Innovations in Biosensor Technologies for Healthcare Diagnostics and Therapeutic Drug Monitoring: Applications, Recent Progress, and Future Research Challenges
Abstract
:1. Introduction
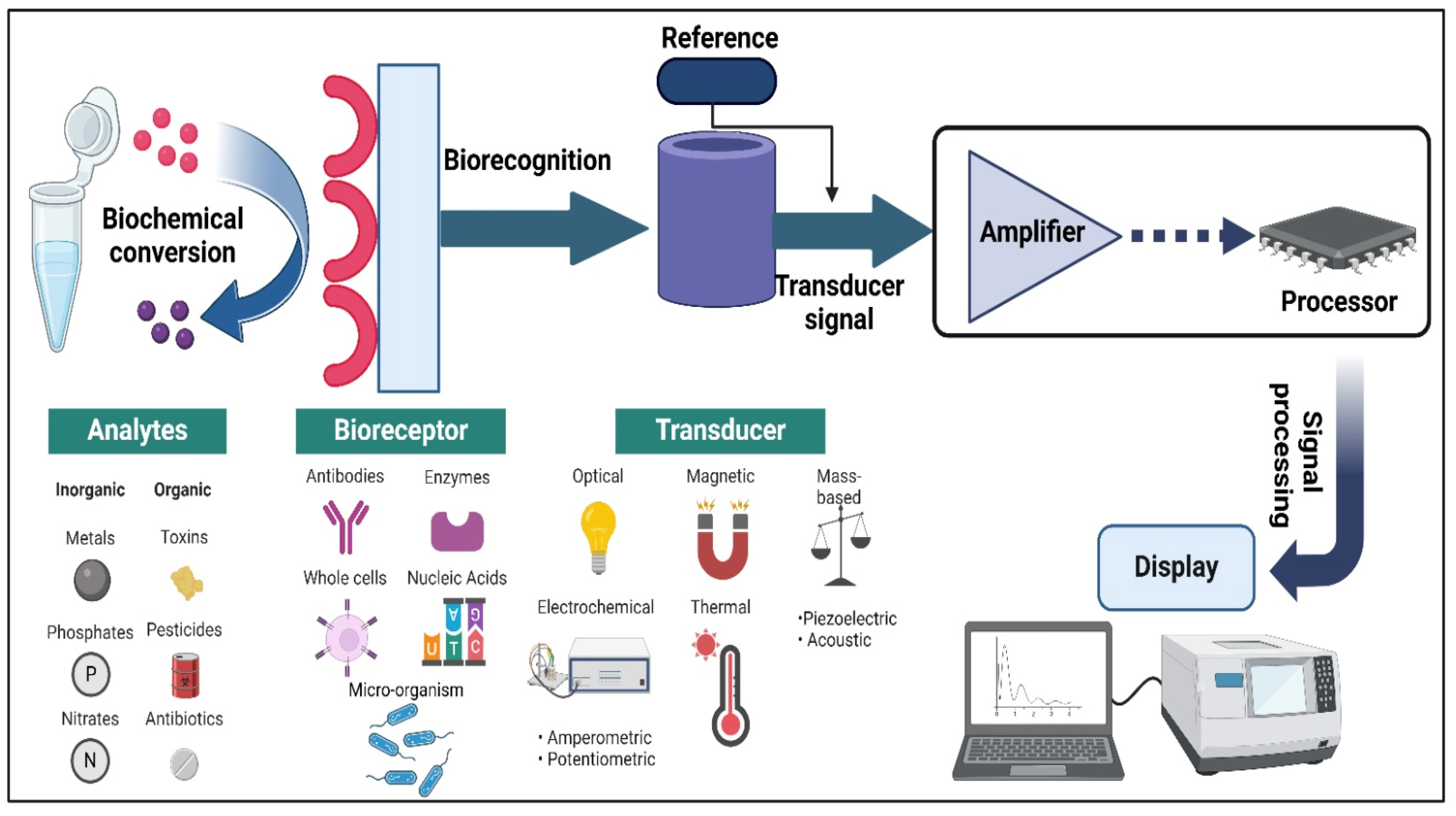
2. Fundamentals of Biosensors: Essential Components, Working Principle and Types
2.1. Essential Components of Biosensors
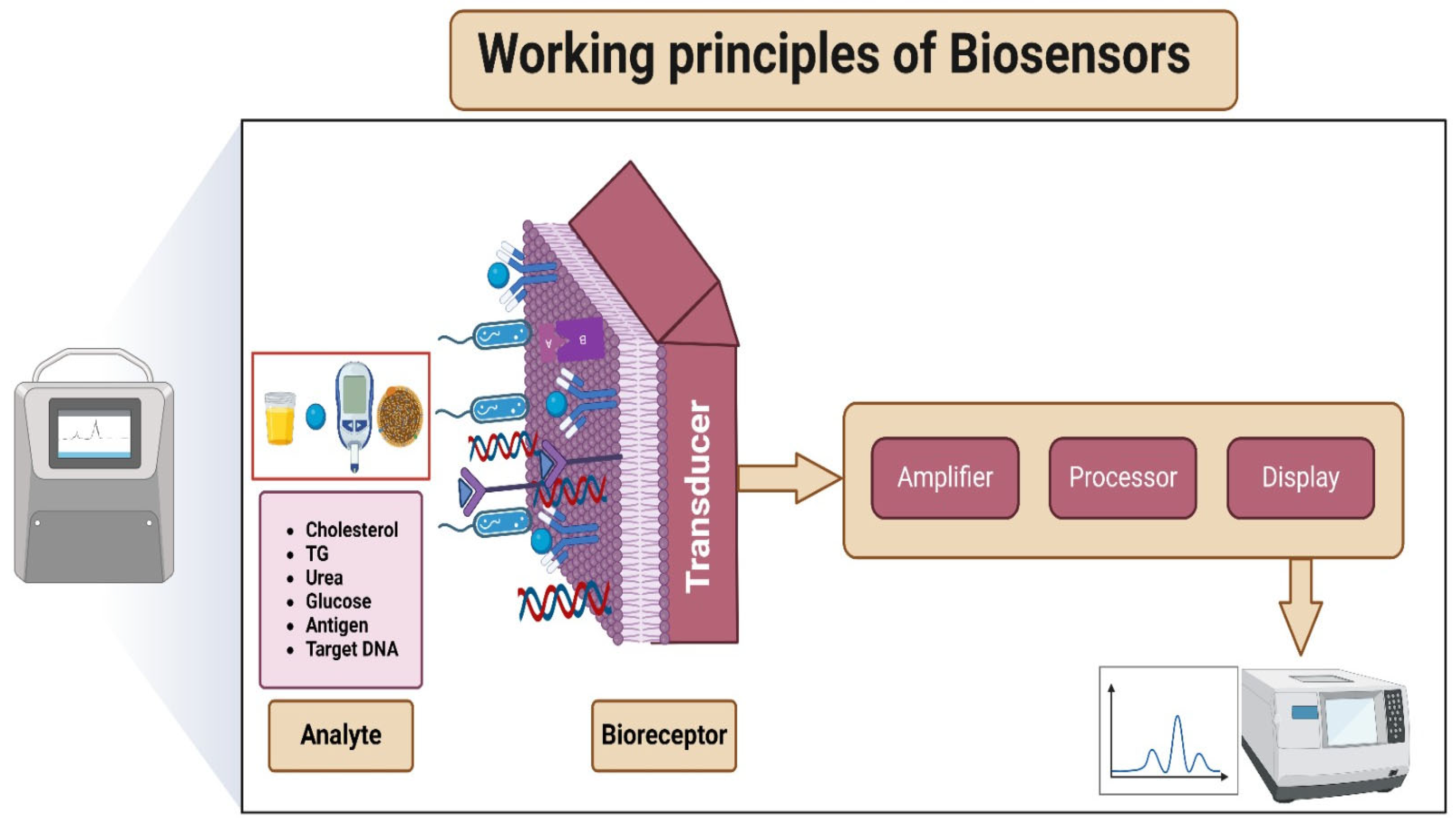
2.2. Working Principles of Biosensors
2.3. Non-Specific Adsorption in Biosensors
2.4. Types of Biosensors
| Biosensor Type | Application | Biosensor Formulation | Ref. |
|---|---|---|---|
| Enzymatic Biosensor | Identification of the endocrine-disrupting chemical Bisphenol A (BPA). | Carbon paste electrode modified with Pd(II)-loaded PAMAM dendrimer and immobilised tyrosinase enzyme via crosslinking using glutaraldehyde. | [84] |
| Glucose monitoring in diabetes patients. | A microneedle composed of polylactic acid (PLA), coated sequentially with gold nanoparticles (AuNPs), glucose oxidase (GOx), overoxidised polypyrrole (OPPy), and Nafion. | [85] | |
| Immunosensor | Quick and precise detection of the antibiotic enrofloxacin (EF) in meat. | Anti-quinolone antibody on carbon electrodes with difloxacin-amino ferrocene electrochemical probe. | [86] |
| Detecting SARS-CoV-2 in saliva using a combined vertical and lateral flow approach. | A container housing immunological reagents attached to magnetic beads alongside a lateral flow device featuring a polyester-based electrode, a magnet, and an absorbent pad. | [87] | |
| DNA biosensor | Detecting Human papillomavirus-16 (HPV-16) | Glass electrodes coated with indium tin oxide (ITO), modified using graphene oxide and gold nanoparticles coated with silver, and immobilised with specific DNA probes for HPV-16 detection. | [88] |
| Colorimetric Aptasensor | Detection of Ochratoxin A (OTA). | DNA circuit driven by entropy (EDC), catalytic hairpin assembly (CHA), and magnesium ion-assisted DNAzyme catalysis (MNAzyme). | [89] |
| Electrochemical Biosensor | HER2 biomarker detection for breast cancer | Cd2+-aptamer@AMNFs@ZIF-67 nanocomposite | [90] |
| Measuring levels of folic acid in expectant mothers | Dihydrofolate reductase (DHFR) anchored on a gold electrode modified with carbon-multiwalled carbon nanotubes (c-MWCNT) and titanium dioxide nanoparticles (TiO2NPs) | [91] | |
| Optical biosensor | Detection of basal cell cancer | Arrays of one-dimensional photonic crystal (PC) structures linked with two metal–insulator–metal (MIM) plasmonic waveguides, utilising tapering techniques for enhanced alignment. | [92] |
| Tracking biomarkers (MUC-1), utilising fluorescence imaging, and delivering targeted curcumin | A nanosystem of mesoporous silica, chitosan, and gold (MCM@CS@Au) targeted by aptamers for MUC-1 positive tumour cells. | [93] | |
| Piezoelectric biosensor | Self-monitoring smart vascular grafts. | A biocompatible piezo composite with a low filler content of 5% volume and high piezoelectric sensitivity (g33~130 mV m N−1) features structured sodium niobate (NaNbO3) fibres embedded in an elastomeric matrix through dielectrophoresis. | [94] |
3. Recent Advancements in Biosensor Technology
3.1. Nanotechnology in Biosensors
| Biosensor Type | Used Nanoparticle | Application | Ref. |
|---|---|---|---|
| Label-free, ultrasensitive electrochemical biosensor | Gold nanoparticles (AuNPs) | Detection of transferrin (Tf), a crucial serum biomarker for atransferrinemia. | [99] |
| Electrochemical biosensor | Iron oxide nanoparticles capped with L-cysteine. | Determination of amino acids using L-Phenylalanine as the representative analyte. | [100] |
| Ultrafast and ultrasensitive DNA biosensor | Nanocubic architecture of MnFe@Pt crystals | Fast and precise determination of SARS-CoV-2 RNA via RNA reverse transcription to DNA (rtDNA). | [101] |
| Multiple-signal amplification PEC biosensor | Carboxylated graphitic carbon nitride (g-C3N4) paired with avidin-functionalised ruthenium-coated silica nanoparticles (Ru@SiO2). | Ultrasensitive detection of kanamycin residues in foods | [102] |
| Electrochemical biosensor | Multi-walled carbon nanotubes are functionalised with acid and tungsten wires coated with gold. | Rapid and easy detection of Escherichia coli (E. coli) | [103] |
| Optical biosensor | Gold nanoparticles (AuNPs) from green tea leaves | Biosensing of CD44 cancer biomarkers with improved sensitivity. | [104] |
| MicroRNA biosensor based on magnetic rod carbon paste electrodes | Carbon nanofibers combined with CuBTC-AIA (Copper-based Metal-Organic Framework) and Fe@rGO (Iron-reduced Graphene Oxide). | Detection of microRNA 155 for the diagnosis of breast cancer. | [105] |
| Nano immunosensor | Gold nanoparticles (AuNPs) | Detection and discrimination of Zika and Dengue viruses | [106] |
| Electrochemical aptasensor | Polypyrrole (PPy)-gold nanoparticles (AuNPs) | Detection of cardiac troponin I (cTnI) without labels for diagnosing acute myocardial infarction (AMI). | [107] |
| Fluorescence biosensor without enzymes or labels, operating on a ratiometric principle | DNA-silver nanoclusters (DNA-AgNCs) | Susceptible detection of tetracycline (TET) for antibiotic detection in food security | [108] |
| Dual-mode nano-biosensor combining ratiometric fluorescence and calorimetry | Carbon dots (BCDs) and Manganese dioxide nanosheets (MnO2 NSs) | Determination of Staphylococcus aureus (S. aureus). | [109] |
| Surface plasmon resonance biosensor | TiO2/Au/graphene | A combined TiO2/Au/graphene layer-based surface plasmon resonance (SPR) sensor showed superior sensitivity (210 to 292.86 deg/RIU) for detecting cervical, skin, adrenal gland, breast, and blood cancer cells. | [110] |
3.2. Integration with Wearable Devices
3.3. Miniaturisation and Portability
4. Biosensors Application in Disease Detection
4.1. Biosensors in Cancer Detection
4.2. Biosensors in Infectious Disease Detection
4.3. Biosensors in Diabetes Management
4.4. Biosensors in Neurological Disorder Detection
4.5. Biosensors in Cardiovascular Disease Detection
4.6. Biosensors in Autoimmune Disorder Detection
4.7. Biosensors in Respiratory Disease Detection
| Target Disease | Biosensor | Transducer Type | Application | LOD | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| Prostate cancer | Maackia amurensis (MAA) lectin immobilised on gold-interdigitated microelectrodes | Electrochemical | Accurate identification of cancer-linked abnormal glycosylation in prostate-specific antigen (PSA). | 3.574 pg/mL | 0.01–100 ng/mL | [141] |
| Gold nanospikes (AuNS) arranged on a quartz crystal microbalance (QCM). | Electrochemical | Detection and assessment of prostate-specific antigen (PSA) for prostate cancer screening | 24 pg/mL | 0.1–100 ng/mL | [142] | |
| Colon and rectal cancer | Electrochemiluminescence immunosensor with Ru@TiO2-MXene as the energy donor and Pd@UiO-66-NH2 as the energy acceptor | ECL-RET (Electrochemiluminescence Resonance Energy Transfer) | Detection of carcinoembryonic antigen (CEA) for diagnosis of colon and rectal cancer | 2.65 fg/mL | 1 × 10−5–80 ng/mL | [143] |
| Colorectal cancer | An electrochemical immunosensor employing nucleic acid aptamer recognition and silver deposition | Electrochemical | Identification of epidermal growth factor receptor (EGFR) for colorectal cancer diagnosis | 0.06 ng/mL | 1 to 1000 ng/mL | [144] |
| COVID-19 and Influenza | Liquid-gated graphene field-effect transistors (GFETs) with quadruple architecture and individual functionalisation | Electrical | Rapid and ultraprecise differentiation and sensing of influenza and SARS-CoV-2 surface proteins | ~50 ag/mL, or 88 zM for COVID-19 and 227 zM for Flu | N/D | [145] |
| Diabetes | Non-invasive tear glucose biosensor based on a photoelectrochemical probe with optical fiber | Photoelectroch-emical | Non-invasive glucose detection. | 4.1 nM | 10 nM to 100 μM | [146] |
| Polyaniline/nickel oxide nanohybrid modified graphite sheet (PANI/NiO/GS) biosensor | Electrochemical | Non-enzymatic electrochemical detection of methylglyoxal in human saliva. | 2.64 nM | 1 to 10 μM | [147] | |
| Phenylboronic acid (PBA)-based glucose biosensor with HCNT/PEDOT:PSS dual conductive structure | Electrochemical | Glucose detection with exceptional sensitivity, stability, and wide linearity range | N/D | 0.20 mM to 2.0 mM | [148] | |
| Alzheimer’s disease | Label-free impedimetric immunosensor using indium tin oxide polyethylene terephthalate (ITO-PET) electrodes modified with anti-Aβ42 antibodies | Electrochemical | Fast, specific, and highly sensitive quantitative assessment of Aβ42 protein for diagnosing Alzheimer’s disease | 0.37 pg/mL | 1–100 pg/mL | [127] |
| A dual “turn-on” fluorescence biosensor using aggregation-induced emission fluorogen (AIEgen)-labelled oligonucleotide (TPET-DNA) probes attached to cationic dextran-modified molybdenum disulfide (TPET-DNA@Dex-MoS2). | Fluorescence | Fast, specific, and highly sensitive quantitative analysis of miR-125b for diagnosing Alzheimer’s disease and monitoring in real-time in PC12 cells and brain tissues of a mouse model of Alzheimer’s disease. | N/D | N/D | [149] | |
| Parkinson’s disease | A biosensor based on a thin-film transistor (TFT) utilising indium gallium zinc oxide (IGZO) thin film technology | Electrochemical | Early detection of Parkinson’s disease using surface-functionalised IGZO TFT biosensor | N/D | 1 pg mL−1 to 100 ng mL−1 | [150] |
| Cardiovascular disease | An electrochemical immunosensor utilising nanostructured molybdenum tetraselenide reduced graphene oxide (nMo3Se4-rGO) for detecting cardiac troponin I (cTnI), offering a broader linear range, increased sensitivity, and lower detection limit. | Electrochemical | Early detection of cardiovascular disease using nMoSe-rGO-based biosensor for cTnI detection | 1 fg mL−1 | 1 fg mL−1–100 ng mL−1 | [151] |
| Rheumatoid arthritis (RA) | Tilted-fiber Bragg grating (TFBG) biosensor bonded with cyclic citrullinated peptide (CCP) antigens and antibodies | Optical | Laboratory-based detection of rheumatoid arthritis (RA) using cyclic citrullinated peptide (CCP) antibodies and antigens | 1 ng/mL | N/D | [152] |
| Magnetic microbeads functionalised with Neutravidin (NA-MBs) and modified with biotinylated anti-double-stranded DNA (dsDNA) for amperometric detection of anti-dsDNA autoantibodies (IgG, IgA, and IgM AAbs). | Amperometric | Detection of anti-dsDNA autoantibodies in the sera of rheumatoid arthritis patients | 0.3 IU mL−1 | 1–200 IU mL−1 | [153] | |
| Chronic obstructive pulmonary disease (COPD) | Point-of-care biosensors for the multiplexed detection of interleukin (IL)-6, IL-8, matrix metalloproteinase (MMP)-8, MMP-9, C-reactive protein (CRP), tumour necrosis factor-alpha (TNF-α), and neutrophil elastase (NE) | Electrochemical | Identification of protein biomarkers in saliva and sputum for the management of chronic obstructive pulmonary disease (COPD). | N/D | N/D | [139] |
5. Biosensors in Therapeutic Drug Monitoring
| Drug Monitored | Biosensor | Transducer | Matrix | LOD | Linearity Range | Ref. |
|---|---|---|---|---|---|---|
| Amoxicillin | Enzyme Biosensor with Microfiber Interferometer (MFI) and Fiber Gratings (FBGs) Power Variation | Optical | Deionised water, real food, urine samples | 0.04 nM | 0.01–100 nM | [179] |
| Tenofovir (TFV) | Alkaline phosphatase (ALP) Enzyme with BaTiO3 Nanoparticles | Electrochemical | Human blood serum | 0.09 nM | N/A | [180] |
| Kanamycin | Ru@MOF/Ag+-Dependent DNAzyme ECL Biosensor | Electrochemiluminescence | Seawater, Milk | 13.7 pM | 30 pM–300 μM | [181] |
| DNA Aptamer-Modified Portable Gold Electrode Biosensor | Electrochemical | Environmental water samples | 0.40 μmol/L | 1–1000 μmol/L | [182] | |
| Thionine functionalised graphene and hierarchical nanoporous (HNP) PtCu Aptasensor | Electrochemical | Animal-derived food | 0.42 pg mL−1 | 5 × 10−7– 5 × 10−2 μg mL−1 | [183] | |
| Streptomycin | Colorimetric Casein hydrolysate peptides-functionalised silver nanoparticles (CHPs@AgNPs) | Colourimetric | Tap water, Dairy whey | ~98 nM (tap water) ~56 nM (dairy whey) | 200–650 nM (tap water); 100–700 nM (dairy whey) | [184] |
| FK506 | Spiky Fe3O4@SiO2@Ag flower magnetic superstructure and hollow Ag@Au superstructure enhanced SERS biosensor | Magnetic/Plasmonic | Blood of transplant patients | 0.33 ng/mL | 0.5–20 ng/mL | [185] |
| Vincristine | DNA (ds-DNA), polypyrrole (PP), peony-like CuO:Tb3+ nanostructure (P-L CuO:Tb3+ NS) | Electrochemical | Pharmaceutical preparations, biological fluids | 0.21 nM | 1.0 nM–400.0 μM | [186] |
| Imatinib (IMA) | DNA/AuPt/p-L-Met Coated SPE Electrochemical DNA Biosensor | Electrochemical | Human serum, pharmaceutical samples | 0.18 nM | 2.33–80 nM | [187] |
| Erlotinib (ERL) | Electrochemical | Human serum, pharmaceutical samples | 0.009 nM | 0.032–1.0 nM | ||
| Doxorubicin (DOX) | Gold nanoparticles (AuNPs) and DNA tetrahedron (TDN) nanoprobe bifunctional glassy carbon electrode | Electrochemical | Human serum, cell lysate | 0.3 nM | 1.0 nM–50 μM | [188] |
| Daunorubicin (DNR) | Upconversion nanoparticles (UCNPs) as energy donor and herring sperm DNA (hsDNA) biosensor. | Luminescence | Biological fluids | 0.60 μg·mL−1 | 1–100 μg·mL−1 | [189] |
6. Concentration Ranges and S/N Ratios for Key Biomarkers
| Target Molecule | Healthy Subject Concentration Range | S/N Ratio | Clinical Relevance | Ref. |
|---|---|---|---|---|
| Cardiac Troponin I (cTnI) | 1 pg/mL–100 ng/mL | 10:1 | Indicative of myocardial infarction | [192] |
| Anti-dsDNA Autoantibodies | 1–200 IU/mL | 10:1 | Marker for rheumatoid arthritis | [193] |
| Glucose | 3.9–5.5 mmol/L | 15:1 | Monitoring diabetes mellitus | [194] |
| Prostate-Specific Antigen (PSA) | 0–4 ng/mL | 15:1 | Screening for prostate cancer | [195] |
| Cholesterol (Total) | <200 mg/dL | 10:1 | Assessing cardiovascular health | [196] |
| C-Reactive Protein (CRP) | 0.8–3.0 mg/L | 10:1 | Indicator of inflammation | [197] |
| Hemoglobin A1c (HbA1c) | 4–5.6% | 15:1 | Long-term glucose level monitoring | [198] |
| Thyroid-Stimulating Hormone (TSH) | 0.4–4.0 mIU/L | 10:1 | Assessing thyroid function | [199] |
7. Research Challenges for Future Applications
7.1. Sensitivity, Specificity, and Reproducibility
7.2. Integration with Electronic Health Records (EHR) and Data Security
7.3. Potential Breakthroughs and Future Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, E.R.; Joe, C.; Mitchell, R.J.; Gu, M.B. Biosensors for Healthcare: Current and Future Perspectives. Trends Biotechnol. 2023, 41, 374–395. [Google Scholar] [CrossRef] [PubMed]
- Sadani, K.; Nag, P.; Thian, X.Y.; Mukherji, S. Enzymatic Optical Biosensors for Healthcare Applications. Biosens. Bioelectron. X 2022, 12, 100278. [Google Scholar] [CrossRef]
- Zeng, X.; Peng, R.; Fan, Z.; Lin, Y. Self-Powered and Wearable Biosensors for Healthcare. Mater. Today Energy 2022, 23, 100900. [Google Scholar] [CrossRef]
- Mohankumar, P.; Ajayan, J.; Yasodharan, R.; Devendran, P.; Sambasivam, R. A Review of Micromachined Sensors for Automotive Applications. Measurement 2019, 140, 305–322. [Google Scholar] [CrossRef]
- Huang, H.; Chen, P.-Y.; Hung, C.-H.; Gharpurey, R.; Akinwande, D. A Zero Power Harmonic Transponder Sensor for Ubiquitous Wireless ΜL Liquid-Volume Monitoring. Sci. Rep. 2016, 6, 18795. [Google Scholar] [CrossRef]
- Ponmozhi, J.; Frias, C.; Marques, T.; Frazão, O. Smart Sensors/Actuators for Biomedical Applications. Measurement 2012, 45, 1675–1688. [Google Scholar] [CrossRef]
- Ng, C.L.; Reaz, M.B.I. Evolution of a Capacitive Electromyography Contactless Biosensor: Design and Modelling Techniques. Measurement 2019, 145, 460–471. [Google Scholar] [CrossRef]
- Karunakaran, R.; Keskin, M. Biosensors: Components, Mechanisms, and Applications. In Analytical Techniques in Biosciences: From Basics to Applications; Egbuna, C., Patrick-Iwuanyanwu, K.C., Shah, M.A., Ifemeje, J.C., Rasul, A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 179–190. ISBN 9780128226544. [Google Scholar]
- Qin, J.; Wang, W.; Gao, L.; Yao, S.Q. Emerging Biosensing and Transducing Techniques for Potential Applications in Point-of-Care Diagnostics. Chem. Sci. 2022, 13, 2857–2876. [Google Scholar] [CrossRef]
- Polat, E.O.; Cetin, M.M.; Tabak, A.F.; Bilget Güven, E.; Uysal, B.Ö.; Arsan, T.; Kabbani, A.; Hamed, H.; Gül, S.B. Transducer Technologies for Biosensors and Their Wearable Applications. Biosensors 2022, 12, 385. [Google Scholar] [CrossRef]
- Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, and Applications. Biosensors 2022, 13, 40. [Google Scholar] [CrossRef]
- UPDIKE, S.J.; HICKS, G.P. The Enzyme Electrode. Nature 1967, 214, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Kröger, S.; Turner, A.P.F.; Mosbach, K.; Haupt, K. Imprinted Polymer-Based Sensor System for Herbicides Using Differential-Pulse Voltammetry on Screen-Printed Electrodes. Anal. Chem. 1999, 71, 3698–3702. [Google Scholar] [CrossRef] [PubMed]
- Modak, N.; Friebe, V.M. Amperometric Biosensors: Harnessing Photosynthetic Reaction Centers for Herbicide Detection. Curr. Opin. Electrochem. 2023, 42, 101414. [Google Scholar] [CrossRef]
- Rachkov, A.; McNiven, S.; El’skaya, A.; Yano, K.; Karube, I. Fluorescence Detection of β-Estradiol Using a Molecularly Imprinted Polymer. Anal. Chim. Acta 2000, 405, 23–29. [Google Scholar] [CrossRef]
- Zahraee, S.S.; Alvandi, N.; Ghamari, M.; Esfandiari, N. An Ultra-Sensitive Nano Biosensor for 17β-Estradiol Detection Using Carbon Dots. Nano-Struct. Nano-Objects 2023, 34, 100951. [Google Scholar] [CrossRef]
- Garg, R.; Parwani, K.; Bist, R. Development of optical biosensors for diagnosis of amyloid-β peptide and glial fibrillary acidic protein. J. Anal. Comput. 2023, 17, 322–333. [Google Scholar] [CrossRef]
- Hong, F.; Xiao, R.; Li, L.; Cai, Z.; Ren, L.; Li, N.; Zhang, F.; Xu, X.; Chen, Y. Win-Win Cooperation: Magnetic Relaxation Switching Biosensor for Chloramphenicol Detection Based on the Pipet Platform and Antigen-Driven Enzyme-Free Dual Signal Amplification Strategy. Sens. Actuators B Chem. 2023, 385, 133686. [Google Scholar] [CrossRef]
- McNiven, S.; Kato, M.; Levi, R.; Yano, K.; Karube, I. Chloramphenicol Sensor Based on an in Situ Imprinted Polymer. Anal. Chim. Acta 1998, 365, 69–74. [Google Scholar] [CrossRef]
- Ngoepe, M.; Choonara, Y.E.; Tyagi, C.; Tomar, L.K.; Du Toit, L.C.; Kumar, P.; Ndesendo, V.M.K.; Pillay, V. Integration of Biosensors and Drug Delivery Technologies for Early Detection and Chronic Management of Illness. Sensors 2013, 13, 7680–7713. [Google Scholar] [CrossRef]
- Metkar, S.K.; Girigoswami, K. Diagnostic Biosensors in Medicine—A Review. Biocatal. Agric. Biotechnol. 2019, 17, 271–283. [Google Scholar] [CrossRef]
- Shoaib, A.; Darraj, A.; Khan, M.E.; Azmi, L.; Alalwan, A.; Alamri, O.; Tabish, M.; Khan, A.U. A Nanotechnology-Based Approach to Biosensor Application in Current Diabetes Management Practices. Nanomaterials 2023, 13, 867. [Google Scholar] [CrossRef]
- Pandey, R.; Lu, Y.; McConnell, E.M.; Osman, E.; Scott, A.; Gu, J.; Hoare, T.; Soleymani, L.; Li, Y. Electrochemical DNAzyme-Based Biosensors for Disease Diagnosis. Biosens. Bioelectron. 2023, 224, 114983. [Google Scholar] [CrossRef] [PubMed]
- Zamiri, G.; Babadi, A.A.; Chaudhary, V.; Numan, A.; Khalid, M.; Walvekar, R.; Khosla, A. Infection Management of Virus-Diagnosing Biosensors Based on Mxenes: An Overview. J. Electrochem. Soc. 2023, 170, 37501. [Google Scholar] [CrossRef]
- Bhatia, D.; Paul, S.; Acharjee, T.; Ramachairy, S.S. Biosensors and Their Widespread Impact on Human Health. Sens. Int. 2024, 5, 100257. [Google Scholar] [CrossRef]
- Sarkar, P. Revolutionizing Drug Transport: Unleashing Futuristic Biosensors with Arduino Programming. Eur. Chem. Bull. 2023, 12, 2126–2139. [Google Scholar]
- Lu, T.; Ji, S.; Jin, W.; Yang, Q.; Luo, Q.; Ren, T.-L. Biocompatible and Long-Term Monitoring Strategies of Wearable, Ingestible and Implantable Biosensors: Reform the Next Generation Healthcare. Sensors 2023, 23, 2991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Current Development on Wearable Biosensors towards Biomedical Applications. Front. Bioeng. Biotechnol. 2023, 11, 1264337. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. CNT and Graphene-Based Transistor Biosensors for Cancer Detection: A Review. Biomolecules 2023, 13, 1024. [Google Scholar] [CrossRef]
- Zhao, V.X.T.; Wong, T.I.; Zheng, X.T.; Tan, Y.N.; Zhou, X. Colorimetric Biosensors for Point-of-Care Virus Detections. Mater. Sci. Energy Technol. 2020, 3, 237–249. [Google Scholar] [CrossRef]
- Parihar, A.; Ranjan, P.; Sanghi, S.K.; Srivastava, A.K.; Khan, R. Point-of-Care Biosensor-Based Diagnosis of COVID-19 Holds Promise to Combat Current and Future Pandemics. ACS Appl. Bio Mater. 2020, 3, 7326–7343. [Google Scholar] [CrossRef]
- Kalasin, S.; Surareungchai, W. Challenges of Emerging Wearable Sensors for Remote Monitoring toward Telemedicine Healthcare. Anal. Chem. 2023, 95, 1773–1784. [Google Scholar] [CrossRef]
- Polshettiwar, S.A.; Deshmukh, C.D.; Wani, M.S.; Baheti, A.M.; Bompilwar, E.; Choudhari, S.; Jambhekar, D.; Tagalpallewar, A. Recent Trends on Biosensors in Healthcare and Pharmaceuticals: An Overview. Int. J. Pharm. Investig. 2021, 11, 131–136. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, Y.; Kianfar, E. Nano Biosensors: Properties, Applications and Electrochemical Techniques. J. Mater. Res. Technol. 2021, 12, 1649–1672. [Google Scholar] [CrossRef]
- Shamsuddin, S.H.; Gibson, T.D.; Tomlinson, D.C.; McPherson, M.J.; Jayne, D.G.; Millner, P.A. Reagentless Affimer-and Antibody-Based Impedimetric Biosensors for CEA-Detection Using a Novel Non-Conducting Polymer. Biosens. Bioelectron. 2021, 178, 113013. [Google Scholar] [CrossRef]
- Kucherenko, I.S.; Soldatkin, O.O.; Kucherenko, D.Y.; Soldatkina, O.V.; Dzyadevych, S.V. Advances in Nanomaterial Application in Enzyme-Based Electrochemical Biosensors: A Review. Nanoscale Adv. 2019, 1, 4560–4577. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, J.; Huang, Y.; Du, Y.; Zhang, Y.; Cui, Y.; Kong, D. Development of the DNA-Based Biosensors for High Performance in Detection of Molecular Biomarkers: More Rapid, Sensitive, and Universal. Biosens. Bioelectron. 2022, 197, 113739. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Renugopalakrishnan, V.; Liepmann, D.; Paulmurugan, R.; Malhotra, B.D. Cell-Based Biosensors: Recent Trends, Challenges and Future Perspectives. Biosens. Bioelectron. 2019, 141, 111435. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent Advances in Electrochemical Biosensors: Applications, Challenges, and Future Scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical Biosensors for Pathogen Detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Zeng, H.; Xie, Y.; Liu, T.; Chu, Z.; Dempsey, E.; Jin, W. Conductive Polymer Nanocomposites: Recent Advances in the Construction of Electrochemical Biosensors. Sens. Diagn. 2024, 3, 165–180. [Google Scholar] [CrossRef]
- Taha, B.A.; Al-Jubouri, Q.; Chahal, S.; Al Mashhadany, Y.; Rustagi, S.; Chaudhary, V.; Arsad, N. State-of-the-Art Telemodule-Enabled Intelligent Optical Nano-Biosensors for Proficient SARS-CoV-2 Monitoring. Microchem. J. 2024, 197, 109774. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Acar, T.; Nur Çimen, K.; Özalp, E.; Ilıca, Ö.; Ahlatcıoğlu Özerol, E. Recent Advances in Biosensors Based on Conducting Polymers for Biomedical Applications. ChemistrySelect 2023, 8, e202300819. [Google Scholar] [CrossRef]
- Huang, S.; Gao, Y.; Hu, Y.; Shen, F.; Jin, Z.; Cho, Y. Recent Development of Piezoelectric Biosensors for Physiological Signal Detection and Machine Learning Assisted Cardiovascular Disease Diagnosis. RSC Adv. 2023, 13, 29174–29194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.X.; Huang, L.Q.; Li, S.F.Y. Microgravimetric DNA sensor based on quartz crystal microbalance: Comparison of oligonucleotide immobilization methods and the application in genetic diagnosis. Biosens. Bioelectron. 2001, 16, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Alawajji, R.A.; Alsudani, Z.A.N.; Biris, A.S.; Kannarpady, G.K. Biosensor Design for the Detection of Circulating Tumor Cells Using the Quartz Crystal Resonator Technique. Biosensors 2023, 13, 433. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Shah, M.R.; Barek, J.; Malik, M.I. Cancer Biomarkers and Their Biosensors: A Comprehensive Review. TrAC Trends Anal. Chem. 2023, 158, 116813. [Google Scholar] [CrossRef]
- Singh, A.K.; Mittal, S.; Das, M.; Saharia, A.; Tiwari, M. Optical Biosensors: A Decade in Review. Alexandria Eng. J. 2023, 67, 673–691. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, H.; Yang, X.; Kang, S.; Cai, L.; Tian, T.; Su, R.; Yang, C.; Zhu, Z. Recent Progress in Microfluidic Biosensors with Different Driving Forces. TrAC Trends Anal. Chem. 2023, 158, 116894. [Google Scholar] [CrossRef]
- Yang, L.; Bai, R.; Xie, B.; Zhuang, N.; Lv, Z.; Chen, M.; Dong, W.; Zhou, J.; Jiang, M. A Biosensor Based on Oriented Immobilization of an Engineered L-Glutamate Oxidase on a Screen-Printed Microchip for Detection of l-Glutamate in Fermentation Processes. Food Chem. 2023, 405, 134792. [Google Scholar] [CrossRef]
- Liu, G.; Lv, Z.; Batool, S.; Li, M.; Zhao, P.; Guo, L.; Wang, Y.; Zhou, Y.; Han, S. Biocompatible Material-Based Flexible Biosensors: From Materials Design to Wearable/Implantable Devices and Integrated Sensing Systems. Small 2023, 19, 2207879. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, M.M.; Manasa, G.; Mascarenhas, R.J.; Mondal, K.; Shetti, N.P. Fundamentals of Bio-Electrochemical Sensing. Chem. Eng. J. Adv. 2023, 16, 100516. [Google Scholar] [CrossRef]
- Gao, Z.F.; Zhu, H.; Li, Y.; Yang, X.; Ren, X.; Wu, D.; Ma, H.; Wei, Q.; Xia, F.; Ju, H. Revolutionizing Biosensing with Superwettability: Designs, Mechanisms, and Applications. Nano Today 2023, 53, 102008. [Google Scholar] [CrossRef]
- Ventura-Aguilar, R.I.; Bautista-Baños, S.; Mendoza-Acevedo, S.; Bosquez-Molina, E. Nanomaterials for Designing Biosensors to Detect Fungi and Bacteria Related to Food Safety of Agricultural Products. Postharvest Biol. Technol. 2023, 195, 112116. [Google Scholar] [CrossRef]
- Wei, L.-N.; Luo, L.; Wang, B.-Z.; Lei, H.-T.; Guan, T.; Shen, Y.-D.; Wang, H.; Xu, Z.-L. Biosensors for Detection of Paralytic Shellfish Toxins: Recognition Elements and Transduction Technologies. Trends Food Sci. Technol. 2023, 133, 205–218. [Google Scholar] [CrossRef]
- Xiang, X.; Song, M.; Xu, X.; Lu, J.; Chen, Y.; Chen, S.; He, Y.; Shang, Y. Microfluidic Biosensor Integrated with Signal Transduction and Enhancement Mechanism for Ultrasensitive Noncompetitive Assay of Multiple Mycotoxins. Anal. Chem. 2023, 95, 7993–8001. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, L.; Cui, Y. Transdermal Amperometric Biosensors for Continuous Glucose Monitoring in Diabetes. Talanta 2023, 253, 124033. [Google Scholar] [CrossRef]
- Johnston, L.; Wang, G.; Hu, K.; Qian, C.; Liu, G. Advances in Biosensors for Continuous Glucose Monitoring towards Wearables. Front. Bioeng. Biotechnol. 2021, 9, 733810. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Probst, D.; Klonoff, D.; Sode, K. Continuous Glucose Monitoring Systems-Current Status and Future Perspectives of the Flagship Technologies in Biosensor Research. Biosens. Bioelectron. 2021, 181, 113054. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, J.Y.; Ling, Y.; Kim, S. Non-Specific Adsorption Reduction Methods in Biosensing. Sensors 2019, 19, 2488. [Google Scholar] [CrossRef]
- Lv, Y.; Zhao, M.; Fan, J.; Wu, R.; Xu, Y.; Li, J.; Li, N.; Shen, H.; Guo, F.; Li, L.S. Self-Assembly Strategy to Reduce Non-Specific Adsorption for the Development of High Sensitivity Quantitative Immunoassay. Anal. Chim. Acta 2022, 1229, 340367. [Google Scholar] [CrossRef] [PubMed]
- Karrat, A.; Amine, A. Innovative Approaches to Suppress Non-Specific Adsorption in Molecularly Imprinted Polymers for Sensing Applications. Biosens. Bioelectron. 2024, 250, 116053. [Google Scholar] [CrossRef] [PubMed]
- Daurai, B.; Ramchiary, S.S.; Gogoi, M. Enzymatic Biosensors for Healthcare Applications. In Enzyme-Based Biosensors: Recent Advances and Applications in Healthcare; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–29. [Google Scholar]
- Bi, R.; Ma, X.; Miao, K.; Ma, P.; Wang, Q. Enzymatic Biosensor Based on Dendritic Gold Nanostructure and Enzyme Precipitation Coating for Glucose Sensing and Detection. Enzyme Microb. Technol. 2023, 162, 110132. [Google Scholar] [CrossRef] [PubMed]
- Khalife, M.; Stankovic, D.; Stankovic, V.; Danicka, J.; Rizzotto, F.; Costache, V.; Schwok, A.S.; Gaudu, P.; Vidic, J. Electrochemical Biosensor Based on NAD(P)H-Dependent Quinone Reductase for Rapid and Efficient Detection of Vitamin K3. Food Chem. 2024, 433, 137316. [Google Scholar] [CrossRef]
- Pittman, T.W.; Zhang, X.; Punyadeera, C.; Henry, C.S. Electrochemical Immunosensor for the Quantification of Galectin-3 in Saliva. Sensors Actuators B Chem. 2024, 400, 134811. [Google Scholar] [CrossRef]
- Yuksel, M.; Luo, W.; McCloy, B.; Mills, J.; Kayaharman, M.; Yeow, J.T.W. A Precise and Rapid Early Pregnancy Test: Development of a Novel and Fully Automated Electrochemical Point-of-Care Biosensor for Human Urine Samples. Talanta 2023, 254, 124156. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, M.; Patel, M.; Mondal, D.P.; Srivastava, A.K.; Dwivedi, N.; Dhand, C. Bio-Inspired Graphene Nanocomposite Enabled Electrochemical Immunosensor for Detection and Quantification of NS1 Protein of Dengue Virus. Electrochim. Acta 2024, 475, 143630. [Google Scholar] [CrossRef]
- Ghasemi, L.; Jahani, S.; Ghazizadeh, M.; Foroughi, M.M. A Novel and Ultrasensitive Electrochemical DNA Biosensor for Pralatrexate Detection. Anal. Methods 2023, 15, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Lomae, A.; Preechakasedkit, P.; Hanpanich, O.; Ozer, T.; Henry, C.S.; Maruyama, A.; Pasomsub, E.; Phuphuakrat, A.; Rengpipat, S.; Vilaivan, T. Label Free Electrochemical DNA Biosensor for COVID-19 Diagnosis. Talanta 2023, 253, 123992. [Google Scholar] [CrossRef]
- Ali, M.R.; Bacchu, M.S.; Das, S.; Akter, S.; Rahman, M.M.; Saad Aly, M.A.; Khan, M.Z.H. Label Free Flexible Electrochemical DNA Biosensor for Selective Detection of Shigella Flexneri in Real Food Samples. Talanta 2023, 253, 123909. [Google Scholar] [CrossRef]
- Guo, X.; Wang, M. Recent Progress in Optical and Electrochemical Aptasensor Technologies for Detection of Aflatoxin B1. Crit. Rev. Food Sci. Nutr. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, Z.; Khalife, M.; Costache, V.; Camacho, M.J.; Cardoso, S.; Martins, V.; Gadjanski, I.; Radovic, M.; Vidic, J. Rapid Detection and Identification of Vancomycin-Sensitive Bacteria Using an Electrochemical Apta-Sensor. ACS Omega 2024, 9, 2841–2849. [Google Scholar] [CrossRef]
- Wu, J.; He, B.; Wang, Y.; Zhao, R.; Zhang, Y.; Bai, C.; Wei, M.; Jin, H.; Ren, W.; Suo, Z.; et al. ZIF-8 Labelled a New Electrochemical Aptasensor Based on PEI-PrGO/AuNWs for DON Detection. Talanta 2024, 267, 125257. [Google Scholar] [CrossRef]
- Liu, C.; Guan, C.; Li, Y.; Li, Z.; Wang, Y.; Han, G. Advances in Electrochemical Biosensors for the Detection of Common Oral Diseases. Crit. Rev. Anal. Chem. 2024, 1–21. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Liu, M.; Ni, Y.; Yue, Y.; He, D.; Liu, R. Magnetically Induced Self-Assembly Electrochemical Biosensor with Ultra-Low Detection Limit and Extended Measuring Range for Sensitive Detection of HER2 Protein. Bioelectrochemistry 2024, 155, 108592. [Google Scholar] [CrossRef]
- Hemdan, M.; Ali, M.A.; Amin, A.S. Eco-Friendly Optical Sensor for Precise Detection of Gold Ions in Diverse Matrices through the Integration of β-2-Hydroxybenzyl-3-Methoxy-2-Hydroxyazastyrene in a PVC Membrane. Anal. Bioanal. Chem. 2024, 416, 3835–3846. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, R.F.; Hemdan, M.; Babalghith, A.O.; Amin, A.S.; Darwish, E.R. An Innovative Approach in Titanium Determination Based on Incorporating 2-Amino-4-((4-Nitrophenyl) Diazenyl) Pyridine-3-Ol in a PVC Membrane. RSC Adv. 2024, 14, 712–724. [Google Scholar] [CrossRef]
- Uniyal, A.; Srivastava, G.; Pal, A.; Taya, S.; Muduli, A. Recent Advances in Optical Biosensors for Sensing Applications: A Review. Plasmonics 2023, 18, 735–750. [Google Scholar] [CrossRef]
- Yildizhan, Y.; Driessens, K.; Tsao, H.S.; Boiy, R.; Thomas, D.; Geukens, N.; Hendrix, A.; Lammertyn, J.; Spasic, D. Detection of Breast Cancer-Specific Extracellular Vesicles with Fiber-Optic SPR Biosensor. Int. J. Mol. Sci. 2023, 24, 3764. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Gao, X.; Zhao, S.; Liu, Y.; Zhang, D.; Zhou, K.; Khanbareh, H.; Chen, W.; Zhang, Y.; Bowen, C. Construction of Bio-piezoelectric Platforms: From Structures and Synthesis to Applications. Adv. Mater. 2021, 33, 2008452. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, H. Rapid and Label-Free Analysis of Antigen–Antibody Dynamic Binding of Tumor Markers Using Piezoelectric Quartz Crystal Biosensor. Biosensors 2023, 13, 917. [Google Scholar] [CrossRef]
- Ergezer, E.E.; Bodur, O.C.; Özkan, E.H.; Sarı, N.; Arslan, F. Development of a Novel Biosensor for the Ultra-Sensitive Detection of the Endocrine Disruptor Bisphenol A and Detection of Bisphenol A Leakage from Some Plastic Containers. J. Iran. Chem. Soc. 2024, 21, 767–779. [Google Scholar] [CrossRef]
- Zhang, B.L.; Yang, Y.; Zhao, Z.Q.; Guo, X.D. A Gold Nanoparticles Deposited Polymer Microneedle Enzymatic Biosensor for Glucose Sensing. Electrochim. Acta 2020, 358, 136917. [Google Scholar] [CrossRef]
- Aymard, C.; Kanso, H.; Serrano, M.J.; Pagán, R.; Noguer, T.; Istamboulie, G. Development of a New Dual Electrochemical Immunosensor for a Rapid and Sensitive Detection of Enrofloxacin in Meat Samples. Food Chem. 2022, 370, 131016. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, L.; Fiore, L.; Fillo, S.; D’Amore, N.; De Santis, R.; Lista, F.; Arduini, F. Smartphone-Assisted Paper-Based Electrochemical Immunosensor for SARS-CoV-2 Detection in Saliva. Bioelectrochemistry 2024, 156, 108619. [Google Scholar] [CrossRef]
- Pareek, S.; Jain, U.; Bharadwaj, M.; Saxena, K.; Roy, S.; Chauhan, N. An Ultrasensitive Electrochemical DNA Biosensor for Monitoring Human Papillomavirus-16 (HPV-16) Using Graphene Oxide/Ag/Au Nano-Biohybrids. Anal. Biochem. 2023, 663, 115015. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, H.; Sun, Y.; Liu, W.; Liu, W.; Yu, J.; Jing, G.; Zhang, J.; Li, W. Colorimetric Aptasensor for the Sensitive Detection of Ochratoxin A Based on a Triple Cascade Amplification Strategy. Anal. Chim. Acta 2023, 1237, 340616. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Xu, Y.; Liu, X.; Ma, Y.; Huang, Z.; Luo, H.; Hou, C.; Huo, D. A Novel Electrochemical Biosensor Based on AMNFs@ ZIF-67 Nano Composite Material for Ultrasensitive Detection of HER2. Bioelectrochemistry 2023, 150, 108362. [Google Scholar] [CrossRef] [PubMed]
- Batra, B.; Yadav, S.; Kalra, V.; Sharma, M.; Rana, J.S. An Electrochemical Biosensor for the Determination of Folic Acid in Pregnant Women Based on DHFR/c-MWCNTs/TiO2NPs Modified Gold Electrode. Sens. Int. 2023, 4, 100235. [Google Scholar] [CrossRef]
- Khani, S.; Hayati, M. Optical Biosensors Using Plasmonic and Photonic Crystal Band-Gap Structures for the Detection of Basal Cell Cancer. Sci. Rep. 2022, 12, 5246. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Khavani, M.; Bigham, A.; Sanati, A.; Bidram, E.; Shariati, L.; Zarrabi, A.; Jolfaie, N.A.; Rafienia, M. Mesoporous Silica@chitosan@gold Nanoparticles as “on/off” Optical Biosensor and PH-Sensitive Theranostic Platform against Cancer. Int. J. Biol. Macromol. 2022, 202, 241–255. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrogio, G.; Zahhaf, O.; Le, M.-Q.; Bordet, M.; Lermusiaux, P.; Della Schiava, N.; Liang, R.; Cottinet, P.-J.; Capsal, J.-F. Piezoelectric Biosensor for Smart Cardiovascular Grafts Based on NaNbO3 Fibers/PDMS Structured Composite. Mater. Des. 2022, 223, 111195. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Fawzy, M. Nanosensors and Nanobiosensors for Monitoring the Environmental Pollutants. Waste Recycl. Technol. Nanomater. Manuf. 2021, 229–246. [Google Scholar] [CrossRef]
- Tang, X.; Zhu, Y.; Guan, W.; Zhou, W.; Wei, P. Advances in Nanosensors for Cardiovascular Disease Detection. Life Sci. 2022, 305, 120733. [Google Scholar] [CrossRef] [PubMed]
- Vu, Q.K.; Tran, Q.H.; Vu, N.P.; Anh, T.-L.; Le Dang, T.T.; Matteo, T.; Nguyen, T.H.H. A Label-Free Electrochemical Biosensor Based on Screen-Printed Electrodes Modified with Gold Nanoparticles for Quick Detection of Bacterial Pathogens. Mater. Today Commun. 2021, 26, 101726. [Google Scholar] [CrossRef]
- Singh, P.; Yadava, R.D.S. Nanosensors for Health Care. In Nanosensors for Smart Cities; Elsevier: Amsterdam, The Netherlands, 2020; pp. 433–450. [Google Scholar]
- Rabbani, G.; Khan, M.E.; Khan, A.U.; Ali, S.K.; Zamzami, M.A.; Ahmad, A.; Bashiri, A.H.; Zakri, W. Label-Free and Ultrasensitive Electrochemical Transferrin Detection Biosensor Based on a Glassy Carbon Electrode and Gold Nanoparticles. Int. J. Biol. Macromol. 2024, 256, 128312. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, T.; Brahma, D.; Gupta, A.N. L-Cysteine Capped Fe3O4 Nanoparticles-Functionalized Conductive Carbon Yarn as a Flexible Electrochemical Biosensor for Detection of L-Phenylalanine. Microchem. J. 2024, 196, 109541. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, Y.; Wang, M.; Liu, T.; Chu, Z.; Jin, W. Facile Construction of Nanocubic Mn3[Fe(CN)6]2@Pt Based Electrochemical DNA Sensors for Ultrafast Precise Determination of SARS-CoV-2. Bioelectrochemistry 2024, 156, 108598. [Google Scholar] [CrossRef] [PubMed]
- Yao, J. A Multiple Signal Amplification Photoelectrochemical Biosensor Based on Biotin-Avidin System for Kanamycin Sensing in Fish and Milk via Synergism of g-C3N4 and Ru@SiO2. Anal. Chim. Acta 2024, 1288, 342141. [Google Scholar] [CrossRef]
- Ertaş, T.; Dinç, B.; Üstünsoy, R.; Eraslan, H.; Ergenç, A.F.; Bektaş, M. Novel Electrochemical Biosensor for Escherichia Coli Using Gold-Coated Tungsten Wires and Antibody Functionalized Short Multiwalled Carbon Nanotubes. Instrum. Sci. Technol. 2024, 52, 109–124. [Google Scholar] [CrossRef]
- Ashikbayeva, Z.; Bekmurzayeva, A.; Myrkhiyeva, Z.; Assylbekova, N.; Atabaev, T.S.; Tosi, D. Green-Synthesized Gold Nanoparticle-Based Optical Fiber Ball Resonator Biosensor for Cancer Biomarker Detection. Opt. Laser Technol. 2023, 161, 109136. [Google Scholar] [CrossRef]
- Sadrabadi, E.A.; Benvidi, A.; Azimzadeh, M.; Asgharnejad, L.; Dezfuli, A.S.; Khashayar, P. Novel Electrochemical Biosensor for Breast Cancer Detection, Based on a Nanocomposite of Carbon Nanofiber, Metal–Organic Framework, and Magnetic Graphene Oxide. Bioelectrochemistry 2024, 155, 108558. [Google Scholar] [CrossRef] [PubMed]
- Cuapa-González, M.A.; Santos-López, G.; Orduña-Díaz, A.; Martínez-Gutiérrez, H.; Rojas-López, M. Detection of Zika Virus by the Development of a Colloidal Gold Nanoparticle-Based Immunosensor. Anal. Lett. 2024, 1–20. [Google Scholar] [CrossRef]
- Eshlaghi, S.N.; Syedmoradi, L.; Amini, A.; Omidfar, K. A Label-Free Electrochemical Aptasensor Based on Screen Printed Carbon Electrodes With Gold Nanoparticles-Polypyrrole Composite for Detection of Cardiac Troponin I. IEEE Sens. J. 2023, 23, 3439–3445. [Google Scholar] [CrossRef]
- Yang, S.; Li, C.; Yu, Y.; Zhan, H.; Zhai, J.; Liu, R.; Chen, W.; Zou, Y.; Xu, K. An Enzyme-Free and Label-Free Ratiometric Fluorescence Signal Amplification Biosensor Based on DNA-Silver Nanoclusters and Catalytic Hairpin Assembly for Tetracycline Detection. Sens. Actuators B Chem. 2024, 404, 135216. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, H.; Liu, L.; Jia, M.; Li, X.; Li, J. Nano-Biosensor Based on Manganese Dioxide Nanosheets and Carbon Dots for Dual-Mode Determination of Staphylococcus Aureus. Food Chem. 2024, 432, 137144. [Google Scholar] [CrossRef] [PubMed]
- Mostufa, S.; Akib, T.B.; Rana, M.M.; Islam, M.R. Highly Sensitive TiO2/Au/Graphene Layer-Based Surface Plasmon Resonance Biosensor for Cancer Detection. Biosensors 2022, 12, 603. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Feng, S.; Huang, L.; Bian, S. Recent Progress in Wearable Biosensors: From Healthcare Monitoring to Sports Analytics. Biosensors 2020, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, Z.; Li, J.; Wei, C.; Liu, S.; Hao, W.; Cheng, H.; Burton, C.; Wang, Y.; Huang, Y. Wearable MXene-Graphene Sensing of Influenza and SARS-CoV-2 Virus in Air and Breath: From Lab to Clinic. Adv. Mater. Technol. 2024, 9, 2201787. [Google Scholar] [CrossRef]
- Li, Y.; Luo, L.; Kong, Y.; George, S.; Li, Y.; Guo, X.; Li, X.; Yeatman, E.; Davenport, A.; Li, Y. A Point-of-Care Sensing Platform for Multiplexed Detection of Chronic Kidney Disease Biomarkers Using Molecularly Imprinted Polymers. Adv. Funct. Mater. 2024, 34, 2316865. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. A Short Review on Miniaturized Biosensors for the Detection of Nucleic Acid Biomarkers. Biosensors 2023, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, K.E.; Assaifan, A.K.; Al-Gawati, M.; Alswieleh, A.M.; Albrithen, H.; Alodhayb, A. Microelectromechanical System-based Biosensor for Label-free Detection of Human Cytomegalovirus. IET Nanobiotechnol. 2023, 17, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Biosensors and Microfluidic Biosensors: From Fabrication to Application. Biosensors 2022, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Kumar, S.; Kaushik, B.K. Recent Advancements in Optical Biosensors for Cancer Detection. Biosens. Bioelectron. 2022, 197, 113805. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.R.; Ahommed, M.S.; Daizy, M.; Bacchu, M.S.; Ali, M.R.; Al-Mamun, M.R.; Aly, M.A.S.; Khan, M.Z.H.; Hossain, S.I. Recent Development in Electrochemical Biosensors for Cancer Biomarkers Detection. Biosens. Bioelectron. X 2021, 8, 100075. [Google Scholar] [CrossRef]
- Wan, H.-H.; Zhu, H.; Chiang, C.-C.; Li, J.-S.; Ren, F.; Tsai, C.-T.; Liao, Y.-T.; Neal, D.; Esquivel-Upshaw, J.F.; Pearton, S.J. High Sensitivity Saliva-Based Biosensor in Detection of Breast Cancer Biomarkers: HER2 and CA15-3. J. Vac. Sci. Technol. B 2024, 42, 023202. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Mathew, M.R.; Sam, S.; Keerthi, K.; Kumar, K.G. Recent Advances and Challenges in Electrochemical Biosensors for Emerging and Re-Emerging Infectious Diseases. J. Electroanal. Chem. 2020, 878, 114596. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-Care Diagnostics for Infectious Diseases: From Methods to Devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef]
- Khan, R.; Deshpande, A.S.; Proteasa, G.; Andreescu, S. Aptamer-Based Electrochemical Biosensor with S Protein Binding Affinity for COVID-19 Detection: Integrating Computational Design with Experimental Validation of S Protein Binding Affinity. Sens. Actuators B Chem. 2024, 399, 134775. [Google Scholar] [CrossRef]
- Pullano, S.A.; Greco, M.; Bianco, M.G.; Foti, D.; Brunetti, A.; Fiorillo, A.S. Glucose Biosensors in Clinical Practice: Principles, Limits and Perspectives of Currently Used Devices. Theranostics 2022, 12, 493. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Y.; Sun, C.; Zhou, Z.; Zhang, J.; Xu, Y.; Xiao, X.; Deng, H.; Zhong, Y.; Li, G.; et al. Fully Integrated Wearable Microneedle Biosensing Platform for Wide-Range and Real-Time Continuous Glucose Monitoring. Acta Biomater. 2024, 175, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Mobed, A.; Razavi, S.; Ahmadalipour, A.; Shakouri, S.K.; Koohkan, G. Biosensors in Parkinson’s Disease. Clin. Chim. Acta 2021, 518, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ameri, M.; Shabaninejad, Z.; Movahedpour, A.; Sahebkar, A.; Mohammadi, S.; Hosseindoost, S.; Ebrahimi, M.S.; Savardashtaki, A.; Karimipour, M.; Mirzaei, H. Biosensors for Detection of Tau Protein as an Alzheimer’s Disease Marker. Int. J. Biol. Macromol. 2020, 162, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Altay, D.N.; Yagar, H.; Ozcan, H.M. A New ITO-Based Aβ42 Biosensor for Early Detection of Alzheimer’s Disease. Bioelectrochemistry 2023, 153, 108501. [Google Scholar] [CrossRef] [PubMed]
- Karaboğa, M.N.S.; Ünal, M.A.; Arı, F.; Sezgintürk, M.K.; Özkan, S.A. An Innovative Method for the Detection of Alpha Synuclein, a Potential Biomarker of Parkinson’s Disease: Quartz Tuning Fork-Based Mass Sensitive Immunosensor Design. Phys. Chem. Chem. Phys. 2024, 26, 5106–5114. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yang, J.; Wang, Y.; Deng, R. Recent Advances in Cardiovascular Disease Biosensors and Monitoring Technologies. ACS Sens. 2023, 8, 956–973. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; Tu, D.; Tong, L.; Sarwar, M.; Bhimaraj, A.; Li, C.; Cote, G.L.; Di Carlo, D. A Review of Biosensor Technologies for Blood Biomarkers toward Monitoring Cardiovascular Diseases at the Point-of-Care. Biosens. Bioelectron. 2021, 171, 112621. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-R.; Kiran Boilla, S.; Wang, C.-H.; Lin, P.-C.; Kuo, C.-N.; Tsai, T.-H.; Lee, G.-B. A Self-Driven, Microfluidic, Integrated-Circuit Biosensing Chip for Detecting Four Cardiovascular Disease Biomarkers. Biosens. Bioelectron. 2024, 249, 115931. [Google Scholar] [CrossRef] [PubMed]
- Hui, H.; Gopinath, S.C.B.; Ismail, Z.H.; Chen, Y.; Pandian, K.; Velusamy, P. Cardiovascular Biomarker Troponin I Biosensor: Aptamer-gold-antibody Hybrid on a Metal Oxide Surface. Biotechnol. Appl. Biochem. 2023, 70, 581–591. [Google Scholar] [CrossRef]
- Imas, J.J.; Ruiz Zamarreno, C.; Zubiate, P.; Sanchez-Martín, L.; Campión, J.; Matías, I.R. Optical Biosensors for the Detection of Rheumatoid Arthritis (RA) Biomarkers: A Comprehensive Review. Sensors 2020, 20, 6289. [Google Scholar] [CrossRef]
- Mobed, A.; Dolati, S.; Shakouri, S.K.; Eftekharsadat, B.; Izadseresht, B. Recent Advances in Biosensors for Detection of Osteoarthritis and Rheumatoid Arthritis Biomarkers. Sens. Actuators A Phys. 2021, 331, 112975. [Google Scholar] [CrossRef]
- Golfinopoulou, R.; Kintzios, S. Biosensing for Autoimmune Chronic Disease—A Review. Chemosensors 2023, 11, 366. [Google Scholar] [CrossRef]
- Chen, H.-M.; Tsai, Y.-H.; Hsu, C.-Y.; Wang, Y.-Y.; Hsieh, C.-E.; Chen, J.-H.; Chang, Y.-S.; Lin, C.-Y. Peptide-Coated Bacteriorhodopsin-Based Photoelectric Biosensor for Detecting Rheumatoid Arthritis. Biosensors 2023, 13, 929. [Google Scholar] [CrossRef] [PubMed]
- Jupe, E.R.; Lushington, G.H.; Purushothaman, M.; Pautasso, F.; Armstrong, G.; Sorathia, A.; Crawley, J.; Nadipelli, V.R.; Rubin, B.; Newhardt, R.; et al. Tracking of Systemic Lupus Erythematosus (SLE) Longitudinally Using Biosensor and Patient-Reported Data: A Report on the Fully Decentralized Mobile Study to Measure and Predict Lupus Disease Activity Using Digital Signals—The OASIS Study. BioTech 2023, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.V.; Cordeiro, T.A.R.; E Freitas, G.R.O.; Ferreira, L.F.; Franco, D.L. Biosensors for the Detection of Respiratory Viruses: A Review. Talanta Open 2020, 2, 100007. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Santos, S.; Yang, Z.; Yang, S.; Kirkhus, N.E. Sputum and Salivary Protein Biomarkers and Point-of-Care Biosensors for the Management of COPD. Analyst 2020, 145, 1583–1604. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Zhao, L.; Huang, Y.; Zhao, C.; Liu, H.; Liu, X.; Cheng, Z.; Yu, F. A Microdroplet SERS-RCA Biosensor with Enhanced Specificity and Reproducibility for Profiling Dual MiRNAs in Idiopathic Pulmonary Fibrosis Diagnosis and Monitoring. Chem. Eng. J. 2024, 482, 149012. [Google Scholar] [CrossRef]
- Rahman, S.F.A.; Arshad, M.K.M.; Gopinath, S.C.B.; Fathil, M.F.M.; Sarry, F.; Ibau, C.; Elmazria, O.; Hage-Ali, S. Interdigitated Impedimetric-Based Maackia Amurensis Lectin Biosensor for Prostate Cancer Biomarker. Microchim. Acta 2024, 191, 118. [Google Scholar] [CrossRef] [PubMed]
- Sonklin, T.; Tongpeng, S.; Munthala, D.; Suksaweang, S.; Janphuang, P.; Bharti, A.; Mathur, A.; Avasthi, D.K.; Jiansirisomboon, S.; Pojprapai, S. Development of Gold Nanospikes-Modified Quartz Crystal Microbalance Biosensor for Prostate Specific Antigen Detection. Surf. Interfaces 2024, 45, 103877. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Y.; Ren, H.; Wen, B.; Liang, W.; Zhang, S.; Cong, B.; Jiang, M.; Hong, C. Electrochemiluminescent Biosensor Based on ECL-RET between Ru@TiO2-MXene and Pd@UiO-66-NH2 for the Detection of Carcinoembryonic Antigens. Sens. Actuators B Chem. 2024, 405, 135381. [Google Scholar] [CrossRef]
- Tang, S.; Xu, Q.; Liu, M.; Zhu, Y.; Zhang, G.; Tang, X. Highly Sensitive Electrochemical Immunosensor Based on SiO2 Nanospheres for Detection of EGFR as Colorectal Cancer Biomarker. Alexandria Eng. J. 2024, 89, 53–59. [Google Scholar] [CrossRef]
- Kumar, N.; Towers, D.; Myers, S.; Galvin, C.; Kireev, D.; Ellington, A.D.; Akinwande, D. Graphene Field Effect Biosensor for Concurrent and Specific Detection of SARS-CoV-2 and Influenza. ACS Nano 2023, 17, 18629–18640. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Yang, X.; Ge, Z.; Ma, H.; Wang, R.; Tian, F.; Teng, P.; Gao, S.; Li, K.; Zhang, B.; et al. Self-Powered Optical Fiber Biosensor Integrated with Enzymes for Non-Invasive Glucose Sensing. Biosens. Bioelectron. 2024, 253, 116191. [Google Scholar] [CrossRef] [PubMed]
- Vasanth, S.; Amir, H.; Ponpandian, N.; Viswanathan, C. Non-Enzymatic Electrochemical Detection of Methylglyoxal in Saliva Using a Polyaniline/Nickel Oxide Nanohybrid Biosensor: A Noninvasive Approach for Diabetes Diagnosis. Biosens. Bioelectron. X 2024, 17, 100444. [Google Scholar] [CrossRef]
- Wang, X.; Huo, H.; Xu, C.; Lin, H.; Wang, Q.; Yang, J.; Vogel, F.; Wang, X.; Lin, Z.; Cao, L.; et al. A Sensitive Non-Enzymatic Dual-Conductive Biosensor for Continuous Glucose Monitoring. Anal. Chim. Acta 2023, 1279, 341845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yin, B.; Huang, Y.; Gu, Y.; Yan, J.; Chen, J.; Li, C.; Zhang, Y.; Wong, S.H.D.; Yang, M. A Dual “Turn-on” Biosensor Based on AIE Effect and FRET for in Situ Detection of MiR-125b Biomarker in Early Alzheimer’s Disease. Biosens. Bioelectron. 2023, 230, 115270. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, T.; Yao, Z.; Ding, Y.; Liu, G.; Shan, F. Highly Sensitive Biosensor Based on IGZO Thin-Film Transistors for Detection of Parkinson’s Disease. Appl. Phys. Lett. 2023, 122, 243701. [Google Scholar] [CrossRef]
- Chauhan, D.; Pooja; Nirbhaya, V.; Srivastava, C.M.; Chandra, R.; Kumar, S. Nanostructured Transition Metal Chalcogenide Embedded on Reduced Graphene Oxide Based Highly Efficient Biosensor for Cardiovascular Disease Detection. Microchem. J. 2020, 155, 104697. [Google Scholar] [CrossRef]
- Wen, H.-Y.; Chiang, C.-C.; Chen, R.-Y.; Ni, W.-Z.; Weng, Y.-Q.; Yeh, Y.-T.; Hsu, H.-C. Immunosensing for Early Detection of Rheumatoid Arthritis Biomarkers: Anti-Cyclic Citrullinated Peptide Antibodies Based on Tilted-Fiber Bragg Grating Biosensor. Bioengineering 2023, 10, 261. [Google Scholar] [CrossRef]
- Arévalo, B.; Serafín, V.; Sánchez-Paniagua, M.; Montero-Calle, A.; Barderas, R.; López-Ruíz, B.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Fast and Sensitive Diagnosis of Autoimmune Disorders through Amperometric Biosensing of Serum Anti-DsDNA Autoantibodies. Biosens. Bioelectron. 2020, 160, 112233. [Google Scholar] [CrossRef]
- Liang, W.S.; Beaulieu-Jones, B. Emerging Therapeutic Drug Monitoring Technologies: Considerations and Opportunities in Precision Medicine. Front. Pharmacol. 2024, 15, 1348112. [Google Scholar] [CrossRef] [PubMed]
- Garzón, V.; Bustos, R.-H.G.; Pinacho, D. Personalized Medicine for Antibiotics: The Role of Nanobiosensors in Therapeutic Drug Monitoring. J. Pers. Med. 2020, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Mohankumar, P.; Ajayan, J.; Mohanraj, T.; Yasodharan, R. Recent Developments in Biosensors for Healthcare and Biomedical Applications: A Review. Measurement 2021, 167, 108293. [Google Scholar] [CrossRef]
- Song, L.; Chen, J.; Xu, B.B.; Huang, Y. Flexible Plasmonic Biosensors for Healthcare Monitoring: Progress and Prospects. ACS Nano 2021, 15, 18822–18847. [Google Scholar] [CrossRef] [PubMed]
- Garzón, V.; Pinacho, D.G.; Bustos, R.H.; Garzón, G.; Bustamante, S. Optical Biosensors for Therapeutic Drug Monitoring. Biosensors 2019, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, S.; Chen, J.; Yang, L.; Qiu, Y.; Du, Q.; Wang, C.; Teng, M.; Wang, T.; Dong, Y. A Novel Strategy for Therapeutic Drug Monitoring: Application of Biosensors to Quantify Antimicrobials in Biological Matrices. J. Antimicrob. Chemother. 2023, 78, 2612–2629. [Google Scholar] [CrossRef] [PubMed]
- Habet, S. Narrow Therapeutic Index Drugs: Clinical Pharmacology Perspective. J. Pharm. Pharmacol. 2021, 73, 1285–1291. [Google Scholar] [CrossRef]
- Maiti, R.; Patel, B.; Patel, N.; Patel, M.; Patel, A.; Dhanesha, N. Antibody Drug Conjugates as Targeted Cancer Therapy: Past Development, Present Challenges and Future Opportunities. Arch. Pharm. Res. 2023, 46, 361–388. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.O.; Baptiste, K.E. Vancomycin-Resistant Enterococci: A Review of Antimicrobial Resistance Mechanisms and Perspectives of Human and Animal Health. Microb. Drug Resist. 2018, 24, 590–606. [Google Scholar] [CrossRef]
- Stogios, P.J.; Savchenko, A. Molecular Mechanisms of Vancomycin Resistance. Protein Sci. 2020, 29, 654–669. [Google Scholar] [CrossRef]
- Martin, J.H.; Norris, R.; Barras, M.; Roberts, J.; Morris, R.; Doogue, M.; Jones, G.R.D. Therapeutic Monitoring of Vancomycin in Adult Patients: A Consensus Review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society Of Infectious Diseases Pharmacists. Clin. Biochem. Rev. 2010, 31, 21–24. [Google Scholar] [PubMed]
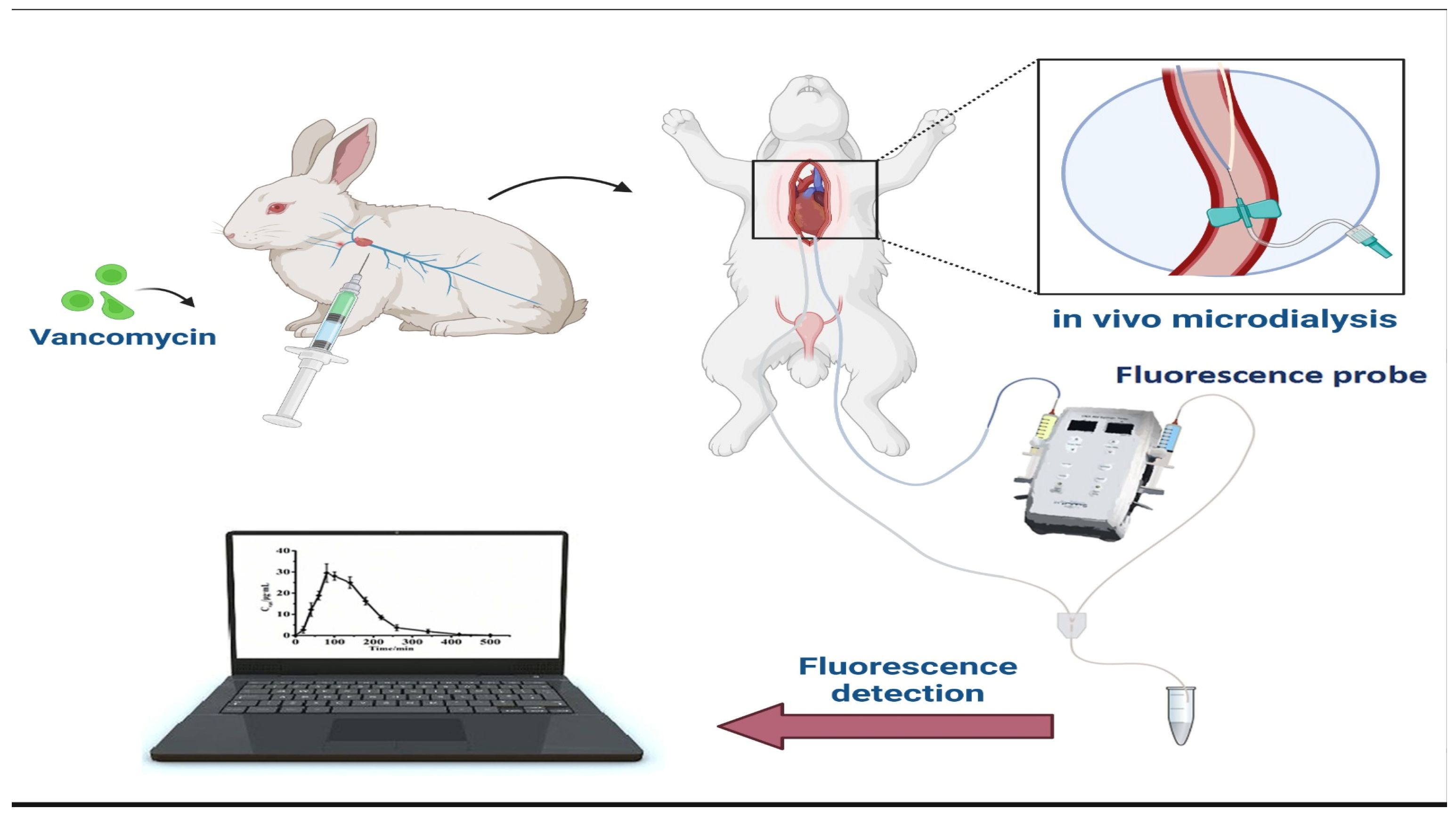
- Mu, F.; Zhou, X.; Fan, F.; Chen, Z.; Shi, G. A Fluorescence Biosensor for Therapeutic Drug Monitoring of Vancomycin Using in Vivo Microdialysis. Anal. Chim. Acta 2021, 1151, 338250. [Google Scholar] [CrossRef]
- Gage, B.F.; Fihn, S.D.; White, R.H. Management and Dosing of Warfarin Therapy. Am. J. Med. 2000, 109, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, I.; Ahmadi, S.; Thompson, M.; Hashemi, P.; Ramezani, Z. Electrochemical Sensor for the Direct Determination of Warfarin in Blood. Chemosensors 2022, 10, 44. [Google Scholar] [CrossRef]
- Nooraee Nia, N.; Hadjmohammadi, M.R. Nanofluid of Magnetic-Activated Charcoal and Hydrophobic Deep Eutectic Solvent: Application in Dispersive Magnetic Solid-Phase Extraction for the Determination and Preconcentration of Warfarin in Biological Samples by High-Performance Liquid Chromatograp. Biomed. Chromatogr. 2021, 35, e5113. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.; Olechowska, P.; Mitoraj, M.; Woźniakiewicz, M.; Kościelniak, P. Determination of Acid Dissociation Constants of Warfarin and Hydroxywarfarins by Capillary Electrophoresis. J. Pharm. Biomed. Anal. 2015, 112, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Özbek, O.; Isildak, Ö.; Isildak, I. A Potentiometric Biosensor for the Determination of Valproic Acid: Human Blood–Based Study of an Anti–Epileptic Drug. Biochem. Eng. J. 2021, 176, 108181. [Google Scholar] [CrossRef]
- Alvau, M.D.; Tartaggia, S.; Meneghello, A.; Casetta, B.; Calia, G.; Serra, P.A.; Polo, F.; Toffoli, G. Enzyme-Based Electrochemical Biosensor for Therapeutic Drug Monitoring of Anticancer Drug Irinotecan. Anal. Chem. 2018, 90, 6012–6019. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Villalona-Calero, M.A. Irinotecan: Mechanisms of Tumor Resistance and Novel Strategies for Modulating Its Activity. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2002, 13, 1841–1851. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Hassani Moghadam, F.; Taher, M.A.; Karimi-Maleh, H. Doxorubicin Anticancer Drug Monitoring by Ds-DNA-Based Electrochemical Biosensor in Clinical Samples. Micromachines 2021, 12, 808. [Google Scholar] [CrossRef] [PubMed]
- Dunvald, A.-C.D.; Iversen, D.B.; Svendsen, A.L.O.; Agergaard, K.; Kuhlmann, I.B.; Mortensen, C.; Andersen, N.E.; Järvinen, E.; Stage, T.B. Tutorial: Statistical Analysis and Reporting of Clinical Pharmacokinetic Studies. Clin. Transl. Sci. 2022, 15, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.-C.; Hong, R.-L.; Tsai, T.; Chen, C.-T. Co-Encapsulation of Chlorin E6 and Chemotherapeutic Drugs in a PEGylated Liposome Enhance the Efficacy of Tumor Treatment: Pharmacokinetics and Therapeutic Efficacy. Pharmaceutics 2019, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-W.; Fu, K.; Correa, S.; Eisenstein, M.; Appel, E.A.; Soh, H.T. Real-Time Monitoring of Drug Pharmacokinetics within Tumor Tissue in Live Animals. Sci. Adv. 2022, 8, eabk2901. [Google Scholar] [CrossRef]
- Jiang, D.; Wei, X.; Zhu, Y.; Qiu, Y.; Liu, X.; Kong, L.; Li, F.; Liu, J.; Zhuang, L.; Wan, H.; et al. Evaluating the Efficacy and Cardiotoxicity of EGFR-TKI AC0010 with a Novel Multifunctional Biosensor. Microsyst. Nanoeng. 2023, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Hou, Z.; Yan, H.; Yang, Y.; Wang, G.; Wu, J.; Ma, J. An All-Fiber System Biosensor for Trace β-Lactam Antibiotics Detection Enhanced by Functionalized Microfiber and Fiber Bragg Grating. J. Colloid Interface Sci. 2024, 658, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Draz, M.U.; Haq, M.Z.U.; Hayat, A.; Ajab, H. An ALP Enzyme-Based Electrochemical Biosensor Coated with Signal-Amplifying BaTiO 3 Nanoparticles for the Detection of an Antiviral Drug in Human Blood Serum. Nanoscale Adv. 2024, 6, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, Q.; Deng, X.; Guo, Q.; Liu, D.; Nie, G. A Novel Electrochemiluminescence Biosensor Based on Ru(Bpy)32+-Functionalized MOF Composites and Cycle Amplification Technology of DNAzyme Walker for Ultrasensitive Detection of Kanamycin. J. Colloid Interface Sci. 2024, 659, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Cai, K.; Liu, Y.; Li, B. Aptamer-Based Antibiotic Electrochemical Detection Platform Using Portable Plastic Gold Electrode and Multichannel Chip. Chin. J. Chem. 2024, 42, 171–176. [Google Scholar] [CrossRef]
- Qin, X.; Yin, Y.; Yu, H.; Guo, W.; Pei, M. A Novel Signal Amplification Strategy of an Electrochemical Aptasensor for Kanamycin, Based on Thionine Functionalized Graphene and Hierarchical Nanoporous PtCu. Biosens. Bioelectron. 2016, 77, 752–758. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Sharma, S.K.; Rasool, K.; Koduru, J.R.; Syed, A.; Ghodake, G. Development of Novel Peptide-Modified Silver Nanoparticle-Based Rapid Biosensors for Detecting Aminoglycoside Antibiotics. J. Agric. Food Chem. 2023, 71, 12883–12898. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ye, J.; Chen, W.; Wang, X.; Li, J.; Su, F.; Ding, C.; Huang, Y. Ultrasensitive Sandwich-Type SERS-Biosensor-Based Dual Plasmonic Superstructure for Detection of Tacrolimus in Patients. ACS Sens. 2022, 7, 3126–3134. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Alsaikhan, F.; Obaid, R.F.; Jahani, S.; Biroudian, S.; Oveisee, M.; Arab, M.R.; Aramesh-Boroujeni, Z.; Foroughi, M.M. Development of the DNA-Based Voltammetric Biosensor for Detection of Vincristine as Anticancer Drug. Front. Chem. 2023, 10, 1060706. [Google Scholar] [CrossRef]
- Eskiköy Bayraktepe, D.; Yıldız, C.; Yazan, Z. The Development of Electrochemical DNA Biosensor Based on Poly-l-Methionine and Bimetallic AuPt Nanoparticles Coating: Picomolar Detection of Imatinib and Erlotinib. Talanta 2023, 257, 124361. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hao, Y.; Cui, M.; Li, N.; Sun, B.; Wang, Y.; Zhao, H.; Zhang, C. An Electrochemical Biosensor Based on DNA Tetrahedron Nanoprobe for Sensitive and Selective Detection of Doxorubicin. Bioelectrochemistry 2024, 157, 108652. [Google Scholar] [CrossRef]
- He, W.; Chen, Z.; Yu, C.; Shen, Y.; Wu, D.; Liu, N.; Zhang, X.; Wu, F.; Chen, J.; Zhang, T. Unlabelled LRET Biosensor Based on Double-Stranded DNA for the Detection of Anthraquinone Anticancer Drugs. Microchim. Acta 2024, 191, 15. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Kurbanoglu, S.; Ozkan, S.A. The Role of Electrochemical Immunosensors in Clinical Analysis. Biosensors 2019, 9, 86. [Google Scholar] [CrossRef]
- Barreiros dos Santos, M.; Rodriguez-Lorenzo, L.; Queirós, R.; Espiña, B. Fundamentals of Biosensors and Detection Methods. In Microfluidics and Biosensors in Cancer Research: Applications in Cancer Modeling and Theranostics; Springer: Berlin/Heidelberg, Germany, 2022; pp. 3–29. [Google Scholar]
- Wang, B.; Wang, C.; Li, Y.; Liu, X.; Wu, D.; Wei, Q. Electrochemiluminescence Biosensor for Cardiac Troponin I with Signal Amplification Based on a MoS2@Cu2O–Ag-Modified Electrode and Ce: ZnO-NGQDs. Analyst 2022, 147, 4768–4776. [Google Scholar] [CrossRef]
- Sohrabian, A.; Parodis, I.; Carlströmer-Berthén, N.; Frodlund, M.; Jönsen, A.; Zickert, A.; Sjöwall, C.; Bengtsson, A.A.; Gunnarsson, I.; Rönnelid, J. Increased Levels of Anti-DsDNA Antibodies in Immune Complexes before Treatment with Belimumab Associate with Clinical Response in Patients with Systemic Lupus Erythematosus. Arthritis Res. Ther. 2019, 21, 259. [Google Scholar] [CrossRef]
- Kai, T.; Hirayama, S.; Soda, S.; Fuwa, F.; Nakagawa, S.; Ueno, T.; Hori, A.; Miida, T. Higher Concentration of 25-Hydroxycholesterol in Treatment-Naïve Patients with Type 2 Diabetes Compared to Healthy Individuals. J. Clin. Lipidol. 2023, 17, 384–391. [Google Scholar] [CrossRef]
- Uruc, S.; Dokur, E.; Gorduk, O.; Sahin, Y. Disposable and Ultrasensitive Label-Free Gold Nanoparticle Patterned Poly (3, 4-Ethylenedioxythiophene-Co-3-Methylthiophene) Electrode for Electrochemical Immunosensing of Prostate-Specific Antigen. New J. Chem. 2024, 48, 10415–10426. [Google Scholar] [CrossRef]
- Bae, H.-J.; Kim, S.-W.; Kim, I.-S. Comparison of Low-Density Lipoprotein Cholesterol Estimation Methods in Individuals with Insulin Resistance: A Cross-Sectional Study. Clin. Chim. Acta 2023, 547, 117393. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhang, L.; Huo, W.; Lian, J.; Jesorka, A.; Shi, X.; Gao, Y. Dynamic Range Expansion of the C-Reactive Protein Quantification with a Tandem Giant Magnetoresistance Biosensor. ACS Omega 2021, 6, 12923–12930. [Google Scholar] [CrossRef] [PubMed]
- Thiruppathi, M.; Lee, J.-F.; Chen, C.C.; Ho, J.A. A Disposable Electrochemical Sensor Designed to Estimate Glycated Hemoglobin (HbA1c) Level in Whole Blood. Sens. Actuators B Chem. 2021, 329, 129119. [Google Scholar] [CrossRef]
- Karami, P.; Gholamin, D.; Johari-Ahar, M. Electrochemical Immunoassay for One-Pot Detection of Thyroxin (T4) and Thyroid-Stimulating Hormone (TSH) Using Magnetic and Janus Nanoparticles. Anal. Bioanal. Chem. 2023, 415, 4741–4751. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Cho, H.-Y.; Shin, M.; Choi, H.K.; Lee, T.; Choi, J.-W. Flexible Electrochemical Biosensors for Healthcare Monitoring. J. Mater. Chem. B 2020, 8, 7303–7318. [Google Scholar] [CrossRef]
- Cernian, A.; Tiganoaia, B.; Sacala, I.; Pavel, A.; Iftemi, A. PatientDataChain: A Blockchain-Based Approach to Integrate Personal Health Records. Sensors 2020, 20, 6538. [Google Scholar] [CrossRef]
- Ebada, A.I.; Abdelrazek, S.; Elhenawy, I. Applying Cloud Based Machine Learning on Biosensors Streaming Data for Health Status Prediction. In Proceedings of the 2020 11th International Conference on Information, Intelligence, Systems and Applications, Piraeus, Greece, 15–17 July 2020; pp. 1–8. [Google Scholar]
- Foroutan, B.; Najafabadi, A.R.A. Capabilities of Bioinformatics Tools for Optimizing Physicochemical Features of Proteins Used in Nano Biosensors: A Short Overview of the Tools Related to Bioinformatics. Biochem. Biophys. Rep. 2021, 27, 101094. [Google Scholar] [CrossRef] [PubMed]
- Preetam, S.; Dash, L.; Sarangi, S.S.; Sahoo, M.M.; Pradhan, A.K. Application of Nanobiosensor in Health Care Sector. Bio-Nano Interface Appl. Food Healthc. Sustain. 2022, 251–270. [Google Scholar] [CrossRef]
- Jin, X.; Liu, C.; Xu, T.; Su, L.; Zhang, X. Artificial Intelligence Biosensors: Challenges and Prospects. Biosens. Bioelectron. 2020, 165, 112412. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemdan, M.; Ali, M.A.; Doghish, A.S.; Mageed, S.S.A.; Elazab, I.M.; Khalil, M.M.; Mabrouk, M.; Das, D.B.; Amin, A.S. Innovations in Biosensor Technologies for Healthcare Diagnostics and Therapeutic Drug Monitoring: Applications, Recent Progress, and Future Research Challenges. Sensors 2024, 24, 5143. https://doi.org/10.3390/s24165143
Hemdan M, Ali MA, Doghish AS, Mageed SSA, Elazab IM, Khalil MM, Mabrouk M, Das DB, Amin AS. Innovations in Biosensor Technologies for Healthcare Diagnostics and Therapeutic Drug Monitoring: Applications, Recent Progress, and Future Research Challenges. Sensors. 2024; 24(16):5143. https://doi.org/10.3390/s24165143
Chicago/Turabian StyleHemdan, Mohamed, Mohamed A. Ali, Ahmed S. Doghish, Sherif S. Abdel Mageed, Ibrahim M. Elazab, Magdy M. Khalil, Mostafa Mabrouk, Diganta B. Das, and Alaa S. Amin. 2024. "Innovations in Biosensor Technologies for Healthcare Diagnostics and Therapeutic Drug Monitoring: Applications, Recent Progress, and Future Research Challenges" Sensors 24, no. 16: 5143. https://doi.org/10.3390/s24165143











