Relationship between Fear-Avoidance Beliefs and Muscle Co-Contraction in People with Knee Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Testing Protocol
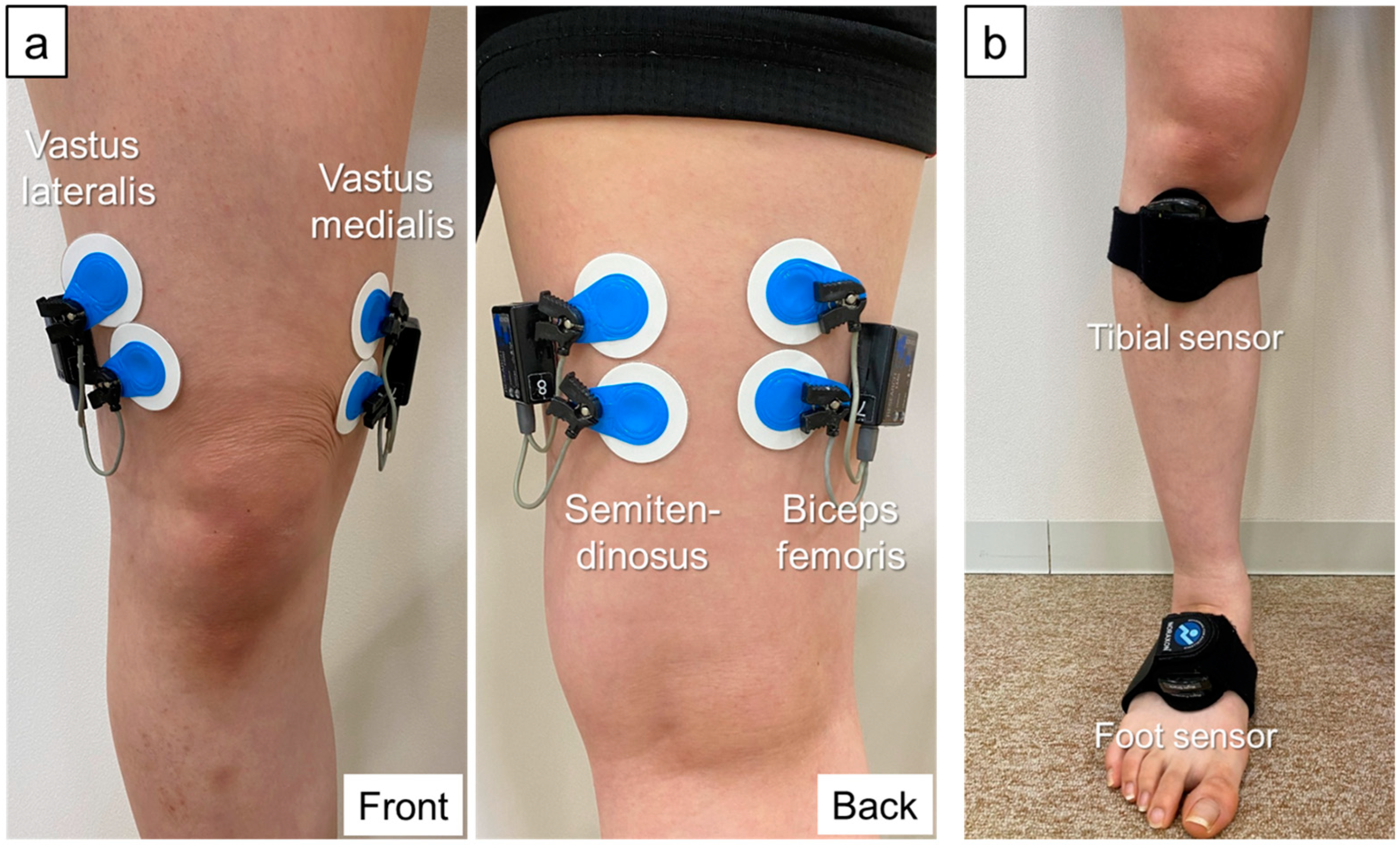
2.3. Data Collection and Processing
2.4. Co-Contraction Data
2.5. Assessment Questionnaires
2.5.1. Tampa Scale for Kinesiophobia-11
2.5.2. Pain Catastrophizing Scale
2.6. Secondary Parameters That May Affect Co-Contraction
2.6.1. Degree of Pain
2.6.2. Lateral Thrust
2.6.3. Weight
2.6.4. Alignment of the Lower Extremities
2.6.5. Knee Extension Strength
2.7. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoshimura, N.; Muraki, S.; Oka, H.; Mabuchi, A.; En-Yo, Y.; Yoshida, M.; Saika, A.; Yoshida, H.; Suzuki, T.; Yamamoto, S.; et al. Prevalence of Knee Osteoarthritis, Lumbar Spondylosis, and Osteoporosis in Japanese Men and Women: The Research on Osteoarthritis/Osteoporosis against Disability Study. J. Bone Miner. Metab. 2009, 27, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.M.; Helmick, C.G.; Renner, J.B.; Luta, G.; Dragomir, A.D.; Woodard, J.; Fang, F.; Schwartz, T.A.; Nelson, A.E.; Abbate, L.M.; et al. Prevalence of Hip Symptoms and Radiographic and Symptomatic Hip Osteoarthritis in African Americans and Caucasians: The Johnston County Osteoarthritis Project. J. Rheumatol. 2009, 36, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Vassão, P.G.; Silva, B.A.; de Souza, M.C.; Parisi, J.R.; de Camargo, M.R.; Renno, A.C.M. Level of Pain, Muscle Strength and Posture: Effects of PBM on an Exercise Program in Women with Knee Osteoarthritis—A Randomized Controlled Trial. Lasers Med. Sci. 2020, 35, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K.L.; Hinman, R.S.; Metcalf, B.R. Association of Sensorimotor Function with Knee Joint Kinematics During Locomotion in Knee Osteoarthritis. Am. J. Phys. Med. Rehabil. 2004, 83, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.H.; Hsieh, R.L.; Lee, W.C. Pain, Physical Function, and Health in Patients with Knee Osteoarthritis. Rehabil. Nurs. 2017, 42, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, G.L.; Costello, K.E.; Astephen Wilson, J.L.; Stanish, W.D.; Hubley-Kozey, C.L. Baseline Gait Muscle Activation Patterns Differ for Osteoarthritis Patients Who Undergo Total Knee Arthroplasty Five to Eight Years Later from Those Who Do Not. Arthritis Care Res. 2021, 73, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; van den Hoorn, W.; Wrigley, T.V.; Hinman, R.S.; Bowles, K.A.; Cicuttini, F.; Wang, Y.; Bennell, K. Increased Duration of Co-Contraction of Medial Knee Muscles Is Associated with Greater Progression of Knee Osteoarthritis. Man. Ther. 2016, 21, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Sirin, A.V.; Patla, A.E. Myoelectric Changes in the Triceps Surae Muscles under Sustained Contractions. Evidence for Synergism. Eur. J. Appl. Physiol. Occup. Physiol. 1987, 56, 238–244. [Google Scholar] [CrossRef]
- Dixon, P.C.; Gomes, S.; Preuss, R.A.; Robbins, S.M. Muscular Co-Contraction Is Related to Varus Thrust in Patients with Knee Osteoarthritis. Clin. Biomech. 2018, 60, 164–169. [Google Scholar] [CrossRef]
- Na, A.; Buchanan, T.S. Self-Reported Walking Difficulty and Knee Osteoarthritis Influences Limb Dynamics and Muscle Co-Contraction during Gait. Hum. Mov. Sci. 2019, 64, 409–419. [Google Scholar] [CrossRef]
- Stefanik, J.J.; Frey-Law, L.; Segal, N.A.; Niu, J.; Lewis, C.E.; Nevitt, M.C.; Neogi, T. The Relation of Peripheral and Central Sensitization to Muscle Co-Contraction: The MOST Study. Osteoarthr. Cartil. 2020, 28, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Boyer, K.A.; Hafer, J.F. Gait Mechanics Contribute to Exercise Induced Pain Flares in Knee Osteoarthritis. BMC Musculoskelet. Disord. 2019, 20, 109. [Google Scholar] [CrossRef]
- Lewek, M.D.; Ramsey, D.K.; Snyder-Mackler, L.; Rudolph, K.S. Knee Stabilization in Patients with Medial Compartment Knee Osteoarthritis. Arthritis Rheum. 2005, 52, 2845–2853. [Google Scholar] [CrossRef]
- Schmitt, L.C.; Rudolph, K.S. Influences on Knee Movement Strategies during Walking in Persons with Medial Knee Osteoarthritis. Arthritis Rheum. 2007, 57, 1018–1026. [Google Scholar] [CrossRef]
- Fallah-Yakhdani, H.R.; Abbasi-Bafghi, H.; Meijer, O.G.; Bruijn, S.M.; Van Den Dikkenberg, N.; Benedetti, M.G.; Van Dieën, J.H. Determinants of Co-Contraction during Walking before and after Arthroplasty for Knee Osteoarthritis. Clin. Biomech. 2012, 27, 485–494. [Google Scholar] [CrossRef]
- Leeuw, M.; Goossens, M.E.J.B.; Linton, S.J.; Crombez, G.; Boersma, K.; Vlaeyen, J.W.S. The Fear-Avoidance Model of Musculoskeletal Pain: Current State of Scientific Evidence. J. Behav. Med. 2007, 30, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Vlaeyen, J.W.S.; Kole-Snijders, A.M.J.; Rotteveel, A.M.; Ruesink, R.; Heuts, P.H.T.G. The Role of Fear of Movement/(Re)Injury in Pain Disability. J. Occup. Rehabil. 1995, 5, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.L.; Bishop, S.R.; Pivik, J. The Pain Catastrophizing Scale: Development and Validation. Psychol. Assess. 1995, 7, 524–532. [Google Scholar] [CrossRef]
- Scopaz, K.A.; Piva, S.R.; Wisniewski, S.; Fitzgerald, G.K. Relationships of Fear, Anxiety, and Depression with Physical Function in Patients with Knee Osteoarthritis. Arch. Phys. Med. Rehabil. 2009, 90, 1866–1873. [Google Scholar] [CrossRef]
- Somers, T.J.; Keefe, F.J.; Pells, J.J.; Dixon, K.E.; Waters, S.J.; Riordan, P.A.; Blumenthal, J.A.; McKee, D.C.; LaCaille, L.; Tucker, J.M.; et al. Pain Catastrophizing and Pain-Related Fear in Osteoarthritis Patients: Relationships to Pain and Disability. J. Pain Symptom Manag. 2009, 37, 863–872. [Google Scholar] [CrossRef]
- Stensdotter, A.K.; Vårbakken, K.; Roeleveld, K. Factors Associated with Self-Rated Difficulty to Descend Stairs in Persons with Knee Osteoarthritis. PM&R 2023, 15, 9–19. [Google Scholar] [CrossRef]
- Sánchez-Herán, Á.; Agudo-Carmona, D.; Ferrer-Peña, R.; López-de-Uralde-Villanueva, I.; Gil-Martínez, A.; Paris-Alemany, A.; La Touche, R. Postural Stability in Osteoarthritis of the Knee and Hip: Analysis of Association with Pain Catastrophizing and Fear-Avoidance Beliefs. PM&R 2016, 8, 618–628. [Google Scholar]
- Suzuki, Y.; Iijima, H.; Aoyama, T. Pain Catastrophizing Affects Stair Climbing Ability in Individuals with Knee Osteoarthritis. Clin. Rheumatol. 2020, 39, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Karayannis, N.V.; Smeets, R.J.E.M.; van den Hoorn, W.; Hodges, P.W. Fear of Movement Is Related to Trunk Stiffness in Low Back Pain. PLoS ONE 2013, 8, e67779. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; Tsao, H.; Sims, K. Gain of Postural Responses Increases in Response to Real and Anticipated Pain. Exp. Brain Res. 2015, 233, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.; Bangerter, C.; Schweinhardt, P.; Meier, M.L. Identifying Motor Control Strategies and Their Role in Low Back Pain: A Cross-Disciplinary Approach Bridging Neurosciences with Movement Biomechanics. Front. Pain Res. 2021, 2, 715219. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.L.; Vrana, A.; Schweinhardt, P. Low Back Pain: The Potential Contribution of Supraspinal Motor Control and Proprioception. Neuroscientist 2019, 25, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Meca, J.; Montilla-Herrador, J.; Gacto-Sánchez, M. Gait Speed in Knee Osteoarthritis: A Simple 10-Meter Walk Test Predicts the Distance Covered in the 6-Minute Walk Test. Musculoskelet. Sci. Pract. 2024, 72, 102983. [Google Scholar] [CrossRef] [PubMed]
- Chandran, V.D.; Calalo, J.A.; Dixon, P.C.; Dennerlein, J.T.; Schiffman, J.M.; Pal, S. Knee Muscle Co-Contractions Are Greater in Old Compared to Young Adults during Walking and Stair Use. Gait Posture 2019, 73, 315–322. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- De Luca, C.J.; Gilmore, L.D.; Kuznetsov, M.; Roy, S.H. Filtering the Surface EMG Signal: Movement Artifact and Baseline Noise Contamination. J. Biomech. 2010, 43, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Balbinot, A.; Favieiro, G. A Neuro-Fuzzy System for Characterization of Arm Movements. Sensors 2013, 13, 2613–2630. [Google Scholar] [CrossRef] [PubMed]
- Ghazwan, A.; Forrest, S.M.; Holt, C.A.; Whatling, G.M. Can Activities of Daily Living Contribute to EMG Normalization for Gait Analysis? PLoS ONE 2017, 12, e0174670. [Google Scholar] [CrossRef] [PubMed]
- Germer, C.M.; Farina, D.; Elias, L.A.; Nuccio, S.; Hug, F.; Del Vecchio, A. Surface EMG Cross Talk Quantified at the Motor Unit Population Level for Muscles of the Hand, Thigh, and Calf. J. Appl. Physiol. 2021, 131, 808–820. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.J.; Merletti, R. Surface Myoelectric Signal Cross-Talk among Muscles of the Leg. Electroencephalogr. Clin. Neurophysiol. 1988, 69, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A.; Fuglevand, A.J.; Archer, S.E. Crosstalk in Surface Electromyography: Theoretical and Practical Estimates. J. Electromyogr. Kinesiol. 1994, 4, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, K.; Takahashi, M.; Tokuda, K.; Sawada, T.; Anan, M.; Shinkoda, K. Lower Limb Kinematics during the Swing Phase in Patients with Knee Osteoarthritis Measured Using an Inertial Sensor. Gait Posture 2017, 57, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Woby, S.R.; Roach, N.K.; Urmston, M.; Watson, P.J. Psychometric Properties of the TSK-11: A Shortened Version of the Tampa Scale for Kinesiophobia. Pain 2005, 117, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Sakano, Y. Assessment of Cognitive Aspect of Pain: Development, Reliability, and Validation of Japanese Version of Pain Catastrophizing Scale. Jpn. J. Psychosom. Med. 2007, 47, 95–102. [Google Scholar]
- Ishii, Y.; Ishikawa, M.; Kurumadani, H.; Hayashi, S.; Nakamae, A.; Nakasa, T.; Sumida, Y.; Tsuyuguchi, Y.; Kanemitsu, M.; Deie, M.; et al. Increase in Medial Meniscal Extrusion in the Weight-Bearing Position Observed on Ultrasonography Correlates with Lateral Thrust in Early-Stage Knee Osteoarthritis. J. Orthop. Sci. 2020, 25, 640–646. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Smits, L.; Kotz, D.; Budé, L.; Spigt, M.; Serroyen, J.; Crutzen, R. A Simple Formula for the Calculation of Sample Size in Pilot Studies. J. Clin. Epidemiol. 2015, 68, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Kang, H. Sample Size Determination and Power Analysis Using the G*Power Software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Markström, J.L.; Grinberg, A.; Häger, C.K. Fear of Reinjury Following Anterior Cruciate Ligament Reconstruction Is Manifested in Muscle Activation Patterns of Single-Leg Side-Hop Landings. Phys. Ther. 2022, 102, pzab218. [Google Scholar] [CrossRef] [PubMed]
- Ford, K.R.; Van Den Bogert, J.; Myer, G.D.; Shapiro, R.; Hewett, T.E. The Effects of Age and Skill Level on Knee Musculature Co-Contraction during Functional Activities: A Systematic Review. Br. J. Sports Med. 2008, 42, 561–566. [Google Scholar] [CrossRef]
- Cabral, A.L.C.E.S.; Jorge, J.G.; Dionisio, V.C. Biomechanical Analysis during Single-Leg Squat in Individuals with Knee Osteoarthritis. Knee 2021, 28, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Meireles, S.; Reeves, N.D.; Jones, R.K.; Smith, C.R.; Thelen, D.G.; Jonkers, I. Patients with Medial Knee Osteoarthritis Reduce Medial Knee Contact Forces by Altering Trunk Kinematics, Progression Speed, and Stepping Strategy During Stair Ascent and Descent: A Pilot Study. J. Appl. Biomech. 2019, 35, 280–289. [Google Scholar] [CrossRef]
- Stacoff, A.; Diezi, C.; Luder, G.; Stüssi, E.; Kramers-De Quervain, I.A. Ground Reaction Forces on Stairs: Effects of Stair Inclination and Age. Gait Posture 2005, 21, 24–38. [Google Scholar] [CrossRef]
- Luc-Harkey, B.A.; Franz, J.R.; Losina, E.; Pietrosimone, B. Association between Kinesiophobia and Walking Gait Characteristics in Physically Active Individuals with Anterior Cruciate Ligament Reconstruction. Gait Posture 2018, 64, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi, S.; Jayabalan, P.; Gustafson, J.A.; Klatt, B.A.; Sowa, G.A.; Piva, S.R. The Influence of Continuous versus Interval Walking Exercise on Knee Joint Loading and Pain in Patients with Knee Osteoarthritis. Gait Posture 2017, 56, 129–133. [Google Scholar] [CrossRef]
- Trinler, U.K.; Baty, F.; Mündermann, A.; Fenner, V.; Behrend, H.; Jost, B.; Wegener, R. Stair Dimension Affects Knee Kinematics and Kinetics in Patients with Good Outcome after TKA Similarly as in Healthy Subjects. J. Orthop. Res. 2016, 34, 1753–1761. [Google Scholar] [CrossRef]
- Chang, A.; Hochberg, M.; Song, J.; Dunlop, D.; Chmiel, J.S.; Nevitt, M.; Hayes, K.; Eaton, C.; Bathon, J.; Jackson, R.; et al. Frequency of Varus and Valgus Thrust and Factors Associated with Thrust Presence in Persons with or at Higher Risk of Developing Knee Osteoarthritis. Arthritis Rheum. 2010, 62, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Kuroyanagi, Y.; Nagura, T.; Kiriyama, Y.; Matsumoto, H.; Otani, T.; Toyama, Y.; Suda, Y. A Quantitative Assessment of Varus Thrust in Patients with Medial Knee Osteoarthritis. Knee 2012, 19, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Vervullens, S.; Meert, L.; Baert, I.; Delrue, N.; Heusdens, C.H.W.; Hallemans, A.; Van Criekinge, T.; Smeets, R.J.E.M.; De Meulemeester, K. The Effect of One Dry Needling Session on Pain, Central Pain Processing, Muscle Co-Contraction and Gait Characteristics in Patients with Knee Osteoarthritis: A Randomized Controlled Trial. Scand. J. Pain 2021, 22, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Preece, S.J.; Jones, R.K.; Brown, C.A.; Cacciatore, T.W.; Jones, A.K.P. Reductions in Co-Contraction Following Neuromuscular Re-Education in People with Knee Osteoarthritis. BMC Musculoskelet. Disord. 2016, 17, 372. [Google Scholar] [CrossRef] [PubMed]
- Vlaeyen, J.W.S.; De Jong, J.; Geilen, M.; Heuts, P.H.T.G.; Van Breukelen, G. The Treatment of Fear of Movement/(Re)Injury in Chronic Low Back Pain: Further Evidence on the Effectiveness of Exposure in Vivo. Clin. J. Pain 2002, 18, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Lüning Bergsten, C.; Lundberg, M.; Lindberg, P.; Elfving, B. Change in Kinesiophobia and Its Relation to Activity Limitation after Multidisciplinary Rehabilitation in Patients with Chronic Back Pain. Disabil. Rehabil. 2012, 34, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Siddall, B.; Ram, A.; Jones, M.D.; Booth, J.; Perriman, D.; Summers, S.J. Short-Term Impact of Combining Pain Neuroscience Education with Exercise for Chronic Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Pain 2022, 163, E20–E30. [Google Scholar] [CrossRef]
- De Jong, J.R.; Vlaeyen, J.W.S.; Onghena, P.; Cuypers, C.; Den Hollander, M.; Ruijgrok, J. Reduction of Pain-Related Fear in Complex Regional Pain Syndrome Type I: The Application of Graded Exposure in Vivo. Pain 2005, 116, 264–275. [Google Scholar] [CrossRef]
- Chowdhury, R.H.; Reaz, M.B.I.; Ali, M.A.B.M.; Bakar, A.A.A.; Chellappan, K.; Chang, T.G. Surface Electromyography Signal Processing and Classification Techniques. Sensors 2013, 13, 12431–12466. [Google Scholar] [CrossRef]

| Variable | Mean ± SD | ||
|---|---|---|---|
| Age (year) | 69.4 ± 7.4 | ||
| Sex (female/male) | 15/5 | ||
| Height (cm) | 155.2 ± 9.0 | ||
| Weight (kg) | 65.5 ± 12.2 | ||
| Kellgren–Lawrence grade | II: 1, III: 8, IV: 11 | ||
| CCR (%) | Gait | Medial muscles | 62.5 ± 23.1 |
| Lateral muscles | 75.6 ± 19.8 | ||
| Stair ascent | Medial muscles | 60.6 ± 18.6 | |
| Lateral muscles | 81.5 ± 28.1 | ||
| Stair descent | Medial muscles | 74.8 ± 23.2 | |
| Lateral muscles | 83.5 ± 20.5 | ||
| TSK-11 score | 23.6 ± 5.3 | ||
| PCS score | 20.6 ± 10.4 | ||
| Pain VAS score (mm) | |||
| Gait | 23.0 ± 18.4 | ||
| Stair ascent | 27.1 ± 22.9 | ||
| Stair descent | 42.2 ± 29.9 | ||
| Lateral acceleration (G) | 0.8 ± 0.4 | ||
| BMI | 27.1 ± 4.0 | ||
| FTA (°) | 184.7 ± 3.7 | ||
| Knee extension strength (N·m/kg) | 27.4 ± 15.7 | ||
| Variable | Female | Male | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TSK-11 score | 24.0 | ± | 5.9 | 22.2 | ± | 3.4 | 0.38 | ||
| PCS score | 20.9 | ± | 10.2 | 19.8 | ± | 12.3 | 0.79 | ||
| CCR (%) | Gait | Medial muscles | 62.2 | ± | 24.5 | 63.4 | ± | 20.8 | 0.83 |
| Lateral muscles | 76.8 | ± | 19.8 | 71.7 | ± | 21.3 | 0.69 | ||
| Stair ascent | Medial muscles | 58.6 | ± | 20.3 | 66.7 | ± | 11.9 | 0.41 | |
| Lateral muscles | 84.1 | ± | 24.1 | 73.8 | ± | 40.2 | 0.90 | ||
| Stair descent | Medial muscles | 77.7 | ± | 24.8 | 65.9 | ± | 16.6 | 0.32 | |
| Lateral muscles | 84.3 | ± | 23.3 | 81.0 | ± | 8.5 | 0.63 | ||
| Variable | CCR | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gait | Stair Ascent | Stair Descent | ||||||||||
| Medial Muscles | Lateral Muscles | Medial Muscles | Lateral Muscles | Medial Muscles | Lateral Muscles | |||||||
| Rho | p-Value | Rho | p-Value | Rho | p-Value | Rho | p-Value | Rho | p-Value | Rho | p-Value | |
| TSK-11 score | 0 | 0.99 | −0.01 | 0.96 | −0.07 | 0.76 | 0.25 | 0.27 | 0.50 | 0.03 * | 0.48 | 0.04 * |
| PCS score | 0.1 | 0.67 | 0.09 | 0.69 | 0.27 | 0.24 | 0.22 | 0.33 | 0.25 | 0.28 | 0.08 | 0.73 |
| Variable | CCR | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gait | Stair Ascent | Stair Descent | ||||||||||
| Medial Muscles | Lateral Muscles | Medial Muscles | Lateral Muscles | Medial Muscles | Lateral Muscles | |||||||
| Rho | p-Value | Rho | p-Value | Rho | p-Value | Rho | p-Value | Rho | p-Value | Rho | p-Value | |
| Pain VAS score | −0.08 | 0.75 | 0.23 | 0.33 | 0.02 | 0.94 | −0.06 | 0.8 | 0.05 | 0.83 | −0.18 | 0.46 |
| Lateral acceleration | 0.12 | 0.62 | 0.07 | 0.78 | ― | ― | ― | ― | ― | ― | ― | ― |
| BMI | −0.19 | 0.43 | 0.19 | 0.42 | 0.03 | 0.89 | 0.17 | 0.47 | 0.3 | 0.21 | 0.52 | 0.02 * |
| FTA | 0.26 | 0.28 | 0.27 | 0.25 | 0.48 | 0.03 * | 0.57 | 0.01 ** | 0.48 | 0.03 * | 0.55 | 0.01 * |
| Knee extension strength | −0.19 | 0.41 | −0.26 | 0.26 | 0.34 | 0.14 | 0.01 | 0.96 | −0.19 | 0.42 | −0.26 | 0.26 |
| Variable | CCR | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gait | Stair Ascent | Stair Descent | ||||||||||
| Medial Muscles | Lateral Muscles | Medial Muscles | Lateral Muscles | Medial Muscles | Lateral Muscles | |||||||
| r | p-Value | r | p-Value | r | p-Value | r | p-Value | r | p-Value | r | p-Value | |
| TSK-11 score | 0.11 | 0.68 | −0.02 | 0.95 | 0.2 | 0.44 | 0.47 | 0.05 | 0.54 | 0.02 * | 0.38 | 0.12 |
| PCS score | −0.13 | 0.6 | 0.03 | 0.89 | 0.27 | 0.29 | 0.26 | 0.29 | 0.31 | 0.21 | 0.24 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taniguchi, T.; Tanaka, S.; Nishigami, T.; Imai, R.; Mibu, A.; Yoshimoto, T. Relationship between Fear-Avoidance Beliefs and Muscle Co-Contraction in People with Knee Osteoarthritis. Sensors 2024, 24, 5137. https://doi.org/10.3390/s24165137
Taniguchi T, Tanaka S, Nishigami T, Imai R, Mibu A, Yoshimoto T. Relationship between Fear-Avoidance Beliefs and Muscle Co-Contraction in People with Knee Osteoarthritis. Sensors. 2024; 24(16):5137. https://doi.org/10.3390/s24165137
Chicago/Turabian StyleTaniguchi, Takanori, So Tanaka, Tomohiko Nishigami, Ryota Imai, Akira Mibu, and Takaaki Yoshimoto. 2024. "Relationship between Fear-Avoidance Beliefs and Muscle Co-Contraction in People with Knee Osteoarthritis" Sensors 24, no. 16: 5137. https://doi.org/10.3390/s24165137
APA StyleTaniguchi, T., Tanaka, S., Nishigami, T., Imai, R., Mibu, A., & Yoshimoto, T. (2024). Relationship between Fear-Avoidance Beliefs and Muscle Co-Contraction in People with Knee Osteoarthritis. Sensors, 24(16), 5137. https://doi.org/10.3390/s24165137






