea, deep-sequencing: Metabarcoding extracellular DNA from sediments of marine canyons. PLoS ONE 2015, 10, e0139633. [Google Scholar] [CrossRef]
- Laroche, O.; Kersten, O.; Smith, C.R.; Goetze, E. From sea surface to seafloor: A benthic allochthonous eDNA survey for the abyssal ocean. Front. Mar. Sci. 2020, 7, 682. [Google Scholar] [CrossRef]
- Edgcomb, V.; Taylor, C.; Pachiadaki, M.; Honjo, S.; Engstrom, I.; Yakimov, M. Comparison of Niskin vs. in situ approaches for analysis of gene expression in deep Mediterranean Sea water samples. Deep Sea Res. Part II Top. Stud. Oceanogr. 2016, 129, 213–222. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, J.; Xu, T.; Qiu, J.-W.; Qian, P.-Y. Phylogenetic relationships and adaptation in deep-sea mussels: Insights from mitochondrial genomes. Int. J. Mol. Sci. 2021, 22, 1900. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-C.; Liang, J.; Cai, Y.; Zhang, S.; Xue, J.; Yang, Z. Deep-sea organisms research oriented by deep-sea technologies development. Sci. Bull. 2022, 67, 1802–1816. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Feng, J.-C.; Zhang, S.; Cai, Y.; Yang, Z.; Ni, T.; Yang, H.-Y. Role of deep-sea equipment in promoting the forefront of studies on life in extreme environments. Iscience 2021, 24, 103299. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, L.; Wu, Q.; Liu, B.; Li, B. Design of a multi-band Raman tweezers objective for in situ studies of deep-sea microorganisms. Opt. Express 2023, 31, 36883–36902. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, J.; Lu, Y.; Wei, Z.; Liu, S.; Wu, L.; Ye, W.; Zheng, R.; Zhang, X. Underwater Raman microscopy—A novel in situ tool for deep-sea microscale target studies. Front. Mar. Sci. 2022, 9, 1018042. [Google Scholar] [CrossRef]
- Mullen, A.D.; Treibitz, T.; Roberts, P.L.; Kelly, E.L.; Horwitz, R.; Smith, J.E.; Jaffe, J.S. Underwater microscopy for in situ studies of benthic ecosystems. Nat. Commun. 2016, 7, 12093. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Lee, S.-Y.; Kim, S.; Lee, S.-H.; Jeong, S.-G. A new DNA chip detection mechanism using optical pick-up actuators. Microsyst. Technol. 2007, 13, 1359–1369. [Google Scholar] [CrossRef]
- Hua, Z.; Zhang, X.; Tu, D. Autofocus methods based on laser illumination. Opt. Express 2023, 31, 29465–29479. [Google Scholar] [CrossRef]
- Bathe-Peters, M.; Annibale, P.; Lohse, M. All-optical microscope autofocus based on an electrically tunable lens and a totally internally reflected IR laser. Opt. Express 2018, 26, 2359–2368. [Google Scholar] [CrossRef]
- Zhang, X.; Kang, K.; Jiang, Y.; He, J.; Qiao, Y. Double-layer focal plane microscopy for high throughput DNA sequencing. Opt. Express 2022, 30, 18496–18504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zeng, F.; Li, Y.; Qiao, Y. Improvement in focusing accuracy of DNA sequencing microscope with multi-position laser differential confocal autofocus method. Opt. Express 2018, 26, 887–896. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Chen, X.; Miao, L.; Kang, K.; Mo, C. High-robustness autofocusing method in the microscope with laser-based arrayed spots. Opt. Express 2024, 32, 4902–4915. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Ma, Y.; Wang, W. Rectangular Amplitude Mask-Based Auto-Focus Method with a Large Range and High Precision for a Micro-LED Wafer Defects Detection System. Sensors 2023, 23, 7579. [Google Scholar] [CrossRef]
- Guo, C.; Ma, Z.; Guo, X.; Li, W.; Qi, X.; Zhao, Q. Fast auto-focusing search algorithm for a high-speed and high-resolution camera based on the image histogram feature function. Appl. Opt. 2018, 57, F44–F49. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Yang, Y.; Wang, Z. Lens-free auto-focusing imaging algorithm for the ultra-broadband light source. Opt. Express 2024, 32, 2619–2630. [Google Scholar] [CrossRef]
- Liu, C.-S.; Lin, Y.-C.; Hu, P.-H. Design and characterization of precise laser-based autofocusing microscope with reduced geometrical fluctuations. Microsyst. Technol. 2013, 19, 1717–1724. [Google Scholar] [CrossRef]
- Montalto, M.C.; McKay, R.R.; Filkins, R.J. Autofocus methods of whole slide imaging systems and the introduction of a second-generation independent dual sensor scanning method. J. Pathol. Inf. 2011, 2, 44. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.A.; Mahecic, D.; Manley, S. Characterization of flat-fielding systems for quantitative microscopy. Opt. Express 2020, 28, 22036–22048. [Google Scholar] [CrossRef]
- Khaw, I.; Croop, B.; Tang, J.; Möhl, A.; Fuchs, U.; Han, K.Y. Flat-field illumination for quantitative fluorescence imaging. Opt. Express 2018, 26, 15276–15288. [Google Scholar] [CrossRef]
- Deschamps, J.; Rowald, A.; Ries, J. Efficient homogeneous illumination and optical sectioning for quantitative single-molecule localization microscopy. Opt. Express 2016, 24, 28080–28090. [Google Scholar] [CrossRef] [PubMed]
- Schröder, D.; Deschamps, J.; Dasgupta, A.; Matti, U.; Ries, J. Cost-efficient open source laser engine for microscopy. Biomed. Opt. Express 2020, 11, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Velsink, M.C.; Lyu, Z.; Pinkse, P.W.; Amitonova, L.V. Comparison of round-and square-core fibers for sensing, imaging, and spectroscopy. Opt. Express 2021, 29, 6523–6531. [Google Scholar] [CrossRef] [PubMed]
- Berry, K.; Taormina, M.; Maltzer, Z.; Turner, K.; Gorham, M.; Nguyen, T.; Serafin, R.; Nicovich, P.R. Characterization of a fiber-coupled EvenField illumination system for fluorescence microscopy. Opt. Express 2021, 29, 24349–24362. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Liu, S.; Wang, G.; Zhang, W.; Sun, C. Recent advances on image edge detection: A comprehensive review. Neurocomputing 2022, 503, 259–271. [Google Scholar] [CrossRef]
- Tariq, N.; Hamzah, R.A.; Ng, T.F.; Wang, S.L.; Ibrahim, H. Quality assessment methods to evaluate the performance of edge detection algorithms for digital image: A systematic literature review. IEEE Access 2021, 9, 87763–87776. [Google Scholar] [CrossRef]
- Saif, J.A.; Hammad, M.H.; Alqubati, I.A. Gradient based image edge detection. Int. J. Eng. Technol. 2016, 8, 153. [Google Scholar] [CrossRef]
- Vincent, O.R.; Folorunso, O. A descriptive algorithm for sobel image edge detection. In Proceedings of the Informing Science & IT Education Conference (InSITE), Macon, GA, USA, 12–15 June 2009; pp. 97–107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Shu, F.; Zhou, W.; Li, H.; Wu, Y.; Wang, Y.; Zhao, S.; Song, Z. A Rapid Nanofocusing Method for a Deep-Sea Gene Sequencing Microscope Based on Critical Illumination. Sensors 2024, 24, 5010. https://doi.org/10.3390/s24155010
Gao M, Shu F, Zhou W, Li H, Wu Y, Wang Y, Zhao S, Song Z. A Rapid Nanofocusing Method for a Deep-Sea Gene Sequencing Microscope Based on Critical Illumination. Sensors. 2024; 24(15):5010. https://doi.org/10.3390/s24155010
Chicago/Turabian StyleGao, Ming, Fengfeng Shu, Wenchao Zhou, Huan Li, Yihui Wu, Yue Wang, Shixun Zhao, and Zihan Song. 2024. "A Rapid Nanofocusing Method for a Deep-Sea Gene Sequencing Microscope Based on Critical Illumination" Sensors 24, no. 15: 5010. https://doi.org/10.3390/s24155010





