Using Flexible-Printed Piezoelectric Sensor Arrays to Measure Plantar Pressure during Walking for Sarcopenia Screening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Piezoelectric Film
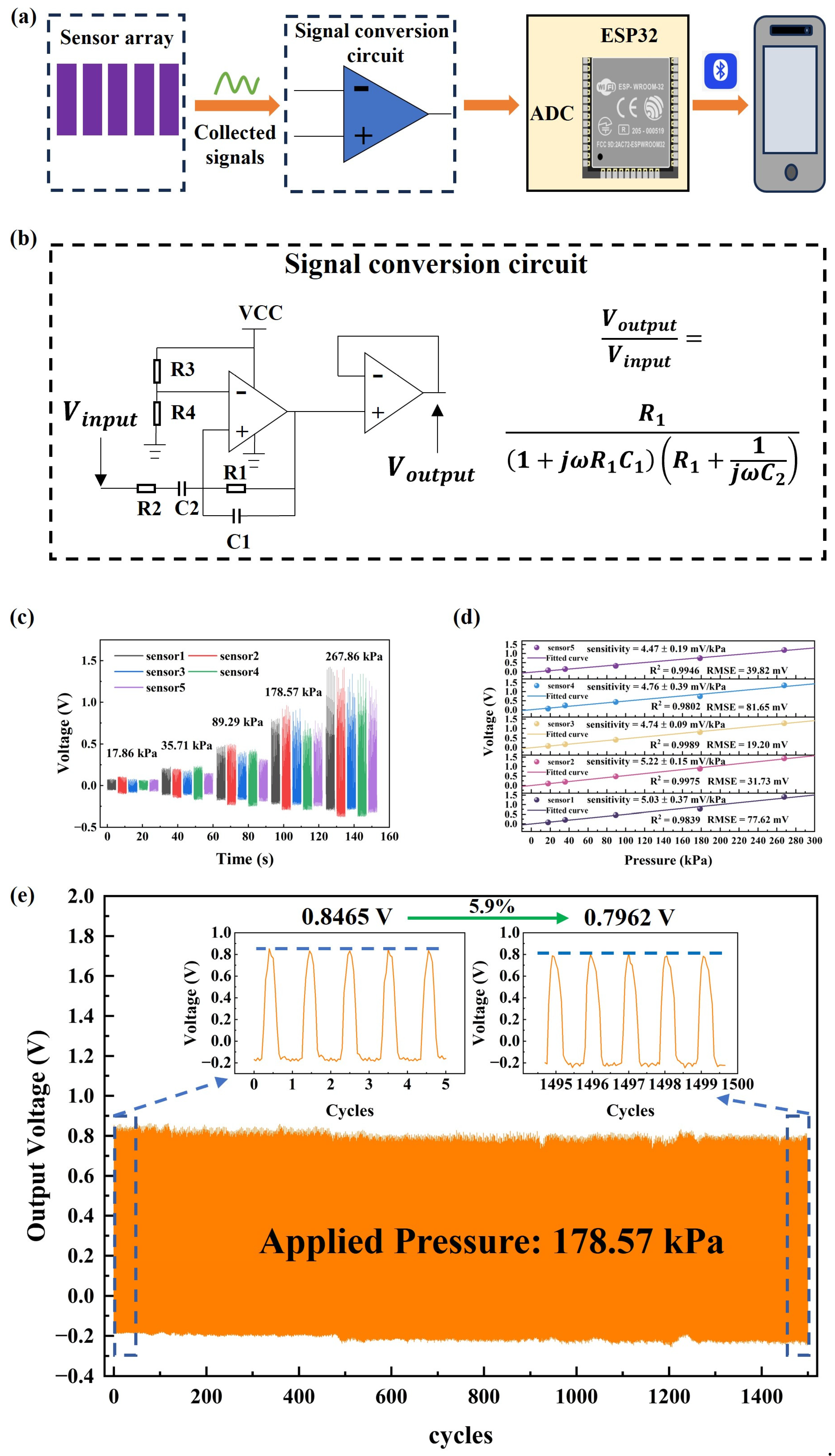
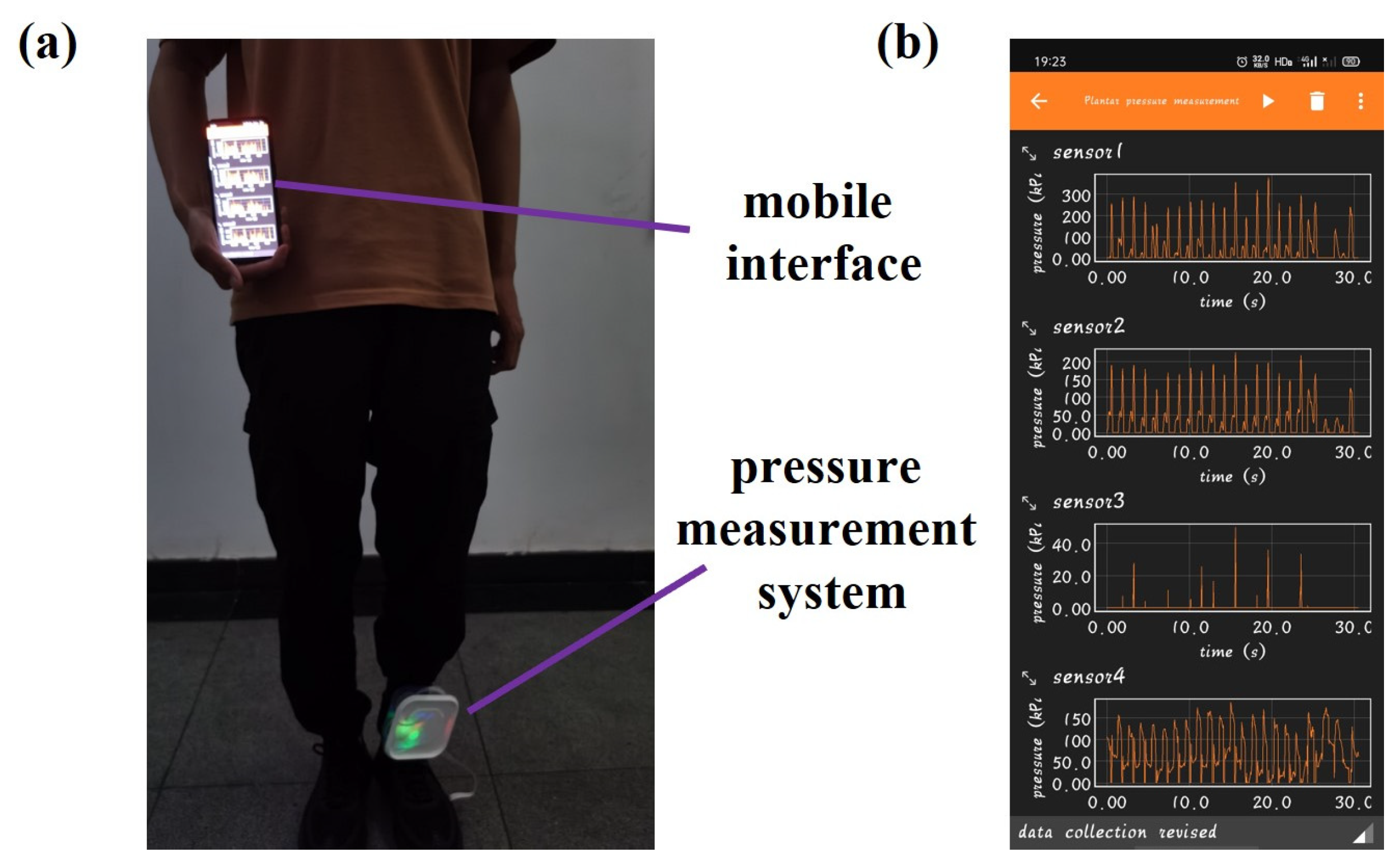
2.2. Fabrication of Plantar Pressure System with Sensor Arrays
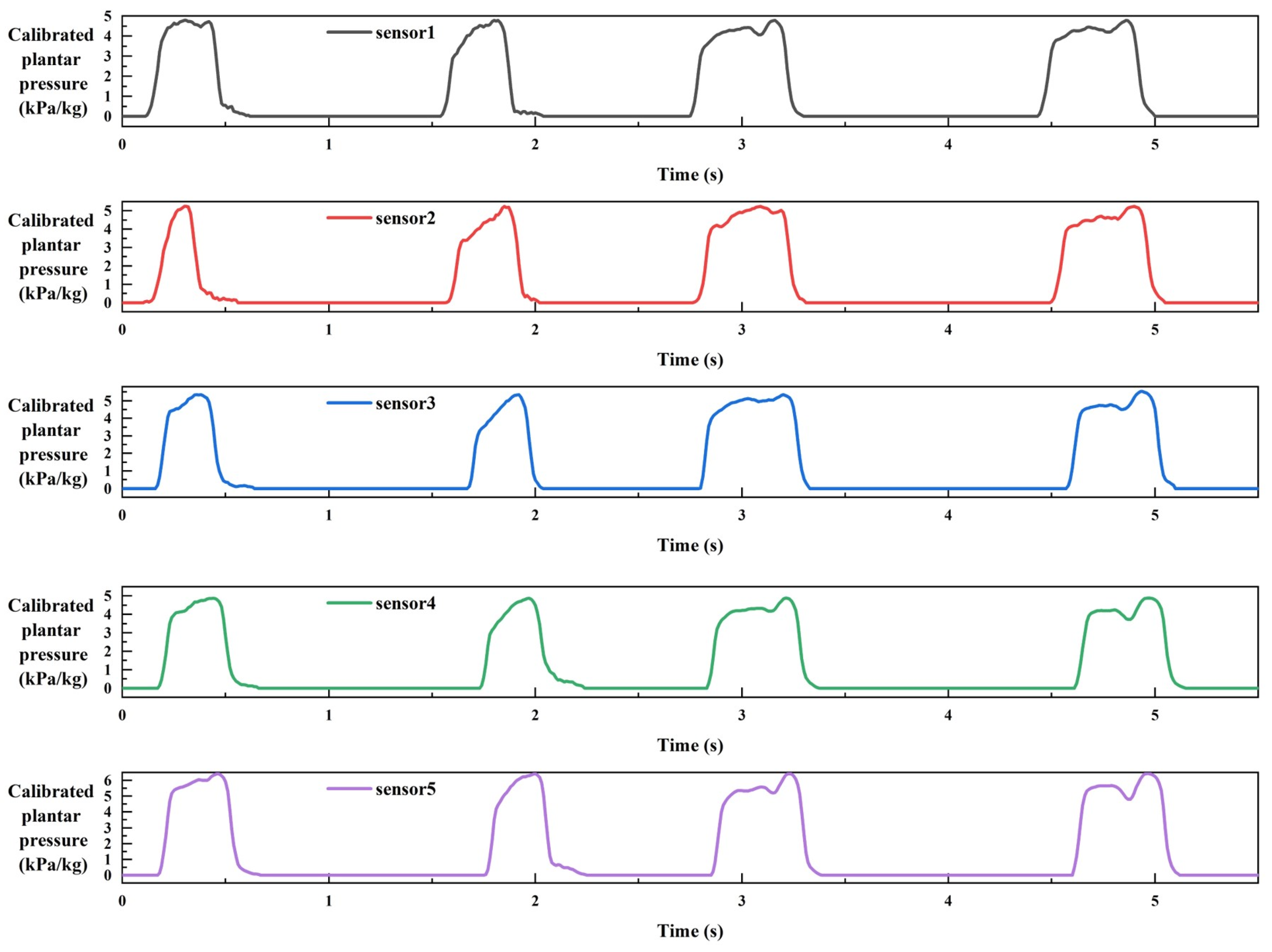
2.3. Data Collection of Plantar Pressure
2.4. Data Processing
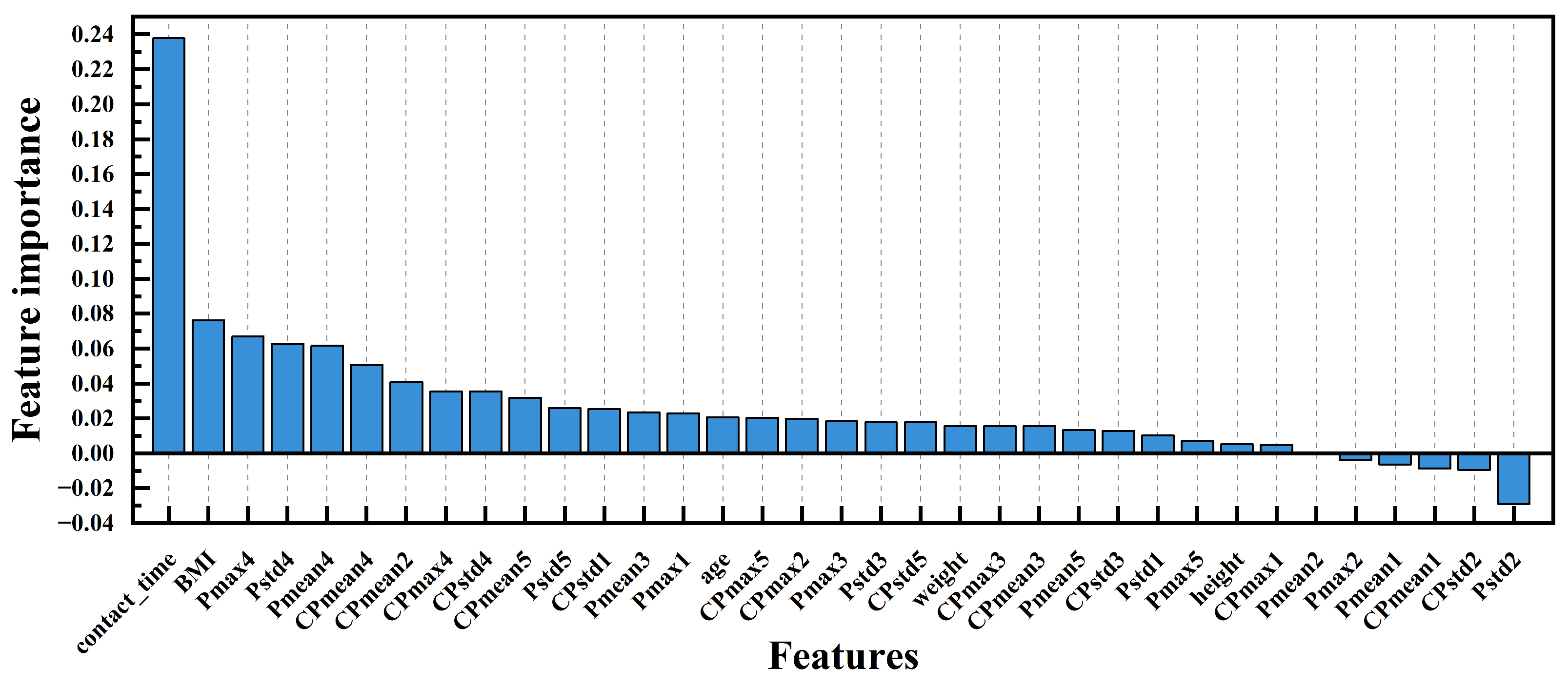
2.5. Feature Extraction and Selection
2.6. Machine Learning Models for Sarcopenia Screening
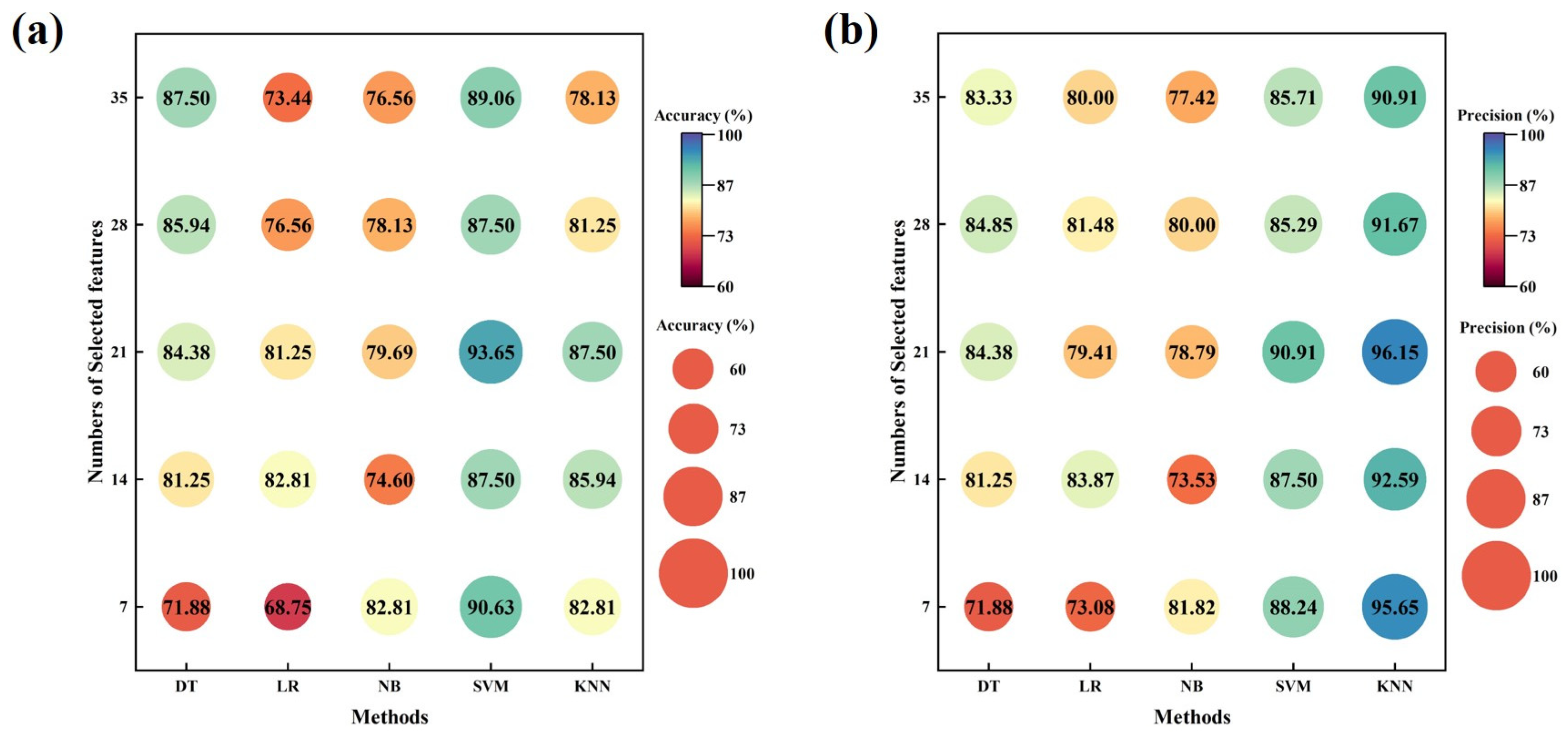
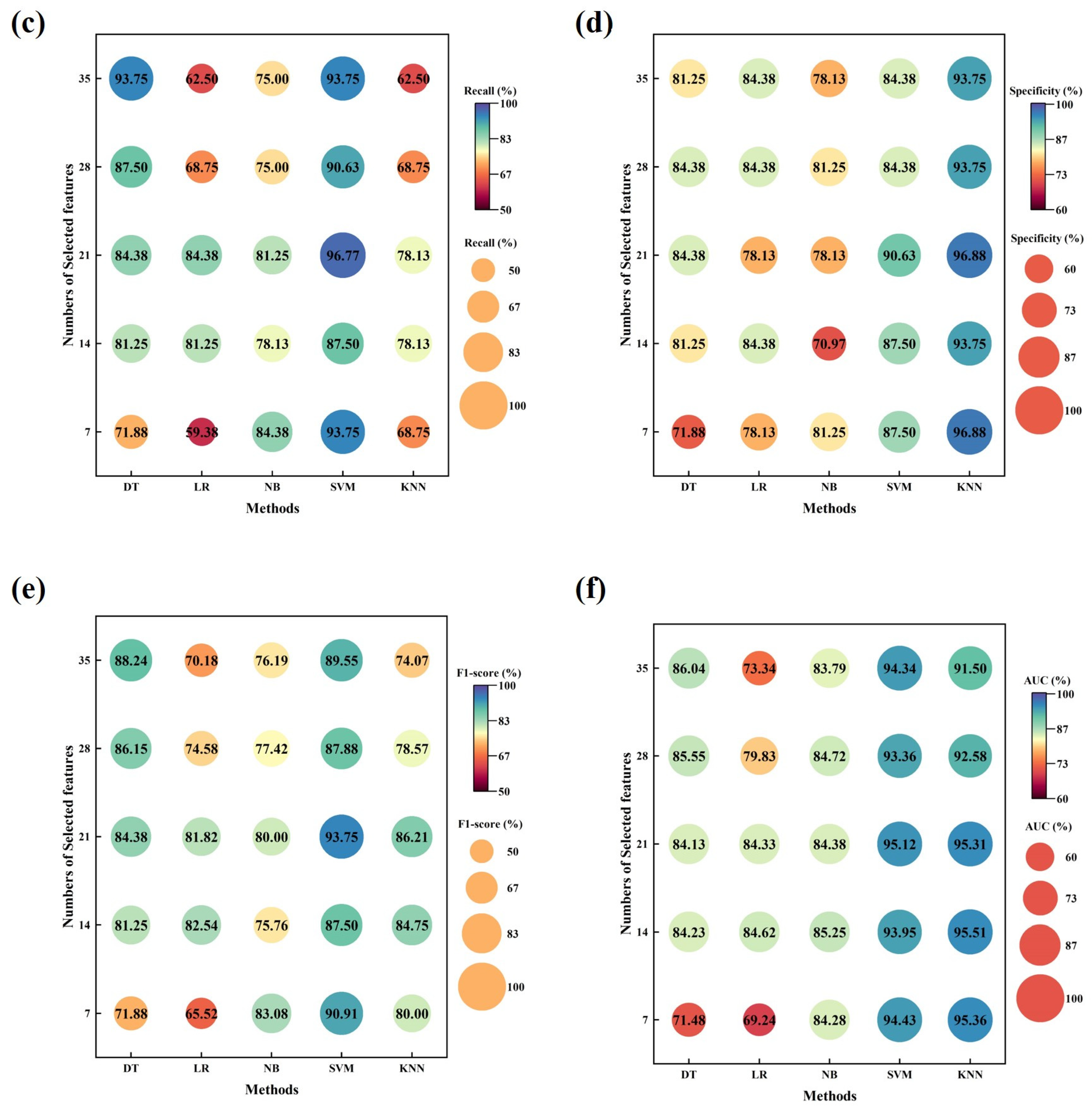
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Angulo, J.; El Assar, M.; Rodriguez-Manas, L. Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol. Asp. Med. 2016, 50, 1–32. [Google Scholar] [CrossRef]
- Supriya, R.; Singh, K.P.; Gao, Y.; Li, F.; Dutheil, F.; Baker, J.S. A Multifactorial Approach for Sarcopenia Assessment: A Literature Review. Biology 2021, 10, 1354. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.E.; Bauer, J.M.; Boirie, Y.; Schneider, S.M.; Sieber, C.C.; Rolland, Y. Toward a definition of sarcopenia. Clin. Geriatr. Med. 2011, 27, 341–353. [Google Scholar] [CrossRef]
- Koliaki, C.; Liatis, S.; Dalamaga, M.; Kokkinos, A. Sarcopenic Obesity: Epidemiologic Evidence, Pathophysiology, and Therapeutic Perspectives. Curr. Obes. Rep. 2019, 8, 458–471. [Google Scholar] [CrossRef]
- Wang, Y.H.; Tee, Y.S.; Wu, Y.T.; Cheng, C.T.; Fu, C.Y.; Liao, C.H.; Hsieh, C.H.; Wang, S.C. Sarcopenia provides extra value outside the PULP score for predicting mortality in older patients with perforated peptic ulcers. BMC Geriatr. 2023, 23, 269. [Google Scholar] [CrossRef] [PubMed]
- Ceniccola, G.D.; Castro, M.G.; Piovacari, S.M.F.; Horie, L.M.; Correa, F.G.; Barrere, A.P.N.; Toledo, D.O. Current technologies in body composition assessment: Advantages and disadvantages. Nutrition 2019, 62, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Gonzalez, M.C.; Lu, J.; Jia, G.; Zheng, J. Skeletal muscle mass and quality: Evolution of modern measurement concepts in the context of sarcopenia. Proc. Nutr. Soc. 2015, 74, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Madden, A.M. Body composition and functional assessment of nutritional status in adults: A narrative review of imaging, impedance, strength and functional techniques. J. Hum. Nutr. Diet. 2016, 29, 714–732. [Google Scholar] [CrossRef]
- Turimov Mustapoevich, D.; Kim, W. Machine Learning Applications in Sarcopenia Detection and Management: A Comprehensive Survey. Healthcare 2023, 11, 2483. [Google Scholar] [CrossRef]
- Kraus, M.; Saller, M.M.; Baumbach, S.F.; Neuerburg, C.; Stumpf, U.C.; Bocker, W.; Keppler, A.M. Prediction of Physical Frailty in Orthogeriatric Patients Using Sensor Insole-Based Gait Analysis and Machine Learning Algorithms: Cross-sectional Study. JMIR Med. Inform. 2022, 10, e32724. [Google Scholar] [CrossRef] [PubMed]
- Anzai, E.; Ren, D.; Cazenille, L.; Aubert-Kato, N.; Tripette, J.; Ohta, Y. Random forest algorithms to classify frailty and falling history in seniors using plantar pressure measurement insoles: A large-scale feasibility study. BMC Geriatr. 2022, 22, 746. [Google Scholar]
- Zulkifli, S.S.; Loh, W.P. A state-of-the-art review of foot pressure. Foot Ankle Surg. 2020, 26, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, D.; Li, Z.; Dey, N.; Crespo, R.G.; Shi, F. Plantar pressure image classification employing residual-network model-based conditional generative adversarial networks: A comparison of normal, planus, and talipes equinovarus feet. Soft Comput. 2021, 27, 1763–1782. [Google Scholar] [CrossRef]
- Dowling, G.J.; Murley, G.S.; Munteanu, S.E.; Smith, M.M.; Neal, B.S.; Griffiths, I.B.; Barton, C.J.; Collins, N.J. Dynamic foot function as a risk factor for lower limb overuse injury: A systematic review. J. Foot Ankle Res. 2014, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Faragó, P.; Grama, L.; Farago, M.-A.; Hintea, S. A Novel Wearable Foot and Ankle Monitoring System for the Assessment of Gait Biomechanics. Appl. Sci. 2020, 11, 268. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, P.; Hou, L.; Bao, H.; Li, J.; Zhao, J. Injury of flexor halluics longus tendon in amateur marathon runners results in abnormal plantar pressure distribution: Observational study. BMC Musculoskelet. Disord. 2024, 25, 77. [Google Scholar] [CrossRef]
- Stewart, S.; Dalbeth, N.; Aiyer, A.; Rome, K. Objectively Assessed Foot and Ankle Characteristics in Patients With Systemic Lupus Erythematosus: A Comparison With Age- and Sex-Matched Controls. Arthritis Care Res. 2020, 72, 122–130. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, Y.; Ma, B.; Wu, H.; Wang, Y.; Wei, B.; Wei, X.; Xu, Y. Three-dimensional gait analysis of orthopaedic common foot and ankle joint diseases. Front. Bioeng. Biotechnol. 2024, 12, 1303035. [Google Scholar] [CrossRef]
- Rosero-Montalvo, P.D.; Fuentes-Hernandez, E.A.; Morocho-Cayamcela, M.E.; Sierra-Martinez, L.M.; Peluffo-Ordonez, D.H. Addressing the Data Acquisition Paradigm in the Early Detection of Pediatric Foot Deformities. Sensors 2021, 21, 4422. [Google Scholar] [CrossRef]
- De Blasiis, P.; Caravaggi, P.; Fullin, A.; Leardini, A.; Lucariello, A.; Perna, A.; Guerra, G.; De Luca, A. Postural stability and plantar pressure parameters in healthy subjects: Variability, correlation analysis and differences under open and closed eye conditions. Front. Bioeng. Biotechnol. 2023, 11, 1198120. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Hong, I.; Kim, T.; Lee, E.; Kim, E.A.; Ryu, J.K.; Jo, Y.; Koo, J.; Han, S.; et al. Foot Plantar Pressure Measurement System Using Highly Sensitive Crack-Based Sensor. Sensors 2019, 19, 5504. [Google Scholar] [CrossRef] [PubMed]
- Samarentsis, A.G.; Makris, G.; Spinthaki, S.; Christodoulakis, G.; Tsiknakis, M.; Pantazis, A.K. A 3D-Printed Capacitive Smart Insole for Plantar Pressure Monitoring. Sensors 2022, 22, 9725. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jones, D.; Chapman, G.J.; Siddle, H.J.; Russell, D.A.; Alazmani, A.; Culmer, P. A Review of Wearable Sensor Systems to Monitor Plantar Loading in the Assessment of Diabetic Foot Ulcers. IEEE Trans. Biomed. Eng. 2020, 67, 1989–2004. [Google Scholar] [CrossRef] [PubMed]
- Nga, D.T.N.; Mattana, G.; Thu, V.T.; Roussel, R.; Piro, B. A simple flexible printed capacitive pressure sensor for chronic wound monitoring. Sens. Actuators A Phys. 2022, 338, 113490. [Google Scholar]
- Maddipatla, D.; Narakathu, B.B.; Atashbar, M. Recent Progress in Manufacturing Techniques of Printed and Flexible Sensors: A Review. Biosensors 2020, 10, 199. [Google Scholar] [CrossRef]
- Chiu, Y.; Chen, Y.-Z.; Hsieh, C.-C.; Hong, H.-C. Design and Characterization of a Flexible Relative Pressure Sensor With Embedded Micro Pressure Channel Fabricated by Flexible Printed Circuit Board Technology. IEEE Sens. J. 2021, 21, 27343–27351. [Google Scholar] [CrossRef]
- Guan, X.; Wang, Z.; Zhao, W.; Huang, H.; Wang, S.; Zhang, Q.; Zhong, D.; Lin, W.; Ding, N.; Peng, Z. Flexible Piezoresistive Sensors with Wide-Range Pressure Measurements Based on a Graded Nest-like Architecture. ACS Appl. Mater. Interfaces 2020, 12, 26137–26144. [Google Scholar] [CrossRef]
- Parameswaran, C.; Gupta, D. Large area flexible pressure/strain sensors and arrays using nanomaterials and printing techniques. Nano Converg. 2019, 6, 28. [Google Scholar] [CrossRef]
- Wang, S.; Shao, H.-Q.; Liu, Y.; Tang, C.-Y.; Zhao, X.; Ke, K.; Bao, R.-Y.; Yang, M.-B.; Yang, W. Boosting piezoelectric response of PVDF-TrFE via MXene for self-powered linear pressure sensor. Compos. Sci. Technol. 2021, 202, 108600. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, Y.; Zheng, H.; Ramakrishna, S. Piezoelectric materials for flexible and wearable electronics: A review. Mater. Des. 2021, 211, 110164. [Google Scholar] [CrossRef]
- Han, S.; Zeng, Q.; Liang, Y.; Xiao, Q.; Chen, Y.; Yan, F.; Xiong, Y.; Yue, J.; Tian, X. Wearable Piezoelectric Sensors Based on BaTiO3 Films for Sarcopenia Recognition. Adv. Mater. Technol. 2024, 9, 2302172. [Google Scholar] [CrossRef]
- Gefen, A. Pressure-sensing devices for assessment of soft tissue loading under bony prominences: Technological concepts and clinical utilization. Wounds Compend. Clin. Res. Pract. 2007, 19, 350–362. [Google Scholar]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, M.; Zhao, S.; Hu, S.; Shi, Z.; Cao, Y. ReliefF-Based EEG Sensor Selection Methods for Emotion Recognition. Sensors 2016, 16, 1558. [Google Scholar] [CrossRef] [PubMed]
- Elreedy, D.; Atiya, A.F. A Comprehensive Analysis of Synthetic Minority Oversampling Technique (SMOTE) for handling class imbalance. Inf. Sci. 2019, 505, 32–64. [Google Scholar] [CrossRef]
- Celik, G. CovidCoughNet: A new method based on convolutional neural networks and deep feature extraction using pitch-shifting data augmentation for COVID-19 detection from cough, breath, and voice signals. Comput. Biol. Med. 2023, 163, 107153. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Ikezoe, T.; Tabara, Y.; Matsuda, F.; Tsuboyama, T.; Ichihashi, N. Differences in lower limb muscle strength and balance ability between sarcopenia stages depend on sex in community-dwelling older adults. Aging Clin. Exp. Res. 2022, 34, 527–534. [Google Scholar] [CrossRef]
- Brown, J.C.; Harhay, M.O.; Harhay, M.N. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J. Cachexia Sarcopenia Muscle 2016, 7, 290–298. [Google Scholar] [CrossRef]
- Burnfield, J.M.; Few, C.D.; Mohamed, O.S.; Perry, J. The influence of walking speed and footwear on plantar pressures in older adults. Clin. Biomech. 2004, 19, 78–84. [Google Scholar] [CrossRef]
- YU, X.; YU, G.-R.; CHEN, Y.-X.; LIU, X.-C. The Characteristics and Clinical Significance of Plantar Pressure Distribution in Patients with Diabetic Toe Deformity: A Dynamic Plantar Pressure Analysis. J. Int. Med. Res. 2011, 39, 2352–2359. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Bae, M.-N.; Lee, K.B.; Hong, S.G. Identification of Patients with Sarcopenia Using Gait Parameters Based on Inertial Sensors. Sensors 2021, 21, 1786. [Google Scholar] [CrossRef] [PubMed]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug. Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, K.; Shen, H.; Zhang, J.; Deng, H.W.; Zhao, L.J. Geographical differences in osteoporosis, obesity, and sarcopenia related traits in white American cohorts. Sci. Rep. 2019, 9, 12311. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Non-Sarcopenia (Numbers = 32) | Sarcopenia (Numbers = 19) |
|---|---|---|
| Age (years, mean ± std) | 69.38 ± 5.48 | 72.63 ± 5.35 |
| Height (cm, mean ± std) | 156.74 ± 7.50 | 152.57 ± 7.97 |
| Weight (kg, mean ± std) | 61.81 ± 8.38 | 54.21 ± 6.36 |
| BMI (kg/m2, mean ± std) | 25.11 ± 2.36 | 23.33 ± 2.59 |
| Features | Non-Sarcopenia | Sarcopenia | p-Value |
|---|---|---|---|
| Age (years) | 69.38 ± 5.48 | 72.63 ± 5.35 | 0.044 * |
| Height (cm) | 156.74 ± 7.50 | 152.57 ± 7.97 | 0.073 |
| Weight (kg) | 60.25 (12.00) | 52.90 (7.50) | <0.001 *** |
| BMI (kg/m2) | 25.11 ± 2.36 | 23.33 ± 2.59 | 0.020 * |
| Pmax1 (kPa) | 210.63 (33.71) | 252.34 (79.04) | 0.016 * |
| Pmax2 (kPa) | 237.16 (28.68) | 260.93 (57.21) | 0.025 * |
| Pmax3 (kPa) | 279.59 (29.96) | 262.13 (13.34) | <0.001 *** |
| Pmax4 (kPa) | 254.14 (21.07) | 246.00 (16.02) | 0.067 |
| Pmax5 (kPa) | 225.77 (46.64) | 243.88 (86.98) | 0.094 |
| Pmean1 (kPa) | 122.64 (27.79) | 142.54 (47.05) | 0.003 ** |
| Pmean2 (kPa) | 145.21 ± 22.39 | 165.32 ± 25.11 | 0.007 ** |
| Pmean3 (kPa) | 166.82 ± 22.75 | 169.38 ± 23.96 | 0.711 |
| Pmean4 (kPa) | 156.86 ± 22.09 | 160.30 ± 21.05 | 0.582 |
| Pmean5 (kPa) | 130.63 (28.75) | 160.30 (21.05) | 0.004 ** |
| Pstd1 (kPa) | 75.80 (15.63) | 90.05 (29.21) | 0.043 * |
| Pstd2 (kPa) | 88.59 ± 9.89 | 94.96 ± 15.82 | 0.082 |
| Pstd3 (kPa) | 101.98 (12.23) | 94.89 (9.18) | <0.001 *** |
| Pstd4 (kPa) | 91.29 (13.66) | 87.98 (11.38) | 0.045 * |
| Pstd5 (kPa) | 84.21 ± 12.20 | 92.11 ± 21.00 | 0.095 |
| CPmax1 (kPa/kg) | 3.59 ± 0.71 | 4.61 ± 0.88 | <0.001 *** |
| CPmax2 (kPa/kg) | 4.04 ± 0.66 | 4.95 ± 0.73 | <0.001 *** |
| CPmax3 (kPa/kg) | 4.73 ± 0.80 | 5.02 ± 0.80 | 0.206 |
| CPmax4 (kPa/kg) | 4.36 ± 0.80 | 4.73 ± 0.71 | 0.089 |
| CPmax5 (kPa/kg) | 3.77 (0.84) | 4.82 (1.66) | <0.001 *** |
| CPmean1 (kPa/kg) | 2.07 ± 0.45 | 2.80 ± 0.59 | <0.001 *** |
| CPmean2 (kPa/kg) | 2.38 ± 0.41 | 3.07 ± 0.46 | <0.001 *** |
| CPmean3 (kPa/kg) | 2.73 ± 0.45 | 3.16 ± 0.59 | 0.009 ** |
| CPmean4 (kPa/kg) | 2.57 ± 0.50 | 3.00 ± 0.50 | 0.006 ** |
| CPmean5 (kPa/kg) | 2.14 (0.61) | 2.96 (1.32) | <0.001 *** |
| CPstd1 (kPa/kg) | 1.32 ± 0.27 | 1.68 ± 0.34 | <0.001 *** |
| CPstd2 (kPa/kg) | 1.46 ± 0.27 | 1.77 ± 0.32 | 0.001 ** |
| CPstd3 (kPa/kg) | 1.73 ± 0.29 | 1.77 ± 0.30 | 0.665 |
| CPstd4 (kPa/kg) | 1.57 ± 0.32 | 1.68 ± 0.29 | 0.260 |
| CPstd5 (kPa/kg) | 1.39 ± 0.27 | 1.71 ± 0.41 | 0.001 ** |
| contact time (s) | 0.35 (0.02) | 0.5 (0.10) | <0.001 *** |
| Comparison | Preparation Methods | Sensitivity | Response Time | Cost | Portability | Complexity | Wireless Transmission | Real-Time Monitoring | |
|---|---|---|---|---|---|---|---|---|---|
| Ref./Co Dev | |||||||||
| [16] | / 1 | n.a. 2 | n.a. | n.a. | √ 3 | low | √ | ||
| [20] | / | n.a. | n.a. | n.a. | √ | medium | √ | √ | |
| [21] | / | n.a. | n.a. | high | √ | medium | √ | ||
| [22] | thermal evaporating | n.a. | n.a. | n.a. | √ | low | √ | ||
| [23] | 3D-printing | 1.19 MPa−1 | 142 ms | low | √ | low | √ | √ | |
| [30] | electrospinning | 2.51 mV/(μm ·N) | n.a. | n.a. | n.a. | n.a. | n.a. | ||
| [42] | / | n.a. | / | n.a. | √ | low | √ | ||
| DXA | / | / | / | high | high | ||||
| BIA | / | / | / | high | high | ||||
| CT | / | / | / | high | high | ||||
| This work | electrospinning and flexible printing | 4.844 mV/kPa | 35 ms | low | √ | low | √ | √ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Xiao, Q.; Liang, Y.; Chen, Y.; Yan, F.; Chen, H.; Yue, J.; Tian, X.; Xiong, Y. Using Flexible-Printed Piezoelectric Sensor Arrays to Measure Plantar Pressure during Walking for Sarcopenia Screening. Sensors 2024, 24, 5189. https://doi.org/10.3390/s24165189
Han S, Xiao Q, Liang Y, Chen Y, Yan F, Chen H, Yue J, Tian X, Xiong Y. Using Flexible-Printed Piezoelectric Sensor Arrays to Measure Plantar Pressure during Walking for Sarcopenia Screening. Sensors. 2024; 24(16):5189. https://doi.org/10.3390/s24165189
Chicago/Turabian StyleHan, Shulang, Qing Xiao, Ying Liang, Yu Chen, Fei Yan, Hui Chen, Jirong Yue, Xiaobao Tian, and Yan Xiong. 2024. "Using Flexible-Printed Piezoelectric Sensor Arrays to Measure Plantar Pressure during Walking for Sarcopenia Screening" Sensors 24, no. 16: 5189. https://doi.org/10.3390/s24165189






