Exploring the Feasibility of Bidirectional Control of Beta Oscillatory Power in Healthy Controls as a Potential Intervention for Parkinson’s Disease Movement Impairment
Abstract
1. Introduction
Related Work
2. Methods
2.1. Participants
2.2. Study Design and Protocol
2.3. EEG Methods
2.3.1. Recordings
2.3.2. Calibration
2.3.3. Neurofeedback Presentation
2.4. Analysis
2.4.1. Neurofeedback and Performance
2.4.2. Perceived Neurofeedback Task Difficulty and Workload
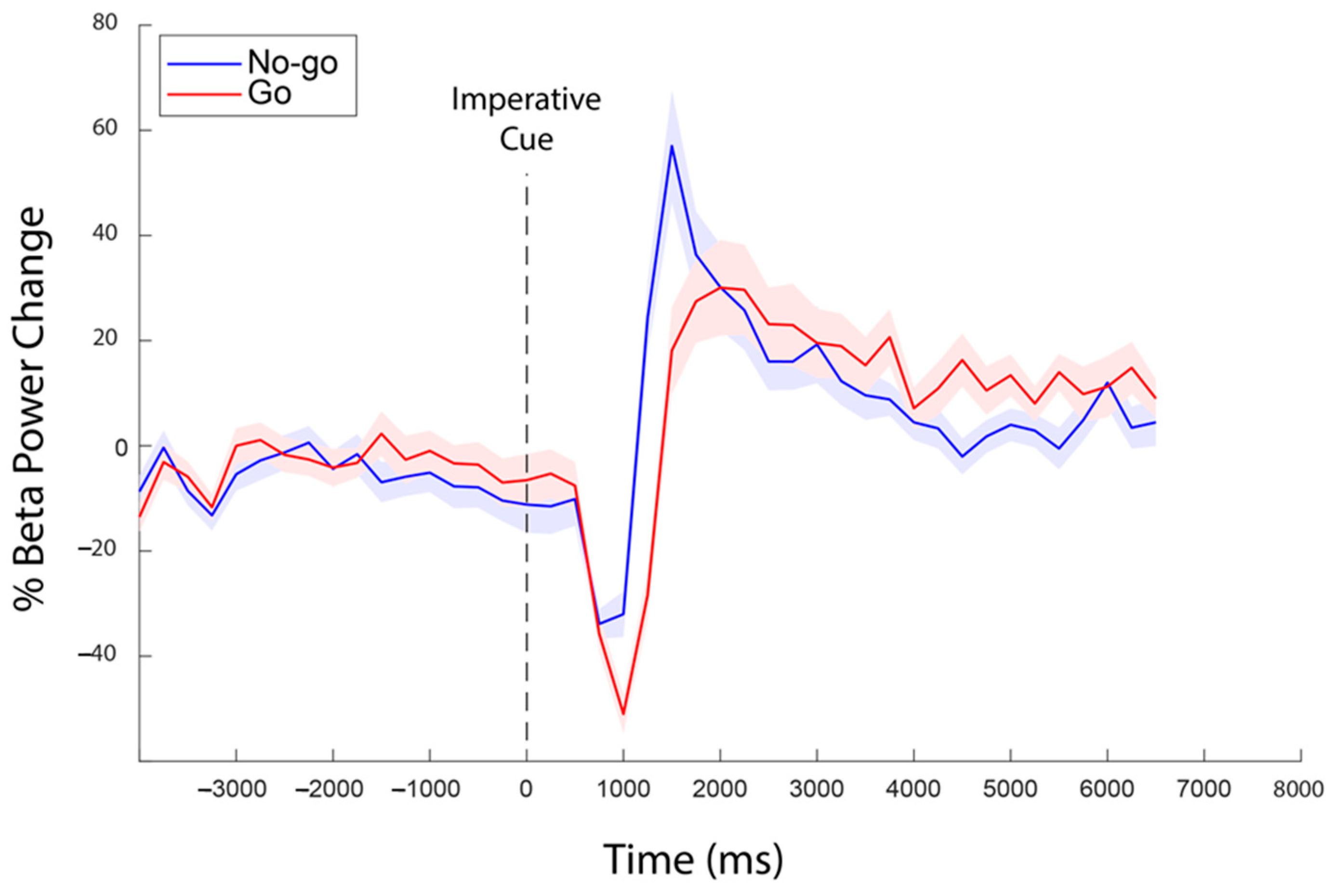
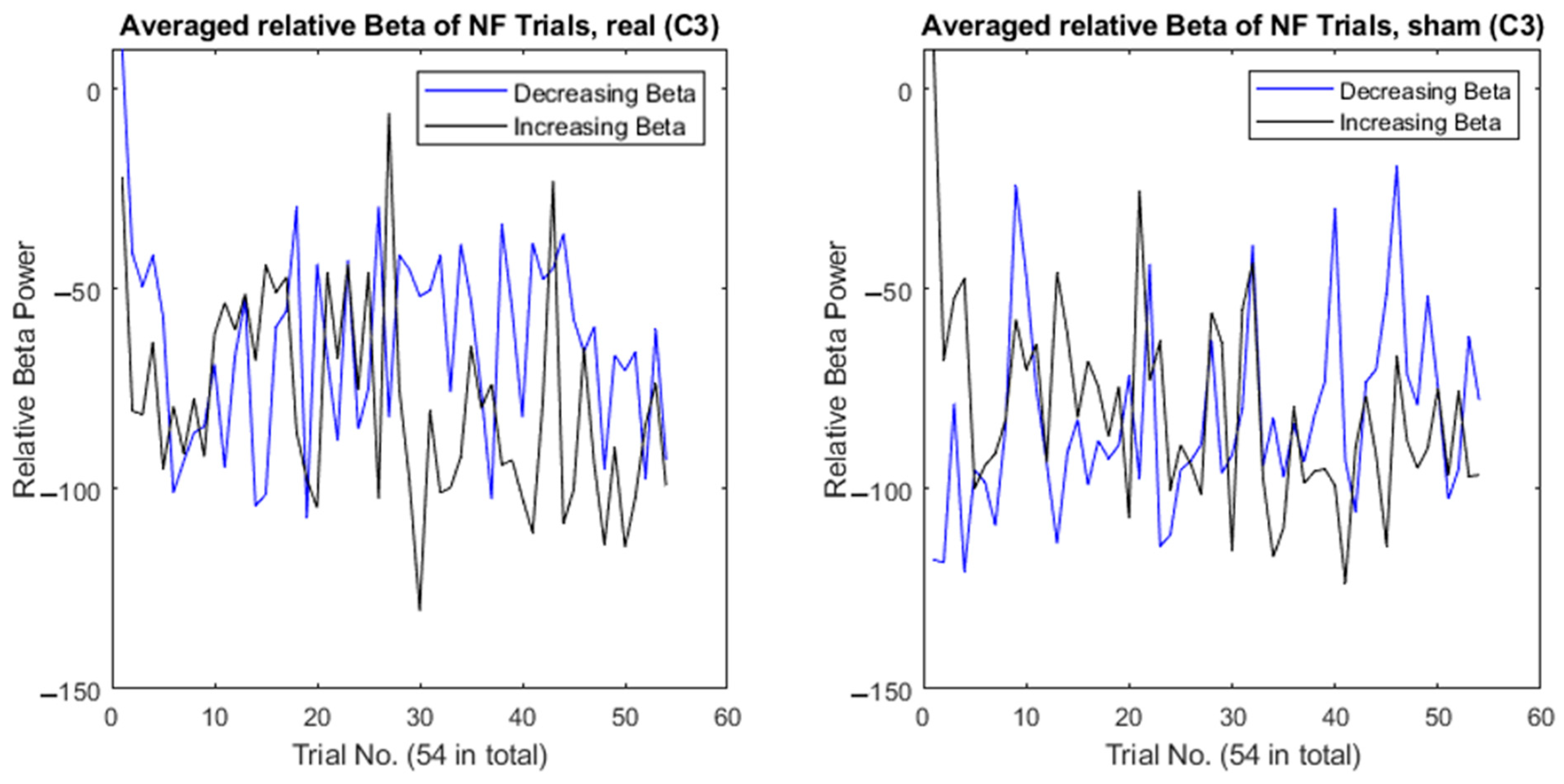
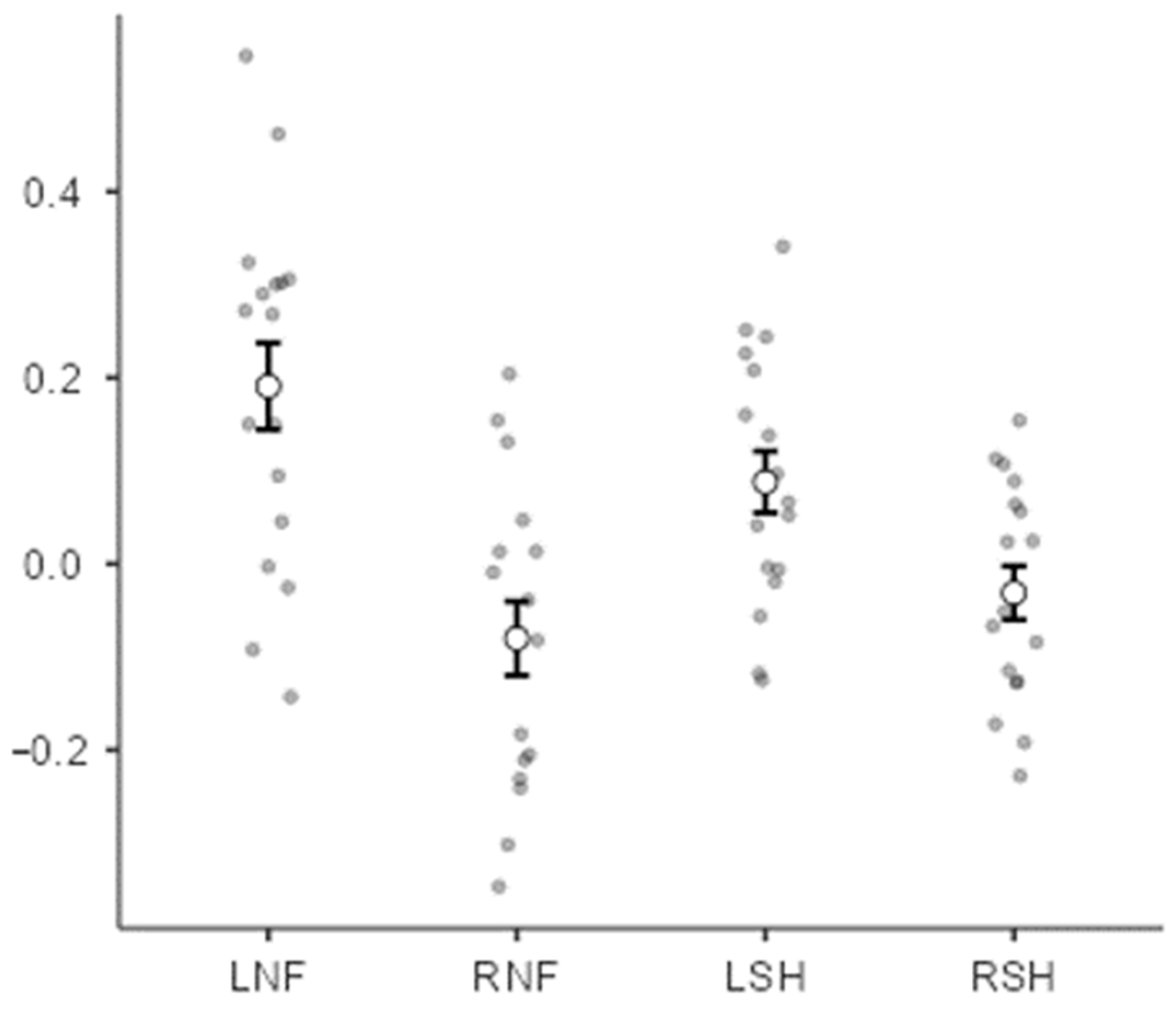
3. Results
Perceived Neurofeedback Task Difficulty and Workload
- “Real” Condition
- Moving the bar to the left was easy (n = 11, 65%).
- Moving the bar to the right was hard (n = 16, 94%).
- The task became harder as time went on (n = 10, 59%).
- “Sham” Condition
- The task was generally hard (n = 12, 71%).
- The task was a mix of easy and hard (n = 5, 29%).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Radad, K.; Gille, G.; Rausch, W.-D. Short review on dopamine agonists: Insight into clinical and research studies relevant to Parkinson’s disease. Pharmacol. Rep. 2005, 57, 701–712. [Google Scholar] [PubMed]
- Eisinger, R.S.; Cagle, J.N.; Opri, E.; Alcantara, J.; Cernera, S.; Foote, K.D.; Okun, M.S.; Gunduz, A. Parkinsonian Beta Dynamics during Rest and Movement in the Dorsal Pallidum and Subthalamic Nucleus. J. Neurosci. 2020, 40, 2859–2867. [Google Scholar] [CrossRef]
- Silberstein, P.; Pogosyan, A.; Kühn, A.A.; Hotton, G.; Tisch, S.; Kupsch, A.; Dowsey-Limousin, P.; Hariz, M.I.; Brown, P. Cortico-cortical coupling in Parkinson’s disease and its modulation by therapy. Brain 2005, 128, 1277–1291. [Google Scholar] [CrossRef]
- Eusebio, A.; Cagnan, H.; Brown, P. Does suppression of oscillatory synchronisation mediate some of the therapeutic effects of DBS in patients with Parkinson’s disease? Front. Integr. Neurosci. 2012, 6, 47. [Google Scholar] [CrossRef]
- Kühn, A.A.; Doyle, L.; Pogosyan, A.; Yarrow, K.; Kupsch, A.; Schneider, G.H.; Hariz, M.I.; Trottenberg, T.; Brown, P. Modulation of beta oscillations in the subthalamic area during motor imagery in Parkinson’s disease. Brain 2006, 129, 695–706. [Google Scholar] [CrossRef]
- Bronstein, J.M.; Tagliati, M.; Alterman, R.L.; Lozano, A.M.; Volkmann, J.; Stefani, A.; Horak, F.B.; Okun, M.S.; Foote, K.D.; Krack, P.; et al. Deep brain stimulation for Parkinson disease: An expert consensus and review of key issues. Arch. Neurol. 2011, 68, 165. [Google Scholar] [CrossRef]
- Limousin, P.; Foltynie, T. Long-term outcomes of deep brain stimulation in Parkinson disease. Nat. Rev. Neurol. 2019, 15, 234–242. [Google Scholar] [CrossRef]
- Brown, P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr. Opin. Neurobiol. 2007, 17, 656–664. [Google Scholar] [CrossRef]
- Cagnan, H.; Mallet, N.; Moll, C.K.; Gulberti, A.; Holt, A.B.; Westphal, M.; Gerloff, C.; Engel, A.K.; Hamel, W.; Magill, P.J.; et al. Temporal evolution of beta bursts in the parkinsonian cortical and basal ganglia network. Proc. Natl. Acad. Sci. USA 2019, 116, 16095–16104. [Google Scholar] [CrossRef]
- Hall, S.D.; Prokic, E.J.; McAllister, C.J.; Ronnqvist, K.C.; Williams, A.C.; Yamawaki, N.; Witton, C.; Woodhall, G.L.; Stanford, I.M. GABA-mediated changes in inter-hemispheric beta frequency activity in early-stage Parkinson’s disease. Neuroscience 2014, 281, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Prokic, E.J.; Stanford, I.M.; Woodhall, G.L.; Williams, A.C.; Hall, S.D. Bradykinesia Is Driven by Cumulative Beta Power During Continuous Movement and Alleviated by Gabaergic Modulation in Parkinson’s Disease. Front. Neurol. 2019, 10, 1298. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs-Graham, E.; Wilson, T.W.; Santamaria, P.M.; Heithoff, S.K.; Torres-Russotto, D.; Hutter-Saunders, J.A.; Estes, K.A.; Meza, J.L.; Mosley, R.L.; Gendelman, H.E. Neuromagnetic evidence of abnormal movement-related beta desynchronization in Parkinson’s disease. Cereb. Cortex 2014, 24, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Berghold, A. Patterns of cortical activation during planning of voluntary movement. Electroencephalogr. Clin. Neurophysiol. 1989, 72, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.D.; Stanford, I.M.; Yamawaki, N.; McAllister, C.J.; Rönnqvist, K.C.; Woodhall, G.L.; Furlong, P.L. The role of GABAergic modulation in motor function related neuronal network activity. Neuroimage 2011, 56, 1506–1510. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Stancák, A., Jr.; Neuper, C. Event-related synchronization (ERS) in the alpha band—An electrophysiological correlate of cortical idling: A review. Int. J. Psychophysiol. 1996, 24, 39–46. [Google Scholar] [CrossRef]
- Jurkiewicz, M.T.; Gaetz, W.C.; Bostan, A.C.; Cheyne, D. Post-movement beta rebound is generated in motor cortex: Evidence from neuromagnetic recordings. Neuroimage 2006, 32, 1281–1289. [Google Scholar] [CrossRef]
- Marzbani, H.; Marateb, H.R.; Mansourian, M. Neurofeedback: A comprehensive review on system design, methodology and clinical applications. Basic Clin. Neurosci. 2016, 7, 143–158. [Google Scholar]
- Anil, K.; Hall, S.D.; Demain, S.; Freeman, J.A.; Ganis, G.; Marsden, J. A Systematic Review of Neurofeedback for the Management of Motor Symptoms in Parkinson’s Disease. Brain Sci. 2021, 11, 1292. [Google Scholar] [CrossRef]
- Fumuro, T.; Matsuhashi, M.; Mitsueda, T.; Inouchi, M.; Hitomi, T.; Nakagawa, T.; Matsumoto, R.; Kawamata, J.; Inoue, H.; Mima, T.; et al. Bereitschaftspotential augmentation by neuro-feedback training in Parkinson’s disease. Clin. Neurophysiol. 2013, 124, 1398–1405. [Google Scholar] [CrossRef]
- Kasahara, K.; Hoshino, H.; Furusawa, Y.; Sayo DaSalla, C.; Honda, M.; Murata, M.; Hanakawa, T. Initial experience with a sensorimotor rhythm-based brain-computer interface in a Parkinson’s disease patient. Brain-Comput. Interfaces 2018, 5, 88–96. [Google Scholar]
- He, S.; Everest-Phillips, C.; Clouter, A.; Brown, P.; Tan, H. Neurofeedback-Linked Suppression of Cortical β Bursts Speeds Up Movement Initiation in Healthy Motor Control: A Double-Blind Sham-Controlled Study. J. Neurosci. 2020, 40, 4021–4032. [Google Scholar] [CrossRef]
- Cook, A.J.; Pfeifer, K.J.; Tass, P.A. A Single Case Feasibility Study of Sensorimotor Rhythm Neurofeedback in Parkinson’s Disease. Front. Neurosci. 2021, 15, 623317. [Google Scholar] [CrossRef] [PubMed]
- Anil, K.; Demain, S.; Burridge, J.; Simpson, D.; Taylor, J.; Cotter, I.; Vuckovic, A. The importance of self-efficacy and negative affect for neurofeedback success for central neuropathic pain after a spinal cord injury. Sci. Rep. 2022, 12, 10949. [Google Scholar] [CrossRef] [PubMed]
- Alkoby, O.; Abu-Rmileh, A.; Shriki, O.; Todder, D. Can we predict who will respond to neurofeedback? A review of the inefficacy problem and existing predictors for successful EEG neurofeedback learning. Neuroscience 2018, 378, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Kadosh, K.C.; Staunton, G. A systematic review of the psychological factors that influence neurofeedback learning outcomes. Neuroimage 2019, 185, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shi, Z.; Pei, G.; Fang, B.; Yan, T. Effect of Neurofeedback Based on Imaginary Movement in Parkinson’s Disease. In Proceedings of the 2023 17th International Conference on Complex Medical Engineering (CME), Suzhou, China, 3–5 November 2023; pp. 83–87. [Google Scholar]
- Homan, R.W.; Herman, J.; Purdy, P. Cerebral location of international 10–20 system electrode placement. Electroencephalogr. Clin. Neurophysiol. 1987, 66, 376–382. [Google Scholar] [CrossRef] [PubMed]
- De Pretto, M.; Mouthon, M.; Debove, I.; Pollo, C.; Schüpbach, M.; Spierer, L.; Accolla, E.A. Proactive inhibition is not modified by deep brain stimulation for Parkinson’s disease: An electrical neuroimaging study. Hum. Brain Mapp. 2021, 42, 3934–3949. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, R.; Danziger-Schragenheim, S.; Possti, D.; Fahoum, F.; Giladi, N.; Hausdorff, J.M.; Mirelman, A.; Maidan, I. Impaired inhibitory control during walking in Parkinson’s disease patients: An EEG study. J. Park. Dis. 2022, 12, 243–256. [Google Scholar] [CrossRef]
- Hart, S.G.; Staveland, L.E. Development of NASA-TLX (Task Load Index): Results of Empirical and Theoretical Research. In Advances in Psychology; Hancock, P.A., Meshkati, N., Eds.; North-Holland: Gilching, Germany, 1988; pp. 139–183. [Google Scholar]
- Medine, D. LSL-actiCHamp. 2021. Available online: https://github.com/brain-products/LSL-actiCHamp (accessed on 1 January 2021).
- Boulay, C. App-LabRecorder. 2021. Available online: https://github.com/labstreaminglayer/App-LabRecorder (accessed on 1 January 2021).
- Brainard, D.H. The Psychophysics Toolbox. Spat. Vis. 1997, 10, 433–436. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Elo, S.; Kyngäs, H. The qualitative content analysis process. J. Adv. Nurs. 2008, 62, 107–115. [Google Scholar] [CrossRef]
- Rhodes, E.; Gaetz, W.C.; Marsden, J.; Hall, S.D. Transient alpha and beta synchrony underlies preparatory recruitment of directional motor networks. J. Cogn. Neurosci. 2018, 30, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Wessel, J.R. β-bursts reveal the trial-to-trial dynamics of movement initiation and cancellation. J. Neurosci. 2020, 40, 411–423. [Google Scholar] [CrossRef]
- Cooke, A.; Hindle, J.; Lawrence, C.; Bellomo, E.; Pritchard, A.W.; MacLeod, C.A.; Martin-Forbes, P.; Jones, S.; Bracewell, M.; Linden, D.E.; et al. Effects of Home-Based EEG Neurofeedback Training as a Non-Pharmacological Intervention for Parkinson’s Disease. medRxiv 2024, 2. [Google Scholar] [CrossRef]
- Khanna, P.; Swann, N.C.; de Hemptinne, C.; Miocinovic, S.; Miller, A.; Starr, P.A.; Carmena, J.M. Neurofeedback control in Parkinsonian patients using electrocorticography signals accessed wirelessly with a chronic, fully implanted device. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 25, 1715–1724. [Google Scholar] [CrossRef]
- Fukuma, R.; Yanagisawa, T.; Tanaka, M.; Yoshida, F.; Hosomi, K.; Oshino, S.; Tani, N.; Kishima, H. Real-time neurofeedback to modulate β-band power in the subthalamic nucleus in Parkinson’s disease patients. Eneuro 2018, 5, 6. [Google Scholar] [CrossRef]
- He, S.; Mostofi, A.; Syed, E.; Torrecillos, F.; Tinkhauser, G.; Fischer, P.; Pogosyan, A.; Hasegawa, H.; Li, Y.; Ashkan, K.; et al. Subthalamic beta-targeted neurofeedback speeds up movement initiation but increases tremor in Parkinsonian patients. Elife 2020, 9, e60979. [Google Scholar] [CrossRef] [PubMed]
- Kober, S.E.; Witte, M.; Ninaus, M.; Neuper, C.; Wood, G. Learning to modulate one’s own brain activity: The effect of spontaneous mental strategies. Front. Hum. Neurosci. 2013, 7, 695. [Google Scholar] [CrossRef]
- Nan, W.; Rodrigues, J.P.; Ma, J.; Qu, X.; Wan, F.; Mak, P.-I.; Mak, P.U.; Vai, M.I.; Rosa, A. Individual alpha neurofeedback training effect on short term memory. Int. J. Psychophysiol. 2012, 86, 83–87. [Google Scholar] [CrossRef]

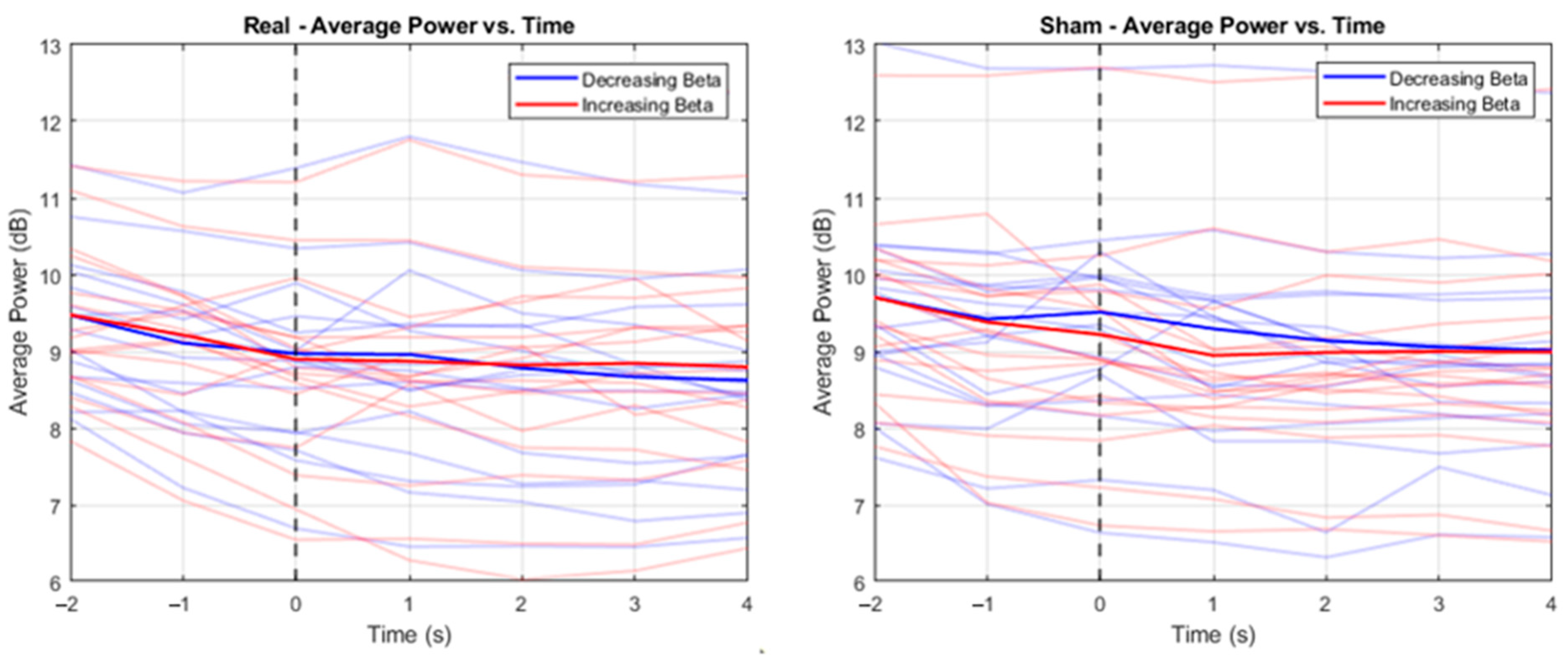
| T1 (−2 s) | T2 (−1 s) | T3 (0 s) | T4 (1 s) | T5 (2 s) | T6 (3 s) | T7 (4 s) | |
|---|---|---|---|---|---|---|---|
| Decreasing beta condition | |||||||
| Mean | 8.85 | 8.14 | 7.89 | 7.87 | 7.56 | 7.36 | 7.29 |
| SD | 2.00 | 1.96 | 2.21 | 2.60 | 2.33 | 2.17 | 2.07 |
| Increasing beta condition | |||||||
| Mean | 8.86 | 8.34 | 7.76 | 7.71 | 7.62 | 7.66 | 7.58 |
| SD | 2.19 | 2.09 | 2.22 | 2.55 | 2.32 | 2.31 | 2.26 |
| T1 (−2 s) | T2 (−1 s) a | T3 (0 s) b | T4 (1 s) c | T5 (2 s) | T6 (3 s) | T7 (4 s) | |
|---|---|---|---|---|---|---|---|
| Decreasing beta condition | |||||||
| Mean | 9.24 | 8.62 | 8.87 | 8.45 | 8.20 | 8.12 | 8.04 |
| SD | 3.29 | 3.12 | 3.16 | 3.27 | 3.20 | 2.92 | 2.91 |
| Increasing beta condition | |||||||
| Mean | 9.19 | 8.46 | 8.32 | 7.90 | 7.97 | 8.00 | 8.01 |
| SD | 2.84 | 3.00 | 3.18 | 3.06 | 3.12 | 2.88 | 2.99 |
| Average Reaction Times—Go Trials (All Errors Taken Out) | ||
| Trial | Bar Direction | Average Reaction Time (s) |
| Real | Left | 0.489 |
| Real | Right | 0.493 |
| Sham | Left | 0.494 |
| Sham | Right | 0.494 |
| Average Errors Made—Go Trials | ||
| Trial | Bar Direction | Average Count |
| Real | Left | 0.056 |
| Real | Right | 0.041 |
| Sham | Left | 0.027 |
| Sham | Right | 0.020 |
| Average Errors Made—No-go Trials | ||
| Trial | Bar Direction | Average Count |
| Real | Left | 0.074 |
| Real | Right | 0.074 |
| Sham | Left | 0.029 |
| Sham | Right | 0.078 |
| Average Timings for Errors—Go Trials (Errors refer to “too slow” reactions; complete “misses” taken out) | ||
| Trial | Bar Direction | Average Reaction Time (s) |
| Real | Left | 0.960 |
| Real | Right | 0.976 |
| Sham | Left | 0.962 |
| Sham | Right | 0.969 |
| Average Timings for Errors—No-go Trials | ||
| Trial | Bar Direction | Average Reaction Time (s) |
| Real | Left | 0.477 |
| Real | Right | 0.485 |
| Sham | Left | 0.395 |
| Sham | Right | 0.410 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anil, K.; Ganis, G.; Freeman, J.A.; Marsden, J.; Hall, S.D. Exploring the Feasibility of Bidirectional Control of Beta Oscillatory Power in Healthy Controls as a Potential Intervention for Parkinson’s Disease Movement Impairment. Sensors 2024, 24, 5107. https://doi.org/10.3390/s24165107
Anil K, Ganis G, Freeman JA, Marsden J, Hall SD. Exploring the Feasibility of Bidirectional Control of Beta Oscillatory Power in Healthy Controls as a Potential Intervention for Parkinson’s Disease Movement Impairment. Sensors. 2024; 24(16):5107. https://doi.org/10.3390/s24165107
Chicago/Turabian StyleAnil, Krithika, Giorgio Ganis, Jennifer A. Freeman, Jonathan Marsden, and Stephen D. Hall. 2024. "Exploring the Feasibility of Bidirectional Control of Beta Oscillatory Power in Healthy Controls as a Potential Intervention for Parkinson’s Disease Movement Impairment" Sensors 24, no. 16: 5107. https://doi.org/10.3390/s24165107
APA StyleAnil, K., Ganis, G., Freeman, J. A., Marsden, J., & Hall, S. D. (2024). Exploring the Feasibility of Bidirectional Control of Beta Oscillatory Power in Healthy Controls as a Potential Intervention for Parkinson’s Disease Movement Impairment. Sensors, 24(16), 5107. https://doi.org/10.3390/s24165107







