Habituation of Central and Electrodermal Responses to an Auditory Two-Stimulus Oddball Paradigm
Abstract
:1. Introduction
1.1. Orienting Reaction
1.2. OR and SC Responses
1.3. Oddball Paradigm(s) and ERPs
1.4. P300 and OR
1.5. Intrinsic Habituation to the Oddball Paradigm
1.6. Aims and Scope
2. Materials and Methods
2.1. Experimental Protocol
2.2. EEG and SC Acquisition
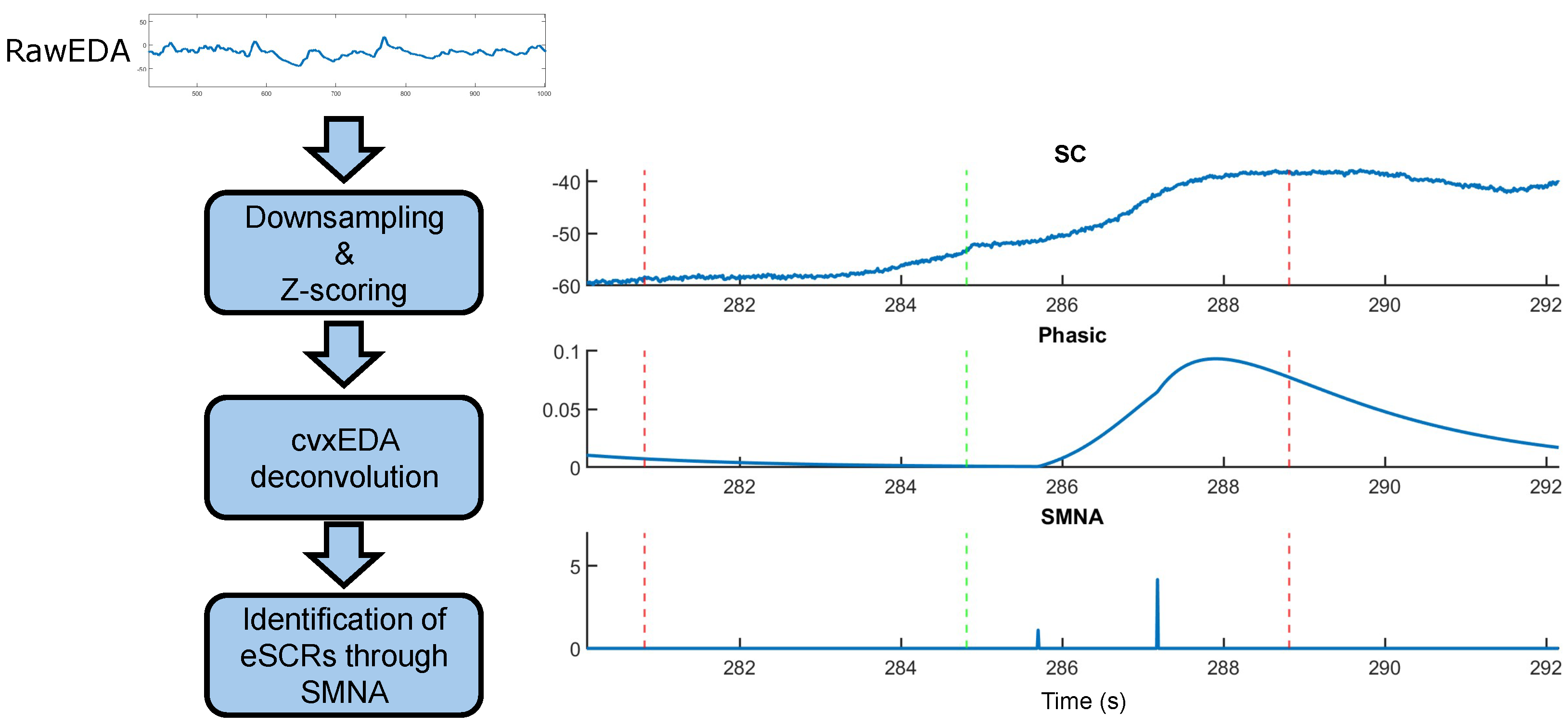
2.3. SC Signal Processing
2.4. EEG Signal Processing
2.5. Temporal PCA
2.6. SC Statistical Analysis
2.7. EEG Statistical Analysis
3. Results
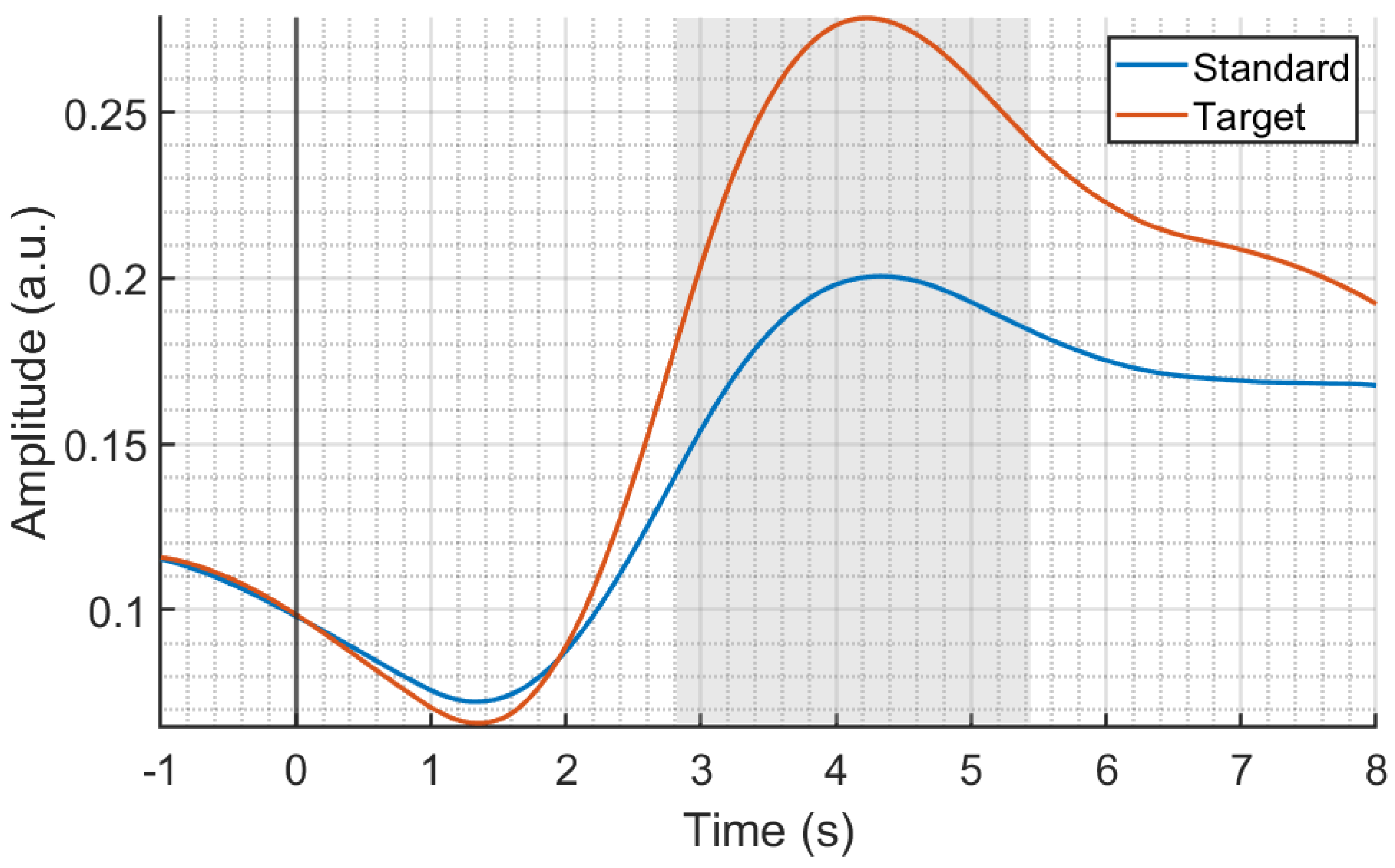
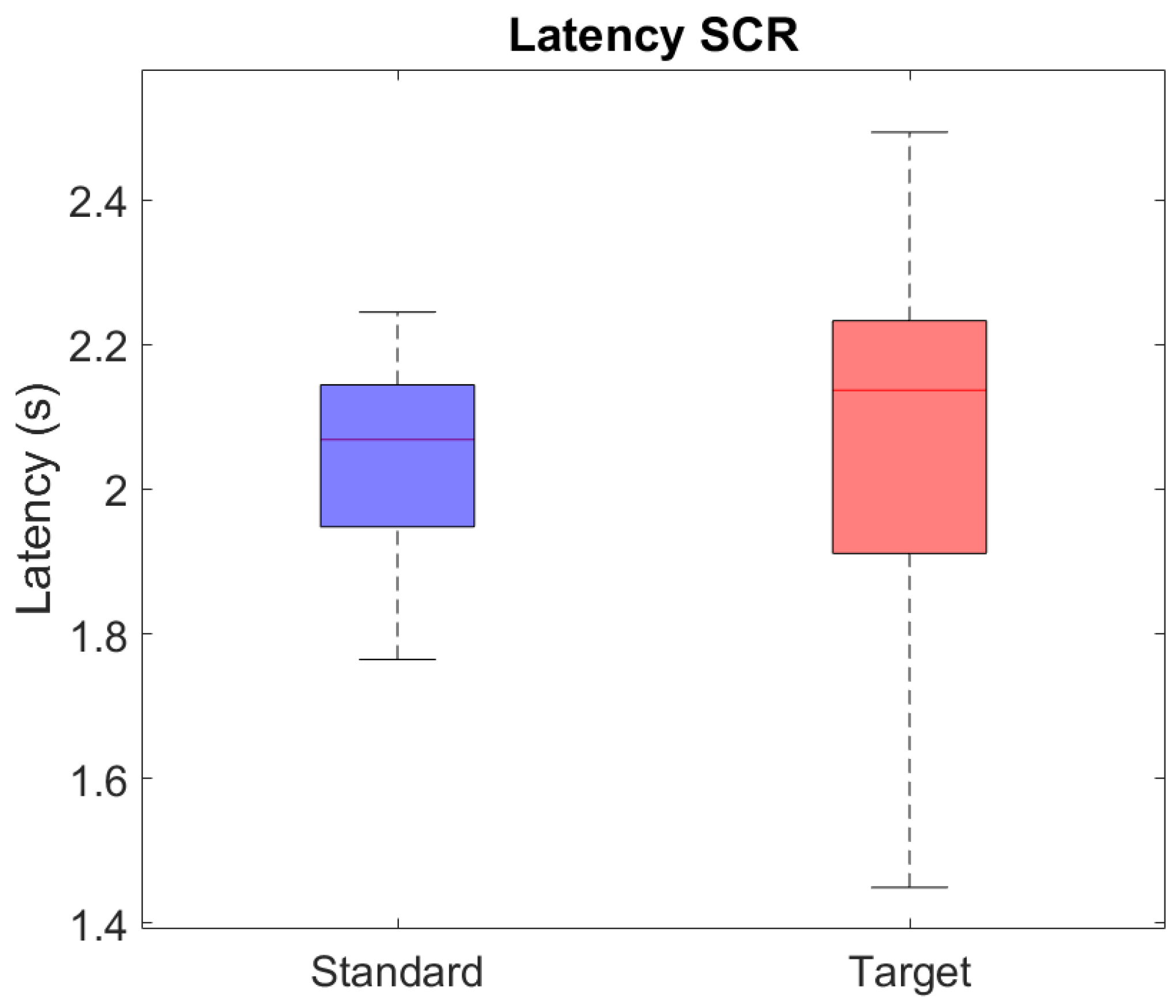
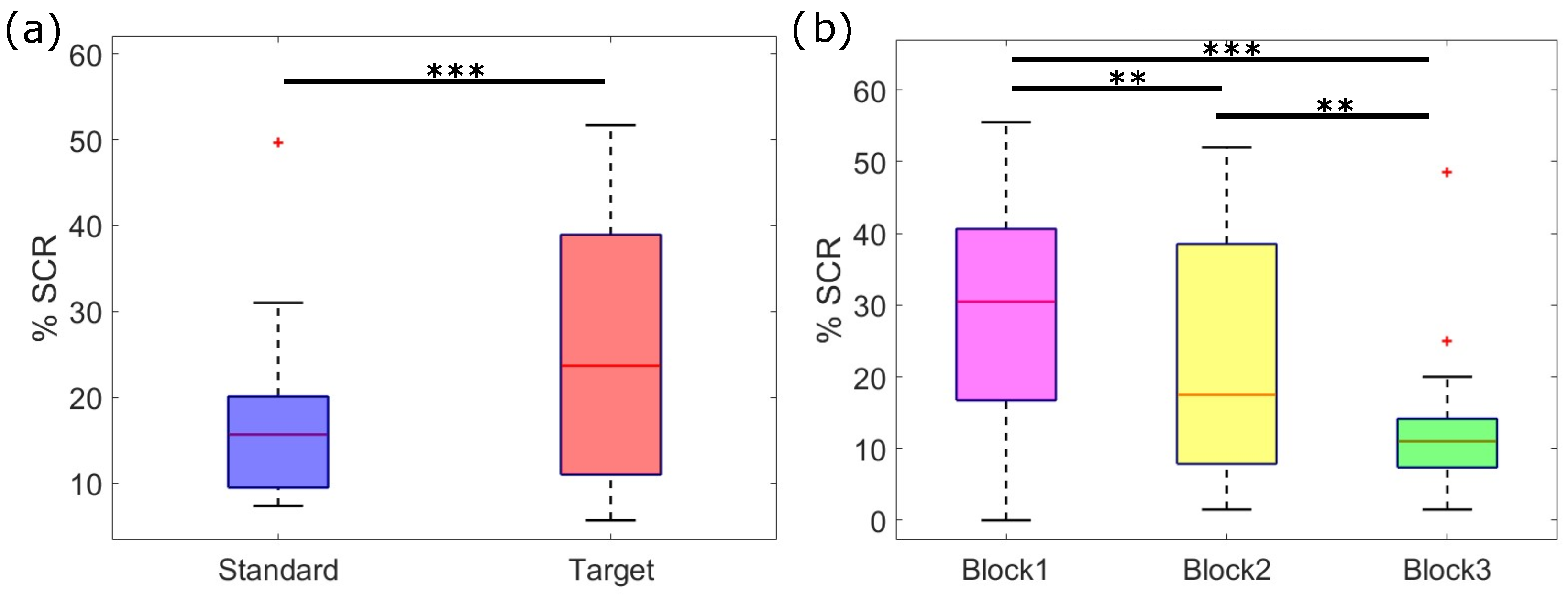
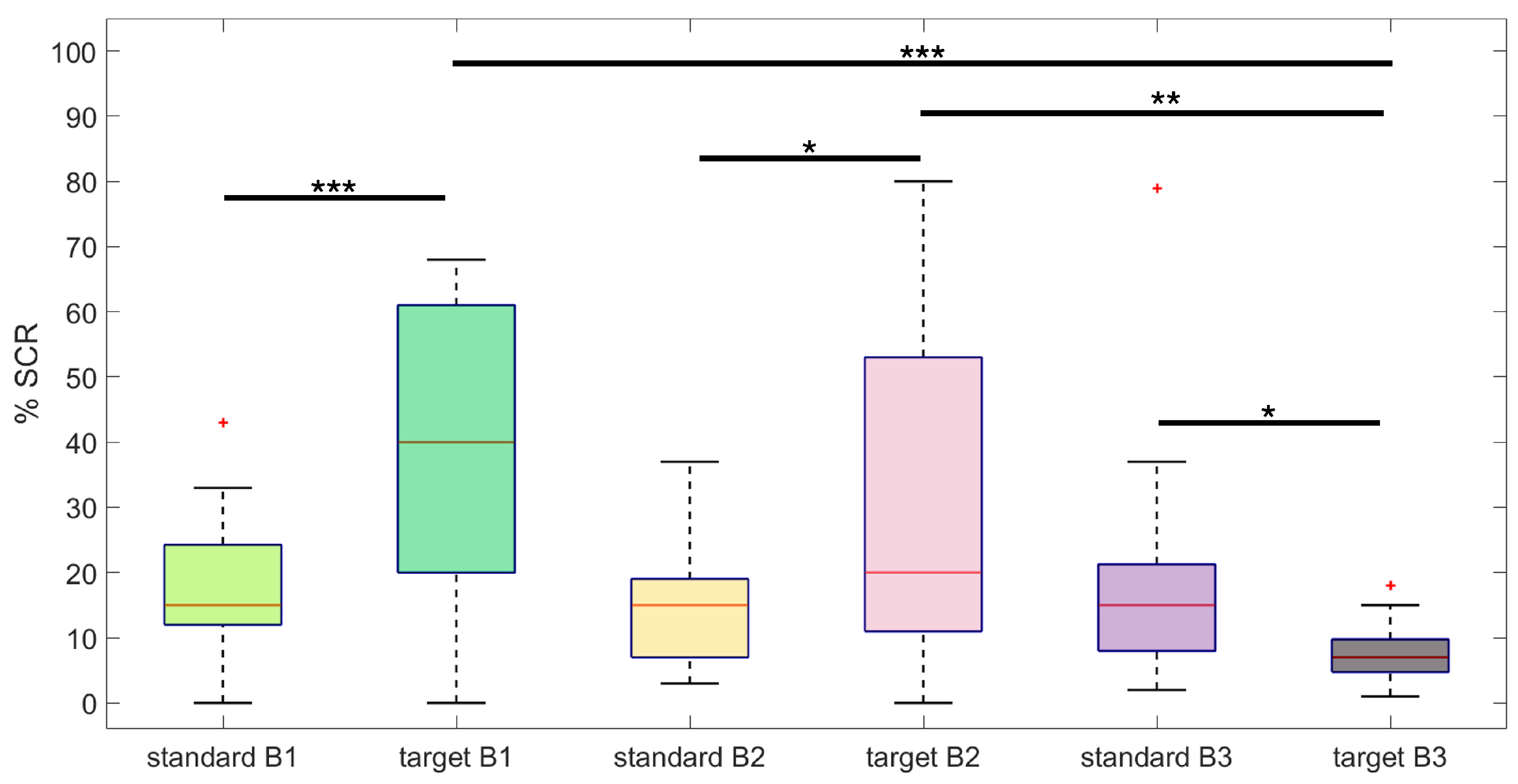
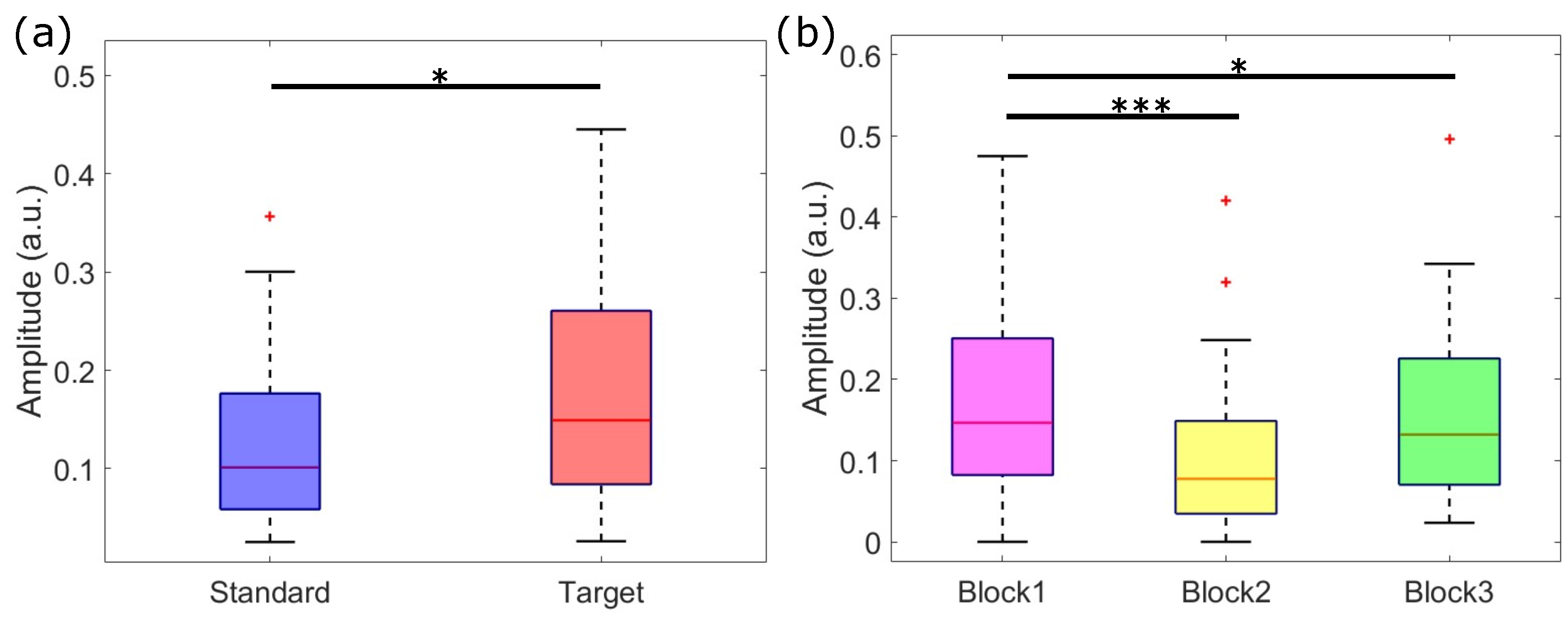
3.1. SC Statistical Analysis Results
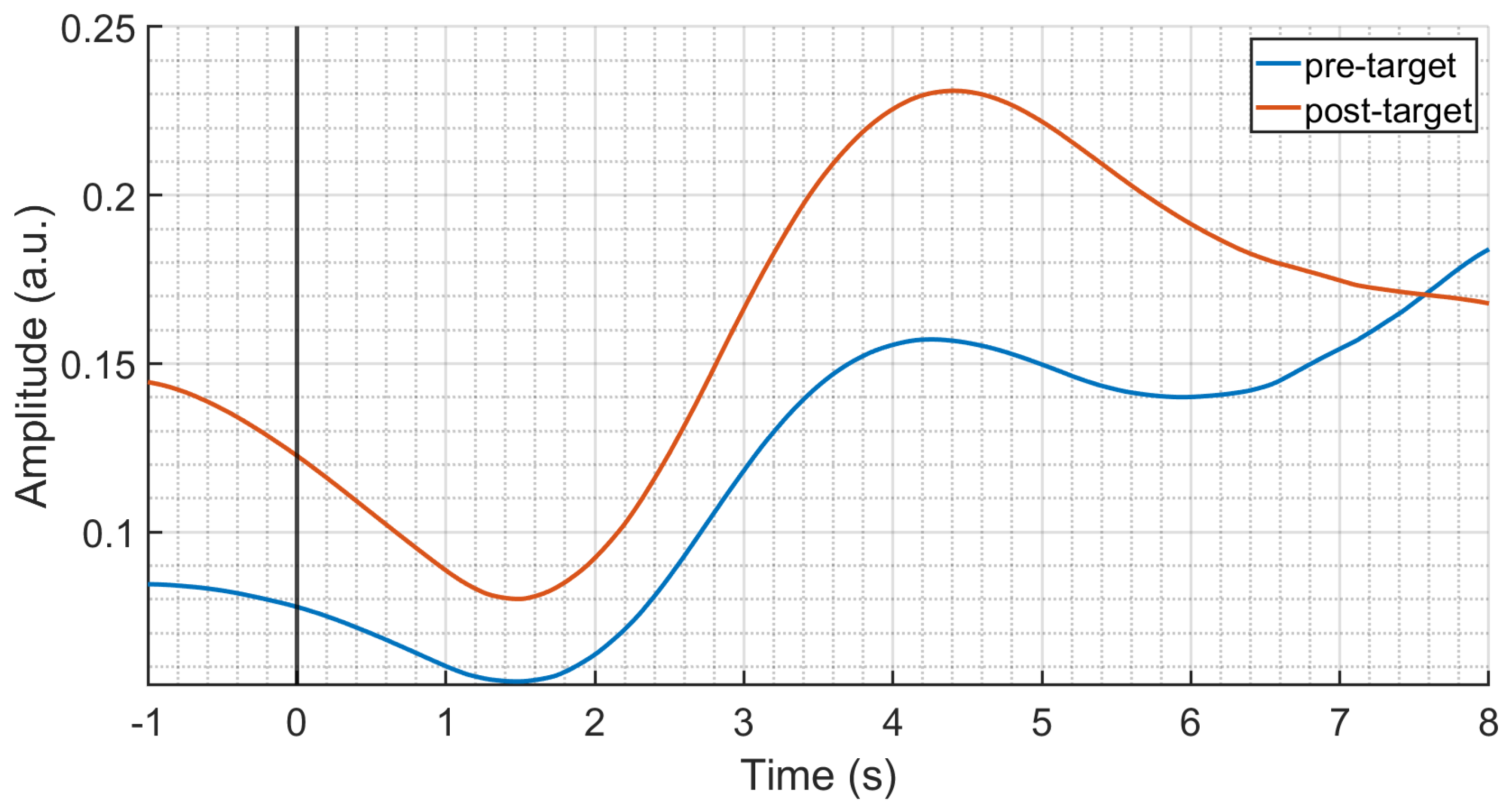
3.2. Temporal PCA Results
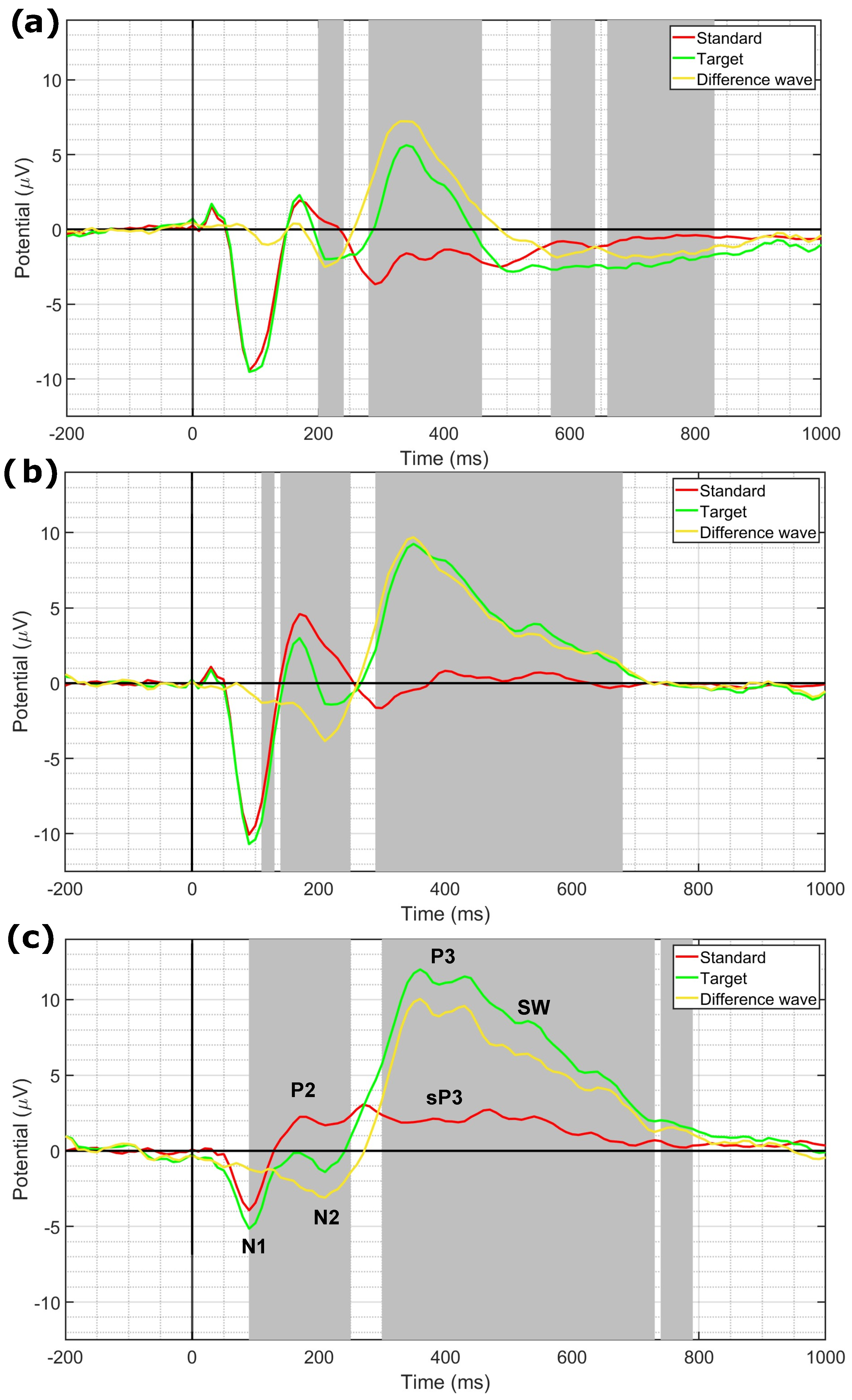
3.3. EEG Statistical Analysis Results
4. Discussion
4.1. Decrease with Stimulus Repetition of Both SCRs Amplitude and Frequency
4.2. P3 Amplitude Decrease with Stimulus Repetition and Recovery with the Original Stimulus Reintroduction
4.3. Dishabituation of ERPs with Stimulus Resumption
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Critchley, H.D. Electrodermal responses: What happens in the brain. Neuroscientist 2002, 8, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, E.; Nezlina, N.; Polyanskii, V.; Evtikhin, D. The orientating reflex: The “targeting reaction” and “searchlight of attention”. Neurosci. Behav. Physiol. 2002, 32, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Polich, J.; Criado, J.R. Neuropsychology and neuropharmacology of P3a and P3b. Int. J. Psychophysiol. 2006, 60, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Rankin, C.H.; Abrams, T.; Barry, R.J.; Bhatnagar, S.; Clayton, D.F.; Colombo, J.; Coppola, G.; Geyer, M.A.; Glanzman, D.L.; Marsland, S.; et al. Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiol. Learn. Mem. 2009, 92, 135–138. [Google Scholar] [CrossRef]
- Barry, R.J.; Sokolov, E.N. Habituation of phasic and tonic components of the orienting reflex. Int. J. Psychophysiol. 1993, 15, 39–42. [Google Scholar] [CrossRef]
- Rushby, J.A.; Barry, R.J. Single-trial event-related potentials to significant stimuli. Int. J. Psychophysiol. 2009, 74, 120–131. [Google Scholar] [CrossRef]
- Thompson, R.F.; Spencer, W.A. Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychol. Rev. 1966, 73, 16. [Google Scholar] [CrossRef] [PubMed]
- Hari, R.; Aittoniemi, K.; Järvinen, M.L.; Katila, T.; Varpula, T. Auditory evoked transient and sustained magnetic fields of the human brain localization of neural generators. Exp. Brain Res. 1980, 40, 237–240. [Google Scholar] [CrossRef]
- Näätänen, R.; Picton, T. The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology 1987, 24, 375–425. [Google Scholar] [CrossRef]
- O’Donnell, B.F.; Hokama, H.; McCarley, R.W.; Smith, R.S.; Salisbury, D.F.; Mondrow, E.; Nestor, P.G.; Shenton, M.E. Auditory ERPs to non-target stimuli in schizophrenia: Relationship to probability, task-demands, and target ERPs. Int. J. Psychophysiol. 1994, 17, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Stapells, D.R. Cortical event-related potentials to auditory stimuli. Handb. Clin. Audiol. 2002, 5, 378–406. [Google Scholar]
- Charest, I.; Pernet, C.R.; Rousselet, G.A.; Quiñones, I.; Latinus, M.; Fillion-Bilodeau, S.; Chartrand, J.P.; Belin, P. Electrophysiological evidence for an early processing of human voices. Bmc Neurosci. 2009, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Capilla, A.; Belin, P.; Gross, J. The early spatio-temporal correlates and task independence of cerebral voice processing studied with MEG. Cereb. Cortex 2013, 23, 1388–1395. [Google Scholar] [CrossRef]
- Paiva, T.O.; Almeida, P.R.; Ferreira-Santos, F.; Vieira, J.B.; Silveira, C.; Chaves, P.L.; Barbosa, F.; Marques-Teixeira, J. Similar sound intensity dependence of the N1 and P2 components of the auditory ERP: Averaged and single trial evidence. Clin. Neurophysiol. 2016, 127, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.C.; Barry, R.J.; Connolly, J.F.; Fischer, C.; Michie, P.T.; Näätänen, R.; Polich, J.; Reinvang, I.; Van Petten, C. Event-related potentials in clinical research: Guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin. Neurophysiol. 2009, 120, 1883–1908. [Google Scholar] [CrossRef]
- Bekinschtein, T.A.; Dehaene, S.; Rohaut, B.; Tadel, F.; Cohen, L.; Naccache, L. Neural signature of the conscious processing of auditory regularities. Proc. Natl. Acad. Sci. USA 2009, 106, 1672–1677. [Google Scholar] [CrossRef]
- Wronka, E.; Kaiser, J.; Coenen, A.M. Neural generators of the auditory evoked potential components P3a and P3b. Acta Neurobiol Exp (Wars) 2012, 72, 51–64. [Google Scholar] [CrossRef]
- Bachiller, A.; Romero, S.; Molina, V.; Alonso, J.F.; Mañanas, M.A.; Poza, J.; Hornero, R. Auditory P3a and P3b neural generators in schizophrenia: An adaptive sLORETA P300 localization approach. Schizophr. Res. 2015, 169, 318–325. [Google Scholar] [CrossRef]
- Krokhine, S.N.; Ewers, N.P.; Mangold, K.I.; Boshra, R.; Lin, C.Y.A.; Connolly, J.F. N2b reflects the cognitive changes in executive functioning after concussion: A scoping review. Front. Hum. Neurosci. 2020, 14, 601370. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, V.D.; Adali, T.; Pearlson, G.D.; Kiehl, K.A. Neuronal chronometry of target detection: Fusion of hemodynamic and event-related potential data. Neuroimage 2006, 30, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Mantini, D.; Corbetta, M.; Perrucci, M.G.; Romani, G.L.; Del Gratta, C. Large-scale brain networks account for sustained and transient activity during target detection. Neuroimage 2009, 44, 265–274. [Google Scholar] [CrossRef]
- Ragazzoni, A.; Di Russo, F.; Fabbri, S.; Pesaresi, I.; Di Rollo, A.; Perri, R.L.; Barloscio, D.; Bocci, T.; Cosottini, M.; Sartucci, F. “Hit the missing stimulus”. A simultaneous EEG-fMRI study to localize the generators of endogenous ERPs in an omitted target paradigm. Sci. Rep. 2019, 9, 3684. [Google Scholar] [CrossRef] [PubMed]
- Verleger, R. Effects of relevance and response frequency on P3b amplitudes: Review of findings and comparison of hypotheses about the process reflected by P3b. Psychophysiology 2020, 57, e13542. [Google Scholar] [CrossRef] [PubMed]
- Rushby, J.A.; Barry, R.J.; Doherty, R.J. Separation of the components of the late positive complex in an ERP dishabituation paradigm. Clin. Neurophysiol. 2005, 116, 2363–2380. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Detection of Change: Event-Related Potential and fMRI Findings; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Barry, R.J.; Steiner-Lim, G.Z.; Cave, A.E.; De Blasio, F.M.; MacDonald, B. Effects of interstimulus interval and significance on electrodermal and central measures of the phasic orienting reflex (OR) in a dishabituation task. Sci. Rep. 2023, 13, 13546. [Google Scholar] [CrossRef]
- Boucsein, W. Electrodermal Activity; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Rho, G.; Callara, A.L.; Bossi, F.; Ognibene, D.; Cecchetto, C.; Lomonaco, T.; Scilingo, E.P.; Greco, A. Combining electrodermal activity analysis and dynamic causal modeling to investigate the visual-odor multimodal integration during face perception. J. Neural Eng. 2024, 21, 016020. [Google Scholar] [CrossRef]
- Greco, A.; Valenza, G.; Lanata, A.; Scilingo, E.P.; Citi, L. cvxEDA: A convex optimization approach to electrodermal activity processing. IEEE Trans. Biomed. Eng. 2015, 63, 797–804. [Google Scholar] [CrossRef]
- Sjouwerman, R.; Lonsdorf, T. Latency of skin conductance responses across stimulus modalities. Psychophysiology 2019, 56, e13307. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Mullen, T.R.; Kothe, C.A.E.; Chi, Y.M.; Ojeda, A.; Kerth, T.; Makeig, S.; Jung, T.P.; Cauwenberghs, G. Real-Time Neuroimaging and Cognitive Monitoring Using Wearable Dry EEG. IEEE Trans. Bio-Med. Eng. 2015, 62, 2553–2567. [Google Scholar] [CrossRef]
- Makeig, S.; Bell, A.; Jung, T.P.; Sejnowski, T.J. Independent component analysis of electroencephalographic data. Adv. Neural Inf. Process. Syst. 1995, 8, 145–151. [Google Scholar]
- Scharf, F.; Widmann, A.; Bonmassar, C.; Wetzel, N. A tutorial on the use of temporal principal component analysis in developmental ERP research–Opportunities and challenges. Dev. Cogn. Neurosci. 2022, 54, 101072. [Google Scholar] [CrossRef] [PubMed]
- Kayser, J.; Tenke, C.E. Optimizing PCA methodology for ERP component identification and measurement: Theoretical rationale and empirical evaluation. Clin. Neurophysiol. 2003, 114, 2307–2325. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar] [CrossRef]
- Demirayak, P.; Kıyı, İ.; İşbitiren, Y.Ö.; Yener, G. Cognitive load associates prolonged P300 latency during target stimulus processing in individuals with mild cognitive impairment. Sci. Rep. 2023, 13, 15956. [Google Scholar] [CrossRef]
- Barry, R.J.; Steiner, G.Z.; De Blasio, F.M.; Fogarty, J.S.; Karamacoska, D.; MacDonald, B. Components in the P300: Don’t forget the Novelty P3! Psychophysiology 2020, 57, e13371. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.J.; Steiner, G.Z.; De Blasio, F.M. Reinstating the novelty P3. Sci. Rep. 2016, 6, 31200. [Google Scholar] [CrossRef] [PubMed]
- Loveless, N.; Simpson, M.; Näätänen, R. Frontal negative and parietal positive components of the slow wave dissociated. Psychophysiology 1987, 24, 340–345. [Google Scholar] [CrossRef]
- Squires, N.K.; Squires, K.C.; Hillyard, S.A. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr. Clin. Neurophysiol. 1975, 38, 387–401. [Google Scholar] [CrossRef]
- Näätänen, R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behav. Brain Sci. 1990, 13, 201–233. [Google Scholar] [CrossRef]
- Courchesne, E.; Hillyard, S.A.; Galambos, R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalogr. Clin. Neurophysiol. 1975, 39, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.M.; Dien, J.; Donchin, E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology 2001, 38, 343–358. [Google Scholar] [CrossRef] [PubMed]
- EN, S. Perception and the Conditioned Reflex; Pergamon Press: Oxford, UK, 1963. [Google Scholar]
- Barry, R.J. Stimulus significance effects in habituation of the phasic and tonic orienting reflex. Integr. Physiol. Behav. Sci. 2004, 39, 166–179. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Knight, R.T. P300 generation by novel somatosensory stimuli. Electroencephalogr. Clin. Neurophysiol. 1991, 78, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Escera, C.; Alho, K.; Winkler, I.; Näätänen, R. Neural mechanisms of involuntary attention to acoustic novelty and change. J. Cogn. Neurosci. 1998, 10, 590–604. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.G.; Gabbay, F.H.; Rietschel, J.C.; Duncan, C.C. Evidence for a new late positive ERP component in an attended novelty oddball task. Psychophysiology 2010, 47, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Kok, A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology 2001, 38, 557–577. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.V.; Kroll, C.F.; Kopp, B. Dopaminergic and norepinephrinergic modulation of endogenous event-related potentials: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2023, 151, 105221. [Google Scholar] [CrossRef] [PubMed]
- Kamp, S.M.; Forester, G.; Vatheuer, C.C.; Domes, G. Stress effects on the oddball P300 and N2 in males and females. Biol. Psychol. 2021, 162, 108095. [Google Scholar] [CrossRef]
- Walpurger, V.; Pietrowsky, R.; Kirschbaum, C.; Wolf, O.T. Effects of the menstrual cycle on auditory event-related potentials. Horm. Behav. 2004, 46, 600–606. [Google Scholar] [CrossRef]
- Martinez-Selva, J.; Gomez-Amor, J.; Olmos, E.; Navarro, N.; Roman, F. Sex and menstrual cycle differences in the habituation and spontaneous recovery of the electrodermal orienting reaction. Personal. Individ. Differ. 1987, 8, 211–217. [Google Scholar] [CrossRef]
- Gómez-Amor, J.; Martínez-Selva, J.; Román, F.; Zamora, S. Electrodermal activity in menstrual cycle phases: A comparison of within-and between-subjects designs. Int. J. Psychophysiol. 1990, 9, 39–47. [Google Scholar] [CrossRef]
- Fleck, K.M.; Polich, J. P300 and the menstrual cycle. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1988, 71, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Waldo, M.C.; Graze, K.; de Graff Bender, S.; Adler, L.E.; Freedman, R. Premenstrual mood changes and gating of the auditory evoked potential. Psychoneuroendocrinology 1987, 12, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kopell, B.S.; Lunde, D.T.; Clayton, R.B.; Moos, R.H. Variations in some measures of arousal during the menstrual cycle. J. Nerv. Ment. Dis. 1969, 148, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Wineman, E. Autonomic balance changes during the human menstrual cycle. Psychophysiology 1971, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, E.; Parlee, M.B. Behavioral Changes Associated with the Menstrual Cycle: An Experimental Investigation 1. J. Appl. Soc. Psychol. 1973, 3, 335–344. [Google Scholar] [CrossRef]
- Strauss, B.; Appelt, H. Psychological concomitants of the menstrual cycle: A prospective longitudinal approach. J. Psychosom. Obstet. Gynecol. 1983, 2, 215–220. [Google Scholar] [CrossRef]



| Block1 | Block2 | Block3 | Sig | |
|---|---|---|---|---|
| eP3a Fz | 0.120 ± 0.274 | −0.102 ± 0.242 | 0.290 ± 0.254 | p > 0.05 |
| eP3a Cz | 0.368 ± 0.343 | 0.125 ± 0.241 | 0.326 ± 0.260 | p > 0.05 |
| lP3a Cz | 0.090 ± 0.303 | −0.076 ± 0.304 | −0.659 ± 0.323 | p < 0.05 |
| lP3a Pz | 0.335 ± 0.170 | 0.275 ± 0.252 | −0.057 ± 0.248 | p > 0.05 |
| P3b Pz | 0.529 ± 0.231 | 0.219 ± 0.265 | −0.229 ± 0.244 | p < 0.05 |
| nP3 Fz | 0.531 ± 0.260 | −0.012 ± 0.196 | 0.512 ± 0.250 | p > 0.05 |
| +SW Fz | 0.137 ± 0.183 | 0.171 ± 0.236 | 0.218 ± 0.289 | p > 0.05 |
| −SW Fz | −0.607 ± 0.146 | −0.700 ± 0.147 | −0.496 ± 0.134 | p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rho, G.; Callara, A.L.; Scilingo, E.P.; Greco, A.; Bonfiglio, L. Habituation of Central and Electrodermal Responses to an Auditory Two-Stimulus Oddball Paradigm. Sensors 2024, 24, 5053. https://doi.org/10.3390/s24155053
Rho G, Callara AL, Scilingo EP, Greco A, Bonfiglio L. Habituation of Central and Electrodermal Responses to an Auditory Two-Stimulus Oddball Paradigm. Sensors. 2024; 24(15):5053. https://doi.org/10.3390/s24155053
Chicago/Turabian StyleRho, Gianluca, Alejandro Luis Callara, Enzo Pasquale Scilingo, Alberto Greco, and Luca Bonfiglio. 2024. "Habituation of Central and Electrodermal Responses to an Auditory Two-Stimulus Oddball Paradigm" Sensors 24, no. 15: 5053. https://doi.org/10.3390/s24155053
APA StyleRho, G., Callara, A. L., Scilingo, E. P., Greco, A., & Bonfiglio, L. (2024). Habituation of Central and Electrodermal Responses to an Auditory Two-Stimulus Oddball Paradigm. Sensors, 24(15), 5053. https://doi.org/10.3390/s24155053






