Gas Chromatography–Sensor System Aids Diagnosis of Inflammatory Bowel Disease, and Separates Crohn’s from Ulcerative Colitis, in Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Screening and Recruitment
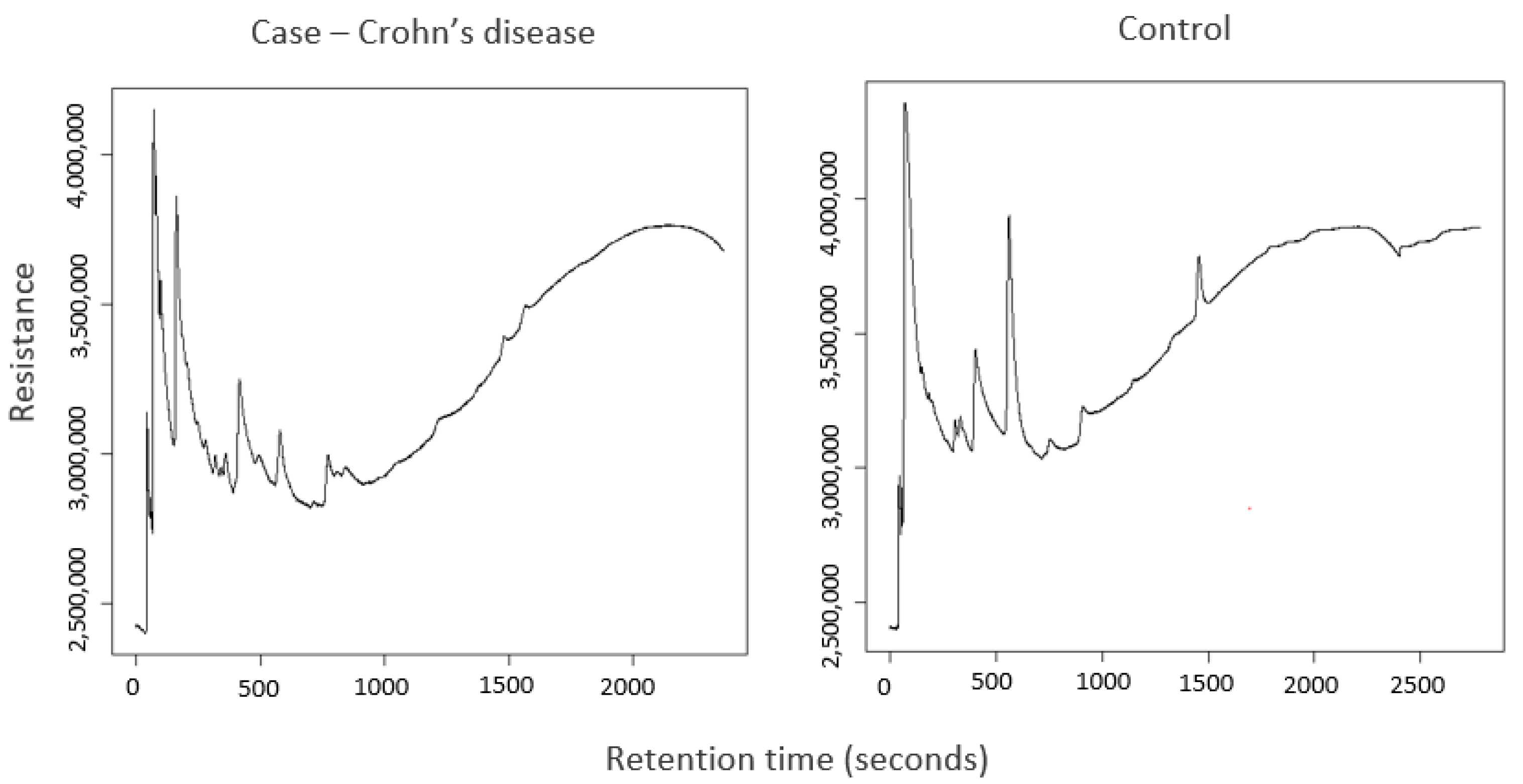
2.2. Analysis of Stool Samples by GC–Sensor
2.3. Statistical Analysis
2.4. Faecal Calprotectin
3. Results
3.1. Patient Characteristics
3.2. GC–Sensor Modelling
3.2.1. IBD vs. Non-IBD Controls
3.2.2. CD/UC vs. Controls
3.2.3. CD vs. UC
3.2.4. Baseline Active vs. 3-Month Follow Up Remission
3.2.5. LASSO Modelling
3.3. Faecal Calprotectin
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosen, M.; Dhawan, A.; Saeed, S. Inflammatory bowel disease in children and adolescents. JAMA Paediatr. 2015, 169, 1053–1060. [Google Scholar] [CrossRef]
- Afzali, A.; Wahbeh, G. Transition of pediatric to adult care in inflammatory bowel disease: Is it as easy as 1, 2, 3? World J. Gastroenterol. 2017, 23, 3624–3631. [Google Scholar] [CrossRef]
- Levine, A.; Koletzko, S.; Turner, D.; Escher, J.C.; Cucchiara, S.; De Ridder, L.; Kolho, K.L.; Veres, G.; Russell, R.K.; Paerregaard, A.; et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 795–806. [Google Scholar] [CrossRef]
- NICE. Faecal Calprotectin Diagnostic Tests for Inflammatory Diseases of the Bowel. NICE Diagnostics Guidance. 2013. Available online: https://www.nice.org.uk/guidance/dg11 (accessed on 8 April 2024).
- Henderson, P.; Casey, A.; Lawrence, S.; Kennedy, N.; Kingston, K.; Rogers, P.; Gillett, P.; Wilson, D. The Diagnostic Accuracy of Fecal Calprotectin During the Investigation of Suspected Pediatric Inflammatory Bowel Disease. Am. J. Gastroenterol. 2012, 106, 941–949. [Google Scholar] [CrossRef]
- Chan, D.; Leggett, C.; Wang, K. Diagnosing gastrointestinal illnesses using fecal headspace volatile organic compounds. World J. Gastroenterol. 2016, 22, 1659. [Google Scholar] [CrossRef]
- Krishnamoorthy, A.; Chandrapalan, S.; Ahmed, M.; Arasaradnam, R.P. The Diagnostic Utility of Volatile Organic Compounds in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Crohn’s Colitis 2024, 18, 320–330. [Google Scholar] [CrossRef]
- Filimoniuk, A.; Daniluk, U.; Samczuk, P.; Wasilewska, N.; Jakimiec, P.; Kucharska, M.; Lebensztejn, D.M.; Ciborowski, M. Metabolomic profiling in children with inflammatory bowel disease. Adv. Med. Sci. 2020, 65, 65–70. [Google Scholar] [CrossRef]
- Jagt, J.Z.; Verburgt, C.M.; De Vries, R.; De Boer, N.K.H.; Benninga, M.A.; De Jonge, W.J.; Van Limbergen, J.E.; De Meij, T.G.J. Faecal Metabolomics in Paediatric Inflammatory Bowel Disease: A Systematic Review. J. Crohn’s Colitis 2022, 16, 1777–1790. [Google Scholar] [CrossRef]
- Bosch, S.; van Gaal, N.; Zuurbier, R.P.; Covington, J.A.; Wicaksono, A.N.; Biezeveld, M.H.; Benninga, M.A.; Mulder, C.J.; de Boer, N.K.H.; de Meij, T.G.J. Differentiation between pediatric irritable bowel syndrome and inflammatory bowel disease based on fecal scent: Proof of principle study. Inflamm. Bowel Dis. 2018, 24, 2468–2475. [Google Scholar] [CrossRef]
- Vermeer, E.; Jagt, J.Z.; Stewart, T.K.; Covington, J.A.; Struys, E.A.; de Jonge, R.; de Boer, N.K.H.; de Meij, T.G.J. Faecal Volatile Organic Compound Analysis in De Novo Paediatric Inflammatory Bowel Disease by Gas Chromatography–Ion Mobility Spectrometry: A Case–Control Study. Sensors 2024, 24, 2727. [Google Scholar] [CrossRef]
- el Manouni el Hassani, S.; Bosch, S.; Lemmen, J.P.M.; Brentar, M.B.; Ayada, I.; Wicaksono, A.N.; Covington, J.A.; Benninga, M.A.; De Boer, N.K.H.; Meij, T.G.J. De Simultaneous assessment of urinary and fecal volatile organic compound analysis in De Novo pediatric IBD. Sensors 2019, 19, 4496. [Google Scholar] [CrossRef]
- van Gaal, N.; Lakenman, R.; Covington, J.; Savage, R.; De Groot, E.; Bomers, M.; Benninga, M.; Mulder, C.; De Boer, N.; De Meij, T. Faecal volatile organic compounds analysis using field asymmetric ion mobility spectrometry: Non-invasive diagnostics in paediatric inflammatory bowel disease. J. Breath Res. 2018, 12, 016006. [Google Scholar] [CrossRef]
- Aggio, R.B.M.; White, P.; Jayasena, H.; de Lacy Costello, B.; Ratcliffe, N.M.; Probert, C.S.J. Irritable bowel syndrome and active inflammatory bowel disease diagnosed by faecal gas analysis. Aliment. Pharmacol. Ther. 2017, 45, 82–90. [Google Scholar] [CrossRef]
- Gould, O.; Wieczorek, T.; de Lacy Costello, B.; Persad, R.; Ratcliffe, N. Assessment of a combined gas chromatography mass spectrometer sensor system for detecting biologically relevant volatile compounds. J. Breath Res. 2018, 12, 016009. [Google Scholar] [CrossRef]
- Belnour, S.; Slater, R.; Tharmaratnam, K.; Auth, M.K.; Muhammed, R.; Spray, C.; Wang, D.; Probert, C.; Allen, S. Faecal volatile organic compounds differ according to disease sub-type, severity and response to treatment in paediatric inflammatory bowel disease. United Eur. Gastroenterol. J. 2024, 12, 780–792. [Google Scholar] [CrossRef]
- Ruemmele, F.M.; Hyams, J.S.; Otley, A.; Griffiths, A.; Kolho, K.L.; Dias, J.A.; Levine, A.; Escher, J.C.; Taminiau, J.; Veres, G.; et al. Outcome measures for clinical trials in paediatric IBD: An evidence-based, expert-driven practical statement paper of the paediatric ECCO committee. Gut 2015, 64, 438–446. [Google Scholar] [CrossRef]
- Turner, D.; Ruemmele, F.M.; Orlanski-Meyer, E.; Griffiths, A.M.; De Carpi, J.M.; Bronsky, J.; Veres, G.; Aloi, M.; Strisciuglio, C.; Braegger, C.P.; et al. Management of paediatric ulcerative colitis, Part 1: Ambulatory Care—An evidence-based guideline from European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 257–291. [Google Scholar] [CrossRef]
- Turner, D.; Ruemmele, F.M.; Orlanski-Meyer, E.; Griffiths, A.M.; De Carpi, J.M.; Bronsky, J.; Veres, G.; Aloi, M.; Strisciuglio, C.; Braegger, C.P.; et al. Management of paediatric ulcerative colitis, Part 2: Acute severe colitis—An evidence-based consensus guideline from the European Crohn’s and Colitis organization and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 292–310. [Google Scholar] [CrossRef]
- van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohn’s Colitis 2021, 15, 171–194. [Google Scholar] [CrossRef]
- Aggio, R.B.M.; De Lacy Costello, B.; White, P.; Khalid, T.; Ratcliffe, N.M.; Persad, R.; Probert, C.S.J. The use of a gas chromatography-sensor system combined with advanced statistical methods, towards the diagnosis of urological malignancies. J. Breath Res. 2016, 10, 017106. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Kursa, M.; Rudnicki, W. Feature selection with the boruta package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Kuhn, M. Classification and Regression Training. R Package, Version 6.0-30; R Foundation for Statistical Computing: Vienna, Austria, 2014.
- Orfei, M.; Gasparetto, M.; Hensel, K.O.; Zellweger, F.; Heuschkel, R.B.; Zilbauer, M. Guidance on the interpretation of faecal calprotectin levels in children. PLoS ONE 2021, 16, e0246091. [Google Scholar] [CrossRef]
- Bosch, S.; Wintjens, D.S.J.; Wicaksono, A.; Kuijvenhoven, J.; van der Hulst, R.; Stokkers, P.; Daulton, E.; Pierik, M.J.; Covington, J.A.; de Meij, T.G.J.; et al. The faecal scent of inflammatory bowel disease: Detection and monitoring based on volatile organic compound analysis. Dig. Liver Dis. 2020, 52, 745–752. [Google Scholar] [CrossRef]
- Bosch, S.; Lemmen, J.P.M.; Menezes, R.; Van Der Hulst, R.; Kuijvenhoven, J.; Stokkers, P.C.F.; De Meij, T.G.J.; De Boer, N.K.H. The influence of lifestyle factors on fecal volatile organic compound composition as measured by an electronic nose. J. Breath Res. 2019, 13, 046001. [Google Scholar] [CrossRef]
- Soomro, S.; Venkateswaran, S.; Vanarsa, K.; Kharboutli, M.; Nidhi, M.; Susarla, R.; Zhang, T.; Sasidharan, P.; Lee, K.H.; Rosh, J.; et al. Predicting disease course in ulcerative colitis using stool proteins identified through an aptamer-based screen. Nat. Commun. 2021, 12, 3989. [Google Scholar] [CrossRef]
- Collins, G.S.; Ogundimu, E.O.; Altman, D.G. Sample size considerations for the external validation of a multivariable prognostic model: A resampling study. Stat. Med. 2016, 35, 214–226. [Google Scholar] [CrossRef]
- Frau, A.; Ijaz, U.; Slater, R.; Jonkers, D.; Penders, J.; Campbell, B.; Kenny, J.; Hall, N.; Lenzi, L.; Burkitt, M.; et al. Inter-kingdom relationships in Crohn’s disease explored using a multi-omics approach. Gut Microbes 2021, 13, 1930871. [Google Scholar] [CrossRef]
- El Manouni el Hassani, S.; Berkhout, D.J.C.; Bosch, S.; Benninga, M.A.; de Boer, N.K.H.; de Meij, T.G.J. Application of fecal volatile organic compound analysis in clinical practice: Current state and future perspectives. Chemosensors 2018, 6, 29. [Google Scholar] [CrossRef]



| Variable Training Set | CD N = 26 | UC N = 18 | IBDU N = 4 | Non-IBD Controls N = 48 | IBD at 3-Months Following Treatment N = 23 (CD = 11, UC = 8, IBDU = 4) |
|---|---|---|---|---|---|
| Age in years (range) | 12.6 (4–16) | 11.4 (5–16) | 10.5 (8–13) | 11.8 (4–16) | 11.5 (4–16) |
| Female n (%) | 12 (46.2) | 8 (44.0) | 1 (25) | 20 (41.7) | 8 (34.8) |
| Received antibiotics in last 3 months: no. (%) | 4 (15.4) | 0 | 0 | 7 (14.6) | - |
| Mean (range) disease activity score | 50.7 (12.5–105) | 45.0 (20–75) | 53.8 (50–60) | - | 16.2 (0–40) |
| Disease location (%) | L1 = 7 (26.9) L2 = 6 (23.1) L3 = 13 (50) | E1 = 4 (14.4) E2 = 2 (11.1) E3 = 1 (5.6) E4 = 11 (61.1) | E1 = 1 (25) E4 = 3 (75) | - | - |
| Variable Validation set | CD N = 16 | UC N = 10 | IBDU N = 2 | Non-IBD Controls N = 28 | IBD at 3-Months Following Treatment N = 14 (CD = 9, UC = 4, IBDU = 1) |
| Age in years (range) | 10.7 (7–15) | 12.7 (8–16) | 7–9 | 10.8 (4–16) | 10.9 (7–15) |
| Female n (%) | 9 (56.3) | 3 (30.0) | 1 (50.0) | 11 (39.3) | 5 (35.7) |
| Received antibiotics in last 3 months: no. (%) | 3 (18.8) | 1 (10.0) | 0 | 1 (3.6) | - |
| Mean (range) disease activity score | 54.2 (25–90) | 52 (30–85) | 50 (50–50) | - | 12.8 (0–35) |
| Disease location: no. (%) | L1 = 5 (31.3) L2 = 5 (31.3) L3 = 6 (37.5) | E1 = 4 (40) E2 = 1 (10) E4 = 5 (50) | E4 = 2 | - | - |
| Calprotectin (100 µg/g Cut Off) | Calprotectin (250 µg/g Cut Off) | Sensor Model SVML with LOOCV | Best Sensor Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparison | Accuracy % (CI) | Sensitivity % (CI) | Specificity % (CI) | Accuracy % (CI) | Sensitivity % (CI) | Specificity % (CI) | Accuracy % (CI) | Sensitivity % (CI) | Specificity % (CI) | Accuracy % (CI) | Sensitivity % (CI) | Specificity % (CI) |
| IBD vs. control | 90 (78–96) | 96 (82–100) | 82 (63–94) | 90 (78–96) | 93 (77–99) | 86 (67–96) | 75 (70–80) | 82 (75–89) | 71 (61–80) | 75 (70–80) | 82 (75–89) * | 71 (61–80) * |
| CD vs. control | 75 (57–89) | 94 (70–100) | 56 (30–80) | 81 (64–93) | 88 (62–98) | 75 (48–93) | 75 (69–81) | 83 (75–92) | 70 (60–80) | 75 (69–81) | 83 (75–92) * | 70 (60–80) * |
| UC vs. control | 90 (68–99) | 100 (69–100) | 80 (44–97) | 95 (75–100) | 100 (69–100) | 90 (56–100) | 50 (38–62) | 50 (38–62) | 50 (32–68) | 68 (54- 82) | 80 (68–92) † | 50 (28–72) † |
| UC and IBDU vs. control | 92 (73–99) | 100 (74–100) | 83 (52–98) | 96 (79–100) | 100 (74–100) | 92 (62–100) | 75 (65–86) | 71 (52–91) | 80 (64–96) | 75 (65–86) | 71 (52–91) * | 80 (64–96) * |
| CD vs. UC | NA | NA | NA | NA | NA | NA | 73 (69–77) | 80 (75–85) | 64 (54–74) | 85 (79–90) | 88 ‡ (82–93) | 80 ‡ (71–89) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slater, R.; Tharmaratnam, K.; Belnour, S.; Auth, M.K.-H.; Muhammed, R.; Spray, C.; Wang, D.; de Lacy Costello, B.; García-Fiñana, M.; Allen, S.; et al. Gas Chromatography–Sensor System Aids Diagnosis of Inflammatory Bowel Disease, and Separates Crohn’s from Ulcerative Colitis, in Children. Sensors 2024, 24, 5079. https://doi.org/10.3390/s24155079
Slater R, Tharmaratnam K, Belnour S, Auth MK-H, Muhammed R, Spray C, Wang D, de Lacy Costello B, García-Fiñana M, Allen S, et al. Gas Chromatography–Sensor System Aids Diagnosis of Inflammatory Bowel Disease, and Separates Crohn’s from Ulcerative Colitis, in Children. Sensors. 2024; 24(15):5079. https://doi.org/10.3390/s24155079
Chicago/Turabian StyleSlater, Rachael, Kukatharmini Tharmaratnam, Salma Belnour, Marcus Karl-Heinz Auth, Rafeeq Muhammed, Christine Spray, Duolao Wang, Ben de Lacy Costello, Marta García-Fiñana, Stephen Allen, and et al. 2024. "Gas Chromatography–Sensor System Aids Diagnosis of Inflammatory Bowel Disease, and Separates Crohn’s from Ulcerative Colitis, in Children" Sensors 24, no. 15: 5079. https://doi.org/10.3390/s24155079





