One-Pot Preparation of Ratiometric Fluorescent Molecularly Imprinted Polymer Nanosensor for Sensitive and Selective Detection of 2,4-Dichlorophenoxyacetic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Characterization
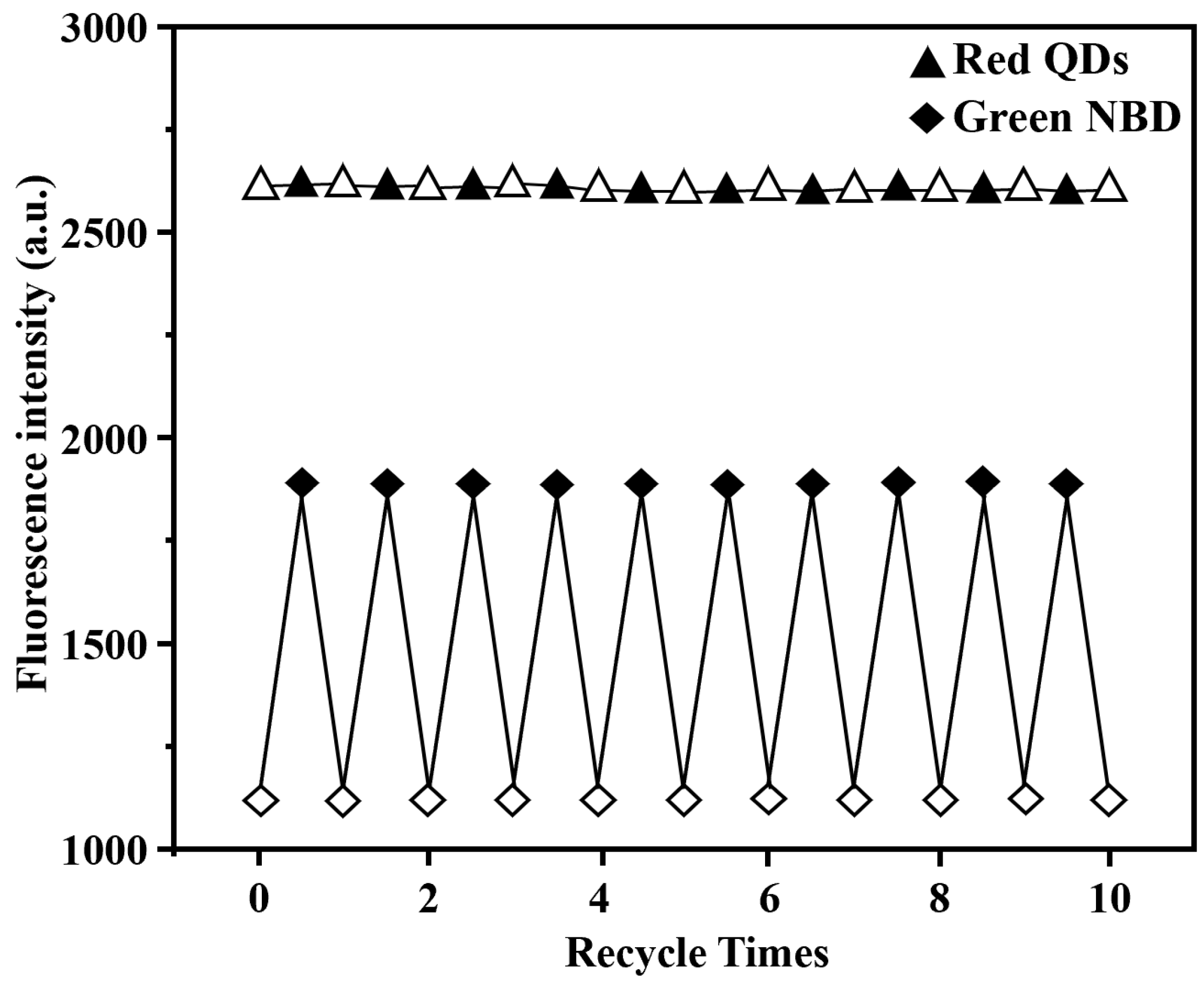
3. Results and Discussion
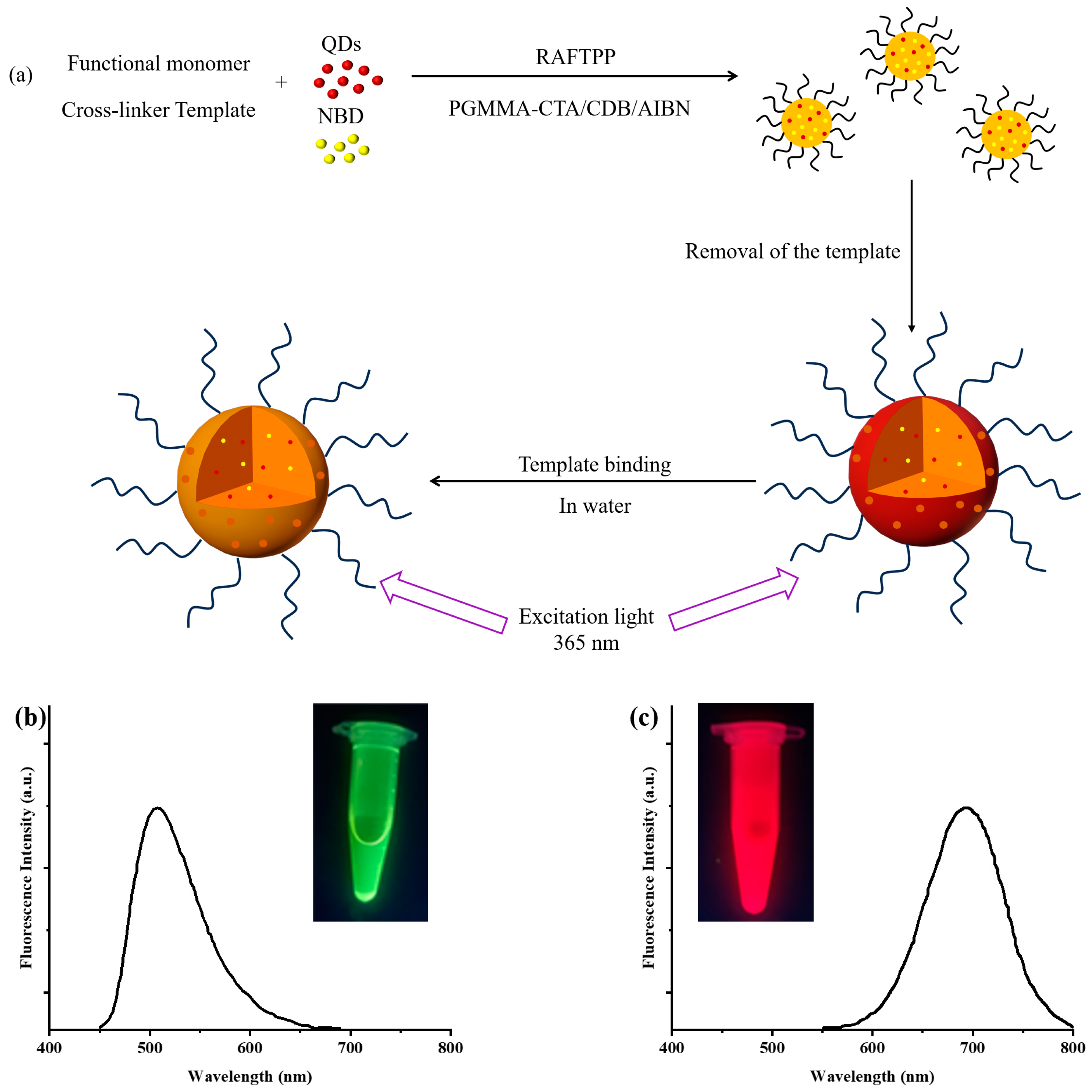
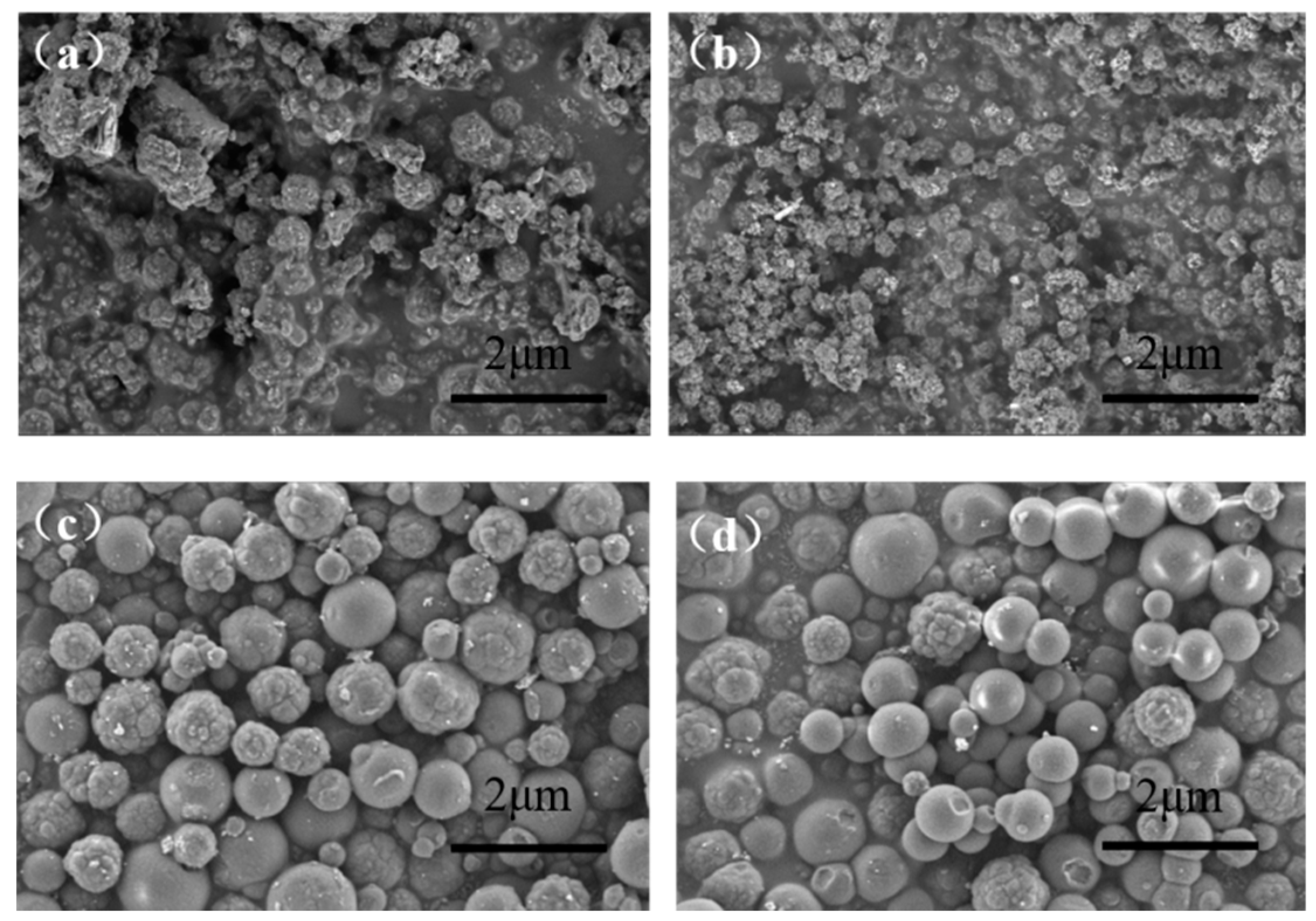
3.1. Synthesis of Hydrophilic 2,4-D-MIP/2,4-D-CP Colloidal Nanoparticles with Dual-Fluorescent Labels
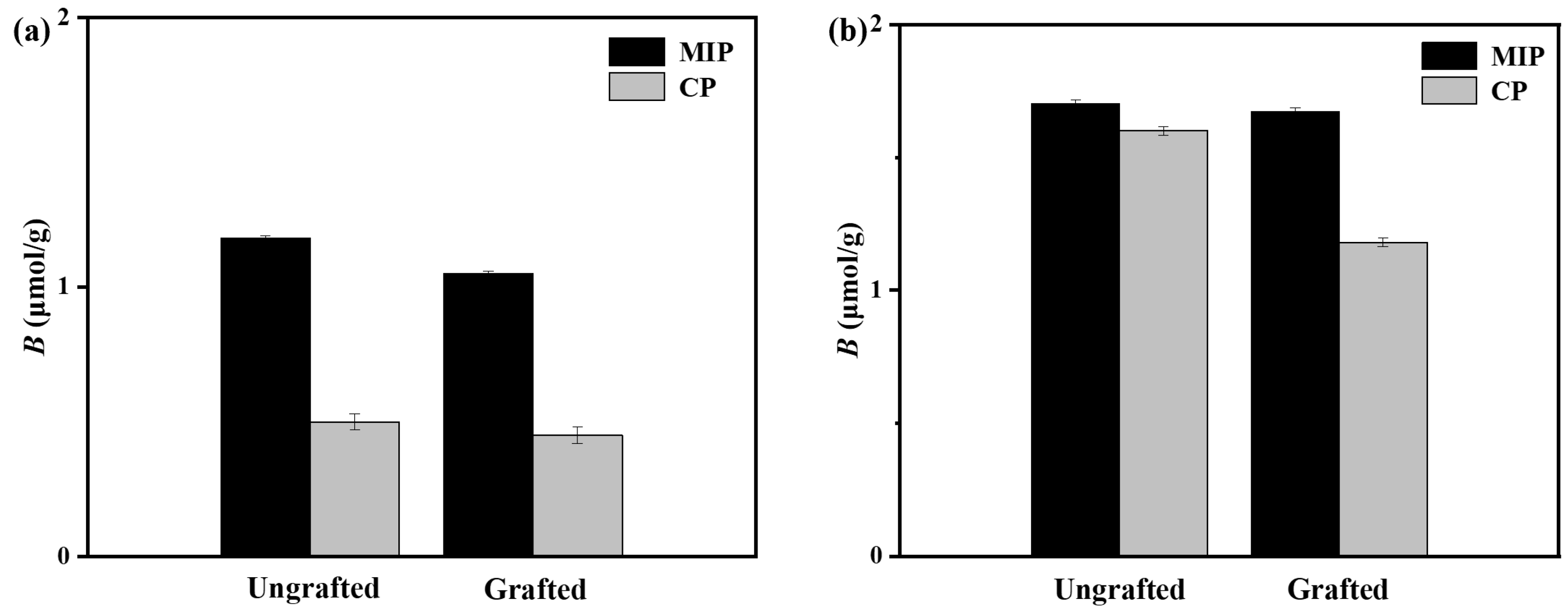
3.2. Equilibrium Adsorption Performance of Grafted and Ungrafted Dual-Fluorescently Labeled Molecularly Imprinted Polymers
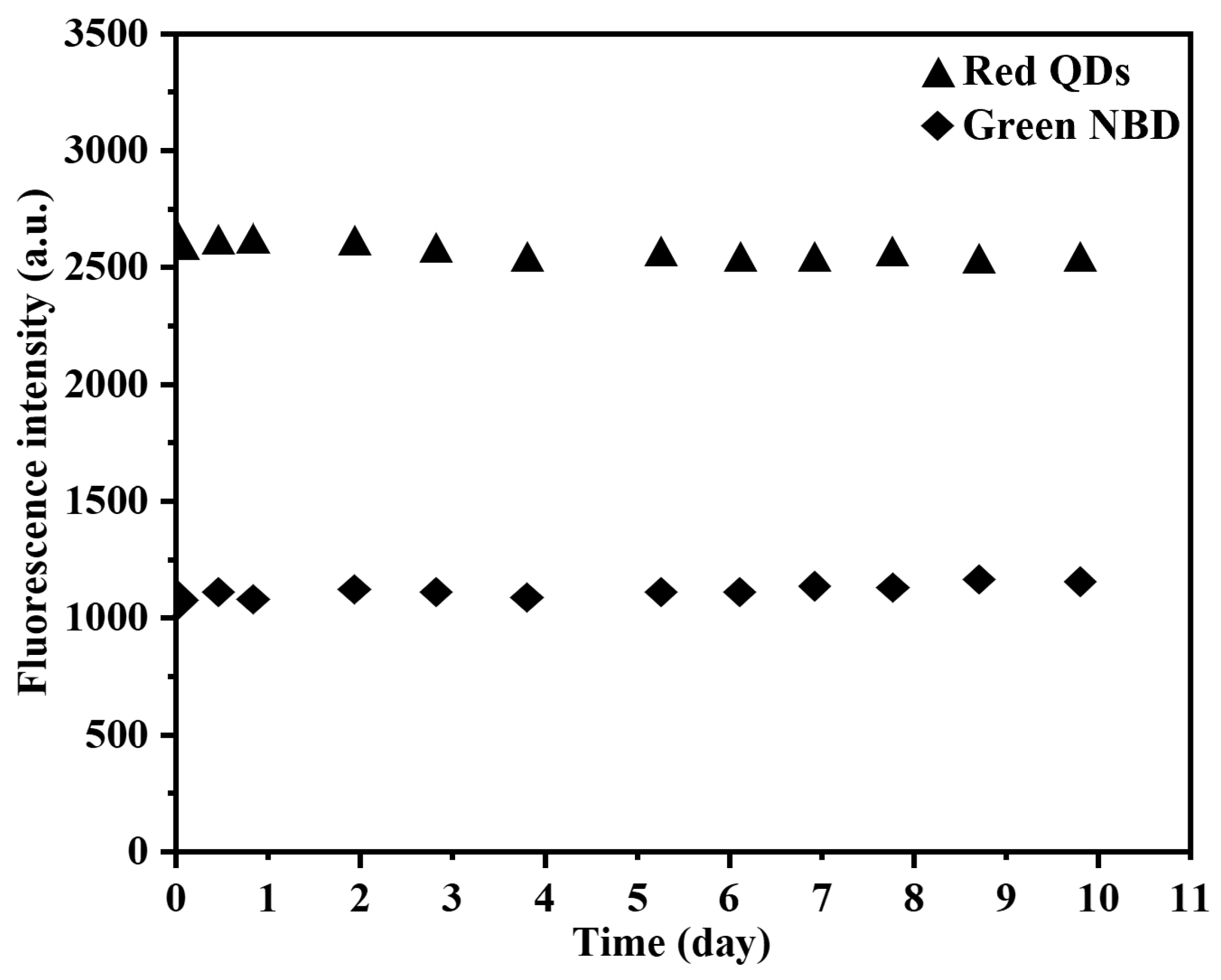
3.3. Optical Sensing Properties of Grafted NBD,QD-Labeled 2,4-D-MIP/2,4-D-CP Nanoparticles in Aqueous System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Q.; Li, J.H.; Wang, X.Y.; Peng, H.L.; Xiong, H.; Chen, L.X. Strategies of molecular imprinting-based fluorescence sensors for chemical and biological analysis. Biosens. Bioelectron. 2018, 112, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Zhao, L.S. A novel nanocomposite optosensing sensor based on porous molecularly imprinted polymer and dual emission quantum dots for visual and high selective detection of bovine serum albumin. Colloids Surf. 2022, 632, 127843. [Google Scholar] [CrossRef]
- Adegoke, O.; Zolotovskaya, S.; Abdolvand, A.; Daeid, N.N. Fabrication of a near-infrared fluorescence-emitting SiO2-AuZnFeSeS quantum dots-molecularly imprinted polymer nanocomposite for the ultrasensitive fluorescence detection of levamisole. Colloids Surf. 2022, 646, 129013. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.; Park, H.; Nam, M.S.; Heo, Y.J.; Kim, S. Batteryless, Miniaturized Implantable Glucose Sensor Using a Fluorescent Hydrogel. Sensors 2021, 21, 8464. [Google Scholar] [CrossRef]
- Chen, L.X.; Wang, X.Y.; Lu, W.H.; Wu, X.Q.; Li, J.H. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Hang, Y.J.; Boryczka, J.; Wu, N.Q. Visible-light and near-infrared fluorescence and surface-enhanced Raman scattering point-of-care sensing and bio-imaging: A review. Chem. Soc. Rev. 2022, 51, 329–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Lin, W.H.; Cao, E.; Xu, X.F.; Liang, W.J.; Zhang, X.F. Surface Plasmon Resonance Sensors on Raman and Fluorescence Spectroscopy. Sensors 2017, 17, 2719. [Google Scholar] [CrossRef]
- Chu, C.Y.; Brazier, J.J.; Yan, M.D.; Bargo, P.R.; Prahl, S.A. Fluorescence-based optical sensor design for molecularly imprinted polymers. Sens. Actuators B Chem. 2004, 102, 107–116. [Google Scholar]
- Ansari, S.; Masoum, S. Recent advances and future trends on molecularly imprinted polymer-based fluorescence sensors with luminescent carbon dots. Talanta 2021, 223, 121411. [Google Scholar] [CrossRef]
- Sun, B.W.; Wang, C.S.; Han, S.H.; Hu, Y.F.; Zhang, L.J. Metal-enhanced fluorescence-based multilayer core–shell Ag-nanocube@SiO2@PMOs nanocomposite sensor for Cu2+ detection. RSC Adv. 2016, 6, 61109–61118. [Google Scholar] [CrossRef]
- Xu, X.Y.; Yan, B.; Lian, X. Wearable glove sensor for non-invasive organophosphorus pesticide detection based on a double-signal fluorescence strategy. Nanoscale 2018, 10, 13722–13729. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Zhang, Y.W.; Lv, X.J.; Fan, D.Q.; Dong, S.J. A facile, low-cost bimetallic iron-nickel MOF nanozyme-propelled ratiometric fluorescent sensor for highly sensitive and selective uric acid detection and its smartphone application. Nanoscale 2024, 16, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Zarejousheghani, M.; Rahimi, P.; Borsdorf, H.; Zimmermann, S.; Joseph, Y. Molecularly Imprinted Polymer-Based Sensors for Priority Pollutants. Sensors 2021, 21, 2406. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, S.; Singh, S.P.; Patel, S.S. Recent advancement of carbon nanomaterials engrained molecular imprinted polymer for environmental matrix. Trends Environ. Anal. Chem. 2020, 27, e00092. [Google Scholar] [CrossRef]
- Cao, Y.R.; Feng, T.Y.; Xu, J.; Xue, C.H. Recent advances of molecularly imprinted polymer-based sensors in the detection of food safety hazard factors. Biosens. Bioelectron. 2019, 141, 111447. [Google Scholar] [CrossRef] [PubMed]
- Barla, L.S.; Pattnaik, G.P.; Meher, G.; Padhan, S.K.; Sahu, S.N.; Chakraborty, H. Fluorescence-based ion sensing in lipid membranes: A simple method of sensing in aqueous medium with enhanced efficiency. RSC Adv. 2019, 9, 31030–31034. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.N.; Chen, H.; Zheng, X.M.; Cao, J.S.; Liu, X.Y. Ratiometric fluorescent nanosensor based on water soluble carbon nanodots with multiple sensing capacities. Nanoscale 2013, 5, 5514–5518. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.R.; Chen, S.; Liu, Y.L.; Zhuang, J.L.; Lei, B.F. Synthesis of molecularly imprinted carbon dot grafted YVO4:Eu3+ for the ratiometric fluorescent determination of paranitrophenol. Biosens. Bioelectron. 2016, 86, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Q.; Huang, T.; Meng, M.J.; Ya, Y.S. Fluorescent polydopamine based molecularly imprinted sensor for ultrafast and selective detection of p-nitrophenol in drinking water. Mikrochim. Acta 2021, 189, 25. [Google Scholar] [CrossRef]
- Surya, S.G.; Khatoon, S.; Lahcen, A.A.; Nguyen, A.T.H.; Dzantiev, B.B.; Tarannum, N.; Salama, K.N. A chitosan gold nanoparticles molecularly imprinted polymer based ciprofloxacin sensor. RSC Adv. 2020, 10, 12823–12832. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Hardwick, S.A.; Sun, T.; Grattan, K.T.V. Intrinsic Fluorescence-Based Optical Fiber Sensor for Cocaine Using a Molecularly Imprinted Polymer as the Recognition Element. IEEE Sens. J. 2012, 12, 255–260. [Google Scholar] [CrossRef]
- Chao, M.R.; Hu, C.W.; Che, J.L. Glass substrates crosslinked with tetracycline-imprinted polymeric silicate and CdTe quantum dots as fluorescent sensors. Anal. Chim. Acta 2016, 925, 61–69. [Google Scholar] [CrossRef]
- Bai, J.W.; Chen, L.Q.; Zhu, Y.W.; Wang, X.Q.; Wu, X.D.; Fu, Y.J. A novel luminescence sensor based on porous molecularly imprinted polymer-ZnS quantum dots for selective recognition of paclitaxel. Colloids Surf. 2021, 610, 125696. [Google Scholar] [CrossRef]
- Yang, J.; Lin, Z.Z.; Nur, A.Z.; Lu, Y.; Wu, M.H.; Zeng, J.; Chen, X.M.; Huang, Z.Y. Detection of trace tetracycline in fish via synchronous fluorescence quenching with carbon quantum dots coated with molecularly imprinted silica. Spectrochim. Acta Part A 2018, 190, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.H.; Cheng, K.K.; Wu, Y.T.; Yu, P.F. Environment-friendly ZnO-based molecularly imprinting polymers fluorescence sensor for direct detection of sulfadimidine. J. Mater. Sci. Mater. Electron. 2020, 31, 9550–9558. [Google Scholar] [CrossRef]
- Zhu, L.; Shi, L.J.; Tan, Y.P.; Zhang, H.Y.; Yin, J.C.; Xu, C.; Wua, D.L.; Ma, Y.S. Dual-emissive ratiometric fluorescent nanosensor based on multi-nanomaterials for Ag+ determination in lake water. RSC Adv. 2022, 12, 30113–30119. [Google Scholar] [CrossRef]
- Qi, J.; Li, B.W.; Wang, X.Y.; Fu, L.W.; Luo, L.Q.; Chen, L.X. Rotational Paper-Based Microfluidic-Chip Device for Multiplexed and Simultaneous Fluorescence Detection of Phenolic Pollutants Based on a Molecular-Imprinting Technique. Anal. Chem. 2018, 90, 11827–11834. [Google Scholar] [CrossRef]
- Liu, G.Y.; Li, T.F.; Yang, X.; She, Y.X.; Wang, M.; Wang, J.; Zhang, M.; Wang, S.S.; Jin, F.; Jin, M.J.; et al. Competitive fluorescence assay for specific recognition of atrazine by magnetic molecularly imprinted polymer based on Fe3O4-chitosan. Carbohydr. Polym. 2016, 137, 75–81. [Google Scholar] [CrossRef]
- Xu, H.Q.; Gao, Y.H.; Tao, Q.T.; Li, A.P.; Liu, Z.C.; Jiang, Y.H.; Liu, H.W.; Yang, R.G.; Liu, Y. Synthesizing a surface-imprinted polymer based on the nanoreactor SBA-15 for optimizing the adsorption of salicylic acid from aqueous solution by response surface methodology. New J. Chem. 2021, 45, 6192–6205. [Google Scholar] [CrossRef]
- Wu, H.Y.; Zheng, W.; Jiang, Y.; Xu, J.C.; Qiu, F.X. Construction of a selective non-enzymatic electrochemical sensor based on hollow nickel nanospheres/carbon dots–chitosan and molecularly imprinted polymer film for the detection of glucose. New J. Chem. 2021, 45, 21676–21683. [Google Scholar] [CrossRef]
- Whitcombe, M.J.; Chianella, I.; Larcombe, L.; Piletsky, S.A.; Noble, J.; Porter, R.; Horgan, A. The rational development of molecularly imprinted polymer-based sensors for protein detection. Chem. Soc. Rev. 2011, 40, 1547–1571. [Google Scholar] [CrossRef]
- Xu, S.J.; Zou, Y.W.; Zhang, H.Q. Well-defined hydrophilic “turn-on”-type ratiometric fluorescent molecularly imprinted polymer microspheres for direct and highly selective herbicide optosensing in the undiluted pure milks. Talanta 2020, 211, 120711. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Fang, G.Z.; Liu, Y.Y.; Zhang, D.D.; Liu, J.M.; Wang, S. Fluorescent Sensing Probe for the Sensitive Detection of Histamine Based on Molecular Imprinting Ionic Liquid-Modified Quantum Dots. Food Anal. Methods 2017, 10, 2585–2592. [Google Scholar] [CrossRef]
- Cheubong, C.; Takano, E.; Kitayama, Y.; Sunayama, H.; Minamoto, K.; Takeuchi, R.; Furutani, S.; Takeuchi, T. Molecularly imprinted polymer nanogel-based fluorescence sensing of pork contamination in halal meat extracts. Biosens. Bioelectron. 2021, 172, 112775. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, C.Y.; Li, J.H.; Wang, X.Y.; Arabi, M.; Peng, H.L.; Xiong, H.; Chen, L.X. Rational construction of a triple emission molecular imprinting sensor for accurate naked-eye detection of folic acid. Nanoscale 2020, 12, 6529–6536. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.E.; Chang, Y.W.; Sharma, A.; Tutt, S. One-Step Assembly of Fluorescence-Based Cyanide Sensors from Inexpensive, Off-The-Shelf Materials. Sensors 2020, 20, 4488. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.F.; Lu, H.Z. One-pot synthesis of mesoporous structured ratiometric fluorescence molecularly imprinted sensor for highly sensitive detection of melamine from milk samples. Biosens. Bioelectron. 2015, 73, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Jia, M.X.; Zhao, H.P.; Kang, L.Z.; Shi, L.C.; Zhou, L.D.; Kong, W.J. Molecularly imprinted polymer-based optical sensors for pesticides in foods: Recent advances and future trends. Trends Food Sci. Technol. 2021, 116, 387–404. [Google Scholar] [CrossRef]
- Hasaneen, N.; Akhtarian, S.; Pulicharla, R.; Brar, S.K.; Rezai, P. Surface molecularly imprinted polymer-based sensors for antibiotic detection. TrAC Trends Anal. Chem. 2024, 170, 117389. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Sun, T.; Grattan, K.T.V. A Turn-On Fluorescence-Based Fibre Optic Sensor for the Detection of Mercury. Sensors 2019, 19, 2142. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Gong, Z.J.; Yang, S.W.; Xiong, T.T.; Wang, D.M.; Fan, M.K. Fluorescent and visual detection of norfloxacin in aqueous solutions with a molecularly imprinted polymer coated paper sensor. Talanta 2020, 208, 120435. [Google Scholar] [CrossRef] [PubMed]
- Limaee, N.Y.; Rouhani, S.; Olya, M.E.; Najaf, F. Selective Recognition of Herbicides in Water Using a Fluorescent Molecularly Imprinted Polymer Sensor. J. Fluoresc. 2020, 30, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, Y.; Peng, R.; Cui, Q.L.; Luo, Y.F.; Li, L.D. Co-precipitation method to prepare molecularly imprinted fluorescent polymer nanoparticles for paracetamol sensing. Colloids Surf. 2020, 587, 124342. [Google Scholar] [CrossRef]
- Du, Q.Z.; Wu, P.; Hu, F.; Li, G.Y.; Shi, J.R.; He, H. A novel molecularly imprinted polymers on metal–organic frameworks as sensors for the highly selective detection of zearalenone in wheat. New J. Chem. 2019, 43, 7044–7050. [Google Scholar] [CrossRef]
- Li, P.P.; Du, Y.X.; Ma, M.X.; Zhang, J. Nitrogen-doped graphene quantum dots coated with molecularly imprinted polymers as a fluorescent sensor for selective determination of warfarin. New J. Chem. 2022, 46, 7537–7544. [Google Scholar] [CrossRef]
- Shaw, S.E.; Russo, T.; Solomon, D.H.; Qiao, G.G. An alternative pathway for the hydrolysis of epoxy ester compounds. Polymer 2006, 47, 8247–8252. [Google Scholar] [CrossRef]
- Dijk-Wolthuis, W.N.E.V.; Franssen, O.; Talsma, H.; Steenbergen, M.J.V.; Bosch, J.J.K.V.D.; Hennink, W.E. Synthesis, Characterization, and Polymerization of Glycidyl Methacrylate Derivatized Dextran. Macromolecules 1995, 28, 6317–6322. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.P.; Xu, J.T.; Fan, D.Q.; Tang, W.; Yang, Y.L. Thermal Decomposition of Cumyl Dithiobenzoate. Macromolecules 2005, 38, 10332–10335. [Google Scholar] [CrossRef]
- Wang, S.S.; Wen, Y.R.; Wang, Y.J.; Ma, Y.Y.; Liu, Z. Pattern Recognition of Cells via Multiplexed Imaging with Monosaccharide-Imprinted Quantum Dots. Anal. Chem. 2017, 89, 5646–5652. [Google Scholar] [CrossRef]
- Xie, C.G.; Gao, S.; Guo, Q.B.; Xu, K. Electrochemical sensor for 2,4-dichlorophenoxy acetic acid using molecularly imprinted polypyrrole membrane as recognition element. Microchim. Acta 2010, 169, 145–152. [Google Scholar] [CrossRef]
- Liang, C.D.; Peng, H.; Nie, L.H.; Yao, S.Z. Bulk acoustic wave sensor for herbicide assay based on molecularly imprinted polymer. Freseniu’s J. Anal. Chem. 2000, 367, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Yu, J.L.; Wu, X.Q.; Fu, J.Q.; Kang, Q.; Shen, D.Z.; Li, J.H.; Chen, L.X. A molecular imprinting-based turn-on Ratiometric fluorescence sensor for highly selective and sensitive detection of 2,4-dichlorophenoxyacetic acid (2,4-D). Biosens. Bioelectron. 2016, 81, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.F.; Lu, H.Z. Ratiometric fluorescence and mesoporous structure dual signal amplification for sensitive and selective detection of TNT based on MIP@QD fluorescence sensors. Chem. Commun. 2015, 51, 3200–3203. [Google Scholar] [CrossRef]
- Islam, F.; Wang, J.; Farooq, M.A.; Khan, M.S.S.; Xu, L.; Zhu, J.W.; Zhao, M.; Muñose, S.; Li, Q.X.; Zhou, W.J. Potential impact of the herbicide 2,4-dichlorophenoxyacetic acid on human and ecosystems. Environ. Int. 2018, 111, 332–351. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, S.; Singh, A.; Mohit; Devi, A.; Gupta, S.; Malik, P.; Khurana, S.; Soni, S. Detection of 2,4-dichlorophenoxyacetic acid in water sample by organosilane based silica nanocomposites. Sci. Total Environ. 2023, 858, 159594. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality: 4th Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022; pp. 1–489. [Google Scholar]



| Entry | MIP/CP a | Yield/(%) | Dn/(nm) b | Contact angle c/(°) |
|---|---|---|---|---|
| 1 | Grafted QD, NBD-labeled 2,4-D-MIP | 15 | 215 | 70.3 |
| 2 | Grafted QD, NBD-labeled 2,4-D-CP | 14 | 232 | 72.1 |
| 3 | Ungrafted QD, NBD-labeled 2,4-D-MIP | 25 | 966 | 120.2 |
| 4 | Ungrafted QD, NBD-labeled 2,4-D-CP | 24 | 865 | 120.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Li, X.; Wang, X.; Liu, Y.; Hu, X.; Chen, S.; Qu, X. One-Pot Preparation of Ratiometric Fluorescent Molecularly Imprinted Polymer Nanosensor for Sensitive and Selective Detection of 2,4-Dichlorophenoxyacetic Acid. Sensors 2024, 24, 5039. https://doi.org/10.3390/s24155039
Cui Y, Li X, Wang X, Liu Y, Hu X, Chen S, Qu X. One-Pot Preparation of Ratiometric Fluorescent Molecularly Imprinted Polymer Nanosensor for Sensitive and Selective Detection of 2,4-Dichlorophenoxyacetic Acid. Sensors. 2024; 24(15):5039. https://doi.org/10.3390/s24155039
Chicago/Turabian StyleCui, Yuhong, Xintai Li, Xianhong Wang, Yingchun Liu, Xiuli Hu, Shengli Chen, and Xiongwei Qu. 2024. "One-Pot Preparation of Ratiometric Fluorescent Molecularly Imprinted Polymer Nanosensor for Sensitive and Selective Detection of 2,4-Dichlorophenoxyacetic Acid" Sensors 24, no. 15: 5039. https://doi.org/10.3390/s24155039





