Sensing with Molecularly Imprinted Membranes on Two-Dimensional Solid-Supported Substrates
Abstract
:1. Introduction
1.1. Molecular Imprinting Technology
1.2. Development of Molecular Imprinting Membranes
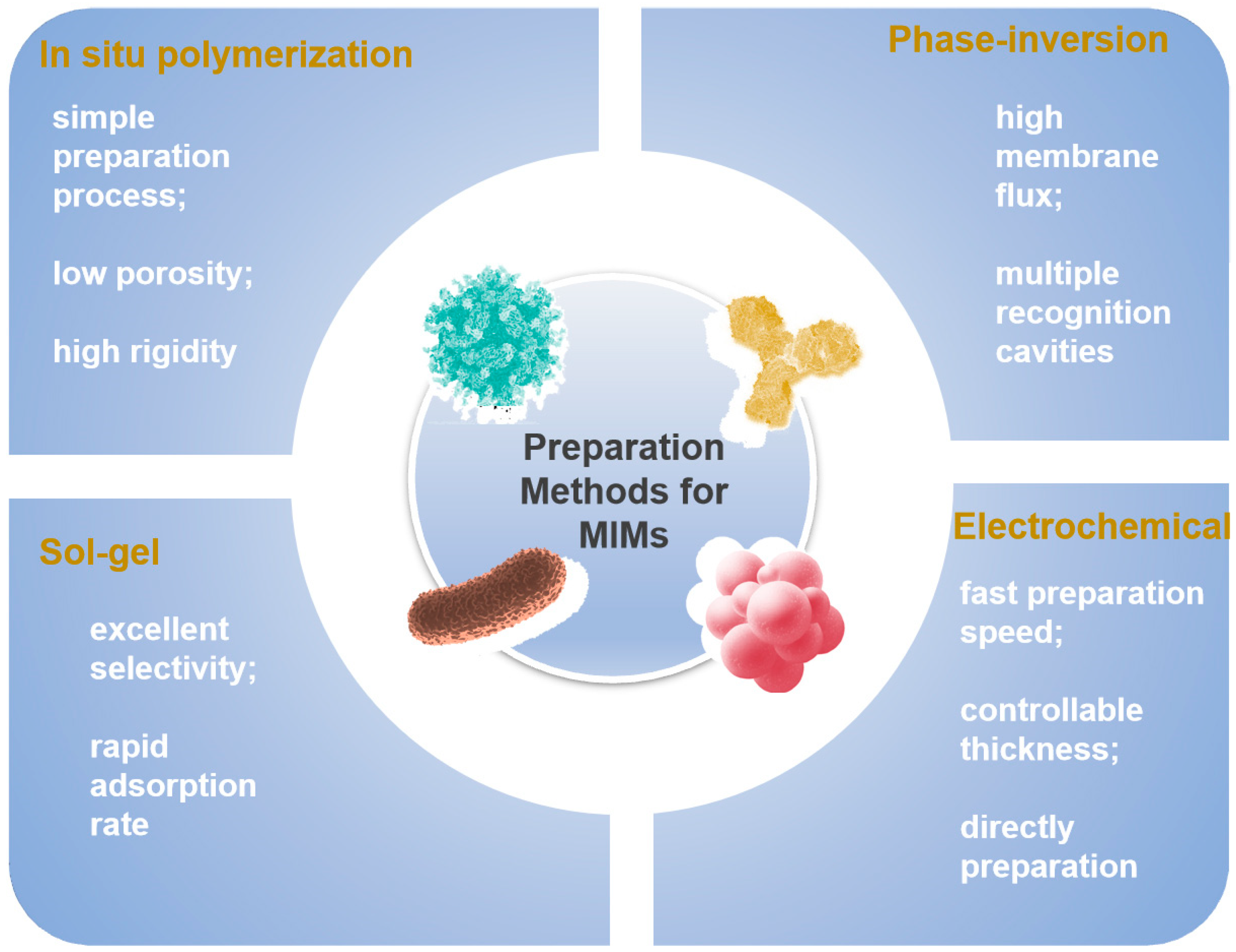
2. Preparation Methods for MIMs
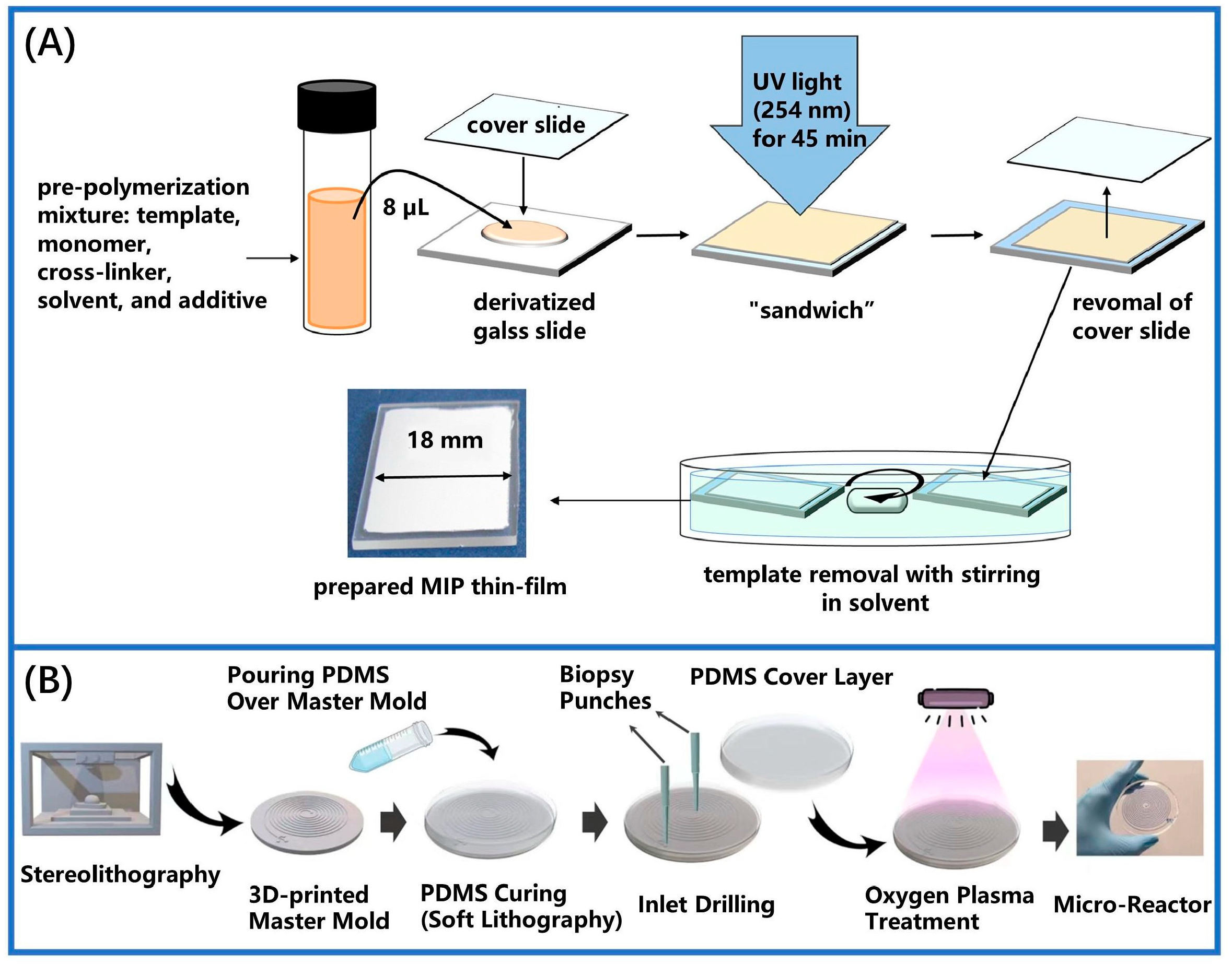
2.1. In Situ Polymerization Method
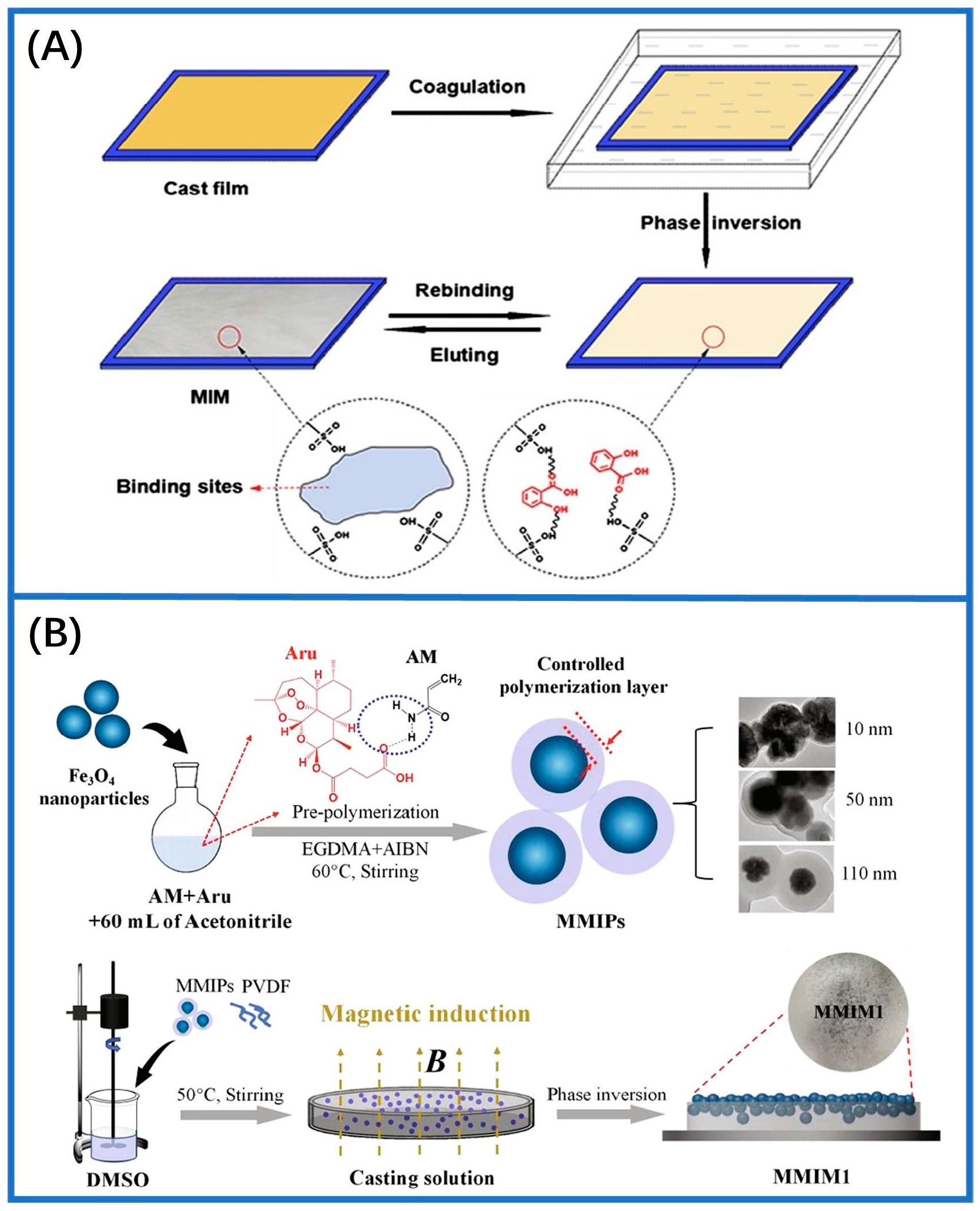
2.2. Phase-Inversion Method
2.3. Sol-Gel Method
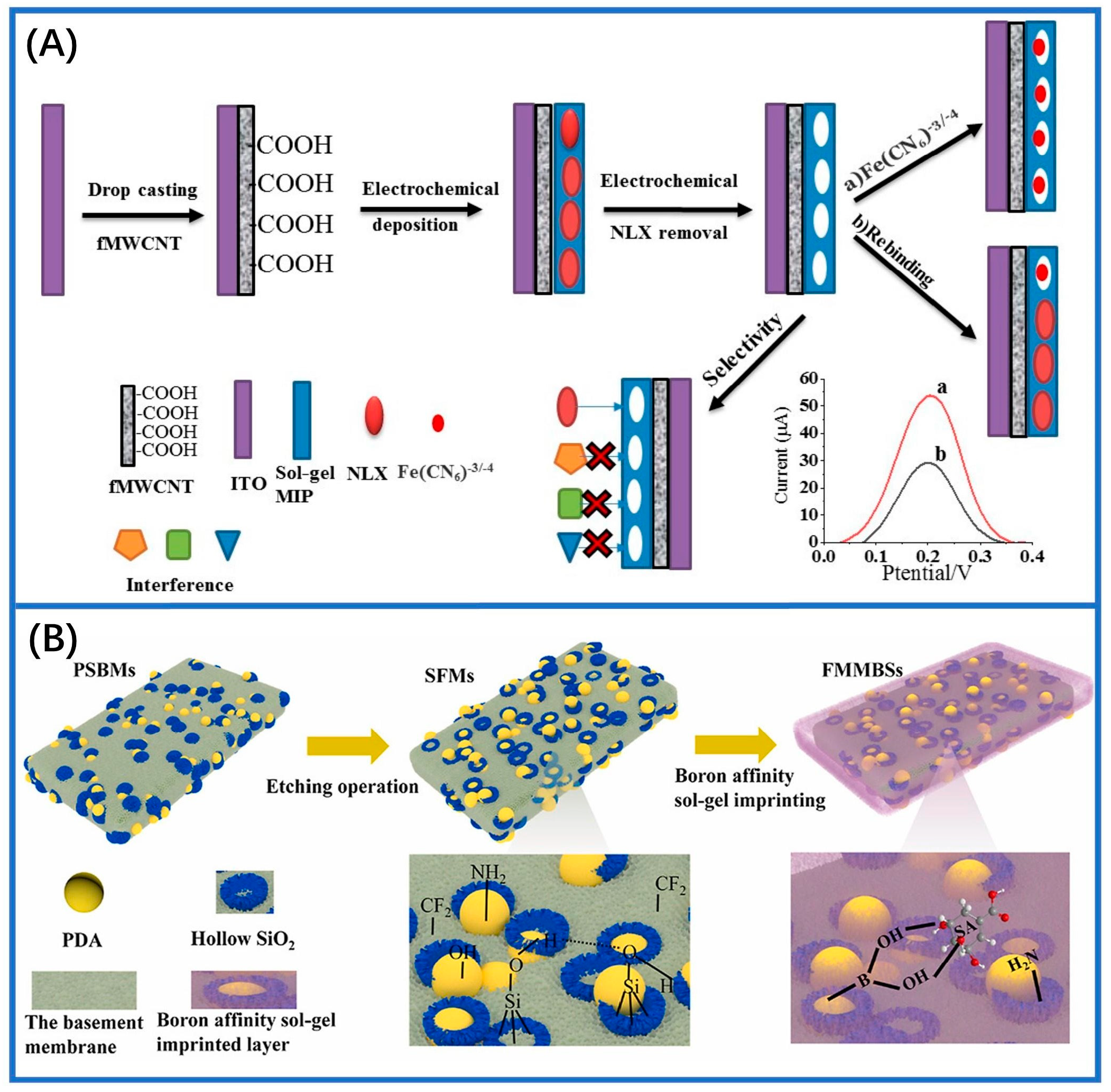
2.4. Electrochemical Method
3. Important Applications of MIMs in Various Fields
3.1. Electrochemical Biochemical Sensors

3.2. Surface-Enhanced Raman Scattering
3.3. Surface Plasmon Resonance
3.4. Quartz Crystal Microbalance
3.5. Ion-Sensitive Field-Effect Transistors
4. Challenging and Limitations
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DiMaio, J.T.M.; Doran, T.M.; Ryan, D.M.; Raymond, D.M.; Nilsson, B.L. Modulating supramolecular peptide hydrogel viscoelasticity using biomolecular recognition. Biomacromolecules 2017, 18, 3591–3599. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.T.; Yan, Z.; Chu, X.; Zheng, X.; Liu, Z.; Xu, L.; Zhang, K.; Wang, J. Physics of biomolecular recognition and conformational dynamics. Rep. Prog. Phys. 2021, 84, 126601. [Google Scholar] [CrossRef]
- Lara, S.; Alnasser, F.; Polo, E.; Garry, D.; Lo Giudice, M.C.; Hristov, D.R.; Rocks, L.; Salvati, A.; Yan, Y.; Dawson, K.A. Identification of receptor binding to the biomolecular corona of nanoparticles. ACS Nano 2017, 11, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Gianneli, M.; Polo, E.; Lopez, H.; Castagnola, V.; Aastrup, T.; Dawson, K.A. Label-free in-flow detection of receptor recognition motifs on the biomolecular corona of nanoparticles. Nanoscale 2018, 10, 5474–5481. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Li, H.; Zhao, L.; Ma, Y.; Zhang, Y.; Liu, J.; Wei, Y. Rigorous recognition mode analysis of molecularly imprinted polymers—Rational design, challenges, and opportunities. Prog. Polym. Sci. 2024, 150, 101790. [Google Scholar] [CrossRef]
- Mirarefi, P.; Lee, C.T., Jr. Reversible control of enzyme-inhibitor interactions with light illumination using a photoresponsive surfactant. Proteins Struct. Funct. Bioinform. 2019, 87, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Adamson, H.; Ajayi, M.O.; Campbell, E.; Brachi, E.; Tiede, C.; Tang, A.A.; Adams, T.L.; Ford, R.; Davidson, A.; Johnson, M.; et al. Affimer–enzyme–inhibitor switch sensor for rapid wash-free assays of multimeric proteins. ACS Sens. 2019, 4, 3014–3022. [Google Scholar] [CrossRef] [PubMed]
- Hang, Y.; He, X.P.; Yang, L.; Hua, J. Probing sugar–lectin recognitions in the near-infrared region using glyco-diketopyrrolopyrrole with aggregation-induced-emission. Biosens. Bioelectron. 2015, 65, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Guo, Z.; Xu, S.; Lu, H.; Wang, L.; Gu, Z.; Liu, Z. Molecularly imprinted nanobeacons redirect innate immune killing towards triple negative breast cancer. Angew. Chem. Int. Ed. 2023, 62, e202301202. [Google Scholar] [CrossRef]
- Bülbül, G.; Hayat, A.; Andreescu, S. Biomolecular recognition: ssDNA-functionalized nanoceria: A redox-active aptaswitch for biomolecular recognition. Adv. Healthc. Mater. 2016, 5, 864. [Google Scholar] [CrossRef]
- Karimi, A.; Hayat, A.; Andreescu, S. Biomolecular detection at ssDNA-conjugated nanoparticles by nano-impact electrochemistry. Biosens. Bioelectron. 2017, 87, 501–507. [Google Scholar] [CrossRef]
- Guest, J.D.; Vreven, T.; Zhou, J.; Moal, I.; Jeliazkov, J.R.; Gray, J.J.; Weng, Z.; Pierce, B.G. An expanded benchmark for antibody-antigen docking and affinity prediction reveals insights into antibody recognition determinants. Structure 2021, 29, 606–621.e5. [Google Scholar] [CrossRef]
- Mujahid, A.; Afzal, A.; Dickert, F.L. Transitioning from supramolecular chemistry to molecularly imprinted polymers in chemical sensing. Sensors 2023, 23, 7457. [Google Scholar] [CrossRef] [PubMed]
- Ostovan, A.; Arabi, M.; Wang, Y.; Li, J.; Li, B.; Wang, X.; Chen, L. Greenificated molecularly imprinted materials for advanced applications. Adv. Mater. 2022, 34, 2203154. [Google Scholar] [CrossRef]
- Haupt, K.; Medina Rangel, P.X.; Bui, B.T.S. Molecularly imprinted polymers: Antibody mimics for bioimaging and therapy. Chem. Rev. 2020, 120, 9554–9582. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J. Molecular imprinting with functional DNA. Small 2019, 15, 1805246. [Google Scholar] [CrossRef] [PubMed]
- Dinc, M.; Esen, C.; Mizaikoff, B. Recent advances on core–shell magnetic molecularly imprinted polymers for biomacromolecules. TrAC Trends Anal. Chem. 2019, 114, 202–217. [Google Scholar] [CrossRef]
- Wulff, G.; Sarhan, A. The use of polymers with enzyme-analogous structures for the resolution of racemates. Angew. Chem. Int. Ed. 1972, 11, 341–346. [Google Scholar]
- Gong, H.; Hajizadeh, S.; Jiang, L.; Ma, H.; Ye, L. Dynamic assembly of molecularly imprinted polymer nanoparticles. J. Colloid Interface Sci. 2018, 509, 463–471. [Google Scholar] [CrossRef]
- Nsibande, S.A.; Forbes, P.B.C. Development of a quantum dot molecularly imprinted polymer sensor for fluorescence detection of atrazine. Luminescence 2019, 34, 480–488. [Google Scholar] [CrossRef]
- Lin, C.C.; Lin, H.Y.; Thomas, J.L.; Yu, J.X.; Lin, C.Y.; Chang, Y.H.; Lee, M.H.; Wang, T.L. Embedded upconversion nanoparticles in magnetic molecularly imprinted polymers for photodynamic therapy of hepatocellular carcinoma. Biomedicines 2021, 9, 1923. [Google Scholar] [CrossRef] [PubMed]
- Ratnaningsih, E.; Kadja, G.T.M.; Putri, R.M.; Alni, A.; Khoiruddin, K.; Djunaidi, M.C.; Ismadji, S.; Wenten, I.G. Molecularly imprinted affinity membrane: A review. ACS Omega 2022, 7, 23009–23026. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Tharpa, K.; Dima, Ş.O. Molecularly imprinted membranes: Past, present, and future. Chem. Rev. 2016, 116, 11500–11528. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.C.; Sánchez, L.T.; Valencia, G.A.; Ahmed, S.; Gutiérrez, T.J. Molecularly imprinted polymers for food applications: A review. Trends Food Sci. Technol. 2021, 111, 642–669. [Google Scholar] [CrossRef]
- Yang, W.; Ma, Y.; Sun, H.; Huang, C.; Shen, X. Molecularly imprinted polymers based optical fiber sensors: A review. TrAC Trends Anal. Chem. 2022, 152, 116608. [Google Scholar] [CrossRef]
- Ahmad, O.S.; Bedwell, T.S.; Esen, C.; Garcia Cruz, A.; Piletsky, S.A. Molecularly imprinted polymers in electrochemical and optical sensors. Trends Biotechnol. 2019, 37, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Zhu, X.; Kang, A.; Li, X. Molecular imprinted electrospun chromogenic membrane for l-tyrosine specific recognition and visualized detection. Talanta 2019, 204, 647–654. [Google Scholar] [CrossRef]
- Chen, J.; Wei, M.; Meng, M. Advanced development of molecularly imprinted membranes for selective separation. Molecules 2023, 28, 5764. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fan, L.; Wang, Y.; Huang, X.; Xu, J.; Lu, J.; Zhang, M.; Xu, W. Molecularly imprinted membrane electrospray ionization for direct sample analyses. Anal. Chem. 2017, 89, 1453–1458. [Google Scholar] [CrossRef]
- Seraj, S.; Lotfollahi, M.N.; Nematollahzadeh, A. Synthesis and sorption properties of heparin imprinted zeolite beta/polydopamine composite nanoparticles. React. Funct. Polym. 2020, 147, 104462. [Google Scholar] [CrossRef]
- Tawfik, S.M.; Elmasry, M.R.; Sharipov, M.; Azizov, S.; Lee, C.H.; Lee, Y.-I. Dual emission nonionic molecular imprinting conjugated polythiophenes-based paper devices and their nanofibers for point-of-care biomarkers detection. Biosens. Bioelectron. 2020, 160, 112211. [Google Scholar] [CrossRef] [PubMed]
- Nontawong, N.; Ngaosri, P.; Chunta, S.; Jarujamrus, P.; Nacapricha, D.; Lieberzeit, P.A.; Amatatongchai, M. Smart sensor for assessment of oxidative/nitrative stress biomarkers using a dual-imprinted electrochemical paper-based analytical device. Anal. Chim. Acta 2022, 1191, 339363. [Google Scholar] [CrossRef] [PubMed]
- Piletsky, S.A.; Dubey, I.; Fedoryak, D.; Kukhar, V.P. Substrate-selective polymeric membranes. Selective transfer of nucleic acid components. Biopolym. Cell 1990, 6, 55–58. [Google Scholar] [CrossRef]
- Wang, H.Y.; Kobayashi, T.; Fujii, N. Molecular imprint membranes prepared by the phase inversion precipitation technique. Langmuir 1996, 12, 4850–4856. [Google Scholar] [CrossRef]
- Wang, H.Y.; Kobayashi, T.; Fukaya, T.; Fujii, N. Molecular imprint membranes prepared by the phase inversion precipitation technique. 2. Influence of coagulation temperature in the phase inversion process on the encoding in polymeric membranes. Langmuir 1997, 13, 5396–5400. [Google Scholar] [CrossRef]
- Yang, H.; Liu, H.B.; Tang, Z.S.; Qiu, Z.D.; Zhu, H.X.; Song, Z.X.; Jia, A.L. Synthesis, performance, and application of molecularly imprinted membranes: A review. J. Environ. Chem. Eng. 2021, 9, 106352. [Google Scholar] [CrossRef]
- Xing, R.; Ma, Y.; Wang, Y.; Wen, Y.; Liu, Z. Specific recognition of proteins and peptides via controllable oriented surface imprinting of boronate affinity-anchored epitopes. Chem. Sci. 2019, 10, 1831–1835. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qian, D.; Xiao, X.; Gao, S.; Cheng, J.; He, B.; Liao, L.; Deng, J. A highly sensitive and selective sensor based on a graphene-coated carbon paste electrode modified with a computationally designed boron-embedded duplex molecularly imprinted hybrid membrane for the sensing of lamotrigine. Biosens. Bioelectron. 2017, 94, 663–670. [Google Scholar] [CrossRef] [PubMed]
- El Hani, O.; García Guzmán, J.J.; Palacios Santander, J.M.; Digua, K.; Amine, A.; Cubillana Aguilera, L. Development of a molecularly imprinted membrane for selective, high-sensitive, and on-site detection of antibiotics in waters and drugs: Application for sulfamethoxazole. Chemosphere 2024, 350, 141039. [Google Scholar] [CrossRef]
- Yazdanian, N.; Akbari Adergani, B.; Kazemipour, M.; Ahmad Panahi, H.; Javanbakht, M. Improving the determination of celecoxib in body fluids and pharmaceuticals using a new selective and thermosensitive molecularly imprinted poly(vinylidene fluoride) membrane. Anal. Methods 2020, 12, 2185–2195. [Google Scholar] [CrossRef]
- Chen Legrand, D.; Mas, S.; Jugeau, B.; David, A.; Barus, C. Silicate marine electrochemical sensor. Sens. Actuators B Chem. 2021, 335, 129705. [Google Scholar] [CrossRef]
- del Campo, F.J. Self-powered electrochemical sensors. Curr. Opin. Electrochem. 2023, 41, 101356. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Ma, Y.; Li, Y.; Shao, J.; Li, H. High transparent Ag NPs/PVC SERS membrane combined with molecular imprinting technology for selective detection of norfloxacin. J. Environ. Chem. Eng. 2022, 10, 108916. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Li, Y.T.; Li, X.J.; Zhang, L.; Kouadio Fodjo, E.; Han, S. Controllable in situ fabrication of portable AuNP/mussel-inspired polydopamine molecularly imprinted SERS substrate for selective enrichment and recognition of phthalate plasticizers. Chem. Eng. J. 2020, 402, 125179. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, L.; Yan, H.; Lu, Z.; Yin, W.; Han, H. Enzyme induced molecularly imprinted polymer on SERS substrate for ultrasensitive detection of patulin. Anal. Chim. Acta 2020, 1101, 111–119. [Google Scholar] [CrossRef]
- Dabrowski, M.; Cieplak, M.; Noworyta, K.; Heim, M.; Adamkiewicz, W.; Kuhn, A.; Sharma, P.S.; Kutner, W. Surface enhancement of a molecularly imprinted polymer film using sacrificial silica beads for increasing l-arabitol chemosensor sensitivity and detectability. J. Mater. Chem. B 2017, 5, 6292–6299. [Google Scholar] [CrossRef]
- Chul Yang, J.; Won Hong, S.; Jeon, S.; Ik Park, W.; Byun, M.; Park, J. Molecular imprinting of hemispherical pore-structured thin films via colloidal lithography for gaseous formaldehyde Gravimetric sensing. Appl. Surf. Sci. 2021, 570, 151161. [Google Scholar] [CrossRef]
- Ikegami, T.; Mukawa, T.; Nariai, H.; Takeuchi, T. Bisphenol A-recognition polymers prepared by covalent molecular imprinting. Anal. Chim. Acta 2004, 504, 131–135. [Google Scholar] [CrossRef]
- Wulff, G. Selective binding to polymers via covalent bonds. The construction of chiral cavities as specific receptor sites. Angew. Chem. Int. Ed. 1982, 54, 2093–2102. [Google Scholar] [CrossRef]
- Awino, J.K.; Gunasekara, R.W.; Zhao, Y. Selective recognition of d-Aldohexoses in water by boronic acid-functionalized, molecularly imprinted cross-linked micelles. J. Am. Chem. Soc. 2016, 138, 9759–9762. [Google Scholar] [CrossRef]
- Mayes, A.G.; Whitcombe, M.J. Synthetic strategies for the generation of molecularly imprinted organic polymers. Adv. Drug Deliv. Rev. 2005, 57, 1742–1778. [Google Scholar] [CrossRef]
- Ye, L.; Mosbach, K. Molecular imprinting: Synthetic materials as substitutes for biological antibodies and receptors. Chem. Mater. 2008, 20, 859–868. [Google Scholar] [CrossRef]
- Arshady, R.; Mosbach, K. Synthesis of substrate-selective polymers by host-guest polymerization. Die Makromol. Chem. 1981, 182, 687–692. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, L.; Mosbach, K. Non-covalent molecular imprinting with emphasis on its application in separation and drug development. J. Mol. Recognit. 2006, 19, 248–259. [Google Scholar] [CrossRef]
- Mosbach, K.; Haupt, K. Some new developments and challenges in non-covalent molecular imprinting technology. J. Mol. Recognit. 1998, 11, 62–68. [Google Scholar] [CrossRef]
- Dong, C.; Shi, H.; Han, Y.; Yang, Y.; Wang, R.; Men, J. Molecularly imprinted polymers by the surface imprinting technique. Eur. Polym. J. 2021, 145, 110231. [Google Scholar] [CrossRef]
- Shen, H.; Wang, S.; Xu, H.; Zhou, Y.; Gao, C. Preparation of polyamide thin film nanocomposite membranes containing silica nanoparticles via an in-situ polymerization of SiCl4 in organic solution. J. Membr. Sci. 2018, 565, 145–156. [Google Scholar] [CrossRef]
- Hijazi, H.Y.; Bottaro, C.S. Molecularly imprinted polymer thin-film as a micro-extraction adsorbent for selective determination of trace concentrations of polycyclic aromatic sulfur heterocycles in seawater. J. Chromatogr. A 2020, 1617, 460824. [Google Scholar] [CrossRef]
- Prabakaran, K.; Jandas, P.J.; Luo, J.; Fu, C. A highly sensitive surface acoustic wave sensor modified with molecularly imprinted hydrophilic PVDF for the selective amino acid detection. Sens. Actuators A Phys. 2022, 341, 113525. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, P.; Yun, Y. Preparing molecularly imprinted membranes by phase inversion to separate kaempferol. Polym. Adv. Technol. 2017, 28, 373–378. [Google Scholar] [CrossRef]
- Che Lah, N.F.; Ahmad, A.L.; Low, S.C. Molecular imprinted membrane biosensor for pesticide detection: Perspectives and challenges. Polym. Adv. Technol. 2021, 32, 17–30. [Google Scholar] [CrossRef]
- Bai, M.; Qiang, L.; Meng, M.; Li, B.; Wang, S.; Wu, Y.; Chen, L.; Dai, J.; Liu, Y.; Pan, J. Upper surface imprinted membrane prepared by magnetic guidance phase inversion method for highly efficient and selective separation of Artemisinin. Chem. Eng. J. 2021, 405, 126899. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, G.; Liu, G.; Pan, M.; Wang, X.; Kong, L.; He, X.; Wang, S. Electrochemical sensor based on molecularly imprinted polymer film via sol–gel technology and multi-walled carbon nanotubes-chitosan functional layer for sensitive determination of quinoxaline-2-carboxylic acid. Biosens. Bioelectron. 2013, 47, 475–481. [Google Scholar] [CrossRef]
- Queirós, R.B.; Silva, S.O.; Noronha, J.P.; Frazão, O.; Jorge, P.; Aguilar, G.; Marques, P.V.S.; Sales, M.G.F. Microcystin-LR detection in water by the Fabry–Pérot interferometer using an optical fibre coated with a sol–gel imprinted sensing membrane. Biosens. Bioelectron. 2011, 26, 3932–3937. [Google Scholar] [CrossRef]
- Dunn, B.; Zink, J.I. Optical properties of sol–gel glasses doped with organic molecules. J. Mater. Chem. 1991, 1, 903–913. [Google Scholar] [CrossRef]
- Moein, M.M.; Javanbakht, M.; Karimi, M.; Akbari-adergani, B.; Abdel-Rehim, M. Three-phase molecularly imprinted sol–gel based hollow fiber liquid-phase microextraction combined with liquid chromatography–tandem mass spectrometry for enrichment and selective determination of a tentative lung cancer biomarker. J. Chromatogr. B 2015, 995–996, 38–45. [Google Scholar] [CrossRef]
- Giwa, A.; Dindi, A.; Kujawa, J. Membrane bioreactors and electrochemical processes for treatment of wastewaters containing heavy metal ions, organics, micropollutants and dyes: Recent developments. J. Hazard. Mater. 2019, 370, 172–195. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Komathi, S.; Muthuchamy, N.; Lee, K.; Whitcombe, M.; Lakshmi, K.; Gopalan, S. Functionalized conjugated polymers for sensing and molecular imprinting applications. Prog. Polym. Sci. 2018, 88, 1–129. [Google Scholar]
- Fu, K.; Zhang, R.; He, J.; Bai, H.; Zhang, G. Sensitive detection of ketamine with an electrochemical sensor based on UV-induced polymerized molecularly imprinted membranes at graphene and MOFs modified electrode. Biosens. Bioelectron. 2019, 143, 111636. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, R.; Lin, H.; Wei, Q.; Hu, F.; Yang, X. Novel plant flavonoid electrochemical sensor based on in-situ and controllable double-layered membranes modified electrode. PLoS ONE 2020, 15, e0237583. [Google Scholar] [CrossRef]
- Kim, S.; Fornasiero, F.; Park, H.G.; In, J.B.; Meshot, E.; Giraldo, G.; Stadermann, M.; Fireman, M.; Shan, J.; Grigoropoulos, C.; et al. Fabrication of flexible, aligned carbon nanotube/polymer composite membranes by in-situ polymerization. J. Membr. Sci. 2014, 460, 91–98. [Google Scholar] [CrossRef]
- Nasreen, S.A.A.N.; Sundarrajan, S.; Syed Nizar, S.A.; Balamurugan, R.; Ramakrishna, S. In situ polymerization of PVDF-HEMA polymers: Electrospun membranes with improved flux and antifouling properties for water filtration. Polym. J. 2014, 46, 167–174. [Google Scholar] [CrossRef]
- Erdem, Ö.; Eş, I.; Saylan, Y.; Atabay, M.; Gungen, M.A.; Ölmez, K.; Denizli, A.; Inci, F. In situ synthesis and dynamic simulation of molecularly imprinted polymeric nanoparticles on a micro-reactor system. Nat. Commun. 2023, 14, 4840. [Google Scholar] [CrossRef]
- Bai, H.; Wang, S.; Liu, P.; Xiong, C.; Zhang, K.; Cao, Q. Electrochemical sensor based on in situ polymerized ion-imprinted membranes at graphene modified electrode for palladium determination. J. Electroanal. Chem. 2016, 771, 29–36. [Google Scholar] [CrossRef]
- Bai, H.; Wang, C.; Chen, J.; Peng, J.; Cao, Q. A novel sensitive electrochemical sensor based on in-situ polymerized molecularly imprinted membranes at graphene modified electrode for artemisinin determination. Biosens. Bioelectron. 2015, 64, 352–358. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Shimada, A.; Izumi, J.I. Novel polymeric membranes having chiral recognition sites converted from tripeptide derivatives. Analyst 2001, 126, 775–780. [Google Scholar] [CrossRef]
- Ramamoorthy, M.; Ulbricht, M. Molecular imprinting of cellulose acetate-sulfonated polysulfone blend membranes for Rhodamine B by phase inversion technique. J. Membr. Sci. 2003, 217, 207–214. [Google Scholar] [CrossRef]
- He, Z.; Meng, M.; Yan, L.; Zhu, W.; Sun, F.; Yan, Y.; Liu, Y.; Liu, S. Fabrication of new cellulose acetate blend imprinted membrane assisted with ionic liquid ([BMIM]Cl) for selective adsorption of salicylic acid from industrial wastewater. Sep. Purif. Technol. 2015, 145, 63–74. [Google Scholar]
- Zeng, J.; Lv, C.; Liu, G.; Zhang, Z.; Dong, Z.; Liu, J.; Wang, Y. A novel ion-imprinted membrane induced by amphiphilic block copolymer for selective separation of Pt(IV) from aqueous solutions. J. Membr. Sci. 2018, 572, 428–441. [Google Scholar] [CrossRef]
- Wu, J.; Gao, J.; Hou, Z.; Xing, W.; Dai, J.; Yan, Y.; Wu, Y. Accelerating the design of β-CD-PVDF-based molecularly imprinted nanocomposite membrane for selective separation: A surface functional monomer-directing strategy. Nano 2020, 15, 2050138. [Google Scholar] [CrossRef]
- Shaabani, N.; Chan, N.W.C.; Jemere, A.B. A molecularly imprinted sol-gel electrochemical sensor for naloxone determination. Nanomaterials 2021, 11, 631. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Y.; Diao, Z.; Cui, H.; Ma, F.; Yan, M.; Wu, Y. Precise identification and transport of specific molecules through framework-functionalized membranes with multiple binding sites. J. Membr. Sci. 2023, 670, 121327. [Google Scholar] [CrossRef]
- Blanco López, M.C.; Lobo Castañón, M.J.; Miranda Ordieres, A.J.; Tuñón Blanco, P. Voltammetric sensor for vanillylmandelic acid based on molecularly imprinted polymer-modified electrodes. Biosens. Bioelectron. 2003, 18, 353–362. [Google Scholar] [CrossRef]
- You, Z.; Fu, Y.; Xiao, A.; Liu, L.; Huang, S. Magnetic molecularly imprinting polymers and reduced graphene oxide modified electrochemical sensor for the selective and sensitive determination of luteolin in natural extract. Arab. J. Chem. 2021, 14, 102990. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Ma, X.; Pang, C.; Wang, M.; Xu, Z.; Li, B. Monitoring levamisole in food and the environment with high selectivity using an electrochemical chiral sensor comprising an MOF and molecularly imprinted polymer. Food Chem. 2024, 430, 137105. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, J.; Zhou, P.; Chen, J.; Wang, R.; Wen, T.; Li, Y.; Zhou, X.; Jiang, H. Electrochemical sensor based on molecularly imprinted membranes at platinum nanoparticles-modified electrode for determination of 17β-estradiol. Biosens. Bioelectron. 2011, 29, 29–33. [Google Scholar] [CrossRef]
- Gui, R.; Jin, H.; Guo, H.; Wang, Z. Recent advances and future prospects in molecularly imprinted polymers-based electrochemical biosensors. Biosens. Bioelectron. 2018, 100, 56–70. [Google Scholar] [CrossRef]
- Zheng, C.; Ling, Y.; Chen, J.; Yuan, X.; Li, S.; Zhang, Z. Design of a versatile and selective electrochemical sensor based on dummy molecularly imprinted PEDOT/laser-induced graphene for nitroaromatic explosives detection. Environ. Res. 2023, 236, 116769. [Google Scholar] [CrossRef]
- Mei, X.; Yang, J.; Yu, X.; Peng, Z.; Zhang, G.; Li, Y. Wearable molecularly imprinted electrochemical sensor with integrated nanofiber-based microfluidic chip for in situ monitoring of cortisol in sweat. Sens. Actuators B Chem. 2023, 381, 133451. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef]
- Karimi Maleh, H.; Orooji, Y.; Karimi, F.; Alizadeh, M.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K.; et al. A critical review on the use of potentiometric based biosensors for biomarkers detection. Biosens. Bioelectron. 2021, 184, 113252. [Google Scholar] [CrossRef] [PubMed]
- Vu, O.T.; Nguyen, Q.H.; Nguy Phan, T.; Luong, T.T.; Eersels, K.; Wagner, P.; Truong, L.T.N. Highly sensitive molecularly imprinted polymer-based electrochemical sensors enhanced by gold nanoparticles for norfloxacin detection in aquaculture water. ACS Omega 2023, 8, 2887–2896. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.T.; Do, M.N.; Dang, T.N.H.; Tran, Q.H.; Le, V.T.; Dao, A.Q.; Vasseghian, Y. A state-of-the-art review on graphene-based nanomaterials to determine antibiotics by electrochemical techniques. Environ. Res. 2022, 208, 112744. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.W.; Najeeb, J.; Naeem, S.; Usman, S.M.; Nahvi, I.; Alismail, F.; Abuzir, A.; Farhan, M.; Nawaz, A. Electrochemical methodologies for investigating the antioxidant potential of plant and fruit extracts: A review. Antioxidants 2022, 11, 1205. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, R.; Chen, J.; Li, S.; Ling, Y.; Zhang, Z. Development of a selective electrochemical microsensor based on molecularly imprinted polydopamine/ZIF-67/laser-induced graphene for point-of-care determination of 3-nitrotyrosine. Biosens. Bioelectron. 2024, 255, 116246. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Zhang, C.; Deng, Y.; Yang, G.; Li, S.; Tang, C.; He, N. A novel α-fetoprotein-MIP immunosensor based on AuNPs/PTh modified glass carbon electrode. Chin. Chem. Lett. 2019, 30, 160–162. [Google Scholar] [CrossRef]
- Meskher, H.; Belhaouari, S.B.; Deshmukh, K.; Hussain, C.M.; Sharifianjazi, F. A magnetite composite of molecularly imprinted polymer and reduced graphene oxide for sensitive and selective electrochemical detection of catechol in water and milk samples: An artificial neural network (ANN) application. J. Electrochem. Soc. 2023, 170, 047502. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Lee, C.K.; Lin, C.T. Improving sensitivity of a miniaturized label-free electrochemical biosensor using zigzag electrodes. Biosens. Bioelectron. 2018, 103, 130–137. [Google Scholar] [CrossRef]
- Hassan Oghli, A.; Soleymanpour, A. Ultrasensitive electrochemical sensor for simultaneous determination of sumatriptan and paroxetine using molecular imprinted polymer/sol-gel/polyoxometalate/rGO modified pencil graphite electrode. Sens. Actuators B Chem. 2021, 344, 130215. [Google Scholar] [CrossRef]
- Zhang, X.; Yarman, A.; Kovács, N.; Bognár, Z.; Gyurcsányi, R.E.; Bier, F.F.; Scheller, F.W. Specific features of epitope-MIPs and whole-protein MIPs as illustrated for AFP and RBD of SARS-CoV-2. Microchim. Acta 2024, 191, 242. [Google Scholar] [CrossRef] [PubMed]
- Bognár, Z.; Kozma, J.; Kovács, N.; Gyurcsányi, R.E. Novel functional monomer for the electrochemical synthesis of highly affine epitope-imprinted polymers. Electroanalysis 2023, 35, e202300025. [Google Scholar] [CrossRef]
- Ratautaite, V.; Boguzaite, R.; Brazys, E.; Plausinaitis, D.; Ramanavicius, S.; Samukaite-Bubniene, U.; Bechelany, M.; Ramanavicius, A. Evaluation of the interaction between SARS-CoV-2 spike glycoproteins and the molecularly imprinted polypyrrole. Talanta 2023, 253, 123981. [Google Scholar] [CrossRef]
- Raziq, A.; Kidakova, A.; Boroznjak, R.; Reut, J.; Öpik, A.; Syritski, V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021, 178, 113029. [Google Scholar] [CrossRef] [PubMed]
- Rawool, C.R.; Srivastava, A.K. A dual template imprinted polymer modified electrochemical sensor based on Cu metal organic framework/mesoporous carbon for highly sensitive and selective recognition of rifampicin and isoniazid. Sens. Actuators B Chem. 2019, 288, 493–506. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.T.; Liu, Y.; Qi, X.M.; Jin, H.G.; Yang, C.; Liu, J.; Li, G.L.; He, Q.G. Recent advances in black phosphorus-based electrochemical sensors: A review. Anal. Chim. Acta 2021, 1170, 338480. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Huang, H.; Guo, W.; Zhang, C.; Chen, Z.; Li, S.; Ma, L.; Deng, Y. Recent progress in black phosphorus sensors. J. Biomed. Nanotechnol. 2020, 16, 1045–1064. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Huang, H.; Xiao, Z.; Zhang, C.; Guo, W.; Ma, T.; Ma, L.; Chen, Z.; Deng, Y. A novel strategy for liquid exfoliation of ultrathin black phosphorus nanosheets. J. Biomed. Nanotechnol. 2020, 16, 548–552. [Google Scholar] [CrossRef]
- Li, G.; Qi, X.; Wu, J.; Xu, L.; Wan, X.; Liu, Y.; Chen, Y.; Li, Q. Ultrasensitive, label-free voltammetric determination of norfloxacin based on molecularly imprinted polymers and Au nanoparticle-functionalized black phosphorus nanosheet nanocomposite. J. Hazard. Mater. 2022, 436, 129107. [Google Scholar] [CrossRef]
- Olorunyomi, J.F.; Geh, S.T.; Caruso, R.A.; Doherty, C.M. Metal–organic frameworks for chemical sensing devices. Mater. Horiz. 2021, 8, 2387–2419. [Google Scholar] [CrossRef] [PubMed]
- Hatamluyi, B.; Rezayi, M.; Beheshti, H.R.; Boroushaki, M.T. Ultra-sensitive molecularly imprinted electrochemical sensor for patulin detection based on a novel assembling strategy using Au@Cu-MOF/N-GQDs. Sens. Actuators B Chem. 2020, 318, 128219. [Google Scholar] [CrossRef]
- Sun, Y.; He, J.; Waterhouse, G.I.N.; Xu, L.; Zhang, H.; Qiao, X.; Xu, Z. A selective molecularly imprinted electrochemical sensor with GO@COF signal amplification for the simultaneous determination of sulfadiazine and acetaminophen. Sens. Actuators B Chem. 2019, 300, 126993. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Xu, Y.; Pan, H.; Guo, K.; Zhang, Y.; Chen, Y.; Liu, D.; Zhang, Y.; Yao, C.; et al. A novel molecularly imprinted polymer composite based on polyaniline nanoparticles as sensitive sensors for parathion detection in the field. Food Control 2022, 133, 108638. [Google Scholar] [CrossRef]
- Yang, B.; Fu, C.; Li, J.; Xu, G. Frontiers in highly sensitive molecularly imprinted electrochemical sensors: Challenges and strategies. TrAC Trends Anal. Chem. 2018, 105, 52–67. [Google Scholar] [CrossRef]
- Lee, H.K.; Lee, Y.H.; Koh, C.S.L.; Phan Quang, G.C.; Han, X.; Lay, C.L.; Sim, H.Y.F.; Kao, Y.C.; An, Q.; Ling, X.Y. Designing surface-enhanced Raman scattering (SERS) platforms beyond hotspot engineering: Emerging opportunities in analyte manipulations and hybrid materials. Chem. Soc. Rev. 2019, 48, 731–756. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.C.; Auguié, B. Enhancement factors: A central concept during 50 years of surface-enhanced Raman spectroscopy. ACS Nano 2024, 18, 9773–9783. [Google Scholar] [CrossRef]
- Fang, S.; Wu, S.; Chen, Z.; He, C.; Lin, L.L.; Ye, J. Recent progress and applications of Raman spectrum denoising algorithms in chemical and biological analyses: A review. TrAC Trends Anal. Chem. 2024, 172, 117578. [Google Scholar] [CrossRef]
- Hang, Y.; Wang, A.; Wu, N. Plasmonic silver and gold nanoparticles: Shape- and structure-modulated plasmonic functionality for point-of-caring sensing, bio-imaging and medical therapy. Chem. Soc. Rev. 2024, 53, 2932–2971. [Google Scholar] [CrossRef] [PubMed]
- Vázquez Iglesias, L.; Stanfoca Casagrande, G.M.; García Lojo, D.; Ferro Leal, L.; Ngo, T.A.; Pérez Juste, J.; Reis, R.M.; Kant, K.; Pastoriza Santos, I. SERS sensing for cancer biomarker: Approaches and directions. Bioact. Mater. 2024, 34, 248–268. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Arabi, M.; Wang, X.; Wang, Y.; Chen, L. Molecular-imprinting-based surface-enhanced Raman scattering sensors. ACS Sens. 2020, 5, 601–619. [Google Scholar] [CrossRef]
- Feng, S.; Hu, Y.; Ma, L.; Lu, X. Development of molecularly imprinted polymers-surface-enhanced Raman spectroscopy/colorimetric dual sensor for determination of chlorpyrifos in apple juice. Sens. Actuators B Chem. 2017, 241, 750–757. [Google Scholar] [CrossRef]
- Lv, Y.; Qin, Y.; Svec, F.; Tan, T. Molecularly imprinted plasmonic nanosensor for selective SERS detection of protein biomarkers. Biosens. Bioelectron. 2016, 80, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Grant, E.; Lu, X. Determination of histamine in canned tuna by molecularly imprinted polymers-surface enhanced Raman spectroscopy. Anal. Chim. Acta 2015, 901, 68–75. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Sun, L.; Bao, S.; Liu, D.; Li, H.; Liu, Y. Self-assembly flexible SERS imprinted membrane based on Ag nanocubes for selective detection of microcystin-LR. Microchim. Acta 2023, 191, 19. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Wen, Y.; Dong, Y.; Wang, Y.; Zhang, Q.; Liu, Z. Dual molecularly imprinted polymer-based plasmonic immunosandwich assay for the specific and sensitive detection of protein biomarkers. Anal. Chem. 2019, 91, 9993–10000. [Google Scholar] [CrossRef]
- Ma, J.; Yan, M.; Feng, G.; Ying, Y.; Chen, G.; Shao, Y.; She, Y.; Wang, M.; Sun, J.; Zheng, L.; et al. An overview on molecular imprinted polymers combined with surface-enhanced Raman spectroscopy chemical sensors toward analytical applications. Talanta 2021, 225, 122031. [Google Scholar] [CrossRef]
- Tu, X.; Muhammad, P.; Liu, J.; Ma, Y.; Wang, S.; Yin, D.; Liu, Z. Molecularly imprinted polymer-based plasmonic immunosandwich assay for fast and ultrasensitive determination of trace glycoproteins in complex samples. Anal. Chem. 2016, 88, 12363–12370. [Google Scholar] [CrossRef]
- He, Q.; Wang, D.; Shao, J.; Li, Y.; Cheng, M.; Dong, L.; Li, Y.; Zhu, J.; Li, H. Multicomponent SERS imprinted bio-membrane based on eggshell membrane for selective detection of spiramycin in water. J. Mol. Struct. 2023, 1289, 135883. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Zhang, Z.; Wang, Y.; Mei, R.; Fu, L.; Wang, X.; Ma, J.; Chen, L. Label-free SERS detection of Raman-inactive protein biomarkers by Raman reporter indicator: Toward ultrasensitivity and universality. Biosens. Bioelectron. 2021, 174, 112825. [Google Scholar] [CrossRef]
- Su, K.; Zhang, Y.; Chen, S.; Zuo, S.; Ha, Y.; Dan, J.; Chen, W.; Sun, C.; Dai, Z.; Shi, X. Selectively encapsulating Ag nanoparticles on the surface of two-dimensional graphene for surface-enhanced Raman scattering. Appl. Surf. Sci. 2019, 492, 108–115. [Google Scholar] [CrossRef]
- Ye, J.; Chen, Y.; Liu, Z. A boronate affinity sandwich assay: An appealing alternative to immunoassays for the determination of glycoproteins. Angew. Chem. Int. Ed. 2014, 53, 10386–10389. [Google Scholar] [CrossRef]
- Zhang, X.; Gan, T.; Xu, Z.; Zhang, H.; Wang, D.; Zhao, X.; Huang, Y.; Liu, Q.; Fu, B.; Dai, Z.; et al. Immune-like sandwich multiple hotspots SERS biosensor for ultrasensitive detection of NDKA biomarker in serum. Talanta 2024, 271, 125630. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Y.; Wang, L.; Lu, Y.; Li, J.; Lu, D.; Zhou, T.; Huang, Z.; Huang, J.; Huang, H.; et al. Interference-free and high precision biosensor based on surface enhanced Raman spectroscopy integrated with surface molecularly imprinted polymer technology for tumor biomarker detection in human blood. Biosens. Bioelectron. 2019, 143, 111599. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, S.; Gao, F.; Li Chan, E.C.Y.; Grant, E.; Lu, X. Detection of melamine in milk using molecularly imprinted polymers–surface enhanced Raman spectroscopy. Food Chem. 2015, 176, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Tang, R.; Xu, J.; Lu, F. Chemical nanosensors based on molecularly-imprinted polymers doped with silver nanoparticles for the rapid detection of caffeine in wastewater. Anal. Chim. Acta 2018, 1034, 176–183. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Wang, X.; Yu, H.; Ji, L.; Zhou, T.; Liu, C.; Che, G.; Wang, D. A high-performance SERS imprinted membrane based on Ag/CNTs for selective detection of spiramycin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 281, 121587. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Fang, H.; Xu, H.; Yu, H.; Zhou, T.; Liu, C.; Che, G.; Wang, D. Hydrophilic modification of PVDF-based SERS imprinted membrane for the selective detection of L-tyrosine. J. Environ. Manag. 2022, 304, 114260. [Google Scholar] [CrossRef] [PubMed]
- Akgönüllü, S.; Yavuz, H.; Denizli, A. SPR nanosensor based on molecularly imprinted polymer film with gold nanoparticles for sensitive detection of aflatoxin B1. Talanta 2020, 219, 121219. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.; Kanwal, T.; Ahmad, N.; Fatima, B.; Najam-ul-Haq, M.; Hussain, D. Advances and challenges in portable optical biosensors for onsite detection and point-of-care diagnostics. TrAC Trends Anal. Chem. 2024, 173, 117640. [Google Scholar] [CrossRef]
- D’Agata, R.; Bellassai, N.; Spoto, G. Exploiting the design of surface plasmon resonance interfaces for better diagnostics: A perspective review. Talanta 2024, 266, 125033. [Google Scholar] [CrossRef]
- Cennamo, N.; Arcadio, F.; Seggio, M.; Maniglio, D.; Zeni, L.; Bossi, A.M. Spoon-shaped polymer waveguides to excite multiple plasmonic phenomena: A multisensor based on antibody and molecularly imprinted nanoparticles to detect albumin concentrations over eight orders of magnitude. Biosens. Bioelectron. 2022, 217, 114707. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, P.; Qin, S.; Huang, Z.; Cao, Y.; Liu, X. A highly sensitive tetracycline sensor based on a combination of magnetic molecularly imprinted polymer nanoparticles and surface plasmon resonance detection. Microchim. Acta 2019, 186, 637. [Google Scholar] [CrossRef] [PubMed]
- Uzun, L.; Turner, A.P.F. Molecularly-imprinted polymer sensors: Realising their potential. Biosens. Bioelectron. 2016, 76, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Erdem, Ö.; Saylan, Y.; Cihangir, N.; Denizli, A. Molecularly imprinted nanoparticles based plasmonic sensors for real-time Enterococcus faecalis detection. Biosens. Bioelectron. 2019, 126, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Torrini, F.; Palladino, P.; Baldoneschi, V.; Scarano, S.; Minunni, M. Sensitive ‘two-steps’ competitive assay for gonadotropin-releasing hormone detection via SPR biosensing and polynorepinephrine-based molecularly imprinted polymer. Anal. Chim. Acta 2021, 1161, 338481. [Google Scholar] [CrossRef] [PubMed]
- Torrini, F.; Battaglia, F.; Palladino, P.; Scarano, S.; Minunni, M. Imprinted biopolymers as green abiotic route in immunoglobulin affinity plasmonic sensing. Biosens. Bioelectron. 2022, 217, 114706. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.; Sullivan, M.V.; Hand, R.A.; Turner, N.W. Detection of selective androgen receptor modulators (SARMs) in serum using a molecularly imprinted nanoparticle surface plasmon resonance sensor. J. Mater. Chem. B 2022, 10, 6792–6799. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, T.S.C.R.; Costa, R.; Brandão, A.T.S.C.; Silva, A.F.; Sales, M.G.F.; Pereira, C.M. Molecularly imprinted polymer SPE sensor for analysis of CA-125 on serum. Anal. Chim. Acta 2019, 1082, 126–135. [Google Scholar] [CrossRef]
- Torrini, F.; Goletta, G.; Palladino, P.; Scarano, S.; Minunni, M. A LysLysLys-tag as trigger in polynorepinephrine epitope imprinting: The case study of soluble PD-L1 detection in serum by optical-based sensing. Biosens. Bioelectron. 2023, 220, 114806. [Google Scholar] [CrossRef]
- Battaglia, F.; Torrini, F.; Palladino, P.; Scarano, S.; Minunni, M. Serotonin: A new super effective functional monomer for molecular imprinting. The case of TNF-α detection in real matrix by surface plasmon resonance. Biosens. Bioelectron. 2023, 242, 115713. [Google Scholar] [CrossRef]
- Baldoneschi, V.; Palladino, P.; Banchini, M.; Minunni, M.; Scarano, S. Norepinephrine as new functional monomer for molecular imprinting: An applicative study for the optical sensing of cardiac biomarkers. Biosens. Bioelectron. 2020, 157, 112161. [Google Scholar] [CrossRef] [PubMed]
- Ertürk Bergdahl, G.; Andersson, T.; Allhorn, M.; Yngman, S.; Timm, R.; Lood, R. In vivo detection and absolute quantification of a secreted bacterial factor from skin using molecularly imprinted polymers in a surface plasmon resonance biosensor for improved diagnostic abilities. ACS Sens. 2019, 4, 717–725. [Google Scholar] [CrossRef]
- Fauzi, F.; Rianjanu, A.; Santoso, I.; Triyana, K. Gas and humidity sensing with quartz crystal microbalance (QCM) coated with graphene-based materials—A mini review. Sens. Actuators A Phys. 2021, 330, 112837. [Google Scholar] [CrossRef]
- Wang, P.; Su, J.; Gong, L.; Shen, M.; Ruths, M.; Sun, H. Numerical simulation and experimental study of resonance characteristics of QCM-P devices operating in liquid and their application in biological detection. Sens. Actuators B Chem. 2015, 220, 1320–1327. [Google Scholar] [CrossRef]
- Wang, L.; Song, J.; Yu, C. The utilization and advancement of quartz crystal microbalance (QCM): A mini review. Microchem. J. 2024, 199, 109967. [Google Scholar] [CrossRef]
- Adel, M.; Allam, A.; Sayour, A.E.; Ragai, H.F.; Umezu, S.; Fath El-Bab, A.M.R. Design and development of a portable low-cost QCM-based system for liquid biosensing. Biomed. Microdevices 2024, 26, 11. [Google Scholar] [CrossRef] [PubMed]
- Migoń, D.; Wasilewski, T.; Suchy, D. Application of QCM in peptide and protein-based drug product development. Molecules 2020, 25, 3950. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, S.; Liu, Q.; Masliyah, J.; Xu, Z. QCM-D study of nanoparticle interactions. Adv. Colloid Interface Sci. 2016, 233, 94–114. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, Y.; Wu, X.; Pan, M.; Hu, N.; Wang, J.; Wang, S. Quartz crystal microbalance sensor based on covalent organic framework composite and molecularly imprinted polymer of poly(o-aminothiophenol) with gold nanoparticles for the determination of aflatoxin B1. Sens. Actuators B Chem. 2019, 291, 293–297. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Battal, D.; Yalcin, M.S.; Yavuz, H.; Denizli, A. Rapid and sensitive detection of synthetic cannabinoids JWH-018, JWH-073 and their metabolites using molecularly imprinted polymer-coated QCM nanosensor in artificial saliva. Microchem. J. 2020, 153, 104454. [Google Scholar] [CrossRef]
- Prabakaran, K.; Jandas, P.J.; Luo, J.; Fu, C.; Wei, Q. Molecularly imprinted poly(methacrylic acid) based QCM biosensor for selective determination of L-tryptophan. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125859. [Google Scholar] [CrossRef]
- Ma, X.; He, X.W.; Li, W.Y.; Zhang, Y.K. Oriented surface epitope imprinted polymer-based quartz crystal microbalance sensor for cytochrome c. Talanta 2019, 191, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Chunta, S.; Suedee, R.; Singsanan, S.; Lieberzeit, P.A. Sensing array based on molecularly imprinted polymers for simultaneous assessment of lipoproteins. Sens. Actuators B Chem. 2019, 298, 126828. [Google Scholar] [CrossRef]
- LariMojarad, I.; Mousavi, M.; Moeini Manesh, M.M.; Bouloorchi Tabalvandani, M.; Badieirostami, M. Electric field-assisted molecularly imprinted polymer-modified QCM sensor for enhanced detection of immunoglobulin. ACS Omega 2024, 9, 16026–16034. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Saha, T.; Tey, B.T.; Tan, W.S.; Hassan, S.S.; Ooi, C.W. Quartz crystal microbalance-based biosensing of hepatitis B antigen using a molecularly imprinted polydopamine film. Talanta 2022, 249, 123659. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Liu, J.; Liang, D.; Tang, S.; Xu, B. Construction of a QCM sensor for detecting diethylstilbestrol in water based on the computational design of molecularly imprinted polymers. Arab. J. Chem. 2023, 16, 104601. [Google Scholar] [CrossRef]
- Chunta, S.; Boonsriwong, W.; Wattanasin, P.; Naklua, W.; Lieberzeit, P.A. Direct assessment of very-low-density lipoprotein by mass sensitive sensor with molecularly imprinted polymers. Talanta 2021, 221, 121549. [Google Scholar] [CrossRef]
- Chunta, S.; Suedee, R.; Boonsriwong, W.; Lieberzeit, P.A. Biomimetic sensors targeting oxidized-low-density lipoprotein with molecularly imprinted polymers. Anal. Chim. Acta 2020, 1116, 27–35. [Google Scholar] [CrossRef]
- Kartal, F.; Çimen, D.; Bereli, N.; Denizli, A. Molecularly imprinted polymer based quartz crystal microbalance sensor for the clinical detection of insulin. Mater. Sci. Eng. C 2019, 97, 730–737. [Google Scholar] [CrossRef]
- Guha, A.; Ahmad, O.S.; Guerreiro, A.; Karim, K.; Sandström, N.; Ostanin, V.P.; van der Wijngaart, W.; Piletsky, S.A.; Ghosh, S.K. Direct detection of small molecules using a nano-molecular imprinted polymer receptor and a quartz crystal resonator driven at a fixed frequency and amplitude. Biosens. Bioelectron. 2020, 158, 112176. [Google Scholar] [CrossRef]
- Kang, H.; Wang, X.; Guo, M.; Dai, C.; Chen, R.; Yang, L.; Wu, Y.; Ying, T.; Zhu, Z.; Wei, D.; et al. Ultrasensitive detection of SARS-CoV-2 antibody by graphene field-effect transistors. Nano Lett. 2021, 21, 7897–7904. [Google Scholar] [CrossRef]
- De Simoni, G.; Paolucci, F.; Solinas, P.; Strambini, E.; Giazotto, F. Metallic supercurrent field-effect transistor. Nat. Nanotechnol. 2018, 13, 802–805. [Google Scholar] [CrossRef]
- Si, M.; Saha, A.K.; Gao, S.; Qiu, G.; Qin, J.; Duan, Y.; Jian, J.; Niu, C.; Wang, H.; Wu, W.; et al. A ferroelectric semiconductor field-effect transistor. Nat. Electron. 2019, 2, 580–586. [Google Scholar] [CrossRef]
- Fu, W.; Jiang, L.; van Geest, E.P.; Lima, L.M.C.; Schneider, G.F. Sensing at the surface of graphene field-effect transistors. Adv. Mater. 2017, 29, 1603610. [Google Scholar] [CrossRef] [PubMed]
- Béraud, A.; Sauvage, M.; Bazán, C.M.; Tie, M.; Bencherif, A.; Bouilly, D. Graphene field-effect transistors as bioanalytical sensors: Design, operation and performance. Analyst 2021, 146, 403–428. [Google Scholar] [CrossRef]
- Fan, H.; Sasaki, Y.; Zhou, Q.; Tang, W.; Nishina, Y.; Minami, T. Non-enzymatic detection of glucose levels in human blood plasma by a graphene oxide-modified organic transistor sensor. Chem. Commun. 2023, 59, 2425–2428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rupakula, M.; Bellando, F.; Garcia Cordero, E.; Longo, J.; Wildhaber, F.; Herment, G.; Guérin, H.; Ionescu, A.M. Sweat biomarker sensor incorporating picowatt, three-dimensional extended metal gate ion sensitive field effect transistors. ACS Sens. 2019, 4, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Rayanasukha, Y.; Pratontep, S.; Porntheeraphat, S.; Bunjongpru, W.; Nukeaw, J. Non-enzymatic urea sensor using molecularly imprinted polymers surface modified based-on ion-sensitive field effect transistor (ISFET). Surf. Coat. Technol. 2016, 306, 147–150. [Google Scholar] [CrossRef]
- Nazeri, M.; Ghalamboran, M.; Grau, G. Laser-induced graphene electrodes for organic electrochemical transistors (OECTs). Adv. Mater. Technol. 2023, 8, 2300188. [Google Scholar] [CrossRef]
- Majak, D.; Fan, J.; Kang, S.; Gupta, M. Delta-9-tetrahydrocannabinol (Δ9-THC) sensing using an aerosol jet printed organic electrochemical transistor (OECT). J. Mater. Chem. B 2021, 9, 2107–2117. [Google Scholar] [CrossRef]
- Ait Yazza, A.; Blondeau, P.; Andrade, F.J. Simple approach for building high transconductance paper-based organic electrochemical transistor (OECT) for chemical sensing. ACS Appl. Electron. Mater. 2021, 3, 1886–1895. [Google Scholar] [CrossRef]
- Diemer, P.J.; Harper, A.F.; Niazi, M.R.; Petty Ii, A.J.; Anthony, J.E.; Amassian, A.; Jurchescu, O.D. Laser-printed organic thin-film transistors. Adv. Mater. Technol. 2017, 2, 1700167. [Google Scholar] [CrossRef]
- Wang, N.; Yang, A.; Fu, Y.; Li, Y.; Yan, F. Functionalized organic thin Film transistors for biosensing. Acc. Chem. Res. 2019, 52, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Cui, N.; Tang, Q.; Tong, Y.; Zhao, X.; Liu, Y. High-performance, ultrathin, ultraflexible organic thin-film transistor array via solution process. Small 2018, 14, 1801020. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, M.; Lach, P.; Cieplak, M.; Kutner, W. Nanostructured molecularly imprinted polymers for protein chemosensing. Biosens. Bioelectron. 2018, 102, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Q.; Chang, C.C.; Liu, Y.; Yang, Z.; Guo, Y.; Wang, Y.; Galanakis, D.K.; Levon, K.; Rafailovich, M. Design of a molecular imprinting biosensor with multi-scale roughness for detection across a broad spectrum of biomolecules. Analyst 2016, 141, 5607–5617. [Google Scholar] [CrossRef] [PubMed]
- Bartold, K.; Iskierko, Z.; Borowicz, P.; Noworyta, K.; Lin, C.Y.; Kalecki, J.; Sharma, P.S.; Lin, H.Y.; Kutner, W. Molecularly imprinted polymer-based extended-gate field-effect transistor (EG-FET) chemosensor for selective determination of matrix metalloproteinase-1 (MMP-1) protein. Biosens. Bioelectron. 2022, 208, 114203. [Google Scholar] [CrossRef] [PubMed]
- Kugimiya, A.; Kohara, K. Biomimetic sensor for cAMP using an ion-sensitive field-effect transistor. Mater. Sci. Eng. C 2009, 29, 959–962. [Google Scholar] [CrossRef]
- Zayats, M.; Lahav, M.; Kharitonov, A.B.; Willner, I. Imprinting of specific molecular recognition sites in inorganic and organic thin layer membranes associated with ion-sensitive field-effect transistors. Tetrahedron 2002, 58, 815–824. [Google Scholar] [CrossRef]
- Rani, D.; Singh, Y.; Salker, M.; Vu, X.T.; Ingebrandt, S.; Pachauri, V. Point-of-care-ready nanoscale ISFET arrays for sub-picomolar detection of cytokines in cell cultures. Anal. Bioanal. Chem. 2020, 412, 6777–6788. [Google Scholar] [CrossRef]
- Lee, W.I.; Subramanian, A.; Mueller, S.; Levon, K.; Nam, C.Y.; Rafailovich, M.H. Potentiometric biosensors based on molecular-imprinted self-assembled monolayer films for rapid detection of influenza a virus and SARS-CoV-2 spike protein. ACS Appl. Nano Mater. 2022, 5, 5045–5055. [Google Scholar] [CrossRef] [PubMed]

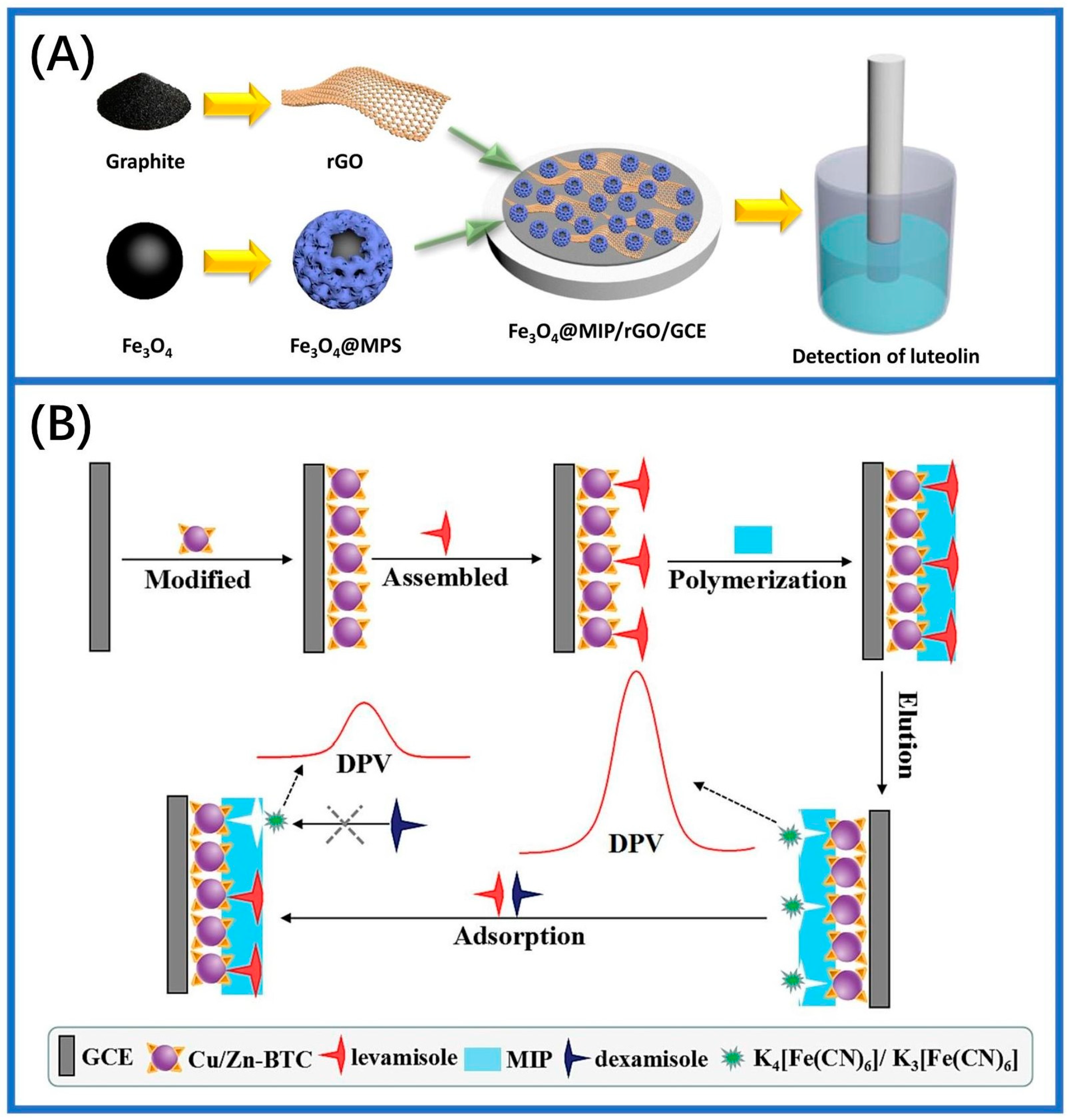
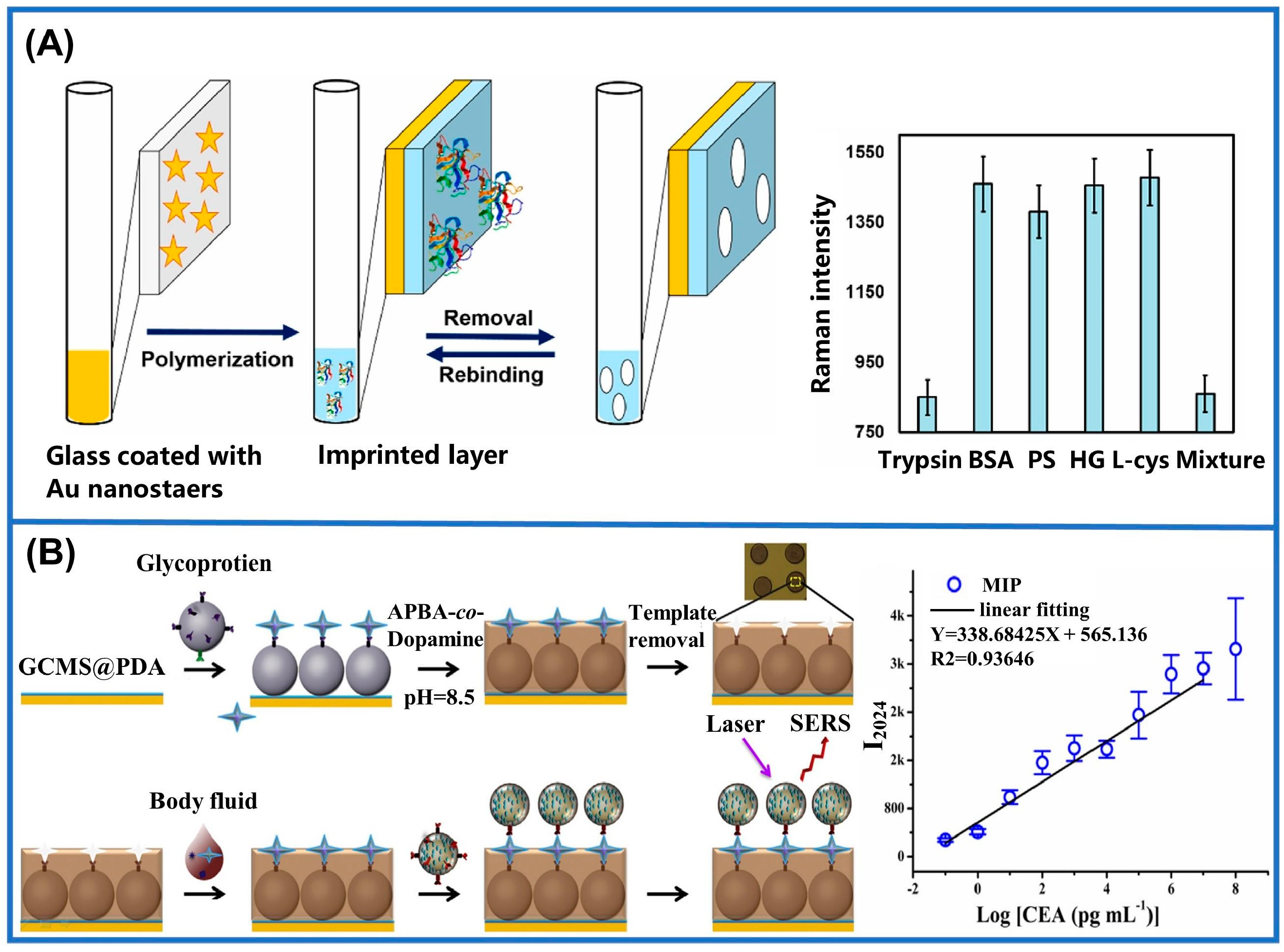
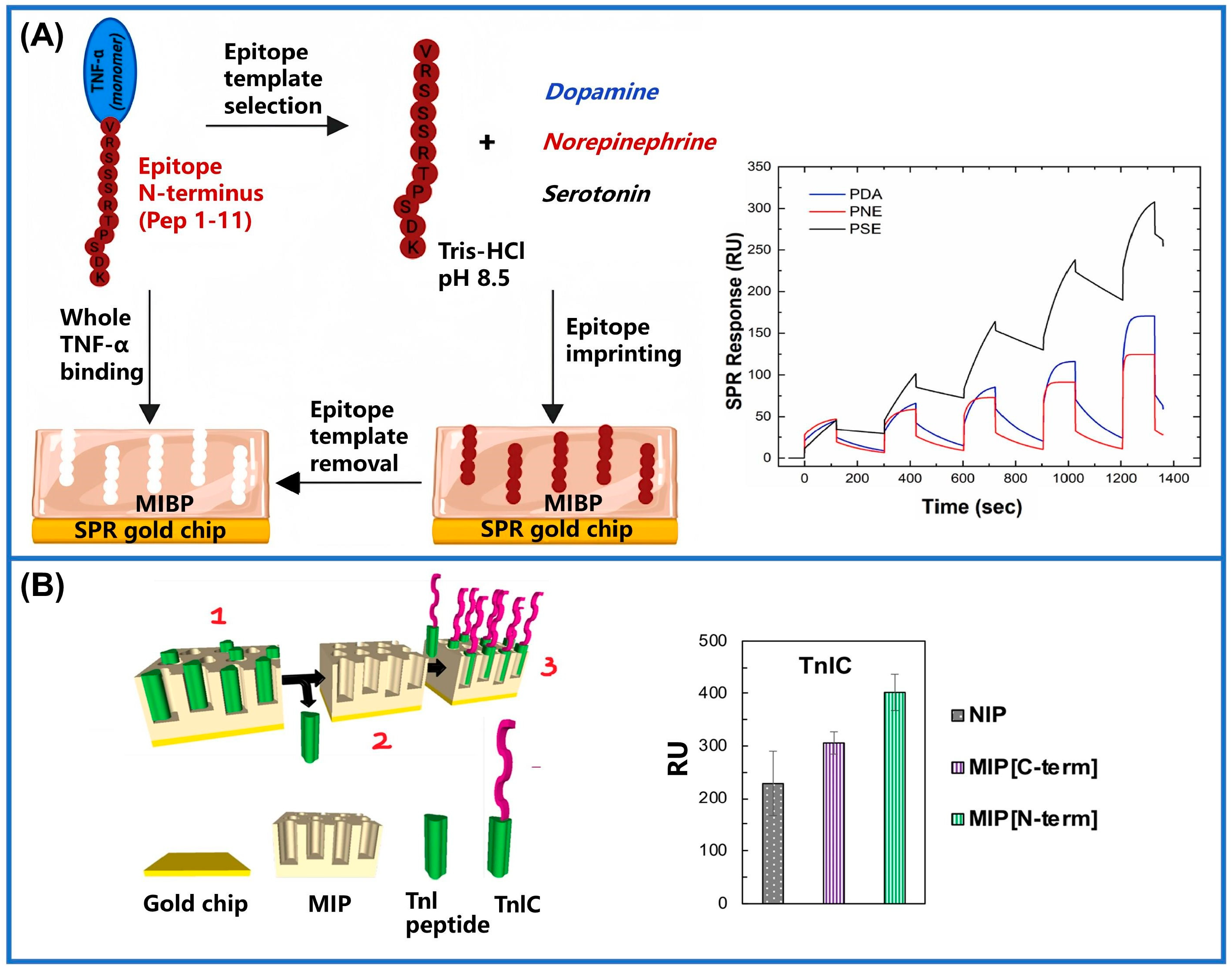


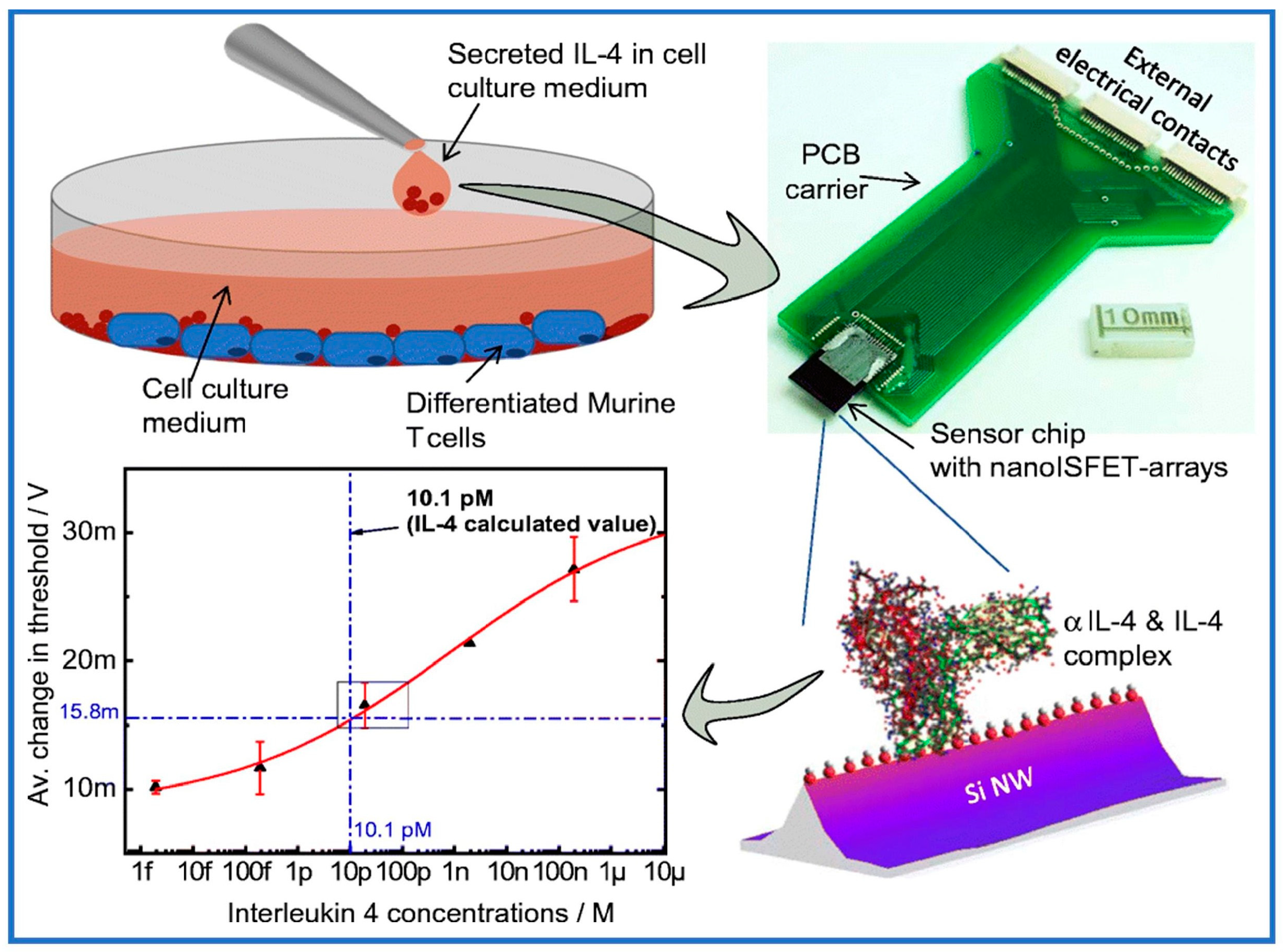
| Methods | Merits and Demerits | Synthetic Process | Imprinted Time | Removal Template | References |
|---|---|---|---|---|---|
| In situ polymerization | Merits: simple preparation process; low porosity of MIMs; high rigidity of MIMs Demerits: poor membrane permeability; the templates are hard to clean | The membrane that is supported by glass or other substances is immersed in the solution, which is composed of template molecules, functional monomers, and cross-linking agents. Following the polymerization reaction, the template molecules are eluted. | 45 min–1 h | The mixture of methanol/acetic acid (90/10) is washed for 4 h | [57,58,59] |
| Phase-inversion | Merits: improves membrane flux; recognition cavities directly in polymeric materials Demerits: complex synthesis process | A mixture of template molecules and functional monomers is dropped on the carrier and placed in a coagulation bath or an inert gas atmosphere. | Almost 12 h | Methanol/acetic acid mixture (9:1, v/v) is repeatedly washed until the UV detector cannot detect the template molecule (ARU). | [60,61,62] |
| Sol-gel | Merits: excellent selectivity; rapid adsorption rate Demerits: longer preparation time; environmentally unfriendly reagents using | Functional monomers and cross-linking agents are initially dissolved in a solvent. Subsequently, a polymer network is formed by polymerization or cross-linking reactions. Finally, the template molecules are removed, resulting in the formation of MIMs. | Almost 24 h | The mixture of methanol:acetic acid (80:20) is washed for 4 h | [63,64,65,66] |
| Electrochemical | Merits: fast preparation speed; controllable thickness; direct preparation on the electrode surface Demerits: MIMs are brittle; template molecules cannot be aligned | The electrodes are cleaned and immersed in an electrolyte solution containing template molecules, which are used to electrochemically polymerize the imprinted film. | A few minutes | Soak in acetic acid/methanol mixture (v/v = 1:1) for 10–30 min | [26,67,68,69,70] |
| Transducer | Nanomaterial | MIM Synthesis Method | Analyte | Detection Range | Detection Limit | References |
|---|---|---|---|---|---|---|
| Electrochemical | ZIF-67/LIG | Electropolymerization | 3-nitrotyrosine | 0.04 μM–100 μM | 6.71 nM | [96] |
| PTh/AuNPs/GCE | Electropolymerization | α-fetoprotein | 0.001 ng/mL–800 ng/mL | 0.8138 pg/mL | [97] | |
| rGO@Fe3O4/GCE | Sol-gel | Catechol | 1 μM–50 μM | 4.18 nM | [98] | |
| Polyoxometalate/rGO/PGE | Sol-gel | Sumatriptan Paroxetine | 0.02 μM–3 μM 0.005 μM–2.2 μM | 4.0 nM 0.7 nM | [100] | |
| Gold wire electrodes | Electropolymerization | SARS-CoV-2 | \ | \ | [101] | |
| Gold SPRi chips | Electropolymerization | Receptor binding domain of SARS-CoV-2 apical protein | 2.5 μM–50 μM | \ | [102] | |
| Pt electrode | Electropolymerization | SARS-CoV-2 spike | 0 μg/mL–25 μg/mL | \ | [103] | |
| Disposable sensor chip/TFE | Electropolymerization | SARS-CoV-2 antigen | 2.22 fM–111 fM | 15 fM | [104] | |
| BPNS-AuNP/GCE | Electropolymerization | Norfloxacin | 0.1 nM–10 μM | 0.012 nM | [109] | |
| Au@Cu-MOF/N-GQDs/GCE | Electropolymerization | Patulin | 0.001 ng/mL–70.0 ng/mL | 0.0007 ng/mL | [111] | |
| FUN-PANI/GC | Sol-gel | Parathion | 0.034 μM–0.0187 mM | 0.0113 μM | [113] | |
| SERS | AuNPs/Glass | In situ polymerization | Protein biomarkers | 0.01 μg/L–1000 μg/L | 4.1 ng/L | [129] |
| G/Ag | Sol-gel | Bovine serum albumin | \ | 10 pM | [130] | |
| Au/PDMS/AAO | In situ polymerization | Patulin | 0.5 nM–1 μM | 0.085 nM | [45] | |
| AuNPs SAM/Glass | In situ polymerization | Protein biomarkers | 0.1 ng/mL–10 μg/mL | \ | [125] | |
| AuNPs/Si | In situ polymerization | Nucleoside diphosphate kinase A | 100 pg/mL–10 ng/mL | 0.25 pg/mL | [132] | |
| GCMS | In situ polymerization | Carcinoembryonic antigen | 0.1 pg/mL–10 μg /mL | 0.064 pg/mL | [133] | |
| AgNPs@MISPE | Precipitate polymerization | Caffeine | 0.1 mmol/L–0.35 mmol/L | 100 ng/L | [135] | |
| AgNPs/PVC | In situ polymerization | Norfloxacin | 1 nM–0.01 mM | 1 nM | [43] | |
| Ag/CNTs | Sol-gel | Spiramycin | 0.01 nM–1 μM | 0.01 nM | [136] | |
| WA/pDA/PVDF | Precipitate polymerization | L-tyrosine | 1 nM–1 mM | 1 nM | [137] | |
| SPR | Au-SPE | Electropolymerization | Carbohydrate antigen 125 | 0.01 U/mL–500 U/mL | 0.01 U/mL | [148] |
| Au chip | Phase-inversion | Soluble programmed cell death protein 1 ligand | 0.25 μg/mL–10 μg/mL | 4.2 ± 0.4 ng/mL | [149] | |
| Au chip | In situ polymerization | Tumor necrosis factor-alpha | \ | 21 ± 4 pmol/L | [150] | |
| Au chip | In situ polymerization | Norepinephrine | \ | 193 ± 30 pM | [151] | |
| Au chip | In situ polymerization | RoxP | \ | 0.23 nM | [152] | |
| QCM | Au-QCM electrodes | Sol-gel | LDL-C HDL-C | 3 mg/dL–400 mg/dL 8 mg/dL–200 mg/dL | 3 mg/dL 8 mg/dL | [163] |
| Au-QCM electrodes | Sol-gel | Immunoglobulin G | \ | \ | [164] | |
| Au-QCM electrodes | In situ polymerization | Hepatitis B core antigen | 0.88 μg/mL–25 μg/mL | 0.88 μg/mL | [165] | |
| QCM electrodes | Sol-gel | Diethylstilbestrol | 50 ng/mL–350 ng/mL | 2.63 ng/mL | [166] | |
| Au-QCM electrodes | Sol-gel | L-tryptophan | 0.0012 μM–0.204 μM | 0.73 ng/mL | [161] | |
| Au-QCM electrodes | Sol-gel | Very-low-density lipoprotein | 2.5 mg/dL–100 mg/dL | 1.5 mg/dL | [167] | |
| Au-QCM electrodes | Sol-gel | Oxidized low-density lipoprotein | 86 μg/dL–5600 μg/dL | 86 μg/dL | [168] | |
| Au-QCM electrodes | Sol-gel | Insulin | 0.008 ng/mL–1.0 ng/mL | 1.58 pg/mL | [169] | |
| QCM electrodes | In situ polymerization | N-hexanoyl-L-homocysteine lactone | 1 μM–50 μM | 1 μM | [170] | |
| FET | Au/Glass | Electropolymerization | Matrix metalloproteinase-1 | 50 nM–500 nM | 20 nM | [187] |
| ISFET electrode | Sol-gel | Urea | 0.1 mM–0.1 M | 0.1 mM | [178] | |
| ISFET electrode | Sol-gel | cAMP | 0.1 mM–1.0 mM | 0.1 mM | [188] | |
| ISFET electrode | Sol-gel | Chloroaromatic acids | 0.08 mM–1 mM | 0.15 mM | [189] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Li, N.; Zhang, X.; Bobrinetskiy, I.; Gadjanski, I.; Fu, W. Sensing with Molecularly Imprinted Membranes on Two-Dimensional Solid-Supported Substrates. Sensors 2024, 24, 5119. https://doi.org/10.3390/s24165119
Wang L, Li N, Zhang X, Bobrinetskiy I, Gadjanski I, Fu W. Sensing with Molecularly Imprinted Membranes on Two-Dimensional Solid-Supported Substrates. Sensors. 2024; 24(16):5119. https://doi.org/10.3390/s24165119
Chicago/Turabian StyleWang, Lishuang, Nan Li, Xiaoyan Zhang, Ivan Bobrinetskiy, Ivana Gadjanski, and Wangyang Fu. 2024. "Sensing with Molecularly Imprinted Membranes on Two-Dimensional Solid-Supported Substrates" Sensors 24, no. 16: 5119. https://doi.org/10.3390/s24165119







