Fluid–Solid Interaction Analysis for Developing In-Situ Strain and Flow Sensors for Prosthetic Valve Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model Geometry
2.2. Simulation
2.2.1. TAVI Structural Simulation
2.2.2. Post-TAVI Two-Way FSI Simulation
2.3. Structural and Flow Measurement
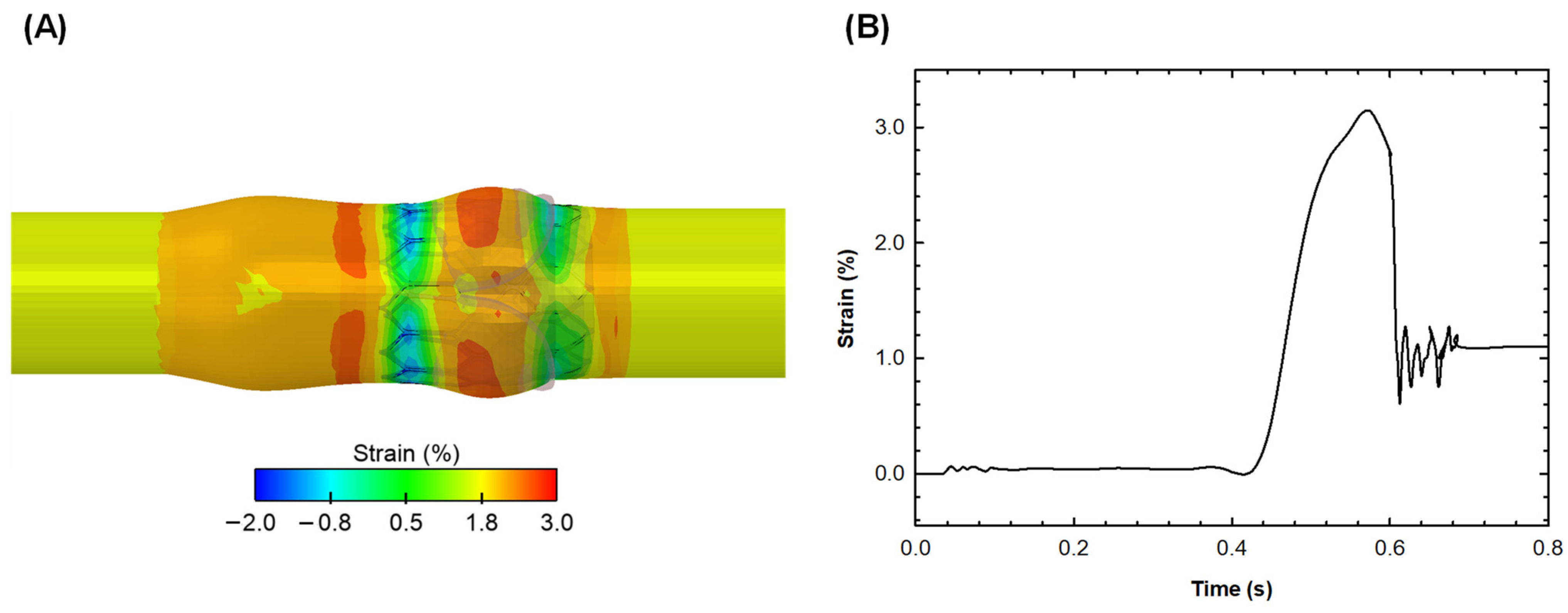
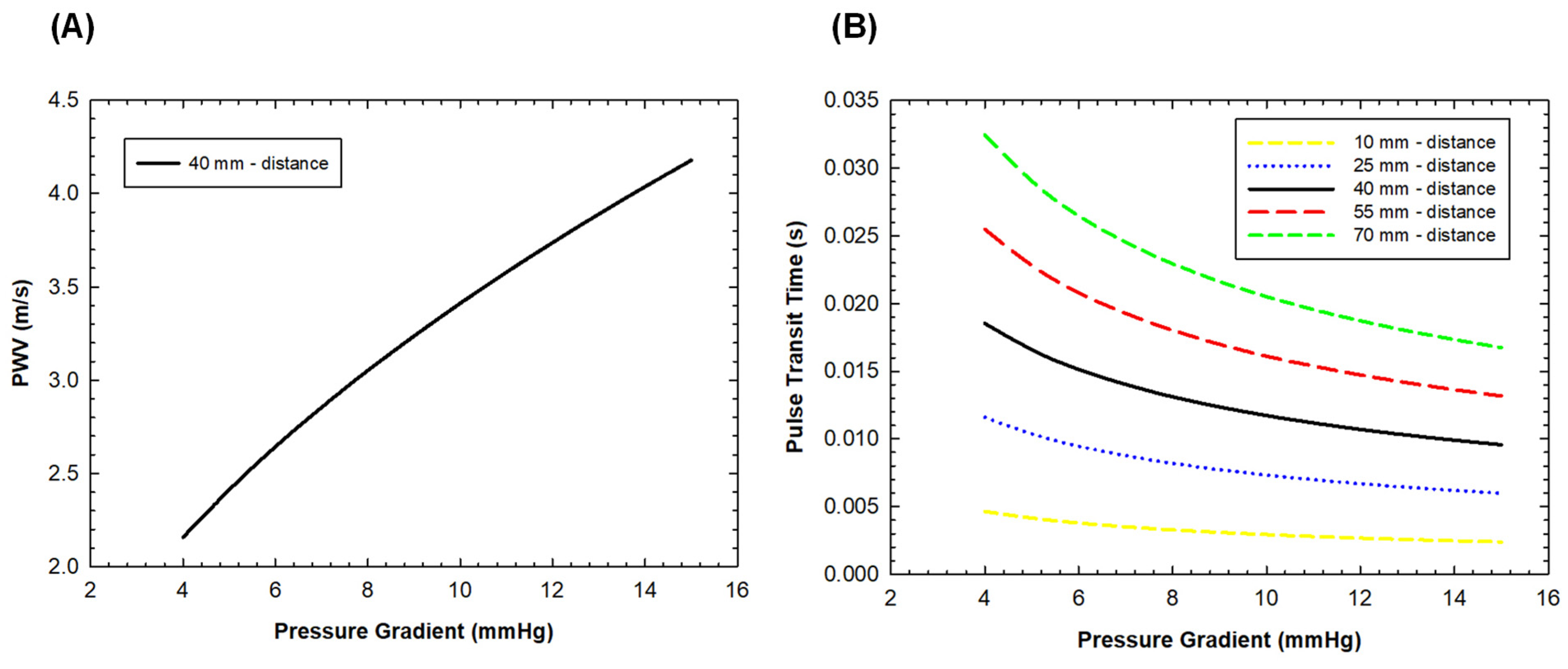
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- De Backer, O.; Landes, U.; Fuchs, A.; Yoon, S.H.; Mathiassen, O.N.; Sedaghat, A.; Kim, W.K.; Pilgrim, T.; Buzzatti, N.; Ruile, P.; et al. Coronary Access After TAVR-in-TAVR as Evaluated by Multidetector Computed Tomography. JACC Cardiovasc. Interv. 2020, 13, 2528–2538. [Google Scholar] [CrossRef] [PubMed]
- Forrestal, B.J.; Case, B.C.; Yerasi, C.; Shea, C.; Torguson, R.; Zhang, C.; Ben-Dor, I.; Deksissa, T.; Ali, S.; Satler, L.F.; et al. Risk of Coronary Obstruction and Feasibility of Coronary Access After Repeat Transcatheter Aortic Valve Replacement With the Self-Expanding Evolut Valve: A Computed Tomography Simulation Study. Circ. Cardiovasc. Interv. 2020, 13, e009496. [Google Scholar] [CrossRef] [PubMed]
- Nai Fovino, L.; Scotti, A.; Massussi, M.; Cardaioli, F.; Rodino, G.; Matsuda, Y.; Pavei, A.; Masiero, G.; Napodano, M.; Fraccaro, C.; et al. Coronary Angiography after Transcatheter Aortic Valve Replacement (TAVR) to Evaluate the Risk of Coronary Access Impairment After TAVR-in-TAVR. J. Am. Heart Assoc. 2020, 9, e016446. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Scardulla, F.; D’Acquisto, L.; Agnese, V.; Gentile, G.; Raffa, G.; Bellavia, D.; Pilato, M.; Pasta, S. Computational Modeling of Bicuspid Aortopathy: Towards Personalized Risk Strategies. J. Mol. Cell. Cardiol. 2019, 4, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Scardulla, F.; Pasta, S.; D’Acquisto, L.; Sciacca, S.; Agnese, V.; Vergara, C.; Quarteroni, A.; Clemenza, F.; Bellavia, D.; Pilato, M. Shear stress alterations in the celiac trunk of patients with a continuous-flow left ventricular assist device as shown by in-silico and in-vitro flow analyses. J. Heart Lung Transpl. 2017, 36, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Agnese, V.; Raffa, G.M.; Gentile, G.; Bellavia, D.; Zingales, M.; Pilato, M.; Pasta, S. On the role of material properties in ascending thoracic aortic aneurysms. Comput. Biol. Med. 2019, 109, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Amat-Santos, I.J.; Messika-Zeitoun, D.; Eltchanino, H.; Kapadia, S.; Lerakis, S.; Cheema, A.N.; Gutiérrez-Ibanes, E.; Munoz-Garcia, A.J.; Pan, M.; Webb, J.G.; et al. Infective Endocarditis After Transcatheter Aortic Valve Implantation Results From a Large Multicenter Registry. Circulation 2015, 131, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Flores-Umanzor, E.; Nogic, J.; Cepas-Guillen, P.; Hascoet, S.; Pysz, P.; Baz, J.A.; Cruz-Gonzalez, I.; Amat-Santos, I.; Antunez-Muinos, P.; Gonzalez, J.C.; et al. Percutaneous paravalvular leak closure after transcatheter aortic valve implantation: The international PLUGinTAVI Registry. EuroIntervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2023, 19, E442–E449. [Google Scholar] [CrossRef]
- Mao, W.; Wang, Q.; Kodali, S.; Sun, W. Numerical Parametric Study of Paravalvular Leak Following a Transcatheter Aortic Valve Deployment Into a Patient-Specific Aortic Root. J. Biomech. Eng. 2018, 140, 101007. [Google Scholar] [CrossRef]
- Liesman, D.R.; Fukuhara, S. Leaflet perforation or tear late after transcatheter aortic valve implantation. JTCVS Tech. 2020, 3, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, T.; Koechlin, L.; Jeger, R.; Leibundgut, G.; Reuthebuch, O. Severe structural valve deterioration after TAVR with ACURATE Neo: Report of two cases. Front. Cardiovasc. Med. 2023, 10, 1135496. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Criscione, E.; Todaro, D.; Tamburino, C.; Barbanti, M. Long-term Transcatheter Aortic Valve Durability. Interv. Cardiol. 2019, 14, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Marcelli, E.; Bortolani, B.; Corazza, I.; Cercenelli, L. A Novel Sensorized Heart Valve Prosthesis: Preliminary In Vitro Evaluation. Sensor 2018, 18, 3905. [Google Scholar] [CrossRef] [PubMed]
- Morany, A.; Bardon, R.G.; Lavon, K.; Hamdan, A.; Bluestein, D.; Haj-Ali, R. Analysis of fibrocalcific aortic valve stenosis: Computational pre-and-post TAVR haemodynamics behaviours. R. Soc. Open Sci. 2024, 11, 230905. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.P.; Marom, G.; Bianchi, M.; D’Souza, K.; Zietak, W.; Bluestein, D. Numerical evaluation of transcatheter aortic valve performance during heart beating and its post-deployment fluid-structure interaction analysis. Biomech. Model. Mechanobiol. 2020, 19, 1725–1740. [Google Scholar] [CrossRef] [PubMed]
- Kuang, R.; Ye, Y.; Chen, Z.; He, R.; Savović, I.; Djordjevich, A.; Savović, S.; Ortega, B.; Marques, C.; Li, X.F.; et al. Low-cost plastic optical fiber integrated with smartphone for human physiological monitoring. Opt. Fiber Technol. 2022, 71, 102947. [Google Scholar] [CrossRef]
- Savović, S.; Djordjevich, A.; Savović, I. Theoretical investigation of bending loss in step-index plastic optical fibers. Opt. Commun. 2020, 475, 126200. [Google Scholar] [CrossRef]
- Garrett, A.; Kim, B.; Sie, E.J.; Gurel, N.Z.; Marsili, F.; Boas, D.A.; Roblyer, D. Simultaneous photoplethysmography and blood flow measurements towards the estimation of blood pressure using speckle contrast optical spectroscopy. Biomed. Opt. Express 2023, 14, 1594–1607. [Google Scholar] [CrossRef] [PubMed]
- Cutugno, S.; Agnese, V.; Gentile, G.; Raffa, G.M.; Wisneski, A.D.; Guccione, J.M.; Pilato, M.; Pasta, S. Patient-Specific Analysis of Ascending Thoracic Aortic Aneurysm with the Living Heart Human Model. Bioengineering 2021, 8, 175. [Google Scholar] [CrossRef]
- Morganti, S.; Conti, M.; Aiello, M.; Valentini, A.; Mazzola, A.; Reali, A.; Auricchio, F. Simulation of transcatheter aortic valve implantation through patient-specific finite element analysis: Two clinical cases. J. Biomech. 2014, 47, 2547–2555. [Google Scholar] [CrossRef] [PubMed]
- D’Humieres, D.; Ginzburg, I.; Krafczyk, M.; Lallemand, P.; Luo, L.S. Multiple-relaxation-time lattice Boltzmann models in three dimensions. Philos. Trans. A Math. Phys. Eng. Sci. 2002, 360, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yu, Y.; Hou, G. Sharp-interface immersed boundary lattice Boltzmann method with reduced spurious-pressure oscillations for moving boundaries. Phys. Rev. E 2013, 87, 053306. [Google Scholar] [CrossRef] [PubMed]
- Pasta, S.; Cannata, S.; Gentile, G.; Agnese, V.; Raffa, G.M.; Pilato, M.; Gandolfo, C. Transcatheter Heart Valve Implantation in Bicuspid Patients with Self-Expanding Device. Bioengineering 2021, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Pasta, S.; Cannata, S.; Gentile, G.; Di Giuseppe, M.; Cosentino, F.; Pasta, F.; Agnese, V.; Bellavia, D.; Raffa, G.M.; Pilato, M.; et al. Simulation study of transcatheter heart valve implantation in patients with stenotic bicuspid aortic valve. Med. Biol. Eng. Comput. 2020, 58, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Qiu, D.; Behnam, Y.; Dvir, D.; Clary, C.; Azadani, A.N. High resolution three-dimensional strain mapping of bioprosthetic heart valves using digital image correlation. J. Biomech. 2018, 76, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, D.; Massaroni, C.; Leitao, C.S.J.; Domingues, M.D.; Sypabekova, M.; Barrera, D.; Floris, I.; Massari, L.; Oddo, C.M.; Sales, S.; et al. Fiber Bragg Gratings for Medical Applications and Future Challenges: A Review. IEEE Access 2020, 8, 156863–156888. [Google Scholar] [CrossRef]
- Scardulla, F.; D’Acquisto, L.; Pasta, S. Steam sterilizations processes affect the stability of clinical thermometers. Opt. Fiber Technol. 2020, 55, 102156. [Google Scholar]
- Scuoppo, R.; Cannata, S.; Gentile, G.; Gandolfo, C.; Pasta, S. Parametric analysis of transcatheter aortic valve replacement in transcatheter aortic valve replacement: Evaluation of coronary flow obstruction. Front. Bioeng. Biotechnol. 2023, 11, 1267986. [Google Scholar] [CrossRef]
- Bailoor, S.; Seo, J.H.; Dasi, L.; Schena, S.; Mittal, R. Prosthetic Valve Monitoring via In Situ Pressure Sensors: In Silico Concept Evaluation using Supervised Learning. Cardiovasc Eng Technol 2022, 13, 90–103. [Google Scholar] [CrossRef]
- Wilson, J.S.; Taylor, W.R.; Oshinski, J. Assessment of the regional distribution of normalized circumferential strain in the thoracic and abdominal aorta using DENSE cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2019, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Satriano, A.; Guenther, Z.; White, J.A.; Merchant, N.; Di Martino, E.S.; Al-Qoofi, F.; Lydell, C.P.; Fine, N.M. Three-dimensional thoracic aorta principal strain analysis from routine ECG-gated computerized tomography: Feasibility in patients undergoing transcatheter aortic valve replacement. BMC Cardiovasc. Disord. 2018, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Bosi, G.M.; Capelli, C.; Cheang, M.H.; Delahunty, N.; Mullen, M.; Taylor, A.M.; Schievano, S. A validated computational framework to predict outcomes in TAVI. Sci. Rep. 2020, 10, 9906. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puleo, S.; Pasta, S.; Scardulla, F.; D’Acquisto, L. Fluid–Solid Interaction Analysis for Developing In-Situ Strain and Flow Sensors for Prosthetic Valve Monitoring. Sensors 2024, 24, 5040. https://doi.org/10.3390/s24155040
Puleo S, Pasta S, Scardulla F, D’Acquisto L. Fluid–Solid Interaction Analysis for Developing In-Situ Strain and Flow Sensors for Prosthetic Valve Monitoring. Sensors. 2024; 24(15):5040. https://doi.org/10.3390/s24155040
Chicago/Turabian StylePuleo, Silvia, Salvatore Pasta, Francesco Scardulla, and Leonardo D’Acquisto. 2024. "Fluid–Solid Interaction Analysis for Developing In-Situ Strain and Flow Sensors for Prosthetic Valve Monitoring" Sensors 24, no. 15: 5040. https://doi.org/10.3390/s24155040





